Abstract
Objective
The objective of this study was to investigate potential pleiotropic effects of rosuvastatin (RSV) in left ventricular (LV) myocardium of dogs with moderate heart failure (HF).
Methods
LV tissue was obtained from HF dogs randomized to 3 months therapy with low dose (LD) RSV (n=7), high dose (HD) RSV (n=7) or to no therapy (Control, n=7), and from 7 normal (NL) dogs. mRNA and protein expression of pro-hypertrophic mediators NGFI-A binding protein 1 (Nab1), phosphatase and tensin homolog (PTEN), phosphoinositide-3 kinase (PI3K) and mammalian target of rapamycin (mTOR); pro-inflammatory cytokine interleukin-6 (IL-6); bone marrow-derived stem cells (BMSCs) markers cKit and Sca1; vascular endothelial (VEGF) and fibroblast (FGF) growth factors and nitric oxide synthase (NOS) isoforms were measured.
Results
Nab1, PTEN, PI3K, mTOR, and IL-6 increased in Controls. HD RSV reduced expression of Nab1, PTEN, PI3K, mTOR, and IL-6 to near normal levels. cKit and Sca1 significantly increased while VEGF and FGF decreased in Controls compared to NL. RSV therapy further increased expression of cKit, Sca1, VEGF and FGF. HD RSV normalized expression of NOS isoforms.
Conclusion
These pleiotropic effects of RSV may account, in part, for the observed beneficial effect of RSV on LV function and structural remodeling.
Keywords: Inflammation, Cytokines, Growth factors, Nitric oxide synthase, Hypertrophy, Stem cells
Introduction
The 3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitors, or statins, have been consistently shown to reduce morbidity and mortality from atherosclerotic cardiovascular disease (1). Beyond their lipid-lowering action, statins possess pleiotropic effects (2) including anti-inflammatory and anti-hypertrophic (3), pro-angiogenic (4) and bone marrow-derived stem cell (BMSCs) mobilizing properties (3,5,6), potentially providing a biologic rationale for their use in the prevention and treatment of left ventricular (LV) dysfunction and heart failure (HF). Albeit partially disappointing (7, 8), overall the gathered evidence on the use of statins in HF due to LV systolic dysfunction suggests that their use is safe and associated with improved LV ejection fraction (EF) and decreased hospitalization for worsening HF (9). Encouraging data have been recently reported with higher statin doses (10).
We have previously shown that early, long-term monotherapy with high-dose (HD) rosuvastatin (RSV) prevents the progressive LV dysfunction and remodeling seen in dogs with moderate HF (3). These beneficial effects on LV function and global cardiac remodeling are associated at the cellular level with reduced myocardial fibrosis and myocyte hypertrophy, and with increased capillary density. HD RSV also showed anti-inflammatory properties by down-regulating TNF-α and gelatinases expression, and stimulated mobilization of circulating Sca1 positive BMSCs. Similar findings have been partly confirmed also in patients with chronic HF (10), yet the underlying mechanisms and the pathways involved in mediating such positive effects are only partially known.
In the present study, we examined the effects, in LV tissue of dogs with moderate HF, of RSV monotherapy on the mRNA and protein expression of specific pro-hypertrophic mediators and pro-inflammatory cytokines and of angiogenetic growth factors, BMSCs markers and nitric oxide synthase (NOS) isoforms in all of which pleiotropic properties potentially account for the observed beneficial effects of HD RSV in this model of HF.
Methods
Experimental Model
The canine model of chronic post-ischemic HF used in this study was previously described in detail (11). In this preparation, LV dysfunction is produced by multiple sequential coronary microembolizations with polystyrene Latex microspheres (70–102 µm in diameter), which results in loss of viable myocardium. The model presents many of the hemodynamic and neurohormonal sequelae of HF observed in humans, as marked and progressive depression of LV systolic and diastolic function, reduced cardiac output, and increased LV filling pressures. In the present study, 21 healthy normocholesterolemic mongrel dogs, weighing between 20 to 30 kg, underwent serial coronary microembolizations to produce HF. To create moderate heart failure, coronary microembolizations were performed 1 to 3 weeks apart and were discontinued when LV ejection fraction (EF), determined angiographically, was between 30 and 40%. All the procedures were performed during cardiac catheterization under general anesthesia and sterile conditions. The anesthesia regimen consisted of a combination of intravenous administration of oxymorphone (0.22 mg/kg), diazepam (0.17 mg/kg), and sodium pentobarbital (150–250 mg to effect). The study was approved by the Henry Ford Hospital Care of Experimental Animals Committee and conformed to the “Position of the American Heart Association on Research Animal Use” (12).
Study Protocol
Two weeks after the last embolization, dogs underwent a pre-randomization left and right cardiac catheterization. One day later, dogs were randomized to 3 months of oral monotherapy with low-dose RSV (0.5 mg once daily, n=7), high-dose RSV (3.0 mg once daily, n=7) or no therapy at all (Control, n=7). At the end of the follow-up period, a final left and right cardiac catheterization was performed, then under general anesthesia the chest was opened and the heart rapidly removed for subsequent histological and biochemical examination. LV tissue samples were obtained and processed alike from 6 normal dogs for comparisons. The results of the hemodynamic, angiographic and histomorphometric analyses performed on the dogs enrolled in the study have been previously published (3).
mRNA and Protein Expression
All tissue samples were submitted for analysis without treatment regimen identifiers. mRNA expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), of pro-hypertrophic mediators NGFI-A binding protein 1 (Nab1), phosphatase and tensin homolog (PTEN), phosphoinositide-3 kinase (PI3K) and mammalian target of rapamycin (mTOR); the pro-inflammatory cytokines interleukin-6 (IL-6); bone marrow-derived stem cells (BMSCs) markers cKit and Sca1 (3) vascular endothelial (VEGF) and fibroblast (FGF) growth factors; and of endothelial (eNOS), inducible (iNOS) and neuronal (nNOS) nitric oxide synthase isoforms was measured. Total RNA with an absorbance ratio (260 nm/280 nm) above 1.7 was isolated from frozen LV tissue and approximately 4–10 µg RNA was reverse-transcribed into cDNA in an assay volume of 80 µl as described previously (13, 14). Protein levels of Nab1, PTEN, PI3K, mTOR, IL-6, cKIT, Sca-1, VEGF, FGF, eNOS, iNOS and nNOS were measured in LV homogenate by Western blots as described previously (13, 14). Primary antibodies specific to each protein were diluted based on the supplier’s instructions (Santa Cruz biotechnology Inc., Santa Cruz, CA; Chemicon, Temecula, CA; Cell Signalling, Danvers, MA and BD Biosciences). In all instances, the antibody was present in excess over the antigen and the density of each protein band was in the linear scale. Band intensities were quantified in densitometric units.
Statistical Analysis
In the original study described in reference 3, a power calculation based on LV ejection fraction as being the primary end-point of interest, a sample size of n=7 per study group was sufficient to provide 80% power to detect effect sizes of 1.52 standard deviations or more by t-test. In the present study, between groups comparisons were performed by using a one way analysis of variance (ANOVA), with α set at 0.05. If significance was attained, pairwise comparisons were performed by means of the Student-Newman-Keuls test, with a P<0.05 considered significant. All the data are reported as the mean ± SEM.
Results
The results of the hemodynamic, angiographic and histomorphometric analyses performed on the dogs enrolled in the study have been previously reported (3). All dogs were normocholesterolemic and no significant differences in total cholesterol and tryglicerides levels were observed at any of the study time-points (3).
Effect of Rosuvastatin on Pro-Hypertrophic Mediators and Pro-Inflammatory Cytokines
Results of mRNA expression of all selected genes and GADPH are shown in Table 1 and Figure 1. The mRNA expression of Nab1, PTEN, PI3K and mTOR was increased in Control HF dogs as compared to Normal dogs. Both doses of RSV reduced the levels of Nab1, mTOR, PTEN and PI3K but the change reached significance for only PTEN with HD RSV. mRNA expression of IL-6 was significantly upregulated in Control HF dogs. LD RSV therapy was associated with a modest but insignificant reduction of IL-6 levels while HD RSV therapy normalized mRNA expression of IL-6.
Table 1.
mRNA expression of all studied genes in densitometric units
| Normal (n=6) |
HF Control (n=7) |
Low-Dose RSV (n=7) |
High-Dose RSV (n=7) |
|
|---|---|---|---|---|
| Nab1 | 6607 ± 485 | 9388 ± 450* | 8396 ± 769 | 7579 ± 940 |
| PTEN | 8024 ± 332 | 11531 ± 493* | 12109 ± 334* | 9442 ± 797† ‡ |
| PI3K | 6336 ± 713 | 9994 ± 480* | 9363 ± 703* | 7830 ± 677 |
| mTOR | 6146 ± 850 | 9181 ± 663 | 8630 ± 1099 | 6710 ± 778 |
| IL-6 | 7102 ± 790 | 15611 ± 2749* | 11717 ± 1865 | 6490 ± 1090† |
| cKit | 624 ± 38 | 911 ± 48* | 960 ± 43* | 1213 ± 86* † ‡ |
| Sca1 | 822 ± 80 | 1104 ± 55* | 1220 ± 71* | 1267 ± 52* |
| VEGF | 10558 ± 924 | 7178 ± 270* | 11874 ± 617† | 10317 ± 1128† |
| FGF | 3914 ± 413 | 1926 ± 391* | 1748 ± 684* | 3740 ± 498† ‡ |
| eNOS | 1347 ± 95 | 876 ± 66* | 1210 ± 95† | 1069 ± 40* |
| iNOS | 1423 ± 129 | 1730 ± 71 | 1511 ± 115 | 1163 ± 97† |
| nNOS | 8381 ± 837 | 16625 ± 2101* | 11522 ± 1534† | 8011 ± 1020† |
| GADPH | 2571 ± 109 | 2637 ± 86 | 2851 ± 133 | 2655 ± 111 |
Nab1=NGFI-A binding protein; PTEN=phosphatase and tensin homolog; PI3K=phosphoinositide-3 kinase; mTOR=mammalian target of rapamycin; IL-6=interleukin-6; VEGF=vascular endothelial growth factor; FGF=fibroblast growth factors; eNOS=endothelial nitric oxide synthase; iNOS=inducible nitric oxide synthase; nNOS=neuronal nitric oxide synthase; GAPDH=glyceraldehydes 3-phosphate dehydrogenase.
P<0.05 vs. Normal;
P<0.05 vs. HF Control;
P<0.05 vs. Low-Dose Rosuvastatin.
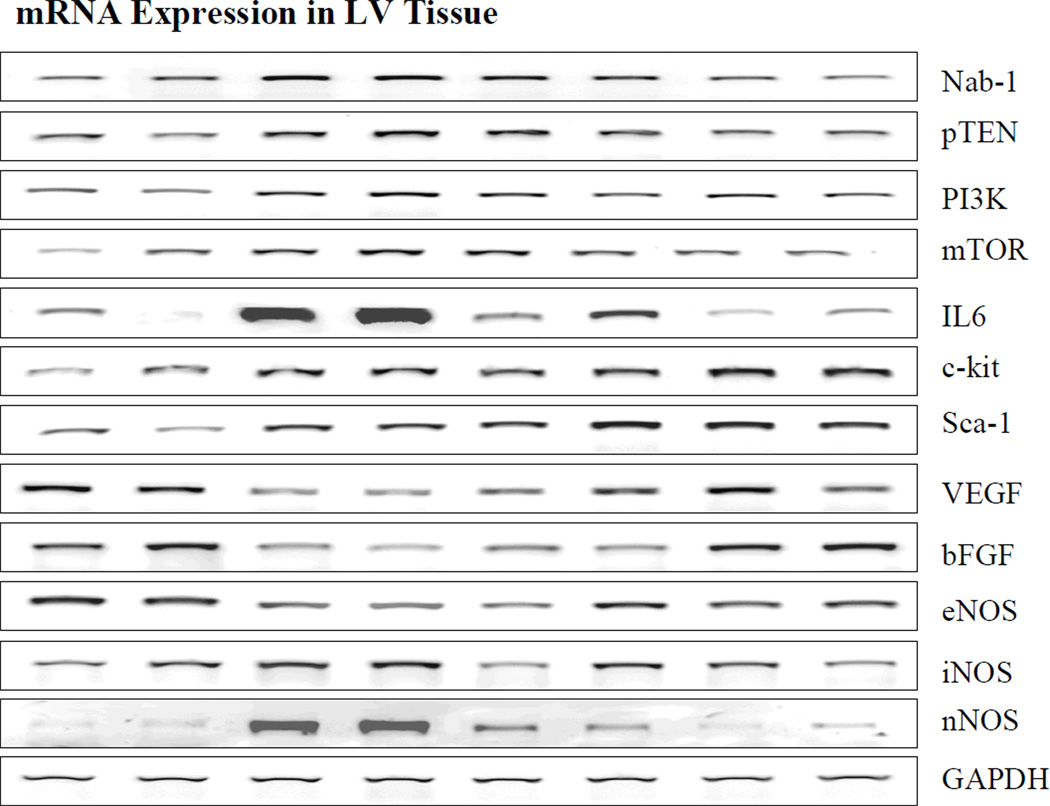
Figure 1.
Bands depicting mRNA expression. From left to right: Bands 1 and 2 are from normal dogs, bands 3 and 4 are from untreated heart failure dogs, bands 5 and 6 are from heart failure dogs treated with low dose rosuvastatin and bands 7 and 8 are from heart failure dogs treated with high dose rosuvastation. Nab1=NGFI-A binding protein; PTEN=phosphatase and tensin homolog; PI3K=phosphoinositide-3 kinase; mTOR=mammalian target of rapamycin; IL-6=interleukin-6; ckit and Sca-1=bone marrow derived stem cells markers; VEGF=vascular endothelial growth factor; bFGF=basic fibroblast growth factors; eNOS=endothelial nitric oxide synthase; iNOS=inducible nitric oxide synthase; nNOS=neuronal nitric oxide synthase; GAPDH=glyceraldehydes 3-phosphate dehydrogenase.
Results of protein expression of pro-hypertrophic and pro-inflammatory mediators are shown in Table 2 and Figure 2. The protein expression of Nab1, PTEN, PI3K and mTOR was significantly increased in Control HF dogs as compared to Normal dogs. Therapy with both doses of RSV significantly decreased levels of PTEN, PI3K and mTOR as compared to Control, with a more significant reduction seen in HD RSV-treated dogs. Conversely, only HD RSV treatment was associated with a significant reduction in Nab1 levels which were restored to near normal values. IL-6 expression was up-regulated in Control HF dogs as compared to Normal dogs.
Table 2 .
Protein expression in densitometric units of pro-hypertrophic and pro-inflammatory mediators
| Normal (n=6) |
HF Control (n=7) |
Low-Dose RSV (n=7) |
High-Dose RSV (n=7) |
|
|---|---|---|---|---|
| Nab1 | 2521 ± 40 | 3154 ± 116* | 3368 ± 148* | 2807 ± 59† ‡ |
| PTEN | 896 ± 101 | 1692 ± 104* | 1350 ±43* † | 1074 ± 54† ‡ |
| PI3K | 2474 ± 94 | 3289 ± 59* | 2722 ± 85* † | 2284 ± 61† ‡ |
| mTOR | 12276 ± 434 | 16414 ± 751* | 11731 ± 715† | 10827 ± 662† |
| IL-6 | 206 ± 4 | 307 ± 20* | 222 ± 9† | 227 ± 10† |
| cKit | 2562±153 | 3424±93* | 3918±162* | 3385±82* |
| Sca1 | 2179±259 | 3107±232* | 3579±99* | 3467±308* |
Abbreviations as in table 1.
P<0.05 vs. Normal;
P<0.05 vs. HF Control;
P<0.05 vs. Low-Dose Rosuvastatin
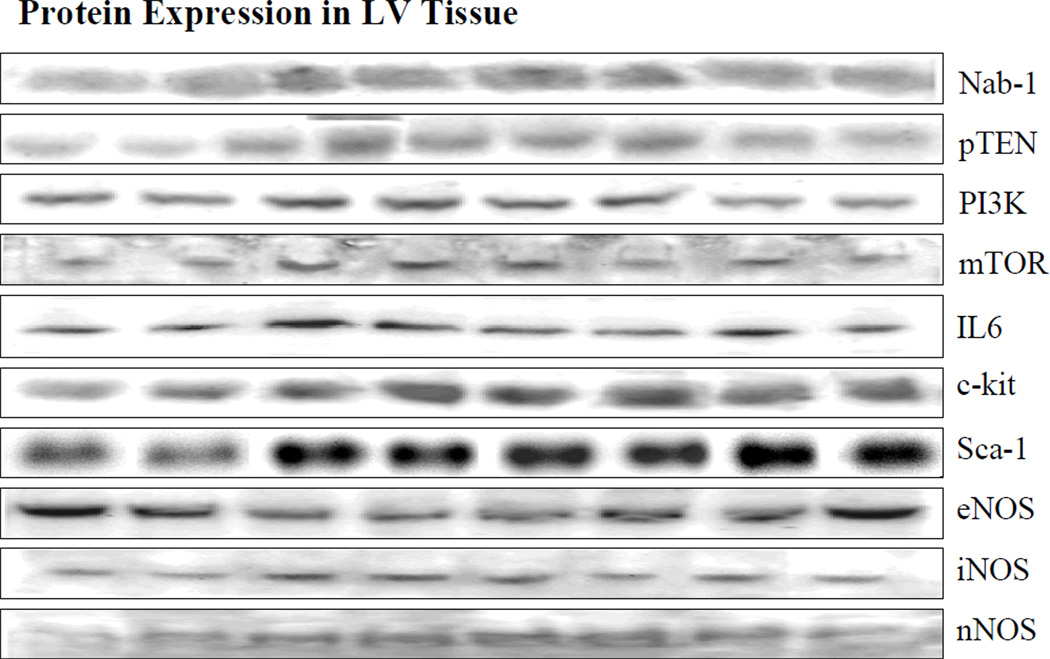
Figure 2.
Bands depicting protein expression. From left to right: Bands 1 and 2 are from normal dogs, bands 3 and 4 are from untreated heart failure dogs, bands 5 and 6 are from heart failure dogs treated with low dose rosuvastatin and bands 7 and 8 are from heart failure dogs treated with high dose rosuvastation. Abbreviations as in Figure 1.
Effect of Rosuvastatin on Growth Factors and Stem Cell Markers
Table 1 and Figure 1 summarize the results of the mRNA expression of VEGF, FGF, cKit and Sca1. VEGF and FGF mRNA expression was significantly reduced while that of cKit and Sca1(3) significantly increased in Control HF dogs as compared to Normal dogs. LD and HD RSV significantly increased mRNA expression of VEGF with near normal levels restored with LD and HD. Only HD RSV was on the other hand associated with normalization of FGF expression. mRNA expression of cKit and Sca1 increased significantly with both HD RSV.
The effects of RSV therapy on the protein expression of VEGF and FGF, and of cKit and Sca1 are shown in Figure 3 and Table 2, respectively. The expression of VEGF and FGF was significantly down-regulated in Control HF dogs as compared to Normal. Three months of RSV therapy restored VEGF and FGF expression to near normal levels. Protein expression of cKit and Sca1 was significantly enhanced in HF Control dogs and further increases, compared to Normal dogs, were attained after long-term treatment with both LD and HD RSV.
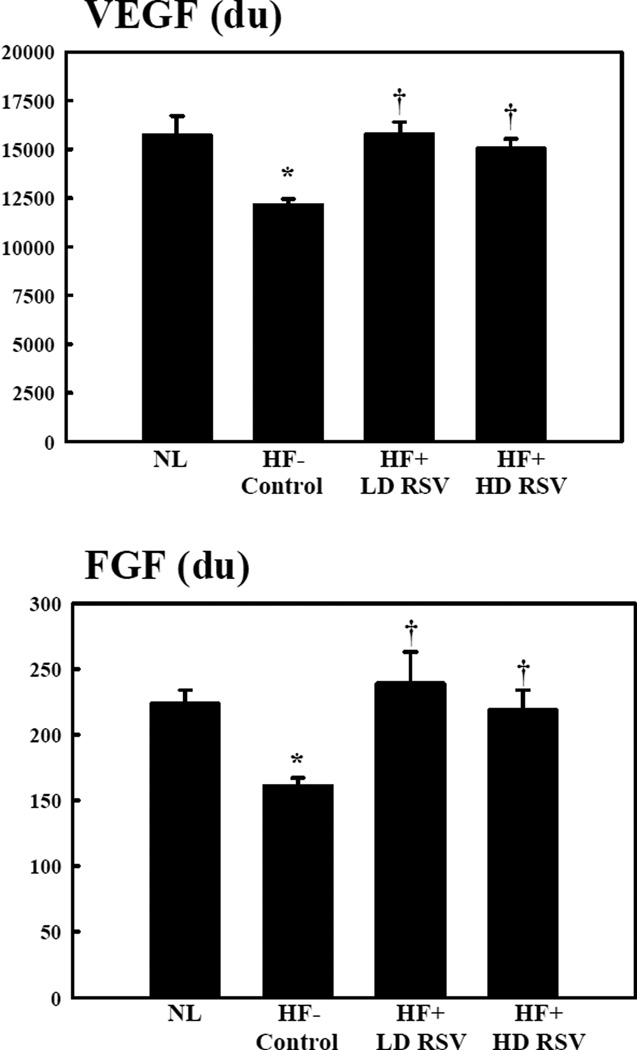
Figure 3.
Vascular endothelial (VEGF) and fibroblast (FGF) growth factor protein levels in densitometric units (du) in normal dogs (NL), heart failure (HF) control dogs, HF dogs randomized to low-dose RSV (LD RSV), and HF dogs randomized to high-dose RSV (HD RSV). *p<0.05 vs. Normal; †p<0.05 vs. Control.
Effect of Rosuvastatin on Nitric Oxide Synthase Isoforms
Results of mRNA expression of NOS isoforms are shown in Table 1 and Figure 1. mRNA expression of eNOS decreased significantly and that of nNOS increased significantly in HF Control dogs compared to Normal dogs. mRNA expression of iNOS tended to increase in HF Controls but the increase did not reach statistical significance. LD RSV restored the expression of eNOS to normal levels and reduced, albeit to a lesser extent than HD, both iNOS and nNOS. HD RSV therapy significantly decreased mRNA expression of nNOS and iNOS, and tended to increase expression of eNOS but not significantly.
Results of protein levels of NOS isoforms are shown in Table 3 and Figure 2. Protein levels of eNOS, decreased significantly and that of iNOS and nNOS increased significantly in HF Control dogs compared to Normal dogs. LD RSV therapy tended to increase the protein expression of eNOS which was restored to near normal values by HD RSV. Treatment with both LD and HD RSV decreased protein expression of nNOS and iNOS but the decrease did not reach statistical significance.
Table 3.
Protein expression in densitometric units of nitric oxide synthase isoforms
| Normal (n=6) |
Control Group (n=7) |
Low-Dose RSV (n=7) |
High-Dose RSV (n=7) |
|
|---|---|---|---|---|
| eNOS | 558 ± 48 | 336± 33* | 400 ± 29* | 518 ± 53† |
| iNOS | 536 ± 37 | 915 ± 89* | 1023 ± 36* | 739 ± 67* ‡ |
| nNOS | 149 ± 6 | 235 ± 12* | 196 ± 22 | 194 ± 17 |
Abbreviations as in table 1.
P<0.05 vs. Normal;
P<0.05 vs. HF Control;
P<0.05 vs. Low-Dose Rosuvastatin.
Discussion
The use of statins in the clinical setting of HF has always been a matter of debate, beginning with the early pathophysiological controversy between their potentially harmful and potentially beneficial biologic effects (15), and reaching its climax in the post-CORONA and post-GISSI-HF era (7, 8). Overall, randomized controlled trials of rosuvastatin, simvastatin and atorvastatin in patients with HF have shown that statin therapy, although exerting neutral effects on cardiovascular and non-cardiovascular mortality, ameliorates LV systolic function and reduces hospitalizations for worsening HF (9). These positive effects may translate into significant benefits in terms of patients’ quality of life and of the socio-economic impact of the syndrome. In the CORONA trial, therapy with RSV in patients with mild to moderate HF showed a positive effects on the primary end-points (7). The CORONA trial also demonstrated an overall reduction in hospitalization (7). More recent evidence with higher doses of RSV (40 mg vs. 10 mg used in the CORONA and GISSI-HF trials), comparable to that used in our studies, are particularly encouraging and suggestive of more extensive anti-remodeling and endothelial function- enhancing effects of more aggressive dosages (10). It appears legitimate, therefore, to further explore the mechanisms potentially underlying the beneficial effects of these agents on the failing LV.
Earlier studies from our group showed that HD RSV prevents progressive LV dysfunction and remodeling in normo-cholesterolemic dogs with moderate HF through non-lipid lowering beneficial effects (3). The present study demonstrates that HD RSV exerts these beneficial effects on global and structural LV remodeling through modulation of several molecular targets including Nab1, PTEN, PI3K, mTOR, IL6, cKit, Sca1, VEGF, FGF, eNOS, iNOS and nNOS. These genes are, in turn, mediators of different signaling pathways involved in the regulation of adaptive and maladaptive hypertrophy (16, 17), inflammation (18), BMSC mobilization, migration and differentiation (5, 6, 19), and cardiac and vascular function (20). Taken together these data do support the hypothesis that RSV possesses pleiotropic properties accounting for the beneficial effects observed in our studies.
Maladaptive cardiac myocyte hypertrophy is one of the key features of the failing heart (21). Statin therapy has been associated with anti-hypertrophic effects in experimental models of HF, likely related to the inhibition of the isoprenylation of small G-proteins, mainly Rac (22, 23). We previously showed that both LD and HD RSV is associated with a significant reduction of myocardial cross-sectional area, a measure of cardiomyocyte hypertrophy (3). In the present study therapy with RSV was associated with favorable modulation of molecular signaling pathways acting upstream of small G-proteins. Nab1 inhibits cardiomyocyte hypertrophy through repression of the transcription factor Egr and is upregulated in conditions of pathological cardiac hypertrophy including experimental and human HF (24). In the present study, HD RSV induced a significant modulation of Nab1 overexpression down to levels similar to that seen in Normal dogs. The PI3K/PTEN signaling is involved in the regulation of several cellular responses to physiological and pathological stimuli acting upstream of several effectors including also Akt and mTOR (16, 17, 25). Cardiovascular effects of the PI3K/PTEN axis include modulation of cell survival and apoptosis, myocyte hypertrophy, myocardial contractility and electrophysiologic properties, energy metabolism and mechanotransduction, and coronary angiogenesis (16, 17). PI3K activity has an essential role in basal cell growth and in adaptive (physiologic) and maladaptive (pathologic) hypertrophy (16, 17). PI3K is activated in many forms of pathological hypertrophy, cardiomyopathy and advanced human HF (26, 27), with Akt and mTOR likely being key mediators of such maladaption. In our study, HD RSV restored near normal levels of both PI3K and mTOR mRNA and significantly reduced protein expression of both genes. This is suggestive of the PI3K/PTEN signaling pathway as a potentially novel molecular target for RSV in HF treatment. Selective inhibition of mTOR also has anti-remodeling effects (28) which may be attained with RSV therapy.
It has been consistently shown that statins reduce inflammatory cytokines in experimental and human HF potentially counteracting the deleterious effects of inflammation on HF pathogenesis and progression (2, 29, 30, 31). In the CORONA trial median levels of high-sensitivity C-reactive protein were reduced by 31.6% in the RSV group and increased by 5.5% in the placebo group with an absolute difference of 37.1% (P<0.001) (7). In the present study, HD RSV significantly decreased the HF-induced upregulation of IL-6. In a previous study, we showed that HD RSV also reduced the expression of TNF-α (3). These cytokines are up-regulated in HF and promote cardiomyocyte hypertrophy, apoptosis, re-induction of the fetal gene program, and endothelial dysfunction, ultimately leading to worsening LV dysfunction and remodeling and reduced functional capacity (18). Reduction of serum markers of inflammation such as TNF-α and IL-6 by means of statin treatment has been largely associated with improved LV EF and attenuated LV remodeling (29).
Myocardial regeneration has been and still is regarded as a potential option to support the failing heart. Evidence is available suggestive of statins as capable of promoting angiogenesis via activation of PI3K/Akt signaling pathway (4). Accordingly, statin therapy may result in increased numbers of endothelial progenitor cells (EPCs), induction of EPC differentiation, improved EPC survival and VEGF-induced migration and myocardial colonization (4, 5). In the present study RSV concomitantly up-regulated the expression of stem cell markers and of VEGF and FGF all of which possibly contribute to the observation of an increased capillary density, a finding potentially consistent with drug-induced myocardial neo-vascularization (3). A limitation of this study is the absence of direct histologic and/or biochemical studies in heart tissue to support a potential causal association between up-regulation of growth factors and the development of neo-vascularization and between up-regulation of stem cell markers and BMSC mobilization, migration and differentiation
Lastly, we observed a near normalization of the expression of NOS isoforms associated with HD RSV therapy. nNOS and eNOS are constitutively present in the heart while iNOS is expressed in disease states in response to inflammatory stimuli (20). NOS isoforms contribute to the control of many cardiac and vascular functions and a perturbation of their balance is seen in cardiac disease. Disregulation of NO and increased oxidative and nitrosative stress are implicated in the genesis of HF (32, 33). Inflammatory cytokine-induced down-regulation of eNOS expression seems to play a major role in the genesis of endothelial dysfunction in HF. Supportive evidence indicates how statin therapy promotes recovery of endothelial function through increased eNOS expression and reduced oxidative stress (34, 35). Nonetheless, in an experimental model of myocardial infarction in wild-type and eNOS-deficient mice, increased eNOS availability was an essential requirement for statin-induced improvement of EPC mobilization, myocardial neovascularization, LV dysfunction, interstitial fibrosis and survival (36).
Despite evidence in support of a biologic rationale for the use of statins in HF in our translational animal model, large randomized clinical trials of RSV in patients with HF failed to show a survival benefit conferred by statin therapy. A major difference between the animal model and the clinical trials is the issue of optimal background medical therapy which was in place in clinical trial patients but not in animal studies. In addition, the study in animals focused primarily on LV functional improvements resulting from RSV therapy whereas large clinical trials focused primarily on mortality. A recent meta-analysis of randomized control trials of statins versus placebo in patients with HF clearly showed that statins improve LV ejection fraction and decrease hospitalization for worsening HF (9). Although LV ejection fraction data are not available from the CORONA and the Effect of Rosuvastatin in Patients with Chronic Heart Failure Trial (the GISSI-HF trial), data from smaller clinical studies with high dose RSV in patients with HF are in-line with findings in our translational animal model of HF. A recent study by Erbs and colleagues reported a significant increase in LV ejection fraction driven primarily by a significant decrease in LV end-systolic volume in patients with systolic HF patients randomized to 3-month therapy with 40 mg RSV as compared to placebo (10). These findings are in-line with findings in our animal model (3).
In conclusion, the results of the present study are suggestive of a pleiotropic effect of RSV, especially at higher doses, in the treatment of dogs with moderate HF. RSV pleiotropic properties include anti-hypertrophic, anti-inflammatory, BMSCs mobilizing and pro-angiogenetic/myocardial regenerative effects elicited through modulation of the expression of several molecular targets. Modulation of the PI3K/PTEN/Akt signaling pathway is a key mediator of the beneficial effects observed with chronic HD RSV monotherapy.
Acknowledgments
Supported, in part, by research grants from AstraZeneca US and National Heart, Lung, and Blood Institute PO1 HL074237-07
References
- 1.Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 2.Ramasubbu K, Estep J, White DL, Deswal A, Mann DL. Experimental and clinical basis for the use of statins in patients with ischemic and nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:415–426. doi: 10.1016/j.jacc.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Zacà V, Rastogi S, Imai M, et al. Chronic monotherapy with rosuvastatin prevents progressive left ventricular dysfunction and remodeling in dogs with heart failure. J Am Coll Cardiol. 2007;50:551–557. doi: 10.1016/j.jacc.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 4.Kureishi Y, Luo Z, Shiojima I, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llevadot J, Murasawa S, Kureishi Y, et al. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 8.Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 9.Lipinski MJ, Cauthen CA, Biondi-Zoccai GG, et al. Meta-analysis of randomized controlled trials of statins versus placebo in patients with heart failure. Am J Cardiol. 2009;10:1708–1716. doi: 10.1016/j.amjcard.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 10.Erbs S, Beck EB, Linke A, et al. High-dose rosuvastatin in chronic heart failure promotes vasculogenesis, corrects endothelial function, and improves cardiac remodeling - Results from a randomized, double-blind, and placebo-controlled study. Int J Cardiol. 2011;146:56–63. doi: 10.1016/j.ijcard.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Sabbah HN, Stein PD, Kono T, et al. A canine model of chronic heart failure produced by multiple sequential intracoronary microembolization. Am J Physiol. 1991;260:H1379–H1384. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 12.Position of the American Heart Association on Research Animal Use. Circulation. 1985;71:849A–850A. No authors listed. [PubMed] [Google Scholar]
- 13.Rastogi S, Imai M, Sharov VG, Mishra S, Sabbah HN. Darbepoetin-alpha prevents progressive left ventricular dysfunction and remodeling in nonanemic dogs with heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H2475–H2482. doi: 10.1152/ajpheart.00074.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rastogi S, Sharov VG, Mishra S, et al. Ranolazine combined with enalapril or metoprolol prevents progressive LV dysfunction and remodeling in dogs with moderate heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H2149–H2155. doi: 10.1152/ajpheart.00728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Harst P, Voors AA, van Gilst WH, Böhm M, van Veldhuisen DJ. Statins in the treatment of chronic heart failure: biological and clinical considerations. Cardiovasc Res. 2006;71:443–454. doi: 10.1016/j.cardiores.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82:250–260. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 18.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 19.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 20.Umar S, van der Laarse A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem. 2010;333:191–201. doi: 10.1007/s11010-009-0219-x. [DOI] [PubMed] [Google Scholar]
- 21.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2118. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 22.Liao JK. Statin therapy for cardiac hypertrophy and heart failure. J Investig Med. 2004;52:248–253. doi: 10.1136/jim-52-04-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laufs U, Kilter H, Konkol C, Wassmann S, Bohm M, Nickenig G. Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovasc Res. 2002;53:911–920. doi: 10.1016/s0008-6363(01)00540-5. [DOI] [PubMed] [Google Scholar]
- 24.Buitrago M, Lorenz K, Maass AH, et al. The transcriptional repressor Nab1 is a specific regulator of pathological cardiac hypertrophy. Nat Med. 2005;11:837–844. doi: 10.1038/nm1272. [DOI] [PubMed] [Google Scholar]
- 25.Sugden PH. Ras, Akt, and mechanotransduction in the cardiac myocyte. Circ Res. 2003;93:1179–1192. doi: 10.1161/01.RES.0000106132.04301.F5. [DOI] [PubMed] [Google Scholar]
- 26.Baba HA, Stypmann J, Grabellus F, et al. Dynamic regulation of MEK/Erks and Akt/GSK-3beta in human end-stage heart failure after left ventricular mechanical support: myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovasc Res. 2003;59:390–399. doi: 10.1016/s0008-6363(03)00393-6. [DOI] [PubMed] [Google Scholar]
- 27.Haq S, Choukroun G, Lim H, et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 28.Buss SJ, Muenz S, Riffel JH, et al. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54:2435–2446. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 29.Sola S, Mir MQS, Lerakis S, Tandon N, Khan BV. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J Am Coll Cardiol. 2006;47:332–337. doi: 10.1016/j.jacc.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 30.Tousoulis D, Antoniades C, Bosinaku E, et al. Effects of atorvastatin on reactive hyperemia and inflammatory process in patients with congestive heart failure. Atherosclerosis. 2005;178:359–363. doi: 10.1016/j.atherosclerosis.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 31.Mozaffarian D, Minami E, Letterer RA, Lawler RL, McDonald GB, Levy WC. The effects of atorvastatin (10 mg) on systemic inflammation in heart failure. Am J Cardiol. 2005;96:1699–1704. doi: 10.1016/j.amjcard.2005.07.092. [DOI] [PubMed] [Google Scholar]
- 32.Pacher P, Schulz R, Liaudet L. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci. 2005;26:302–310. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 34.Ferrari R, Agnoletti L, Comini L, et al. Oxidative stress during myocardial ischaemia and heart failure. Eur Heart J. 1998;19(Suppl B):B2–B11. [PubMed] [Google Scholar]
- 35.Lefer AM, Scalia R, Lefer DJ. Vascular effects of HMG CoA-reductase inhibitors (statins) unrelated to cholesterol lowering: new concepts for cardiovascular disease. Cardiovasc Res. 2001;49:281–287. doi: 10.1016/s0008-6363(00)00247-9. [DOI] [PubMed] [Google Scholar]
- 36.Landmesser U, Engberding N, Bahlmann FH, et al. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]