Abstract
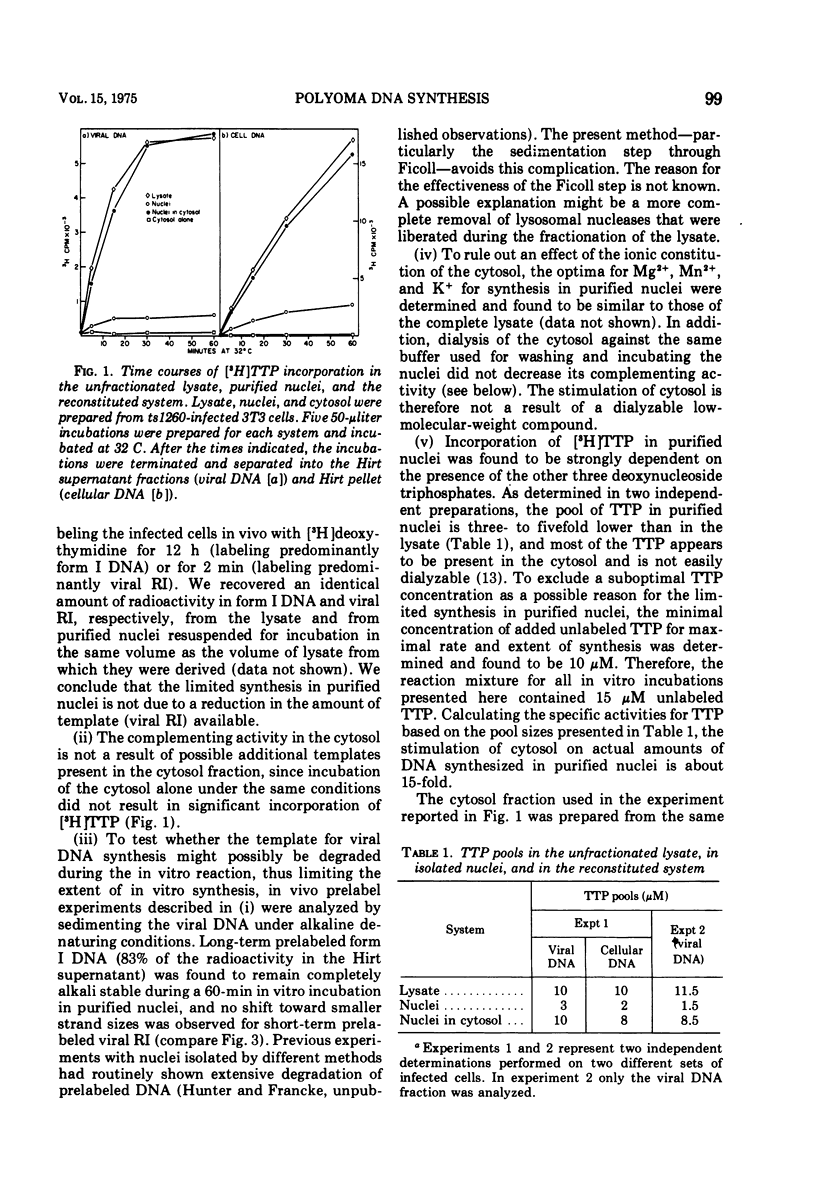
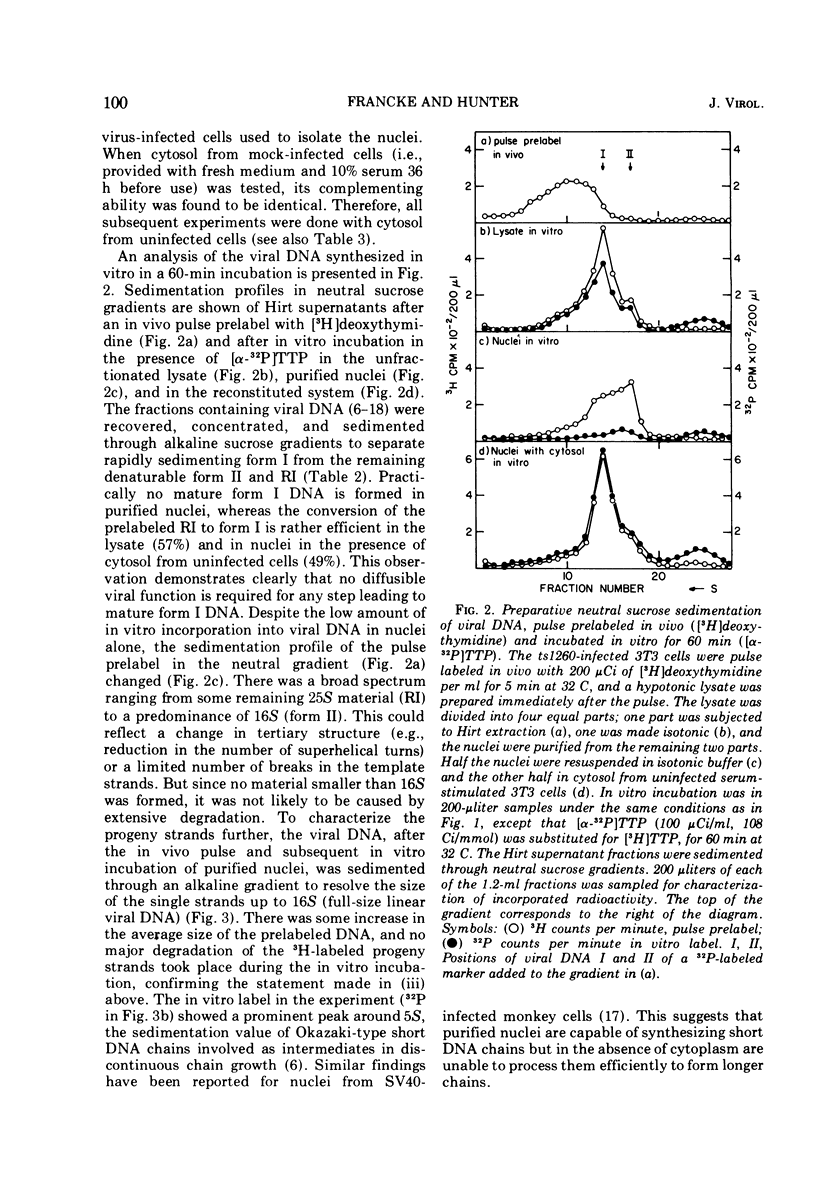
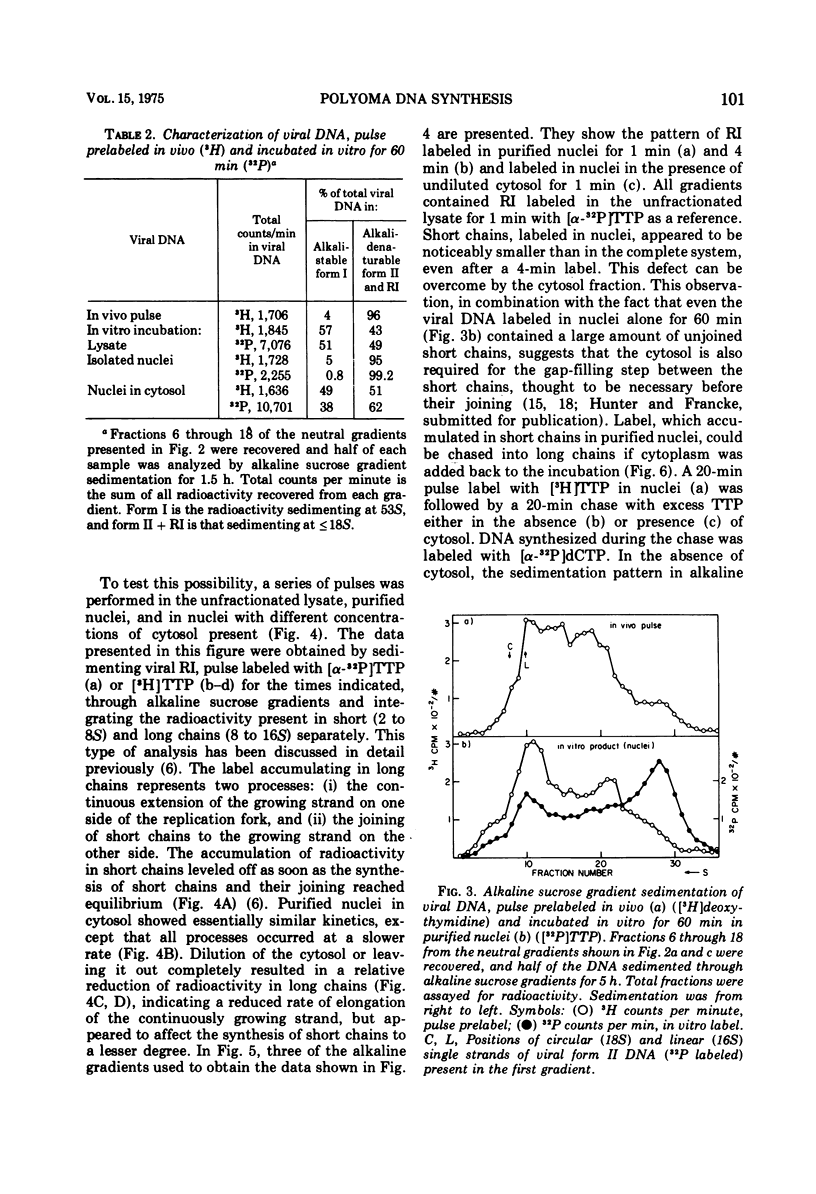
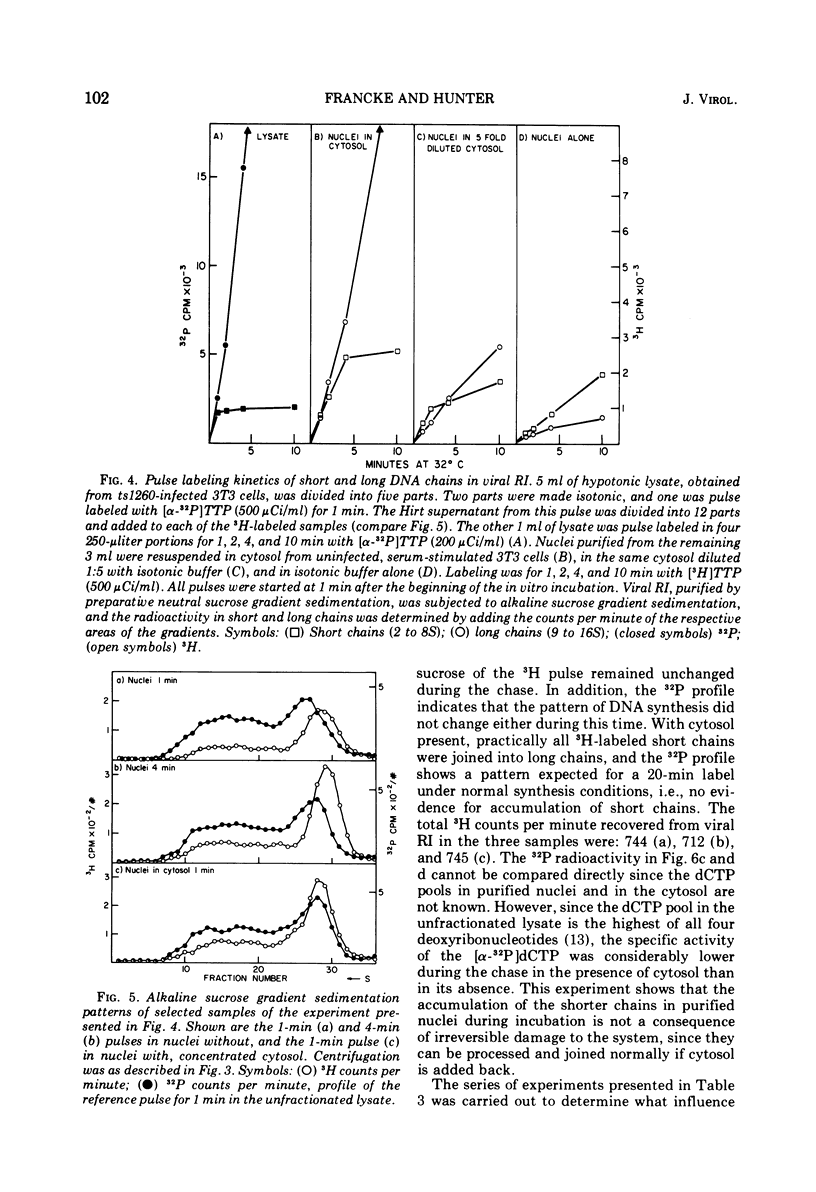
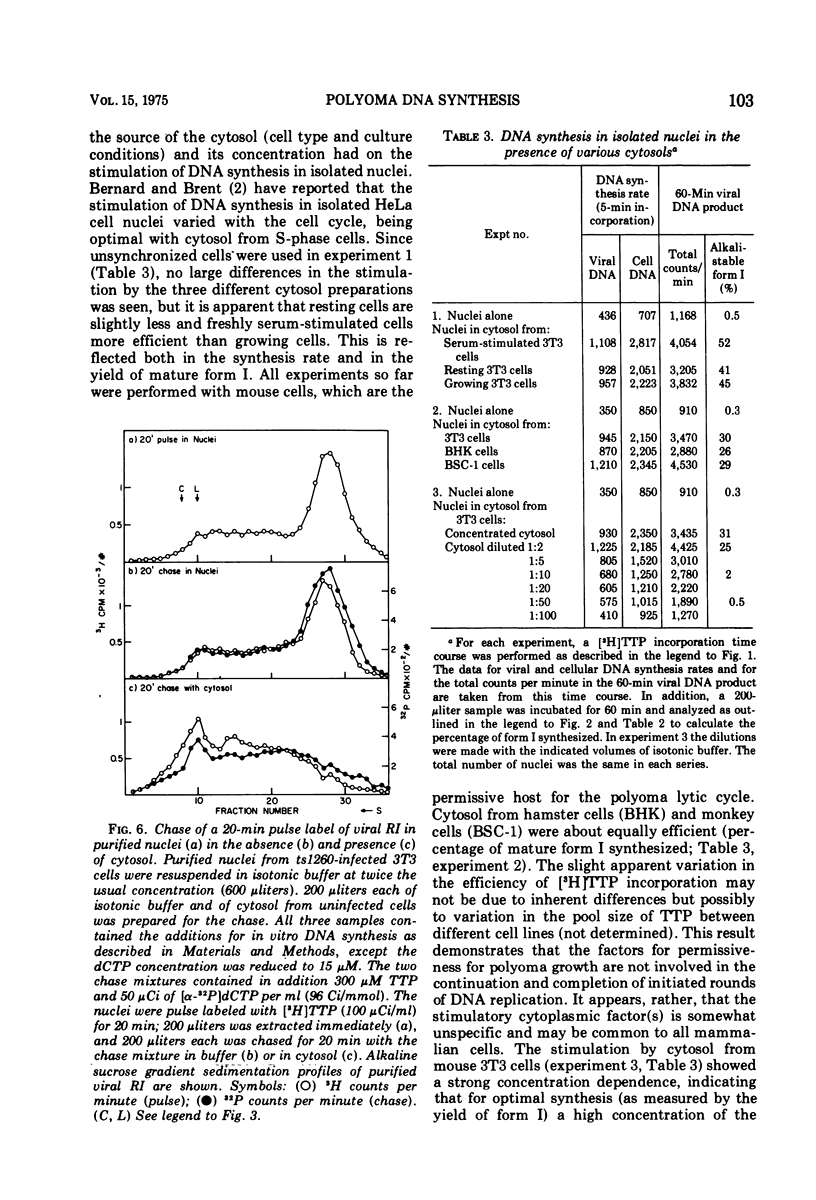
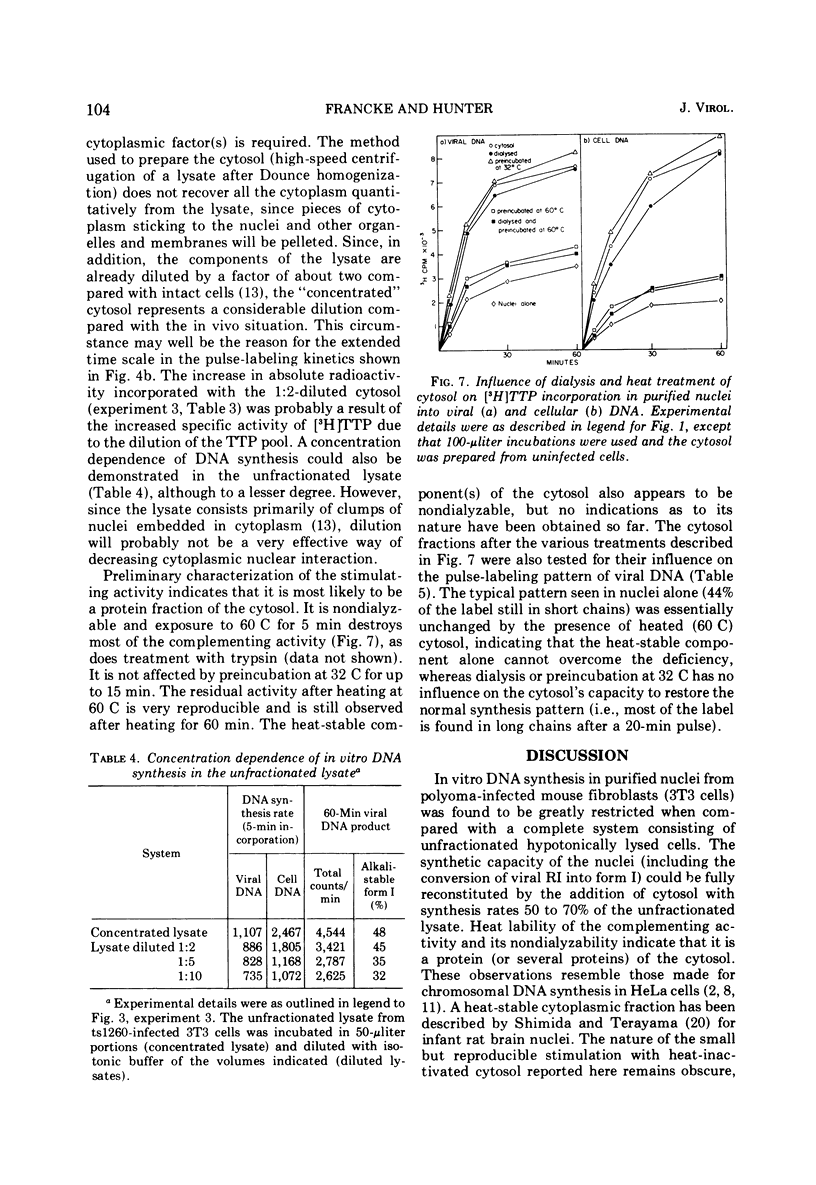
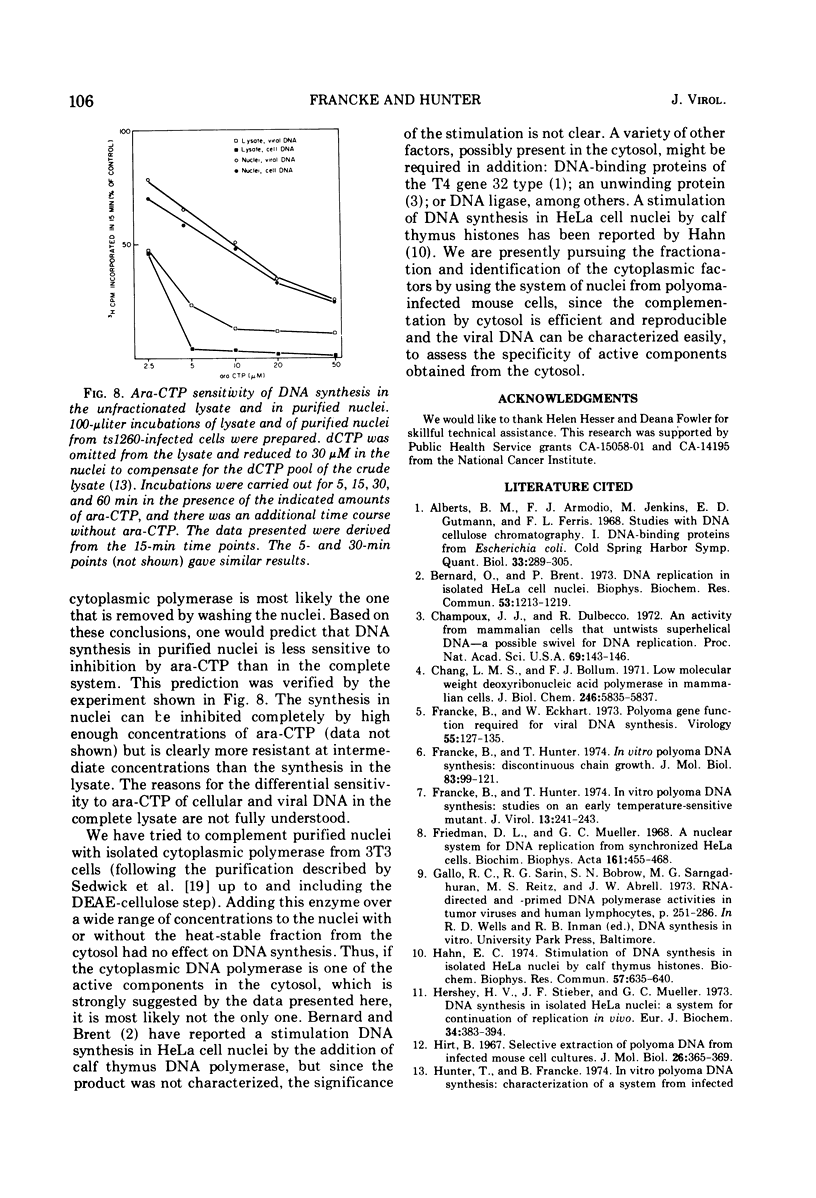
Purified nuclei from polyoma-infected mouse (3T3) cells were found to be greatly reduced in their ability to synthesize viral DNA in vitro when compared with a crude system consisting of an unfractionated hypotonic lysate of the infected cells. The synthetic capacity of the nuclei could be fully reconstituted when a high-speed cytoplasmic supernatant was added back to them. Cytosols from uninfected mouse, monkey, and hamster cells were equally as effective in stimulating purified nuclei as that of virus-infected mouse cells. Optimal complementation required high concentrations of the cytosol, and most of the complementing activity was destroyed by heating to 60 C. Dialysis had no effect on the activity. Analysis of the viral DNA synthesized in purified nuclei showed an accumulation of Okazaki-type short DNA chains, which could be chased into viral progeny DNA strands if cytosol was added back to the nuclei. Kinetic analysis of the pulse-labeling pattern of viral replicative DNA showed a strong dependence of the extension of viral progeny strands and of the processing of Okazaki-type fragments on the amount of cytosol present during the reaction. It is suggested that the cytoplasmic DNA polymerase might be one of the active components in the cytosol, but most likely not the only one.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Amodio F. J., Jenkins M., Gutmann E. D., Ferris F. L. Studies with DNA-cellulose chromatography. I. DNA-binding proteins from Escherichia coli. Cold Spring Harb Symp Quant Biol. 1968;33:289–305. doi: 10.1101/sqb.1968.033.01.033. [DOI] [PubMed] [Google Scholar]
- Bernard O., Brent T. P. DNA replication in isolated HeLa cell nuclei. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1213–1219. doi: 10.1016/0006-291x(73)90594-9. [DOI] [PubMed] [Google Scholar]
- Champoux J. J., Dulbecco R. An activity from mammalian cells that untwists superhelical DNA--a possible swivel for DNA replication (polyoma-ethidium bromide-mouse-embryo cells-dye binding assay). Proc Natl Acad Sci U S A. 1972 Jan;69(1):143–146. doi: 10.1073/pnas.69.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Low molecular weight deoxyribonucleic acid polymerase in mammalian cells. J Biol Chem. 1971 Sep 25;246(18):5835–5837. [PubMed] [Google Scholar]
- Francke B., Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973 Sep;55(1):127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- Francke B., Hunter T. In vitro polyoma DNA synthesis: discontinuous chain growth. J Mol Biol. 1974 Feb 15;83(1):99–121. doi: 10.1016/0022-2836(74)90426-4. [DOI] [PubMed] [Google Scholar]
- Francke B., Hunter T. In vitro polyoma DNA synthesis: studies on an early temperature-sensitive mutant. J Virol. 1974 Jan;13(1):241–243. doi: 10.1128/jvi.13.1.241-243.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. L., Mueller G. C. A nuclear system for DNA replication from synchronized HeLa cells. Biochim Biophys Acta. 1968 Jul 23;161(2):455–468. doi: 10.1016/0005-2787(68)90122-6. [DOI] [PubMed] [Google Scholar]
- Hahn E. C. Stimulation of DNA synthesis in isolated HeLa nuclei by calf thymus histones. Biochem Biophys Res Commun. 1974 Apr 8;57(3):635–640. doi: 10.1016/0006-291x(74)90593-2. [DOI] [PubMed] [Google Scholar]
- Hershey H. V., Stieber J. F., Mueller G. C. Dna synthesis in isolated HeLa nuclei. A system for continuation of replication in vivo. Eur J Biochem. 1973 Apr;34(2):383–394. doi: 10.1111/j.1432-1033.1973.tb02770.x. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Francke B. In vitro polyoma DNA synthesis: characterization of a system from infected 3T3 cells. J Virol. 1974 Jan;13(1):125–139. doi: 10.1128/jvi.13.1.125-139.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Francke B. Letter: In vitro polyoma DNA synthesis: involvement of RNA in discontinuous chain growth. J Mol Biol. 1974 Feb 15;83(1):123–130. doi: 10.1016/0022-2836(74)90427-6. [DOI] [PubMed] [Google Scholar]
- Laipis P. J., Levine A. J. DNA replication in SV40-infected cells. IX. The inhibition of a gap-filling step during discontinuous synthesis of SV40 DNA. Virology. 1973 Dec;56(2):580–594. doi: 10.1016/0042-6822(73)90059-7. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Pigiet V., Winnacker E. L., Abrams R., Reichard P. RNA-linked short DNA fragments during polyoma replication. Proc Natl Acad Sci U S A. 1973 Feb;70(2):412–415. doi: 10.1073/pnas.70.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasba P. K. Synthesis of simian virus 40 DNA in isolated nuclei. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1045–1049. doi: 10.1073/pnas.71.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman N. P., Thoren M. M. Inhibition in the joining of DNA intermediates to growing simian virus 40 chains. J Virol. 1973 May;11(5):721–729. doi: 10.1128/jvi.11.5.721-729.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedwick W. D., Wang T. S., Korn D. Purification and properties of nuclear and cytoplasmic deoxyribonucleic acid polymerases from human KB cells. J Biol Chem. 1972 Aug 25;247(16):5026–5033. [PubMed] [Google Scholar]
- Shimada H., Terayama H. DNA synthesis in isolated nuclei from the brains of rats at different post-partal stages and the infant rat brain cytosol factor stimulating the DNA synthesis in infant rat brain nuclei. Biochim Biophys Acta. 1972 Dec 22;287(3):415–426. doi: 10.1016/0005-2787(72)90285-7. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Replication of polyoma DNA in isolated nuclei. I. Characterization of the system from mouse fibroblast 3T6 cells. J Mol Biol. 1972 Dec 30;72(3):523–537. doi: 10.1016/0022-2836(72)90172-6. [DOI] [PubMed] [Google Scholar]


