Abstract
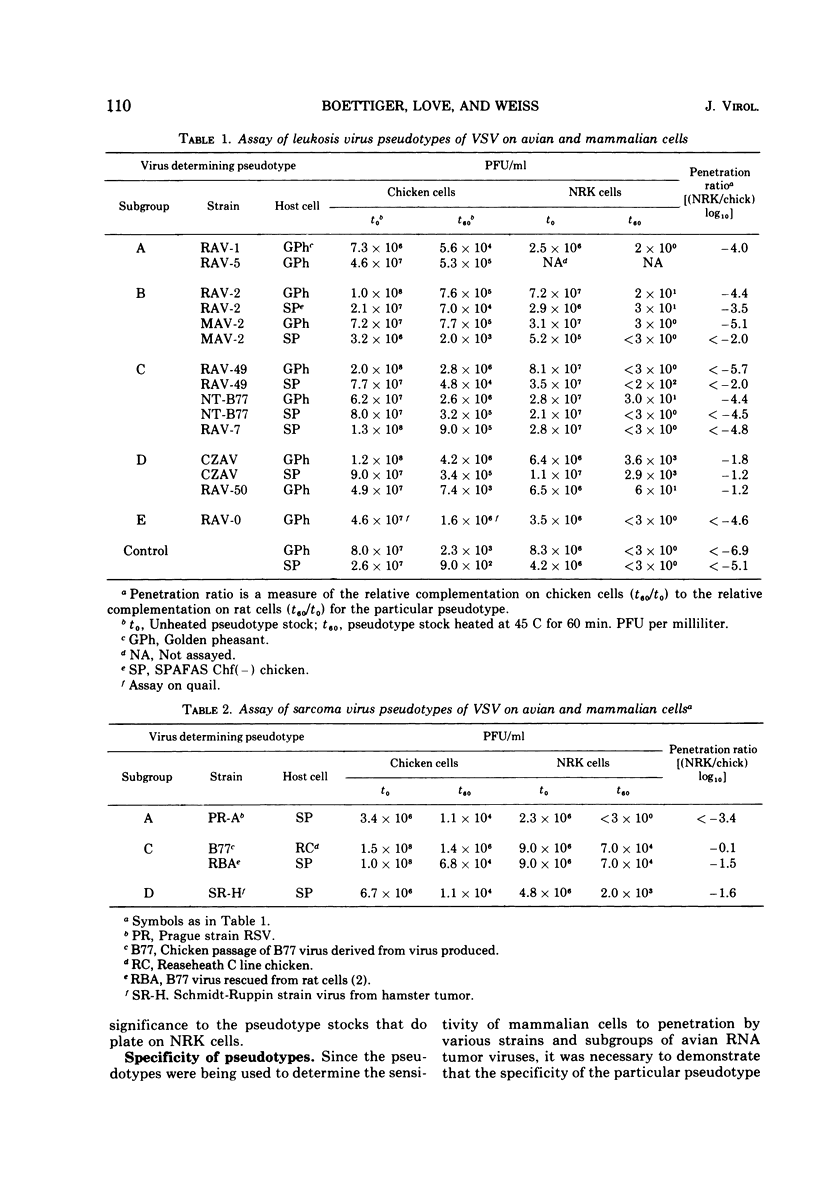
Pseudotypes of vesicular stomatitis virus were prepared with avian sarcoma viruses and avian leukemia viruses representing five different subgroups. These pseudotypes display a host range restricted to that of the avian tumor virus when assayed on avian cells and are neutralized by subgroup-specific antisera. The efficiency of penetration of mammalian cells was assayed by using these vesicular stomatitis virus pseudotypes. Pseudotypes of avian tumor viruses belonging to subgroup D and of B77 virus were able to plate on mammalian cells with a high efficiency, whereas pseudotypes of other strains were not. The efficiency of penetration of the vesicular stomatitis virus pseudotypes was 10-2-to 10-3-fold higher than the efficiency of transformation of the corresponding avian tumor virus strain assayed on mammalian cells, suggesting that there are postpenetration blocks to the expression of transformation in these cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altaner C., Svec F. Virus production in rat tumors induced by chicken sarcoma virus. J Natl Cancer Inst. 1966 Dec;37(6):745–752. [PubMed] [Google Scholar]
- Altaner C., Temin H. M. Carcinogenesis by RNA sarcoma viruses. XII. A quantitative study of infection of rat cells in vitro by avian sarcoma viruses. Virology. 1970 Jan;40(1):118–134. doi: 10.1016/0042-6822(70)90384-3. [DOI] [PubMed] [Google Scholar]
- Boettiger D. Virogenic nontransformed cells isolated following infection of normal rat kidney cells with B77 strain Rous sarcoma virus. Cell. 1974 Sep;3(1):71–76. doi: 10.1016/0092-8674(74)90042-7. [DOI] [PubMed] [Google Scholar]
- Duff R. G., Vogt P. K. Characteristics of two new avian tumor virus subgroups. Virology. 1969 Sep;39(1):18–30. doi: 10.1016/0042-6822(69)90344-4. [DOI] [PubMed] [Google Scholar]
- HLAVAYOVA E., SMIDA J., ALTANER C., SVEC F. PATHOGENICITY OF VIRUS B77 IN RATS. Folia Biol (Praha) 1964;10:301–306. [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Determining factor in the capacity of Rous sarcoma virus to induce tumors in mammals. Proc Natl Acad Sci U S A. 1966 Mar;55(3):532–538. doi: 10.1073/pnas.55.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Miyamoto T., Hanafusa T. A cell-associated factor essential for formation of an infectious form of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):314–321. doi: 10.1073/pnas.66.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Besmer P., Chu L., Baltimore D. Growth of pseudotypes of vesicular stomatitis virus with N-tropic murine leukemia virus coats in cells resistant to N-tropic viruses. J Virol. 1973 Sep;12(3):659–662. doi: 10.1128/jvi.12.3.659-662.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., Soeiro R., Fields B. N. Host restriction of Friend leukemia virus. Role of the viral outer coat. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2549–2553. doi: 10.1073/pnas.70.9.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D. N., Weiss R. A. Pseudotypes of vesicular stomatitis virus determined by exogenous and endogenous avian RNA tumor viruses. Virology. 1974 Jan;57(1):271–278. doi: 10.1016/0042-6822(74)90127-5. [DOI] [PubMed] [Google Scholar]
- SIMKOVIC D., SMIDA J., THURZO V. Release of tumour virus by cells of the B77 fowl virus tumour in vitro. Neoplasma. 1961;8:495–500. [PubMed] [Google Scholar]
- Simkovic D. Characteristics of tumors induced in mammals, especially rodents, by viruses of the avian leukosis sarcoma group. Adv Virus Res. 1972;17:95–127. [PubMed] [Google Scholar]
- Toyoshima K., Friis R. R., Vogt P. K. The reproductive and cell-transforming capacities of avian sarcoma virus B77: inactivation with UV light. Virology. 1970 Sep;42(1):163–170. doi: 10.1016/0042-6822(70)90249-7. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Patterns of viral interference in the avian leukosis and sarcoma complex. Virology. 1966 Nov;30(3):368–374. doi: 10.1016/0042-6822(66)90115-2. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Biggs P. M. Leukosis and Marek's disease viruses of feral red jungle flow and domestic fowl in Malaya. J Natl Cancer Inst. 1972 Dec;49(6):1713–1725. doi: 10.1093/jnci/49.6.1713. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Payne L. N. The heritable nature of the factor in chicken cells which acts as a helper virus for Rous sarcoma virus. Virology. 1971 Aug;45(2):508–515. doi: 10.1016/0042-6822(71)90351-5. [DOI] [PubMed] [Google Scholar]
- Závada J. Pseudotypes of vesicular stomatitis virus with the coat of murine leukaemia and of avian myeloblastosis viruses. J Gen Virol. 1972 Jun;15(3):183–191. doi: 10.1099/0022-1317-15-3-183. [DOI] [PubMed] [Google Scholar]
- Závada J. VSV pseudotype particles with the coat of avian myeloblastosis virus. Nat New Biol. 1972 Nov 22;240(99):122–124. doi: 10.1038/newbio240122a0. [DOI] [PubMed] [Google Scholar]
- Závada J., Závodská E. Complementation and phenotypic stabilization of vesicular stomatitis virus temperature-sensitive and thermolabile mutants by avian myeloblastosis virus. Intervirology. 1974;2(1):25–32. doi: 10.1159/000149401. [DOI] [PubMed] [Google Scholar]


