Abstract
Colonic chloride secretion is regulated via the neurohormonal and immune systems. Exogenous chemicals (e.g., butyrate, propionate) can affect chloride secretion. Capsaicin, the pungent ingredient of the chili peppers, exerts various effects on gastrointestinal function. Capsaicin is known to activate the transient receptor potential vanilloid type 1 (TRPV1), expressed in the mesenteric nervous system. Recent studies have also demonstrated its presence in epithelial cells but its role remains uncertain. Because capsaicin has been reported to inhibit colonic chloride secretion, we tested whether this effect of capsaicin could occur by direct action on epithelial cells. In mouse colon and model T84 human colonic epithelial cells, we found that capsaicin inhibited forskolin-dependent short-circuit current (FSK-Isc). Using PCR and Western blot, we demonstrated the presence of TRPV1 in colonic epithelial cells. In T84 cells, TRPV1 localized at the basolateral membrane and in vesicular compartments. In permeabilized monolayers, capsaicin activated apical chloride conductance, had no effect on basolateral potassium conductance, but induced NKCC1 internalization demonstrated by immunocytochemistry and basolateral surface biotinylation. AMG-9810, a potent inhibitor of TRPV1, did not prevent the inhibition of the FSK-Isc by capsaicin. Neither resiniferatoxin nor N-oleoyldopamine, two selective agonists of TRPV1, blocked the FSK-Isc. Conversely capsaicin, resiniferatoxin, and N-oleoyldopamine raised intracellular calcium ([Ca2+]i) in T84 cells and AMG-9810 blocked the rise in [Ca2+]i induced by capsaicin and resiniferatoxin suggesting the presence of a functional TRPV1 channel. We conclude that capsaicin inhibits chloride secretion in part by causing NKCC1 internalization, but by a mechanism that appears to be independent of TRPV1.
Keywords: T84 cells, AMG-9810, capsazepine, resiniferatoxin, N-oleoyldopamine
chloride secretion by epithelia lining the gastrointestinal tract, lung, and other exocrine organs is the fundamental physiological process that accounts for mucosal surface hydration and driving force for secretory diarrhea. In epithelial cells, the sodium gradient generated by the Na/K-ATPase drives the uptake of sodium, chloride and potassium by the basolateral Na-K-Cl-cotransporter 1 (NKCC1). Chloride is accumulated intracellularly against its electrochemical gradient, while sodium and potassium are recycled by the Na-K-ATPase and basolateral potassium channels respectively. Chloride exits apically in an electrogenic fashion through the regulated activity of various chloride channels such as the cystic fibrosis transmembrane regulator (CFTR) or calcium-activated chloride channels (4).
In the colon, a wide array of molecules (neurotransmitters, hormones, cytokines) elicits chloride secretion by mechanisms that converge on cAMP/cGMP and intracellular calcium signaling (4, 46). Because excessive activation of chloride secretion underlies diarrheal disorders, substances that inhibit this process are of interest for targeted pharmacotherapy. Inhibition of chloride secretion can be accomplished at different levels such as blockade of apical chloride channels (43), blockade of basolateral potassium channels (38, 39) and internalization of NKCC1 (11, 58). Various natural compounds produced by luminal bacteria such as short-chain fatty acids and ammonium ions can inhibit chloride secretion (47, 66). Compounds found in food such as capsaicin (the pungent ingredient of the red chili pepper) have been reported to have various effects on intestinal function. Capsaicin has been shown to 1) block fluid secretion induced by E. coli STa enterotoxin (42), 2) modulate intestinal motility (37), and 3) evoke an increase of the short-circuit current in the guinea pig ileum (63).
Capsaicin was recognized to increase capsaicin-sensitive conductances in primary sensory neurons responsible for the transmission of painful stimuli (27) well before the cloning of its target receptor, the transient receptor potential vanilloid type 1, TRPV1 (9). Currently, capsaicin is one of the most commonly used agonists to study TRPV1 (2, 6, 41, 49). In the gastrointestinal tract TRPV1 function is actively studied for its involvement in gastrointestinal motility (37, 63), visceral pain, and in inflammatory disorders (28, 36). Most studies related to TRPV1 focus on its neuronal function, but recent studies have shown the presence of TRPV1 in various epithelia including gastrointestinal epithelial cells, where its role remains elusive (2, 6, 7, 24, 30, 51). In combination with the findings that capsaicin/TRPV1 affects chloride homeostasis (2, 3, 26, 29), we hypothesized that capsaicin/TRPV1 may affect chloride secretion in the colon by a direct effect on epithelial cells responsible for chloride secretion independent of its effects on enteric neurons.
In the present study we find that capsaicin blocks forskolin-stimulated chloride secretion in both mouse colon and model human T84 colonic epithelial cells. Using biochemistry and immunocytochemistry approaches we demonstrated that T84 cells express TRPV1. Furthermore, in T84 cells, capsaicin was found to induce NKCC1 internalization; however, the pharmacological profile of chloride secretory inhibition argues for a mechanism independent of TRPV1.
MATERIALS AND METHODS
Reagents
Hybridoma cells producing the mouse anti-NKCC1 monoclonal antibody T4 developed by Lytle (34) were purchased from the Developmental Studies Hybridoma Bank operated under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA. The production and purification of the T4 antibody was conducted by the Frank W. Fitch monoclonal facility at the University of Chicago. Rabbit anti-TRPV1 (cat. no. ACC-30, stock 0.8 mg/ml) was purchased from Alomone Labs (Jerusalem, Israel), and goat anti-TRPV1 (cat. nos. SC-12498 and SC-12503, stocks 0.2 μg/μl) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The monoclonal anti-TRPV1, clone N221/17 (cat. no. 75-254, stock 1 mg/ml) was obtained from the UC Davis/NIH NeuroMab facility. Mouse anti-Na/K-ATPase α-1 subunit (cat. no. 05-369, stock 0.91 mg/ml) was from Millipore (Billerica, MA) and the mouse anti-ZO1 (cat. no. 33-9100, stock 0.5 mg/ml) from Invitrogen (Life Technologie, Carlsbad, CA). Donkey anti-mouse horseradish peroxidase (cat. no. 715-035-150, stock 0.8 mg/ml), goat anti-rabbit horseradish peroxidase (cat. no. 111-035-003, stock 0.8 mg/ml), bovine anti-goat horseradish peroxidase (cat. no. 805-035-180, stock 0.8 mg/ml), goat anti-rabbit DyLight 488 (cat. no. 111-485-008, stock 1.5 mg/ml) and donkey anti-mouse DyLight 594 (cat. no. 715-515-150, stock 1.5 mg/ml) were obtained from Jackson ImmunoResearch Laboratory (West Grove, PA).
Gel electrophoresis and immunoblotting reagents were from Bio-Rad (Hercules, CA); ECL detection reagents were purchased from GE Healthcare (Piscataway, NJ). Protease inhibitor, complete Mini, was from Roche (Indianapolis, IN). EZ-Link sulfo N-hydroxysulfosuccinimidobiotin (sulfo-NHS-SS-Biotin), ImmunoPure-immobilized streptavidin-agarose beads, and ECL Western blotting substrate were purchased from Pierce.
Forskolin, capsaicin, capsazepine, indomethacin, phenylmethanesulfonyl fluoride (PMSF), dimethyl sulfoxide, nystatin, and ouabain were purchased from Sigma-Aldrich (St. Louis, MO). AMG-9810, resiniferatoxin (RTX), and N-oleoyldopamine (OLDA) were obtained from Tocris Bioscience (Ellisville, MO). Fura-2 AM was purchased from Molecular Probes (Life Technologies). All other chemicals were of the highest grade available and were purchased from Fisher Scientific (Pittsburgh, PA).
Cell Culture
T84 cells obtained from Dr. James McRoberts (University of California, Los Angeles, CA) were cultured in DMEM/F12 media 50/50 (Invitrogen) supplemented with 10% fetal bovine serum, maintained at 37°C in a 5% CO2 humidified incubator and the media was changed 3 times/wk. For epithelial electrophysiology, T84 cells were seeded on Millicell (Millipore) permeable supports (0.33 cm2, 0.4 μm pores size), coated with rat-tail collagen prepared according to the protocol previously described (60). Cells were fed 3 times/wk, and grown for 8–10 days. For biochemistry experiments, T84 cells were plated on Transwells (4.67 cm2, 0.4 μm pore size membrane, Corning, Corning, NY) and allowed to grow for 8–10 days with culture media changed 3 times/wk. To measure intracellular calcium, cells were plated on 96 well plates clear glass bottom and black wall and were used the next day.
Mouse Colon Preparation
C57Bl/6 mice (Generous gift from Dr. Bissonnette, University of Chicago) were bred and housed at the Animal Resource Center of the University of Chicago. Animals were maintained in 12:12-h light-dark cycle and had free access to food and water. The University of Chicago Animal Care and Use committee approved all experimental procedures. Animals were anesthetized by inhalation of isoflurane followed by cervical dislocation. The colon was removed and placed in HEPES phosphate-buffered solution (HPBSS) containing (in mM) 140 NaCl, 5 KCl, 1.2 MgSO4, 1 CaCl2, 2 NaH2PO4, 10 HEPES, 10 glucose, 2 Na-pyruvate and 2 l-glutamine, pH 7.40 ± 0.03 at 37°C, and an osmolarity of 300 ± 5 mOsm (measured with a vapor pressure osmometer model 5520C, Wescor, Logan, UT) containing 5 μM indomethacin. The proximal and distal colon were cut open, rinsed with HPBSS, mounted on a P2304 mouse slider with 0.3 cm2 aperture and inserted into a P2300 Ussing Chambers (Physiological Instruments, San Diego, CA). Tissues were bathed in 5% CO2/22 mM HCO3− HPBSS (in which 22 mM NaCl was substituted with 22 mM NaHCO3), pH 7.40 ± 0.03 at 37°C and 300 ± 5 mOsm supplemented with 5 μM indomethacin. Solutions were continuously gassed with a 5% CO2-95% O2 mixture and maintained at 37°C.
Short-Circuit Current, Transepithelial Resistance, Basolateral Potassium, and Apical Chloride Conductance Measurements
The short-circuit current (Isc) in T84 cell monolayers was measured as previously described (58). Briefly, the Isc was defined as the external current needed to nullify the spontaneous transepithelial voltage (Vt0), with positive values meaning anion flowing from the basolateral to the apical chamber. The transepithelial resistance (TER) was calculated according to Ohm's law from the voltage deflection measured during the imposition of a 25 μA current pulse (Vt0 − Vt25) injected using a 710C-1 dual-voltage/current-clamp system (Bioengineering, the University of Iowa). Before starting electrical recordings, T84 cell inserts were rinsed three times by dipping the insert into prewarmed HPBSS to wash away culture media and placed into a new plate containing HPBSS at 37°C. Cells were allowed to recover from the change of media for 40–50 min. During the experimental procedure, cells were kept at 37°C by using an aluminum plate holder connected to a circulating water bath.
For mouse colon mounted in Ussing chamber, spontaneous transepithelial voltage (Vt) was measured via Ag-AgCl electrodes connected by 3 M KCl agar salt bridge to a DM MC6 single-channel electrode input module connected to a VCC MC8 multichannel voltage/current clamp system (Physiologic Instruments). Another pair of electrodes was used to pass current (Isc) across the epithelium to clamp the Vt to zero. Current signals were sampled at a frequency of 20 Hz and digitized. Acquire and Analyze software (Physiologic Instruments) on a Window XP computer was used to remotely control the VCC MC8 and analyzed data. In our experiments we rejected tissue that did not display a response larger than 5 μA/cm2 during the application of 10 μM forskolin.
Basolateral potassium (K+) conductance was measured in apical permeabilized T84 cell monolayers as previously described (32, 39). Cells were exposed first to HPBSS, then T84 cells were bathed both side in a low K+ solution (in mM: 10 Na+-gluconate, 135 N-methylglucamine/gluconate, 5 K+-gluconate, 1 Ca2+-gluconate, 1.2 Mg2+-gluconate, 2 NaH2PO4, 10 glucose, 10 HEPES, pH 7.40 ± 0.02 and 300 ± 0.05 mOsm). The apical solution was changed for a high K+ solution (where 135 N-methylglucamine was substituted for 135 K+-gluconate); after stabilization of the Isc, 500 U/ml of nystatin was added to the apical chamber to permeabilize the apical membrane and allow basolateral current measurements (32). The basolateral Na-K-ATPase activity was blocked by addition 100 μM ouabain to the solution. The junction potentials (maximum 6 mV) were small under these circumstances.
Apical chloride (Cl−) conductance was assessed using a similar approach to one described above and published by our group (39). At the beginning of the experiment, cells were bathed in HPBSS then exposed bilaterally to high K+/Cl−-free solution (in mM: 10 Na+-gluconate, 140 K+-gluconate, 1 Ca2+-gluconate, 1.2 Mg2+-gluconate, 2 NaH2PO4, 10 glucose, 10 HEPES, pH 7.40 ± 0.02 and 300 ± 5 mOsm). After equilibration, the basolateral solution was replaced with one containing a high KCl solution (in mM: 140 KCl, 1 CaCl2, 1.2 MgSO4, 2 NaH2PO4, 10 glucose, 10 HEPES, pH 7.40 ± 0.02 and 300 ± 5 mOsm) to generate a basolateral to apical Cl− gradient. When the current was stable, 500 U/ml of nystatin was added to the basolateral chamber to permeabilize the basolateral membrane allowing apical Cl− current measurements (32). In our experimental conditions, we found that basolateral permeabilization by nystatin was more effective when T84 cells were cultured on a collagen-free inserts.
Measurement of Intracellular Calcium in T84 Cells
T84 cells plated on 96 well glass bottom and black wall plates were loaded with the fluorescent calcium indicator fura-2 AM (23) for 45 min, dissolved in HPBSS at a final concentration of 10 μM with 0.5% (vol/vol) pluronic F-127. Cells were washed twice with HPBSS and the fura-2 fluorescence was measured using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA). The fluorescent dye was alternately excited at 340 nm and 380 nm and the emitted light at each excitation wavelength was collected at 510 nm. We computed the fluorescent ratio R340/380 of the emitted light at each excitation wavelength as an indicator of intracellular calcium change as previously described (8). On the plate, 8–24 wells were subjected to the same experimental condition, and in each plate 8 wells were not loaded with fura-2 for background correction. During the analysis of the fluorescence intensity, we removed wells displaying a sudden drop in fluorescence intensity and being close to the background fluorescence.
RT-PCR
T84 cells were plated on a 10-cm dish and harvested at 80% confluency. Total mRNA was extracted using Trizol (Invitrogen) following the manufacturer's directions. Five micrograms of total mRNA was treated with DNase I (Invitrogen) to eliminate contaminating DNA. First-strand cDNA was synthesized by using DNase I-treated mRNA, the SuperScript III reverse transcriptase (Invitrogen), and random primers according to manufacturer's manual. To assay for the presence of TRPV1 (accession no. AJ272063) the following primers were used: forward (VR1F3) 5′-GGAGGTGGCCGACAACACGG-3′, reverse complement (VR1R3) 5′-GCGACGTGGACTCAGACGGC-3′ (expected size 962 bp) as well as (VR1F4) 5′-GGACTCGGTGGGCAACACGG-3′, reverse complement (VR1R4) 5′-CCAGCCCAAGGCCAGGGAGA-3′ (expected size 805 bp) were ordered from Integrated DNA Technologies (Coralville, IA). GAPDH (accession no. NM_001256799.1) and NKCC1 (accession no. NM_001046) were used as positive control. The primers were: forward primer 5′-AGCGAGGGCAGCAGCCTG-3′ and the reverse complement was 5′-ATAGCAAGAAGTAGGATC-3′ (expected size 800 bp for NKCC1), and the forward primer 5′-GAAGATGGTGATGGGATTTC-3′ and the reverse complement 5′-GAAGGTGAAGGTCGGAGTC-3′ (expected size 226 bp for GAPDH). The cycling PCR reaction was denature 5 min at 94°C, followed by a 30-cycle denature 1 min at 94°C, anneal 1 min at 58°C, elongation 1 min 30 at 72°C, and ending with a 5-min elongation at 72°C, using a Platinum Taq DNA polymerase (Invitrogen). PCR reactions were carried out in the DNA Engine Dyad PTC-0220G thermal cycler (Bio-Rad, Hercules, CA). PCR products, stained with SYBRSafe (Invitrogen), were separated on 1.2% agarose gel and visualized with the Gel Doc system (Bio-Rad).
Mouse Colon Epithelial Cell Isolation
To isolate colonic epithelial cells we used a similar protocol to the one described previously by Grossmann et al. (22). Briefly, a mouse was deeply anesthetized by inhalation of isofluorane followed by a cervical dislocation. A ∼5-cm piece of colon was harvested, cut open, and washed in ice-cold calcium, magnesium free Hank's buffered saline solution (CMF-HBSS). The colon was transferred into ice-cold CMF-HBSS containing 10 mM dithiothreitol (DTT) for 30 min on ice. The tube was inverted ∼30 times to dislodge the mucus and the colon was transferred into CMF-HBSS containing 10 mM EDTA for 1 h at 4°C. The tube was vigorously shaken for 30–40 s to dislodge the epithelial cells. Cells were spun down at 2,000 g for 10 min at 4°C and resuspended in RIPA lysis buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and 50 mM Tris-Cl) supplemented with protease inhibitors (Complete, Roche) and 1 mM PMSF and subjected to immunoblotting.
Immunoblotting
T84 cells grown on Transwells were washed twice with a phosphate-buffered saline solution (PBS) and then exposed to RIPA lysis buffer for 30 min under gentle agitation. The insert support membrane was cut to scrap the cells off, transferred into an Eppendorf tube, followed by 3-s sonication (position 3) using a Sonic Dismembrator (model 550, Fisher Scientific). After 10 min centrifugation at 3,000 g, the supernatant was transferred into a new tube and protein concentration was determined using a bicinchoninic acid (BCA) kit from Pierce (Pierce Thermo Fisher Scientific, Rockford, IL). Equal amount of protein was dissolved in a loading sample buffer (10% glycerol, 5% sodium dodecyl sulfate, 0.5% bromophenol blue, 100 mM DTT, 125 mM Tris base pH 6.80), boiled at 95°C for 5 min, and loaded on acrylamide gel (8 to 12% depending on the target protein molecular weight) and subjected to SDS-PAGE. Proteins were transferred onto a PVDF membrane (GE Healthcare Life Science) and the membrane was blocked in blotto composed of 5% wt/vol nonfat dry milk dissolved in 0.1% Tween-20, 85 mM Tris-Cl, 14 mM Tris-base and 145.5 mM NaCl (TBS-T) for 1 h under continuous agitation. The membrane was then incubated in the presence of the primary antibody dissolved in blotto for 1 h (room temperature) or overnight (4°C), followed by three 10-min washes in TBS-T. These washes were followed by 1 h incubation with the secondary antibody (horseradish peroxidase labeled), three 10 min washes in TBS-T. The bound horseradish peroxidase was detected by enhanced chemiluminescence (Pierce) and X-Ray film processor (Alpha Tek AX 200, Broadview, IL).
Surface Biotinylation
Basolateral biotinylation was performed as previously described (11, 21, 58). Briefly, T84 cells grown to confluence on Transwell were treated with vehicle (DMSO), or with 20 μM capsaicin (applied either to the apical or basolateral side) for 1 h at 37°C in HPBSS. Cells were washed two times with ice-cold PBS (pH 8.0), before incubating the basolateral side with 10 μM sulfo-NHS-SS-biotin for 30 min on ice. A fresh aliquot of sulfo-NHS-SS-biotin was then added and cells were incubated for 30 more minutes. Cells were washed three times with ice-cold PBS (pH 8.0); the last wash (15 min incubation) was performed in presence of 100 mM glycine (a quenching agent) dissolved in PBS. To each filter, 500 μl of RIPA buffer was added and cells were incubated for 30 min on ice under gentle agitation. Filters were excised and cells were scraped from the filter and homogenized with a Teflon pestle. Two wells were pooled and the homogenate centrifuged at 3,000 g at 4°C for 10 min. Equal amount of protein (typically 1 mg/ml) was incubated with 35–50 μl streptavidin-agarose beads (Pierce) overnight at 4°C on a tube rotator. The next day the samples were washed two times with a high-salt buffer (0.1% Triton X-100, 500 mM NaCl, 50 mM Tris, 5 mM EDTA, pH 7.50) and once with a low-salt wash buffer (100 mM NaCl, 0.1% Triton X-100, 50 mM Tris, 5 mM EDTA pH 7.50) 10 min wash each time. Beads were resuspended in a loading sample buffer, boiled for 5 min and proteins subjected to SDS-PAGE followed by transfer onto PVDF membrane.
Immunostaining
Ice-cold methanol or 3% paraformaldehyde (PFA) was used to fix cells depending on the antibody used as previously described (40). T84 cells grown on Millicell inserts were subjected to the experimental protocol, washed with PBS containing 1 mM CaCl2 (PBS+), and fixed in 3% PFA dissolved in PBS+ for 30 min. Cells were washed three times in PBS+ and incubated in 50 mM NH4Cl (dissolved in PBS+) to quench the cross-linking reaction. Cells were exposed to a blocking buffer (PBS+ containing 3% goat serum and 0.05% saponin) for 30 min before adding the primary antibody for 2–3 h (dissolved in blocking buffer). In case of NKCC1 staining 0.5% sodium dodecyl sulfate was added during the primary incubation as previously recommended (34). Six washes with the blocking buffer were performed before adding the secondary antibody for 1 h. Cells were washed six times with the blocking buffer and a piece of the insert membrane was cut and mounted on a coverslip in ProLong Gold antifade reagent (Invitrogen).
The second method consisted of fixing the cells in cold methanol for 5 min, followed by extensive wash with PBS+ before 30 min incubation in PBS+ with 0.1% n-octyl-glucopyranoside (PBS+-og) 100 μM bis(sulfosuccinimidyl) suberate. Inserts were washed three times in PBS+-og, subsequently quenched 15 min in 100 mM ethylenediamine at pH 7.50, and rinsed a last time in PBS-og. Cells were blocked in 1% nonfat dry milk, 1% fish gelatin, and 1% bovine serum albumin in PBS-og for 30 min and then primary antibodies were added for 2–3 h. Several washes in blocking buffer were performed before incubation with the secondary antibody for 1 h in blocking buffer. After five washes, the piece of membrane with the cells was rinsed with deionized water dried and mounted in ProLong Gold.
Fluorescence Microscopy
Images acquisition was done as previously described (40). Briefly fluorescent images were captured using an epifluorescence microscope (DM4000; Leica Microsystems, Bannockburn, IL), equipped with a 63× oil immersion objective, NA 1.32 and a Retiga EXi camera (Q Imaging, Burnaby, British Columbia, Canada) controlled by MetaMorph 7 software (Universal Imaging, Downingtown, PA). Filter sets (Chroma Technology, Rockingham, VT) mounted in a filter wheel were used to select the adequate excitation.
Confocal images were collected with a Zeiss LSM 510 confocal scanning microscope using 63× oil immersion, NA 1.35 Plan Apochromat objective. Z-stack images were collected in 0.5 μm steps using a motorized stage. Images stored on an external drive were analyzed using ImageJ software (Rasband WS, ImageJ, National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/).
Statistical Analysis and Curve Fitting
Data are expressed as means ± SE, unless mentioned in the text, with n representing the number of experiments. Each experiment was performed at least in duplicate, and data shown are representative of two or more independent studies on cells grown on different passage and batch. When relevant, data analysis was done by paired and unpaired Student's t-test (2-tailed) or ANOVA and Dunnett's multiple comparison for ANOVA using Kaleidagraph (version 4.03, Synergy Software). P < 0.05 is considered statistically significant. Curve fitting was performed using Kaleidagraph.
RESULTS
Capsaicin Blocks Forskolin-Dependent Short-Circuit Current in Mouse Colon
Previous reports indicated that capsaicin blocked chloride secretion elicited by bradykinin in human colon (3) and fluid secretion in rat jejunum, ileum, and colon induced by E. coli Sta enterotoxin (42). To test whether capsaicin affects chloride secretion in mouse colon, proximal and distal colon were mounted in Ussing chambers and the short-circuit current (Isc) was monitored. In the colon, the Isc elicited by an increase of intracellular cAMP is mainly due to chloride secretion (38). Forskolin (FSK) was used here to activate adenylate cyclase and increase intracellular cAMP content.
In absence of stimulus, the baseline TER for the proximal and distal colon was 124.80 ± 27.23 Ω·cm2 (n = 8) and 90.60 ± 8.11 Ω·cm2 (n = 9), respectively, and the baseline Isc was 11.50 ± 2.36 μA/cm2 and 12.2 ± 2.44 μA/cm2, respectively. In initial experiments, we tested the acute effect of capsaicin (20 and 40 μM) on the forskolin-dependent short-circuit current (FSK-Isc) and found that luminal-side application blocked the FSK-Isc. As shown in Fig. 1A, addition of bilateral FSK caused a rapid increase of the Isc in the mouse proximal colon (a similar response was measured in the distal colon, not shown). When the Isc reached a steady-state, 40 μM capsaicin was added to the apical side rapidly reducing the FSK-Isc to a lower baseline. Subsequent addition of 40 μM capsaicin to the basolateral chamber induced a complete inhibition of the FSK-Isc. The side to which capsaicin was applied first was inverted in some experiments. In three experiments, capsaicin addition to one side then to the other caused the Isc to decrease below the baseline Isc value before addition of FSK. In control tissues (DMSO treated) the baseline TER for proximal and distal colon was 85.50 ± 4.0 Ω·cm2 (n = 5) and 79.90 ± 5.66 Ω·cm2 (n = 8) and the baseline Isc was 13.30 ± 3.74 and 8.90 ± 2.75 μA/cm2, respectively. As depicted in Fig. 1B, addition of FSK caused a sustained increase of the Isc (ΔFSK-Isc 13.22 ± 3.53 and 10.42 ± 1.30 μA/cm2 for proximal and distal colon, respectively, beginning of the plateau phase). At the end (∼25 min) of the experiment the ΔFSK-Isc were 12.66 ± 3.80 and 9.85 ± 1.34 for proximal and distal colon, respectively, which were not significantly different from the beginning of the experiment (P = 0.3 and P = 0.39, paired t-test for proximal and distal colon, respectively).
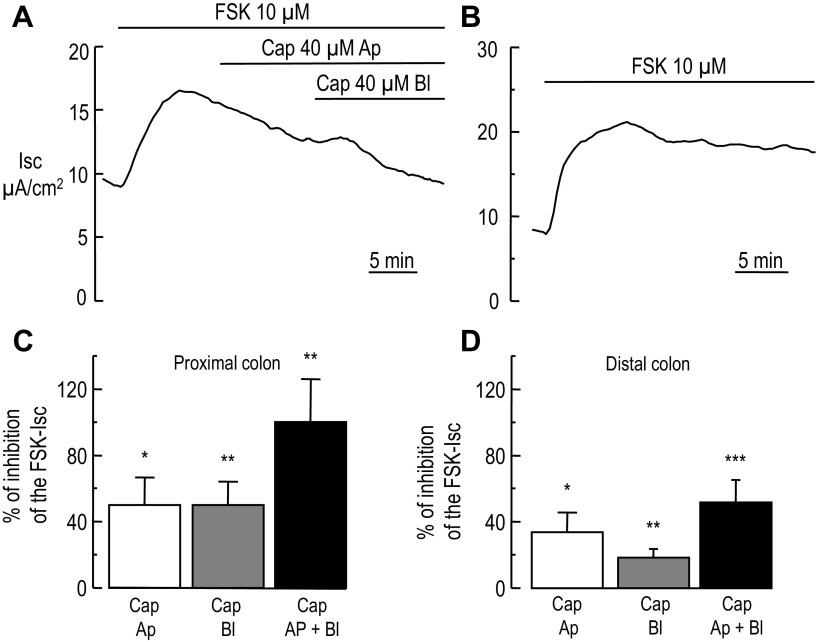
Fig. 1.
Inhibition of the forskolin-stimulated short-circuit current by luminal and basolateral capsaicin in mouse colon. A: representative Ussing chamber recording of the short-circuit (Isc) in mouse proximal colon. When the baseline Isc was stable, addition of forskolin (FSK) activated chloride secretion depicted by an increase of the Isc, which subsequently reaches a steady state. At the indicated time (horizontal bar) the tissue was exposed first to apical (Ap) capsaicin (Cap). When the Isc reached a plateau basolateral (Bl) capsaicin was added causing further inhibition of the forskolin-stimulated short-circuit current (FSK-Isc). B: representative recording of Isc in a distal colon exposed only to FSK. At the indicated time FSK was added causing an increase of the Isc, which remained constant during the time of application of FSK. C and D: summary data (n = 8 and 9 animals for proximal and distal colon, respectively) of the inhibition of the FSK-Isc by apical and basolateral capsaicin in the proximal colon (C) and distal colon (D), respectively. Paired t-test, *P < 0.05, **P < 0.01, ***P < 0.001.
In the proximal colon, apical and basolateral capsaicin caused 50.1 ± 16.76 (P = 0.012, n = 8, paired t-test) and 50.2 ± 13.75% (P = 0.005, n = 8, paired t-test) inhibition, respectively, of the FSK-Isc (Fig. 1C). Conversely, in the distal colon, apical and basolateral capsaicin induced a smaller inhibition than the one measured in the proximal colon (Fig. 1D), 33.7 ± 12.19 (P = 0.011, n = 9, paired t-test) and 18.2 ± 5.28% (P = 0.001, n = 9, paired t-test). Our experiments demonstrate that acute addition of capsaicin blocked the FSK-Isc to greater extent in the proximal colon than in the distal colon. The difference of capsaicin potency to block the FSK-Isc in proximal vs. distal colon may be caused by a different level of expression of the capsaicin receptor, an issue we will explore later.
Capsaicin Blocks the Forskolin-Dependent Short-Circuit Current in the Human Colonic Crypt Cell Line T84
The results from Fig. 1 suggested either that capsaicin blocked the FSK- Isc by acting on enteric nerves or directly on the epithelial cells responsible for chloride secretion, or both. To test the effect of capsaicin in a pure epithelial cell preparation, T84 cells, a well-established human colonic crypt-like cell line widely used to study chloride secretion (3, 12, 58), was used to test the effect of basolateral and apical capsaicin.
T84 cells grown on Millicell inserts were bathed in HPBSS, and TER and Isc were recorded to obtain baseline values (see Supplemental Table S1; supplemental material for this article is available online at the Journal website). Inserts were then transferred to a plate containing different concentrations of capsaicin in the basolateral compartment, or half of the apical solution content was replaced with equal volume of solution containing the double capsaicin concentration and cells were incubated 30 min. After the incubation period capsaicin had only minimal effect on the TER and Isc in unstimulated cells (see Supplemental Tables S1 and S2). Chloride secretion was then stimulated by transferring inserts into a plate containing 10 μM forskolin in the presence or absence of capsaicin and the Isc was recorded every 5 min for 20–25 min until a plateau was reached. Figure 2A depicts a representative experiment of Isc recordings for basolateral capsaicin exposure; similar results were obtained using apical capsaicin (data not shown). Increasing capsaicin concentration in the basolateral chamber from 1 to 300 μM blocked the FSK-Isc in a dose-dependent manner (Fig. 2B). Similar dose-dependent inhibition of the FSK-Isc was recorded after application of apical capsaicin (Fig. 2C). After adjusting our data to a single binding site inhibition curve (45) an IC50 of 23.5 ± 3.1 μM (SD) and 30.7 ± 5.0 μM (SD) for basolateral and apical capsaicin, respectively, was computed.
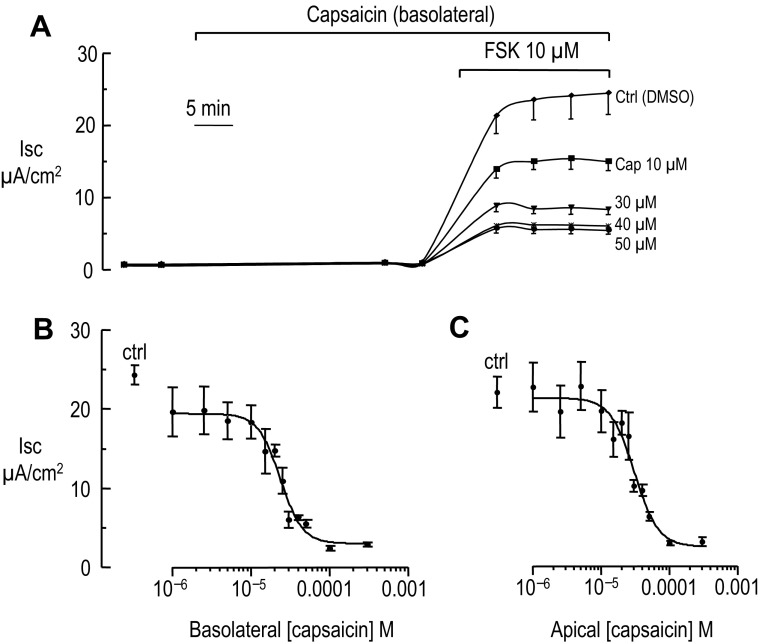
Fig. 2.
Inhibition of the forskolin-stimulated short-circuit current by basolateral and apical capsaicin in T84 cells. A: short-circuit current recording in T84 cells cultured on Millicell inserts (n = 3 inserts for each condition for this experiment). T84 cells were incubated in HPBSS (see materials and methods for the salt composition) and then exposed to basolateral capsaicin (Cap) or vehicle (DMSO) for 30 min. At the indicated time (horizontal bar) 10 μM FSK was added to stimulate chloride secretion. B: each point represents the mean Isc measured at the end of the Isc plateau in A during FSK exposure in presence of increasing basolateral capsaicin concentration (n ≥ 7 for each concentration). Ctrl represents the Isc in absence of capsaicin. From the adjustment of the data to a single-site inhibition curve an IC50 of 23.5 ± 3.1 (SD) was computed. C: same as B except capsaicin was applied to the apical side (n ≥ 8 for each concentration). From the adjustment of the data to a single-site inhibition curve an IC50 of 30.7 ± 5.0 (SD) was computed.
Our results show that capsaicin inhibits the FSK-Isc directly in chloride secreting epithelial cells, therefore suggesting that capsaicin can mediate its effect without acting on the nerves. In the remainder of the study, unless otherwise specified, we used 20 μM capsaicin.
Colonic Intestinal Epithelial Cells Express TRPV1
Capsaicin is known to activate TRPV1 (9, 19) and others have established that TRPV1 is expressed in epithelial cells (6, 7, 24, 30). The presence of TRPV1 and its splice variant TRPV1b (33) shorter by 180 bases pair (bp) was tested using VR1R3-VR1F3 and VR1R4-VR1F4 primers (lanes 1 and 2 in Fig. 3A). Using VR1R3-VR1F3 primers a band of the expected size, 962 bp, was found. On the other hand, using VR1R4-VR1F4 primers (lane 2) three bands were detected, one close to 805 bp, the expected size for TRPV1, a shorter one close to 625 bp (predicted for TRPV1b), as well as a shorter below 400 bp. Conversely, using VR1R3-VR1F4 primers only a band of the expected size, 769 bp, was detected (lane 3). Thus we believe that the extra bands seen in lane 2 are specific for the primer pair VR1R4-VR1F4 and are not representing a potential fragment for TRPV1b (accession no. AY986821.1). GADPH and NKCC1 PCR products were also amplified (lanes 4 and 5) as positive control.
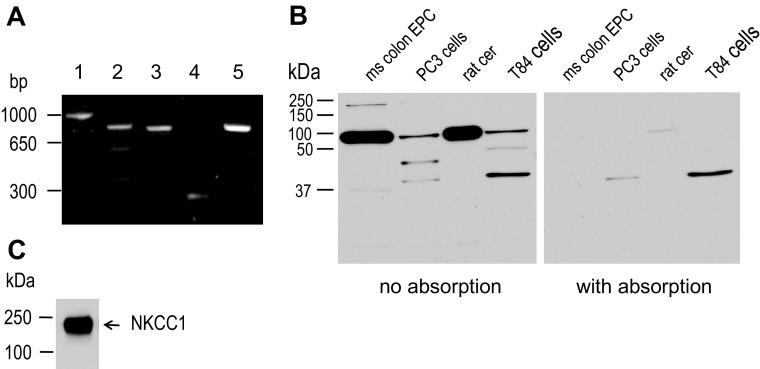
Fig. 3.
Expression of TRPV1 in colonic epithelial cell. A: RT-PCR products of TRPV1, GAPDH, and NKCC1. The agarose gel shows the products of the PCR designed to amplify a 962 bp (lane 1), 805 bp (lane 2), and 769 bp (lane 3) product for TRPV1, 226 bp (lane 4) for GAPDH, and 800 bp (lane 5) NKCC1 from T84 cells cDNA (representative gel of a triplicate experiment). B: TRPV1 expression in mouse colon epithelial cells, PC3 cells (human prostate epithelial line), rat cerebellum (rat cer), and T84 cells. Whole cell lysate was prepared using a RIPA lysis buffer. Proteins were separated by SDS-PAGE and immunoblotted for TRPV1 (left blot) using a rabbit anti-TRPV1 antibody (1:800 dilution), or after antibody preabsorption by the antigen peptide (right blot). Representative image of a triplicate experiment. C: from the mouse colonic epithelial cells harvested by calcium-EDTA chelation method and used in B, NKCC1 expression was tested using T4 mouse anti-NKCC1 (1:7,500 dilution). Representative image of n = 4 experiments.
To further confirm the presence of TRPV1 protein, we performed Western blots. Several antibodies were tested; the goat anti-TRPV1 antibodies (from Santa Cruz) against the NH2 and COOH termini did not immunoreact in our condition (data not shown). The mouse monoclonal (clone N221/17) from NeuroMab detected bands in T84 cells, human prostate PC3 cells (epithelial cell line generously provided by Dr. Vander Griend, University of Chicago), rat cerebellum and weakly immunoreacted with the mouse colon epithelial cell sample with a lower molecular weight than the one predicted (data not shown). Finally, using the rabbit polyclonal antibody from Alomone, TRPV1 could be detected in all tissues tested. Figure 3B shows the results of a triplicate experiment in which we probed for the presence of TRPV1 in mouse colon epithelial cells, T84 cells, PC3 cells, and rat cerebellum. The last two samples were used as positive control (51, 59). A ∼95-kDa-band protein of the predicted molecular weight was detected in the samples. High-molecular-weight bands (∼180 kDa) were detected and probably reflect the dimer form of TRPV1 (59) whereas the lower molecular weight may be degradation products. Preabsorption of the antibody with the antigen abrogated the detection of TRPV1. From the mouse colon epithelial cells NKCC1 was detected to confirm the successful isolation of crypt epithelial cells (Fig. 3C).
Next, we investigated the localization of TRPV1 in T84 cells. Since capsaicin was more effective when applied to the basolateral side, we first tested TRPV1 localization with a basolateral marker, the Na-K-ATPase. T84 cells grown on inserts were fixed with cold methanol and co-stained for TRPV1 and the α-subunit of the Na-K-ATPase. En face view of T84 cells shows that TRPV1 has a membrane-staining pattern similar to the distribution of the Na-K-ATPase (Fig. 4, A and B). In the XZ and YZ sections, TRPV1 staining overlapped, in some regions along the lateral membrane, with the staining of the Na-K-ATPase (yellow arrows). Additionally a large fraction of the TRPV1 staining was localized to a subapical cytoplasmic pool, in a pattern that was distinct from Na-K-ATPase (yellow stars in Fig. 4, A and B).
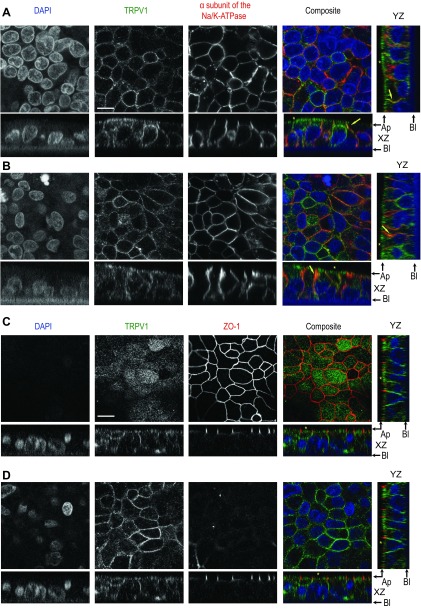
Fig. 4.
Immunolocalization of TRPV1 in polarized T84 cells. A: TRPV1 localization with the Na-K-ATPase. T84 cells grown on Millicell inserts to develop a polarized epithelium were fixed in cold methanol and mounted for confocal microscopy. Cells were dual-stained for TRPV1 (primary antibody 1:200 dilution) using a goat anti-rabbit DyLight 488 (1:200 dilution) and the α-subunit of the Na-K-ATPase (primary antibody 1:200 dilution) using a donkey anti-mouse DyLight 594 (1:200 dilution). Top four planes represent en face views (focal plane adjusted on TRPV1 staining) of the nuclear staining (DAPI), TRPV1, the α-subunit of the Na-K-ATPase, and composite of the three colors for colocalization. Below each en face view is the XZ optical section, with a supplemental YZ section for the composite. The black arrows indicate apical (Ap) and basolateral (Bl) sides. The yellow arrows point to colocalization of TRPV1 and Na-K-ATPase, whereas the yellow stars point to the subapical TRPV1 staining. B: same as A except the focal plane was adjusted to the staining of the Na-K-ATPase. Representative images of n = 6 experiments, 3 inserts for each condition. C: TRPV1 localization with ZO-1 in T84 cells. Cells were dual-stained for TRPV1 using a goat anti-rabbit DyLight 488 and ZO-1 (primary antibody 1:200 dilution) using a donkey anti-mouse DyLight 594 (1:200 dilution). The top four planes represent en face views (focal plane adjusted on ZO-1 staining) of the nuclear staining (DAPI), TRPV1, ZO-1 and composite of the three colors for localization. Below each en face view is the XZ optical section, with a supplemental YZ section for the composite. D: same as C except the focal plane for the en face views was focused on TRPV1 staining. The yellow stars point to the subapical TRPV1 staining. Representative images of n = 6 experiments, 3 inserts for each condition. Scale bar, 10 μm.
In some cells the Na-K-ATPase staining could be seen inside the cells in the subapical region. This staining can be the result of an oblique cross section of basolateral membrane of a neighboring T84 cell since these cells tend to grow in a pseudo-stratified pattern. The other possibility is that we are actually visualizing newly synthesized Na-K-ATPase accumulating in the subapical region as described in the Mardin-Darby canine Kidney cells (16).
To further delineate the localization of TRPV1 pool close to the subapical region, T84 cells were costained with TRPV1 and ZO-1, a tight junction marker. As shown in the en face view (Fig. 4C), when the image was focused on the ZO-1 staining, TRPV1 did not show the chicken wire pattern previously observed in Fig. 4, A and B, but rather showed a diffuse staining throughout the subapical portion of the cells. In the XZ and YZ section TRPV1 was localized to a subapical region at the level of the tight junction (yellow stars in Fig. 4C). The membrane-staining pattern could be found again when we focused below the ZO-1 (Fig. 4D). Therefore, TRPV1 is present at the tight junction, basolateral plasma membrane and within the subapical cytoplasm in T84 cells.
Effect of Capsaicin on the Basolateral Potassium and Apical Chloride Conductance
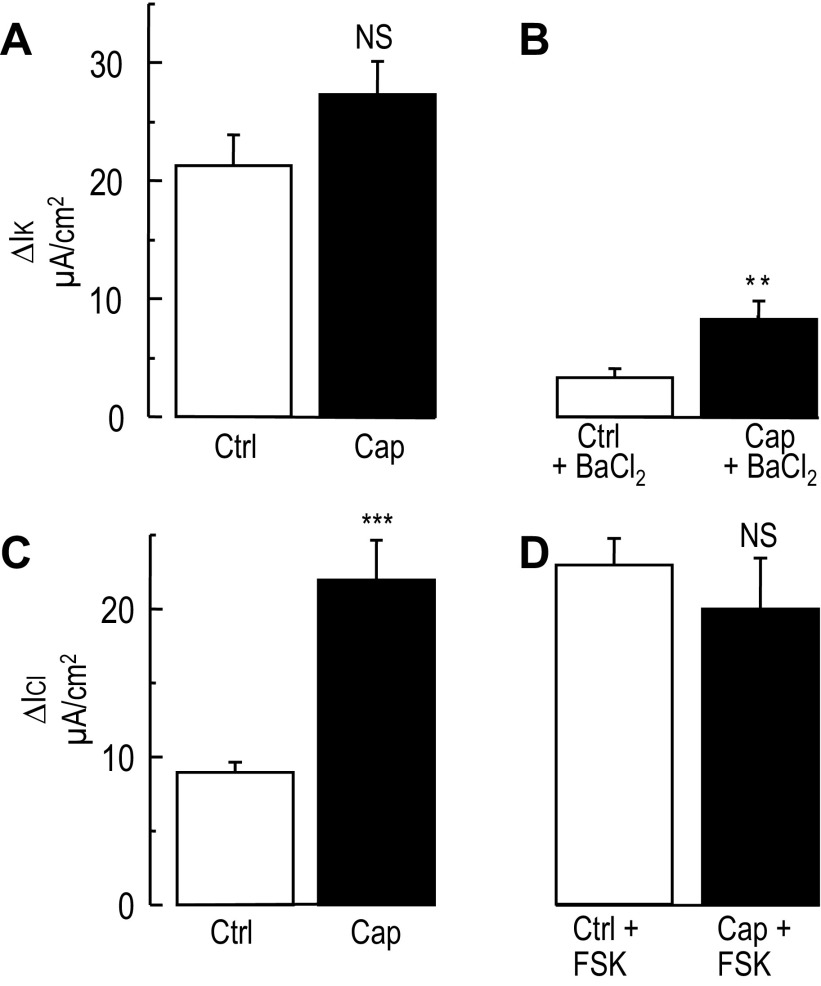
After localizing TRPV1, the mechanism of inhibition of the FSK-Isc by capsaicin was investigated. Potassium (K+) conductance plays a key role in chloride secretion in the colon (4, 35, 39). To test whether capsaicin inhibits chloride secretion by blocking a potassium conductance, T84 cell monolayers were exposed to a high apical-basolateral K+ gradient in presence of nystatin to permeabilize the apical membrane. As shown in Fig. 5A, exposing T84 cells to a high K+ apical-basolateral gradient caused a similar increase of the potassium current in vehicle and capsaicin treated cells (P = 0.12, unpaired t-test). In some experiments, the addition of 3 mM BaCl2 reduced marginally the stimulated potassium current in the control group (Fig. 5B) as previously described (39). In contrast, in the capsaicin-treated group, BaCl2 blocked a larger fraction of the current compared with control (P = 0.004, unpaired t-test), suggesting that capsaicin may stimulate a barium-sensitive potassium conductance in T84 cells.
Fig. 5.
Effect of capsaicin on the basolateral potassium and apical chloride currents in T84 cells. After equilibrium in a bilateral low K+ solution, T84 cells were exposed to high K+ (140 mM K+) apical to low basolateral K+ (5 mM) gradient. The apical membrane was permeabilized using 500 U/ml of nystatin, causing an increase of basolateral potassium current (IK). A: histograms represent the change in IK induced in control (vehicle) and capsaicin (Cap) treated T84 monolayers during the imposition of the K+ gradient. No significant difference was found between Ctrl and Cap-treated cells (n = 48 and 47 for Ctrl and Cap, respectively). B: the histograms represent the inhibition of IK by 3 mM barium in control (n = 24) and capsaicin-treated cells (n = 23). NS, nonsignificant, **P < 0.01 (unpaired t-test). C: T84 cells were exposed to a high basolateral KCl (140 mM) to high K+, Cl−-free (140 mM K+-gluconate) apical gradient. The basolateral membrane was permeabilized with 500 U/ml nystatin, which caused an increase of apical chloride current (ICl). Histograms represent the change in ICl in control (n = 48) and capsaicin-treated cells (n = 48). In this condition capsaicin induced a significant stimulation of the apical ICl compared with control. ***P < 0.001 (unpaired t-test). D: histograms represent the change in ICl at the peak of the forskolin stimulation in control (n = 48) and capsaicin-treated cells (n = 48). No difference between the two conditions was found.
The previous results suggested that the blockade of chloride secretion was not mediated by an inhibition of the basolateral K+ conductance. Therefore we tested whether apical chloride conductance was the target of capsaicin action. Using a similar approach to the one described above, cells were exposed to a basolateral to apical Cl− gradient. Upon addition of nystatin to the basolateral compartment, the apical Cl− conductance was unmasked showing that capsaicin substantially stimulated apical Cl− conductance (Fig. 5C, P < 0.001 unpaired t-test) as reported by others (1, 26). On the other hand, capsaicin did not enhance or block the forskolin-stimulated apical chloride current compared with untreated cells (P = 0.4, unpaired t-test). Together these results showed that the inhibition of chloride secretion during capsaicin treatment was not related to a decrease of the apical chloride conductance.
Capsaicin Reduces NKCC1 Basolateral Membrane Expression
NKCC1 is the major chloride-loading transporter in secretory epithelia; thus we tested whether NKCC1 was the target of capsaicin. We previously showed that NKCC1 internalization represents one of the most effective pathways to reduce chloride secretion (11, 58) and during PKC activation by phorbol 12 myristate 13-acetate (PMA) NKCC1 is internalized by clathrin-mediated pathway (40). Increasing intracellular calcium concentration ([Ca2+]i) in human colonic crypts was reported to induce NKCC1 internalization (48). TRPV1 activation is known to increase [Ca2+]i (9, 14, 41); thus we tested whether capsaicin induced-inhibition of the FSK-Isc was mediated by NKCC1 internalization.
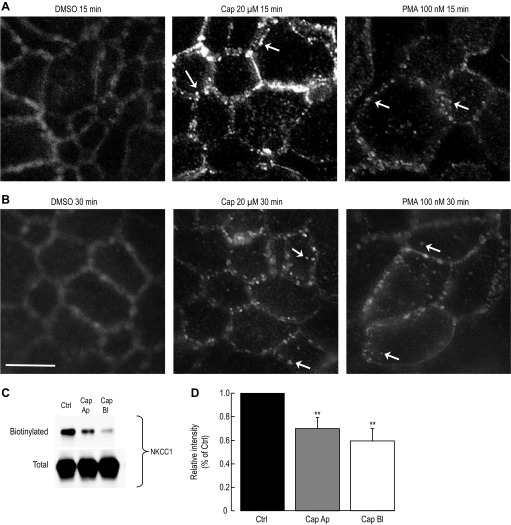
To do so, T84 cells grown on Millicell inserts were exposed to vehicle, 20 μM capsaicin (basolateral side) or 100 nM PMA, an activator of the protein kinase C leading to NKCC1 internalization in T84 cells (11, 58). After exposure to PMA, capsaicin or DMSO, cells were fixed with 3% PFA, stained for NKCC1, and images were acquired using a brightfield microscope equipped for fluorescence. In control (vehicle), NKCC1 expression in the en face view stained the lateral membrane (Fig. 6A) as previously reported (11). On the other hand after 15 min exposure to 20 μM capsaicin, NKCC1 staining no longer delineated the lateral membrane but appeared inside intracellular vesicles (white arrows in Fig. 6, A and B). The staining pattern resembled the one obtained with PMA (Fig. 6) as previously described (40). Prolonged exposure to capsaicin (30 min) led to further disappearance of NKCC1 from the lateral membrane (Fig. 6B).
Fig. 6.
Loss of NKCC1 membrane expression during capsaicin treatment in T84 cells. A: NKCC1 internalization during capsaicin and PMA treatment. T84 cells grown on Millicell inserts to develop a polarized epithelium were exposed to vehicle (DMSO), capsaicin (Cap), or phorbol 12-myristate 13-acetate (PMA), subsequently fixed with 3% paraformaldehyde, and mounted for fluorescent microscopy. Images were captured using a brightfield microscope equipped for fluorescence. Cells were stained for NKCC1 (primary antibody 1:200 dilution) using a donkey anti-mouse DyLight 594 (1:200 dilution). En face view of NKCC1 staining during vehicle, 20 μM capsaicin and 100 nM PMA exposure (15 min). White arrows point to NKCC1 staining in endocytic vesicles. B: same as A except cells were treated for 30 min with vehicle, capsaicin, or PMA (n = 5 experiments, 2 to 3 inserts per condition). Scale bar, 10 μm. C: NKCC1 surface expression during apical and basolateral capsaicin exposure. T84 cells were treated for 1 h with 20 μM capsaicin applied either to the apical (Ap) or basolateral (Bl) side or with DMSO (Ctrl). Basolateral membrane was exposed to sulfo-NHS-SS-biotin and biotinylated proteins were captured by streptavidin and subjected to Western blotting. NKCC1 proteins in the biotinylated and total fraction were detected using a mouse anti-NKCC1 (T4 1:7,500 dilution). D: data summary of the experiment in C. Films were scanned and relative intensity was measured using ImageJ and normalized to control (n = 7 for each condition). **P < 0.01 (unpaired t-test).
The decrease of NKCC1 from the basolateral membrane was further investigated by measuring the effect of capsaicin on NKCC1 membrane expression by surface biotinylation. T84 cells grown to confluence on inserts were incubated with 20 μM capsaicin on either the basolateral or apical side for 60 min (representing approximately the time of incubation of T84 cells with capsaicin in the Isc in Fig. 2). Basolateral membrane proteins were subjected to surface biotinylation, and the biotinylated proteins were captured by streptavidin and were separated by SDS-PAGE, transferred onto PVDF membrane and immunoblotted for NKCC1 using T4 antibody. As shown in Fig. 6C, 60 min exposure of T84 cells to apical and basolateral capsaicin caused a significant loss of NKCC1 from the basolateral membrane without affecting the total amount of NKCC1. Apical capsaicin caused a 30 ± 9% (P = 0.007, n = 7, unpaired t-test) loss of NKCC1 membrane expression, whereas basolateral capsaicin caused a 40 ± 10% loss (P = 0.002, n = 7, unpaired t-test) of NKCC1 membrane expression (Fig. 6D). To test whether we could find a correlation between the decrease of chloride secretion and the amount of NKCC1 membrane expression we repeated biotinylation experiments at 30 min exposure to 20 μM capsaicin on the basolateral side. We found that capsaicin caused a 15 ± 6% (P < 0.05, unpaired t-test) decrease of NKCC1 from the basolateral membrane (data not shown). On the other hand measuring the short-circuit current after 30 min exposure to 20 μM capsaicin already caused a 49.5 ± 1.6% inhibition, while at 1 h exposure we measured a 41.5 ± 3.7% inhibition, almost identical to the 30 min exposure. Therefore the decrease of the FSK-Isc by capsaicin preceded the decrease of NKCC1 membrane expression.
These results imply that capsaicin in the first 30 min alters other mechanisms. Therefore, the loss of NKCC1 from the membrane during capsaicin treatment explains only in part the the decrease of the FSK-Isc measured in Fig. 2.
Pharmacological Profile of Capsaicin-Induced Inhibition of the Forskolin-Stimulated Short-Circuit Current in T84 Cells
AMG-9810 and capsazepine.
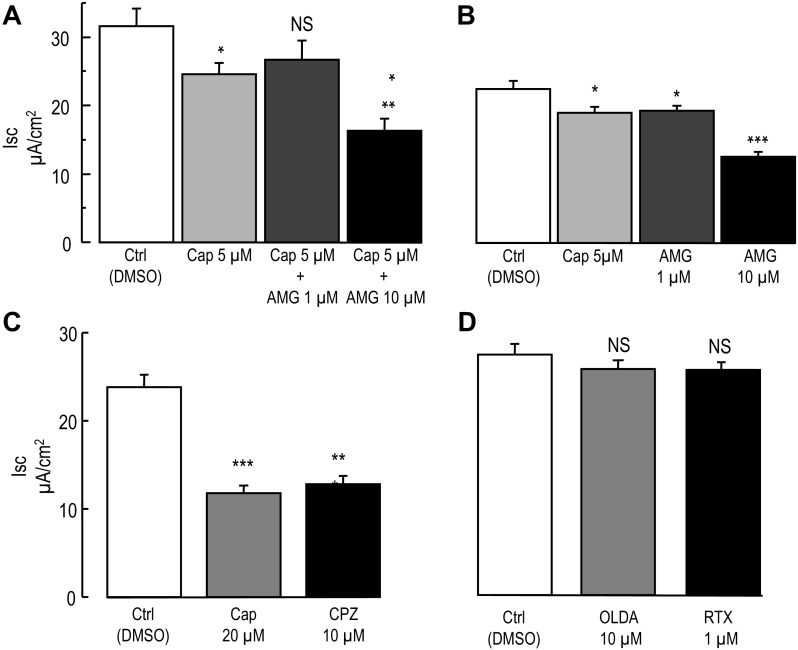
Next we used a pharmacological approach to define the profile of capsaicin-induced inhibition of the FSK-Isc in T84 cells. Capsaicin is a common agonist of TRPV1 (2, 9, 19, 41, 49, 53); therefore in the following experiments we used AMG-9810, a potent inhibitor of TRPV1 that has been shown to block capsaicin-elicited TRPV1 activity (14, 19, 49).
In preliminary experiments 100 nM AMG-9810 (4 × IC50) was applied 10 min before the addition of 20 μM capsaicin followed by 30 min incubation before recording the Isc. In these conditions AMG-9810 failed to prevent the inhibition of the FSK-Isc by capsaicin. In addition, we tested 5 μM capsaicin (basolateral), the lowest dose causing a significant inhibition of the FSK-Isc and increased the AMG-9810 concentration to 1 and 10 μM (above 10 μM, AMG-9810 is no longer soluble in HPBSS). Cells were bathed in HPBSS and the mean TER and Isc were recorded (see Supplemental Table S3). After 10 min incubation in presence of 1 μM AMG-9810 (basolateral side), a slight increase in the TER and Isc was measured (1765. 2 ± 126.6 Ω·cm2, P = 0.005, paired t-test, and 0.93 ± 0.03 μA/cm2 P = 0.008, paired t-test), while in presence of 10 μM AMG-9810 the Isc slightly increased (0.96 ± 0.03 μA/cm2, P = 0.02, paired Student's t-test) without a change in the TER (P = 0.19, paired Student's t-test). Cells were then incubated with 5 μM capsaicin or DMSO for 30 min (see Supplemental Table S3 for TER and Isc values after the incubation period), and chloride secretion was stimulated by FSK and Isc was recorded for 20–25 min. As shown in Fig. 7A the FSK- Isc was significantly inhibited by capsaicin and preincubation with 1 μM AMG-9810 prevented the inhibition of the FSK-Isc by capsaicin. On the other hand increasing the concentration of AMG-9810 to 10 μM had the opposite effect and increased the inhibition of the FSK-Isc by capsaicin (Fig. 7A). When tested alone, 1 μM AMG-9810 decreased the FSK-Isc compared with control (P = 0.048, unpaired t-test, Fig. 7B) to the same extent as did 5 μM capsaicin. At 10 μM AMG-9810 further inhibited the FSK-Isc compared with control (P < 0.001, unpaired t-test, Fig. 7B).
Fig. 7.
Pharmacological characterization of the inhibition of the forskolin-stimulated short-circuit current by capsaicin. A: effect of AMG-9810 on capsaicin-induced inhibition of the FSK-Isc. The experimental design was similar to the one shown in Fig. 2A. T84 cells grown on Millicell inserts were exposed to DMSO (Ctrl), 5 μM capsaicin, or AMG-9810 (AMG) (1 μM or 10 μM) plus capsaicin (AMG-9810 was added 10 min before capsaicin) for 30 min, before stimulation of chloride secretion by FSK (10 μM). Histograms represent the mean Isc values measured at the end of the FSK-Isc plateau (n = 12 for each condition). B: same as A, except that the cells were incubated with either 5 μM capsaicin, AMG-9810 at 1 μM or 10 μM alone for 30 min. Histograms represent the mean Isc values measured at the end of the FSK-Isc plateau (n = 6 for each condition). C: same as A except that cells were incubated in presence of 20 μM capsaicin or 10 μM capsazepine (CPZ). Histograms represent the mean Isc values measured at the end of the FSK-Isc plateau (n = 19 for Ctrl, n = 16 for Cap and n = 19 for CPZ). D: same as A except that cells were incubated in presence of 10 μM N-oleoyldopamine (OLDA) or 1 μM resiniferatoxin (RTX). Histograms represent the mean Isc values measured at the end of the FSK-Isc plateau (n = 24 for each condition). Unpaired t-test between Ctrl and experimental condition are *P < 0.05, **P < 0.01, and ***P < 0.001.
To better understand the failure of the TRPV1 antagonists to prevent the effect of capsaicin, we used capsazepine (CPZ), an inhibitor of TRPV1 unrelated to AMG-9810 (5). The mean TER and Isc recorded during HPBSS are presented in (Supplemental Table S4). T84 cells were incubated with 10 μM CPZ, 20 μM capsaicin, or DMSO added to the basolateral chamber for 30 min. At the end of the incubation period TER and Isc were measured again (Supplemental Table S4) and chloride secretion was stimulated with FSK. In presence of capsazepine the FSK-Isc was substantially decreased (Fig. 7C) compared with control (P < 0.001, unpaired t-test). Surprisingly CPZ blocked the FSK-Isc to the same extent as did 20 μM capsaicin (P = 0.5, unpaired t-test). Therefore CPZ could not be used to test whether capsaicin-mediated inhibition of the FSK-Isc was TRPV1-dependent.
N-oleoyldopamine and resiniferatoxin.
Because each TRPV1 inhibitor used caused an inhibition of the FSK-Isc on its own, we turned to TRPV1 activators. We reasoned that if TRPV1 was mediating the effect of capsaicin, using TRPV1 activators should reproduce the inhibition of capsaicin on the FSK-Isc. N-oleoyldopamine (OLDA) and resiniferatoxin (RTX) are known activators of TRPV1 (2, 10, 49, 53, 57) and were used in the following experiments. The mean TER and Isc values measured at the beginning of the experiment in HPBSS are presented in Supplemental Table S5. T84 cells were then transferred to a plate containing OLDA and RTX (basolateral) and incubated for 30 min, and TER and Isc measurements were acquired (see Supplemental Table S5). Addition of FSK stimulated chloride secretion, and neither the presence of 10 μM OLDA (P = 0.03, unpaired t-test, control vs. OLDA) nor the presence of 1 μM RTX (P = 0.25, unpaired t-test control vs. RTX) significantly altered the FSK-Isc (Fig. 7D). These results strongly support that inhibition of the chloride secretion by capsaicin is TRPV1-independent.
Calcium Measurement in T84 Cells
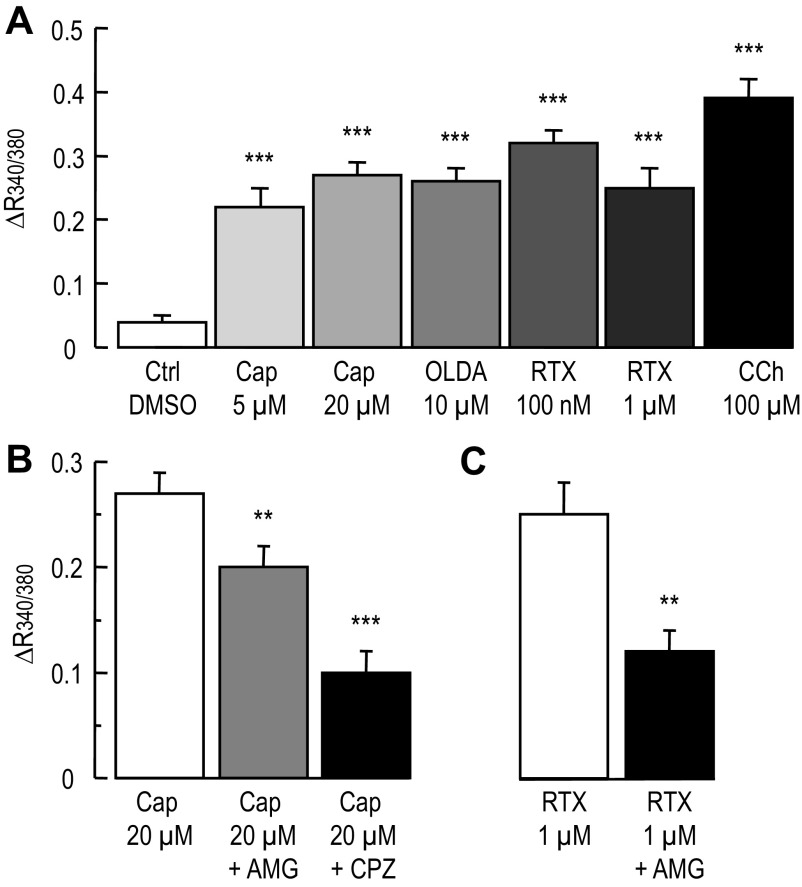
As mentioned earlier, TRPV1 is a cationic permeable channel allowing calcium to enter in the cells (9, 41). To determine whether T84 cells express an active TRPV1 channel we used fura-2 to measure [Ca2+]i changes during exposure to capsaicin, OLDA, and RTX. T84 cells plated on glass bottom, 96-well plates were loaded with fura-2, and the ratio, R340/380, was computed as an index of [Ca2+]i changes. As shown in Fig. 8A, addition of capsaicin, OLDA, and RTX significantly increased R340/380 compared with control (P < 0.001, unpaired t-test for all conditions tested). Carbachol (CCh, 100 μM) also significantly raised [Ca2+]i (P < 0.001 unpaired t-test) and was used as a positive control (65). Furthermore, pretreatment (∼10 min) of T84 cells with either 100 nM AMG-9810 or 10 μM CPZ significantly attenuated the increase in R340/380 induced by 20 μM capsaicin (Fig. 8B) compared with capsaicin alone (P < 0.01 and P < 0.001 unpaired t-test for AMG-9810 and CPZ, respectively). Finally, we also found that AMG-9810 decreased the rise in [Ca2+]i induced by 1 μM RTX (Fig. 8C, P < 0.01 unpaired t-test). Consequently our results suggest that T84 cells express a functional TRPV1 channel and further support that the inhibitory effect of capsaicin on the FSK-Isc is not related to TRPV1 activation by capsaicin.
Fig. 8.
Calcium measurement in T84 cells during exposure to capsaicin, OLDA, and RTX. T84 cells plated on 96 well glass bottom and black wall plates were loaded with the calcium fluorescent dye fura-2. The fluorescent ratio (R340/380) of the emitted light at 510 nm after excitation at 340 and 380 nm was computed and used as an index of change in [Ca2+]i. A: histograms represent the change in R340/380 in control (DMSO), in presence of DMSO (n = 81 wells from five plates), capsaicin 5 μM (n = 62 wells obtained from four plates), capsaicin 20 μM (n = 83 wells obtained from five plates) OLDA (n = 62 wells obtained from four plates), 100 nM RTX (n = 16 wells obtained from two plates) 1 μM RTX (n = 38 wells obtained from two plates) and carbachol (CCh, n = 48 wells obtained from three plates). Unpaired t-test between Ctrl and experimental condition are ***P < 0.001. B: histograms represent the change in R340/380 induced by 20 μM capsaicin alone or in presence of 100 nM AMG-9810 (n = 84 wells obtained from five plates) or 10 μM CPZ (n = 57 wells obtained from four plates). **P < 0.01 and ***P < 0.001 unpaired t-test between 20 μM capsaicin and in presence of AMG-9810 and CPZ respectively. C: Histograms represent the change in R340/380 induced by 1μM RTX alone and in presence of 100 nM AMG-9810 (n = 34 wells obtained from two plates). **P < 0.01, unpaired t-test between RTX alone and in presence of 100 nM AMG-9810.
DISCUSSION
Capsaicin (found in “hot” chili peppers) is a highly potent activator of TRPV1 that exerts diverse actions on the gut and has several potential clinical applications (28, 55, 56). In guinea pig ileum, capsaicin evokes a biphasic response of the Isc that is only presented when nerves are intact (63), while others report that capsaicin alone activates an inward Isc in human colon preparation (3). In rat jejunum, ileum and proximal colon, capsaicin blocked fluid secretion evoked by E coli STa enterotoxin but not by carbachol (42). In our experiments, acute exposure of capsaicin inhibited the forskolin-dependent chloride secretion in mouse colon and T84 cells (Figs. 1 and 2). Contrary to other intestinal preparations, capsaicin alone did not substantially affect Isc or the TER. Variations in TER and Isc after incubation period were also noticed in our previous study (58). It is possible in our experiments with T84 cells that we did not capture a transient effect of capsaicin because of our acquisition rate. However, even in mouse colon, where data points were obtained every 20 s, we did not find an effect on baseline Isc. In both models, capsaicin inhibited the FSK-Isc.
After demonstrating that capsaicin blocked forskolin-dependent chloride secretion in mouse colon and T84 cells, we sought evidence for the presence of TRPV1, the known receptor for capsaicin, in the epithelial cells using RT-PCR and immunoblotting. TRPV1 was cloned in 1997 (9). Its first splice was identified in rat (52), and subsequently the human ortholog was cloned (33). In our experiments using a set of primers encompassing the 180 bases pair deletion, no evidence for the presence of the splice variant in the T84 cells could be found, but RT-PCR amplified a product of the predicted size for TRPV1. The presence of TRPV1 protein was demonstrated in mouse colon epithelial cells, isolated by calcium-EDTA to avoid contamination by other cellular components, as well as in T84 cells (Fig. 3). The molecular weight matched the one detected in PC3 cells (human epithelial prostate cells) and rat cerebellum, two samples reported to express TRPV1 (51, 59).
In T84 cells TRPV1 was present along the basolateral membrane and colocalized with the α-subunit of the Na-K-ATPase. TRPV1 was also found in vesicular type compartments in the cytosol with a predominant pool in the subapical region (Fig. 4). Vesicular localization of TRPV1 has been described in other epithelial cells. For example, in the urothelium TRPV1 stains basal and superficial epithelial cells and was present in large vesicular compartments resembling our cytosolic staining, with no apparent membrane staining (6). In keratinocytes, the Langerhans cells of the epidermis, and sweat gland epithelium, TRPV1 staining also was cytosolic (7). In laryngeal mucosal epithelial cells of the epiglottis, TRPV1 immunoreactivity was observed predominantly in the cytoplasm with faint lateral membrane staining (24) and finally, in the Het1A (human esophageal epithelial cell), TRPV1 staining displayed also a vesicular pattern (30). The vesicle type in which TRPV1 resides has not been so far elucidated and the role of TRPV1 in those vesicles remained unknown. It is also important to note that in the murine and rat epithelia of the bladder several antibodies produce aspecific staining in TRPV1 null animals (15). Such aspecific staining as not been reported for other epithelial cells, but it remains to be tested using TRPV1 null animals whether or not the staining in the other epithelial cells is real.
In the colon potassium conductance sustains chloride secretion (35, 39), and in other preparations capsaicin was shown to inhibit potassium conductance (26, 44). When tested in T84 cells, capsaicin did not block the basolateral potassium conductance. On the contrary it seemed that capsaicin slightly increased a Ba2+-sensitive conductance. Apical chloride conductance is another site of the regulation of colonic chloride secretion (4), but we found that capsaicin as in other preparations (1, 26) stimulated the basal apical chloride conductance and had no effect on the FSK-Isc (Fig. 5).
In isolated colonic crypts, increasing [Ca2+]i induces NKCC1 internalization (48). With the knowledge that TRPV1 also acts as a calcium channel (9), we investigated whether capsaicin would induce NKCC1 internalization. Indeed, capsaicin induced robust internalization of NKCC1, similar to the response we previously reported with the phorbol ester PMA (11, 40, 58). The internalization pathway by which capsaicin induced NKCC1 internalization has not been characterized, but previous work from our laboratory has shown that NKCC1 is endocytosed by a clathrin-mediated pathway during PKC activation by PMA (40). A similar mechanism may be at work with capsaicin. We have evidence that capsaicin increases [Ca2+]i (Fig. 8), and capsazepine, another capsaicinoid, which recapitulate the decrease of the FSK- Isc, caused internalization of FM1-43 through a clathrin-dependent pathway (61). In our experiments we have not tested whether blocking a rise in [Ca2+]i could prevent NKCC1 internalization during capsaicin treatment. The reduction of NKCC1 membrane expression was confirmed by surface biotinylation (Fig. 6). Therefore, loss of NKCC1 membrane expression during capsaicin treatment explains at least in part the inhibition of the FSK-Isc. As we did not investigate whether the rate of NKCC1 delivery to the membrane was altered we cannot conclude for sure that increased internalization by capsaicin vs. delivery rate is the primary mechanism at play. Furthermore performing surface biotinylation at 30 min revealed a 15% loss of NKCC1 from the membrane but capsaicin already caused a similar inhibition of the FSK-Isc as the one measured at 1 h. Earlier effect of capsaicin may alter the transport activity of NKCC1 (e.g., affinity of the transported ions). Besides NKCC1 other components of the chloride secretion (e.g., Na-K-ATPase, apical potassium channels) machinery may also be affected by capsaicin, but they were not addressed in our study.
In an effort to better characterize the inhibition of chloride secretion by capsaicin we used a pharmacological approach involving known TRPV1 antagonists. To our surprise, the effect of capsaicin on the FSK-Isc did not correlate with activation of TRPV1. AMG-9810, a highly potent inhibitor of TRPV1, which blocks the three modes of activation of TRPV1 by capsaicin, temperature, and proton (49), was ineffective to prevent the inhibition of the FSK-Isc by capsaicin. In our experiment AMG-9810 had a biphasic effect; at 1 μM it prevented the inhibition of chloride secretion by capsaicin, but at 10 μM, it potentiated it, which could be a nonspecific effect of the blocker when used above a certain concentration. Capsazepine, another inhibitor of TRPV1, unrelated to AMG-9810 (5), with no effect on the activation of TRPV1 by the proton (18, 49), was equally potent in blocking the FSK-Isc in T84 cells. Similar behavior of capsazepine was described in Calu-3 cells. In this cell model capsaicin and capsazepine blocked the cAMP-stimulated anion secretion (26).
Using TRPV1 agonists also suggests that TRPV1 is not involved in the inhibition of the FSK-Isc. N-oleoyldopamine, a physiological agonist of TRPV1, with equal potency to capsaicin to activate TRPV1 (10), was unable to prevent the FSK- Isc. Finally, resiniferatoxin, an ultra potent activator (49) of TRPV1 (up to 2,000 times more effective that capsaicin to activate TRPV1), did not block the FSK-Isc. Because of the lower dose of resiniferatoxin needed to activate TRPV1, it is unlikely that resiniferatoxin produced nonspecific effect as capsaicin does (13). All together these results strongly suggest that TRPV1 is not involved in the inhibition of the FSK-Isc by capsaicin.
Others studies have reported TRPV1-independent effects of capsaicin. In Calu-3 cells, both capsaicin and capsazepine blocked the FSK-Isc via a Rho-kinase-dependent pathway (26). In guinea pig ileal longitudinal smooth muscle, capsaicin induced a capsazepine-insensitive relaxation that was 4-aminopyridine and high KCl-sensitive (17). Similarly, capsaicin induced a vasodilatation of nasal vasculature that was capsazepine-insensitive, but affected by blockers of COX-2 and the prostanoid receptor (62). In neutrophils capsaicin increased [Ca2+]i that was slightly sensitive to blockers of the phospholipase C or blocker of receptor-gated and store operated Ca2+-channels (64).
To explain our results a rise in [Ca2+]i mediated by capsaicin could be the mediator of NKCC1 internalization as described in human colon (48). Experiments conducted in Fig. 8 showed that capsaicin elicited a rise in [Ca2+]i, but we have not explored this possibility in the current study. Interestingly capsazepine (another vanilloid compound) increased [Ca2+]i in alveolar epithelial cells, and mediated internalization of FM1-43 through a clathrin-dependent pathway (61). This is an interesting finding as we recently demonstrated that NKCC1 endocytosis during PMA exposure is clathrin-dependent (40). The other pathway by which capsaicin could have blocked chloride secretion is by activation of protein kinase C (PKC). The capsaicin analog resiniferatoxin has been shown to activate several PKC isoforms (20, 25, 50), including PKCδ and PKCϵ involved in NKCC1 internalization (58). At the concentration tested in their studies resiniferatoxin showed less than 10% stimulation of PKCδ and PKCϵ (20), and others (50) found similar results. Despite the low level of activation of the PKC it should be kept in mind that in our study we used capsaicin and it had not been reported whether capsaicin is more or less potent than resiniferatoxin in activating PKC.
In conclusion, we have demonstrated the expression of TRPV1 in mouse colonocytes and in the T84 cells. Measuring change in R340/380, we have evidence that capsaicin, OLDA and RTX increased [Ca2+]i in T84 cells and AMG-9810 blocked both the rise in R340/380 caused by capsaicin and RTX supporting the expression of a functional TRPV1 channel in T84 cells. We also show that capsaicin inhibits the FSK-Isc in both the mouse colon and in T84 cells. Decrease of NKCC1 expression from the basolateral membrane during capsaicin exposure explains in part the inhibition of chloride secretion. In T84 cells the lack of effect of the TRPV1 inhibitors and activators strongly suggest that capsaicin inhibits chloride secretion via a TRPV1-independent mechanism.
The functional role of TRPV1 in intestinal epithelial cells remains to be elucidated. One potential role could be in adjustment of energy expenditure by controlling the intake of nutrients and fat. Recently it was shown that intra-abdominal desensitization of TRPV1 blocks the regulation of body temperature (54), and control of adipocyte fat storage by TRPV1 has been documented (31). Because vanilloids are used clinically, a clear characterization of their targets/receptors will be necessary to fully understand their mode of actions (TRPV1 dependent or not) on the different tissues to avoid potential side effects.
GRANTS
This work was supported the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-48010 (to J. B. Matthews). L. Shen is supported by a Career Development Award from the Crohn's and Colitis Foundation of America.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.G.B. and J.B.M. conception and design of research; P.G.B. and X.T. performed experiments; P.G.B. and X.T. analyzed data; P.G.B., X.T., C.R.W., L.S., J.R.T., and J.B.M. interpreted results of experiments; P.G.B. prepared figures; P.G.B. and J.B.M. drafted manuscript; P.G.B., X.T., C.R.W., L.S., J.R.T., and J.B.M. edited and revised manuscript; P.G.B., X.T., C.R.W., L.S., J.R.T., and J.B.M. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We are very grateful to Mary Chaiken-Fedor and Roger Worrell for preliminary discussions and to Juli Hayashi for her technical assistance in part of the project.
We thank the Frank W Fritch Monoclonal Antibody Facility of the University of Chicago Cancer Center for their assistance in the production and purification of the T4 antibody. The Facility is supported by NCI Cancer support grant 5P30CA014599-35.
REFERENCES
- 1. Ai T, Bompadre SG, Wang X, Hu S, Li M, Hwang TC. Capsaicin potentiates wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride-channel currents. Mol Pharmacol 65: 1415–1426, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Auzanneau C, Norez C, Antigny F, Thoreau V, Jougla C, Cantereau A, Becq F, Vandebrouck C. Transient receptor potential vanilloid 1 (TRPV1) channels in cultured rat Sertoli cells regulate an acid sensing chloride channel. Biochem Pharmacol 75: 476–483, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Baird AW, Skelly MM, O′Donoghue DP, Barrett KE, Keely SJ. Bradykinin regulates human colonic ion transport in vitro. Br J Pharmacol 155: 558–566, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62: 535–572, 2000. [DOI] [PubMed] [Google Scholar]
- 5. Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol 107: 544–552, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA 98: 13396–13401, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bodó E, Kovács I, Telek A, Varga A, Paus R, Kovács L, Biró T. Vanilloid receptor-1 (VR1) is widely expressed on various epithelial and mesenchymal cell types of human skin. J Invest Dermatol 123: 410–413, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Bouyer P, Paulais M, Cougnon M, Hulin P, Anagnostopoulos T, Planelles G. Extracellular ATP raises cytosolic calcium and activates basolateral chloride conductance in Necturus proximal tubule. J Physiol 510: 535–548, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- 10. Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, Zipkin RE, Daddario N, Appendino G, Di Marzo V, Walker JM. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem 278: 13633–13639, 2003. [DOI] [PubMed] [Google Scholar]
- 11. Del Castillo IC, Fedor-Chaiken M, Song JC, Starlinger V, Yoo J, Matlin KS, Matthews JB. Dynamic regulation of Na+-K+-2Cl− cotransporter surface expression by PKC-ϵ in Cl−-secretory epithelia. Am J Physiol Cell Physiol 289: C1332–C1342, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol Gastrointest Liver Physiol 246: G204–G208, 1984. [DOI] [PubMed] [Google Scholar]
- 13. Dogan MD, Patel S, Rudaya AY, Steiner AA, Szekely M, Romanovsky AA. Lipopolysaccharide fever is initiated via a capsaicin-sensitive mechanism independent of the subtype-1 vanilloid receptor. Br J Pharmacol 143: 1023–1032, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doherty EM, Fotsch C, Bo Y, Chakrabarti PP, Chen N, Gavva N, Han N, Kelly MG, Kincaid J, Klionsky L, Liu Q, Ognyanov VI, Tamir R, Wang X, Zhu J, Norman MH, Treanor JJ. Discovery of potent, orally available vanilloid receptor-1 antagonists. Structure-activity relationship of N-aryl cinnamides. J Med Chem 48: 71–90, 2005. [DOI] [PubMed] [Google Scholar]
- 15. Everaerts W, Sepulveda MR, Gevaert T, Roskams T, Nilius B, De Ridder D. Where is TRPV1 expressed in the bladder, do we see the real channel? Naunyn Schmiedebergs Arch Pharmacol 379: 421–425, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Farr GA, Hull M, Mellman I, Caplan MJ. Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. J Cell Biol 186: 269–282, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fujimoto S, Mori M, Tsushima H, Kunimatsu M. Capsaicin-induced, capsazepine-insensitive relaxation of the guinea-pig ileum. Eur J Pharmacol 530: 144–151, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Garami A, Shimansky YP, Pakai E, Oliveira DL, Gavva NR, Romanovsky AA. Contributions of different modes of TRPV1 activation to TRPV1 antagonist-induced hyperthermia. J Neurosci 30: 1435–1440, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, Doherty EM, Norman MH, Wild KD, Bannon AW, Louis JC, Treanor JJ. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 313: 474–484, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Geiges D, Meyer T, Marte B, Vanek M, Weissgerber G, Stabel S, Pfeilschifter J, Fabbro D, Huwiler A. Activation of protein kinase C subtypes alpha, gamma, delta, epsilon, zeta, and eta by tumor-promoting and nontumor-promoting agents. Biochem Pharmacol 53: 865–875, 1997. [DOI] [PubMed] [Google Scholar]
- 21. Gottardi CJ, Dunbar LA, Caplan MJ. Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am J Physiol Renal Fluid Electrolyte Physiol 268: F285–F295, 1995. [DOI] [PubMed] [Google Scholar]
- 22. Grossmann J, Maxson JM, Whitacre CM, Orosz DE, Berger NA, Fiocchi C, Levine AD. New isolation technique to study apoptosis in human intestinal epithelial cells. Am J Pathol 153: 53–62, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 24. Hamamoto T, Takumida M, Hirakawa K, Takeno S, Tatsukawa T. Localization of transient receptor potential channel vanilloid subfamilies in the mouse larynx. Acta Otolaryngol (Stockh) 128: 685–693, 2008. [DOI] [PubMed] [Google Scholar]
- 25. Harvey JS, Davis C, James IF, Burgess GM. Activation of protein kinase C by the capsaicin analog resiniferatoxin in sensory neurones. J Neurochem 65: 1309–1317, 1995. [DOI] [PubMed] [Google Scholar]
- 26. Hibino Y, Morise M, Ito Y, Mizutani T, Matsuno T, Ito S, Hashimoto N, Sato M, Kondo M, Imaizumi K, Hasegawa Y. Capsaicinoids regulate airway anion transporters through Rho kinase- and cAMP-dependent mechanisms. Am J Respir Cell Mol Biol 45: 684–691, 2011. [DOI] [PubMed] [Google Scholar]
- 27. Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 43: 143–201, 1991. [PubMed] [Google Scholar]
- 28. Holzer P. TRPV1: a new target for treatment of visceral pain in IBS? Gut 57: 882–884, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacobs JM, Fedor-Chaiken M, Merk LM, Delpire E, Matthews JB, Worrell RT. Epithelial cell specific capsaicin effect on Cl− secretion and NKCC1 (Abstract). FASEB J 21: A542, 2007. [Google Scholar]
- 30. Kishimoto E, Naito Y, Handa O, Okada H, Mizushima K, Hirai Y, Nakabe N, Uchiyama K, Ishikawa T, Takagi T, Yagi N, Kokura S, Yoshida N, Yoshikawa T. Oxidative stress-induced posttranslational modification of TRPV1 expressed in esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol 301: G230–G238, 2011. [DOI] [PubMed] [Google Scholar]
- 31. Leung FW. Capsaicin-sensitive intestinal mucosal afferent mechanism and body fat distribution. Life Sci 83: 1–5, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Lewis SA, Eaton DC, Clausen C, Diamond JM. Nystatin as a probe for investigating the electrical properties of a tight epithelium. J Gen Physiol 70: 427–440, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu G, Henderson D, Liu L, Reinhart PH, Simon SA. TRPV1b, a functional human vanilloid receptor splice variant. Mol Pharmacol 304: 1119–1127, 2005. [DOI] [PubMed] [Google Scholar]
- 34. Lytle C, Xu JC, Biemesderfer D, Forbush B., 3rd Distribution and diversity of Na-K-Cl cotransport proteins: a study with monoclonal antibodies. Am J Physiol Cell Physiol 269: C1496–C1505, 1995. [DOI] [PubMed] [Google Scholar]
- 35. Maguire D, MacNamara B, Cuffe JE, Winter D, Doolan CM, Urbach V, O'Sullivan GC, Harvey BJ. Rapid responses to aldosterone in human distal colon. Steroids 64: 51–63, 1999. [DOI] [PubMed] [Google Scholar]
- 36. Massa F, Sibaev A, Marsicano G, Blaudzun H, Storr M, Lutz B. Vanilloid receptor (TRPV1)-deficient mice show increased susceptibility to dinitrobenzene sulfonic acid induced colitis. J Mol Med 84: 142–146, 2006. [DOI] [PubMed] [Google Scholar]
- 37. Matsumoto K, Kurosawa E, Terui H, Hosoya T, Tashima K, Murayama T, Priestley JV, Horie S. Localization of TRPV1 and contractile effect of capsaicin in mouse large intestine: high abundance and sensitivity in rectum and distal colon. Am J Physiol Gastrointest Liver Physiol 297: G348–G360, 2009. [DOI] [PubMed] [Google Scholar]
- 38. McNamara B, Winter DC, Cuffe JE, O'Sullivan GC, Harvey BJ. Basolateral K+ channel involvement in forskolin-activated chloride secretion in human colon. J Physiol 519: 251–260, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mun EC, Mayol JM, Riegler M, O'Brien TC, Farokhzad OC, Song JC, Pothoulakis C, Hrnjez BJ, Matthews JB. Levamisole inhibits intestinal Cl− secretion via basolateral K+ channel blockade. Gastroenterology 114: 1257–1267, 1998. [DOI] [PubMed] [Google Scholar]
- 40. Mykoniatis A, Shen L, Fedor-Chaiken M, Tang J, Tang X, Worrell RT, Delpire E, Turner JR, Matlin KS, Bouyer P, Matthews JB. Phorbol 12-myristate 13-acetate-induced endocytosis of the Na-K-2Cl cotransporter in MDCK cells is associated with a clathrin-dependent pathway. Am J Physiol Cell Physiol 298: C85–C97, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Nzegwu HC, Levin RJ. Luminal capsaicin inhibits fluid secretion induced by enterotoxin E. coli STa, but not by carbachol, in vivo in rat small and large intestine. Exp Physiol 81: 313–315, 1996. [DOI] [PubMed] [Google Scholar]
- 43. Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J Biol Chem 284: 28698–28703, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park K, Brown PD, Kim YB, Kim JS. Capsaicin modulates K+ currents from dissociated rat taste receptor cells. Brain Res 962: 135–143, 2003. [DOI] [PubMed] [Google Scholar]
- 45. Parsons JD. A high-throughput method for fitting dose-response curves using Microsoft Excel. Anal Biochem 360: 309–311, 2007. [DOI] [PubMed] [Google Scholar]
- 46. Powell DW. Immunophysiology of intestinal electrolyte transport. In: Handbook of Physiology. The Gastrointestinal System. Bethesda, MD: Am. Physiol Soc. 1991, p. 591–641. [Google Scholar]
- 47. Prasad M, Matthews JB, He XD, Akbarali HI. Monochloramine directly modulates Ca2+-activated K+ channels in rabbit colonic muscularis mucosae. Gastroenterology 117: 906–917, 1999. [DOI] [PubMed] [Google Scholar]
- 48. Reynolds A, Parris A, Evans LA, Lindqvist S, Sharp P, Lewis M, Tighe R, Williams MR. Dynamic and differential regulation of NKCC1 by calcium and cAMP in the native human colonic epithelium. J Physiol 582: 507–524, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev 61: 228–261, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ryves WJ, Evans AT, Olivier AR, Parker PJ, Evans FJ. Activation of the PKC-isotypes alpha, beta 1, gamma, delta and epsilon by phorbol esters of different biological activities. FEBS Lett 288: 5–9, 1991. [DOI] [PubMed] [Google Scholar]
- 51. Sánchez MG, Sánchez AM, Collado B, Malagarie-Cazenave S, Olea N, Carmena MJ, Prieto JC, Díaz-Laviada II. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur J Pharmacol 515: 20–27, 2005. [DOI] [PubMed] [Google Scholar]
- 52. Schumacher MA, Moff I, Sudanagunta SP, Levine JD. Molecular cloning of an N-terminal splice variant of the capsaicin receptor. Loss of N-terminal domain suggests functional divergence among capsaicin receptor subtypes. J Biol Chem 275: 2756–2762, 2000. [DOI] [PubMed] [Google Scholar]
- 53. Smart D, Jerman JC, Gunthorpe MJ, Brough SJ, Ranson J, Cairns W, Hayes PD, Randall AD, Davis JB. Characterisation using FLIPR of human vanilloid VR1 receptor pharmacology. Eur J Pharmacol 417: 51–58, 2001. [DOI] [PubMed] [Google Scholar]
- 54. Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, Gavva NR, Romanovsky AA. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 27: 7459–7468, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sterner O, Szallasi A. Novel natural vanilloid receptor agonists: new therapeutic targets for drug development. Trends Pharmacol Sci 20: 459–465, 1999. [DOI] [PubMed] [Google Scholar]
- 56. Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev 51: 159–212, 1999. [PubMed] [Google Scholar]
- 57. Szolcsanyi J, Szallasi A, Szallasi Z, Joo F, Blumberg PM. Resiniferatoxin: an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther 255: 923–928, 1990. [PubMed] [Google Scholar]
- 58. Tang J, Bouyer P, Mykoniatis A, Buschmann M, Matlin KS, Matthews JB. Activated PKCδ and PKCϵ inhibit epithelial chloride secretion response to cAMP via inducing internalization of the Na+-K+-2Cl− cotransporter NKCC1. J Biol Chem 285: 34072–34085, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tóth A, Boczán J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Édes I, Csiba L, Blumberg PM. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res 135: 162–168, 2005. [DOI] [PubMed] [Google Scholar]
- 60. Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol Cell Physiol 273: C1378–C1385, 1997. [DOI] [PubMed] [Google Scholar]
- 61. Usmani SM, Fois G, Albrecht S, von Aulock S, Dietl P, Wittekindt OH. 2-APB and capsazepine-induced Ca2+ influx stimulates clathrin-dependent endocytosis in alveolar epithelial cells. Cell Physiol Biochem 25: 91–102, 2010. [DOI] [PubMed] [Google Scholar]
- 62. Van Crombruggen K, Van Nassauw L, Derycke L, Timmermans JP, Holtappels G, Hall D, Bachert C. Capsaicin-induced vasodilatation in human nasal vasculature is mediated by modulation of cyclooxygenase-2 activity and abrogated by sulprostone. Naunyn Schmiedebergs Arch Pharmacol 383: 613–626, 2011. [DOI] [PubMed] [Google Scholar]
- 63. Vanner S, MacNaughton WK. Capsaicin-sensitive afferent nerves activate submucosal secretomotor neurons in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 269: G203–G209, 1995. [DOI] [PubMed] [Google Scholar]
- 64. Wang JP, Tseng CS, Sun SP, Chen YS, Tsai CR, Hsu MF. Capsaicin stimulates the non-store-operated Ca2+ entry but inhibits the store-operated Ca2+ entry in neutrophils. Toxicol Appl Pharmacol 209: 134–144, 2005. [DOI] [PubMed] [Google Scholar]
- 65. Wong SM, Tesfaye A, DeBell MC, Chase HS., Jr Carbachol increases basolateral K+ conductance in T84 cells. Simultaneous measurements of cell [Ca2+] and gK explore calcium's role. J Gen Physiol 96: 1271–1285, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yajima T. Luminal propionate-induced secretory response in the rat distal colon in vitro. J Physiol 403: 559–575, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.