Abstract
Aggregation of the 140 amino acid protein α-synuclein (α-syn) is linked to the development of Parkinson’s disease (PD). α-Syn is a copper binding protein with potential function as a regulator of metal dependent redox activity. Epidemiological studies suggest that human exposure to excess copper increases the incidence of PD. α-Syn exists in both solution and membrane bound forms. Previous work evaluated the Cu2+ uptake for α-syn in solution and identified Met1 Asp2 and His50 as primary contributors to the coordination shell, with a dissociation constant of approximately 0.1 nM. When bound to the membrane bilayer, α-syn takes on a predominantly helical conformation, which spatially separates His50 from the protein N-terminus and is therefore incompatible with the copper coordination geometry of the solution state. Here we use circular dichroism and electron paramagnetic resonance (continuous wave and pulsed) to evaluate copper coordination to the membrane bound form of α-syn. In this molecular environment, Cu2+ binds exclusively to the protein N-terminus (Met1-Asp2) with no participation from His50. Copper does not alter the membrane bound α-syn conformation, or enhance the protein’s release from the bilayer. The Cu2+ affinity is similar to that identified for solution α-syn suggesting that copper coordination is retained in the membrane. Consideration of these results demonstrates that copper exerts its greatest conformational affect on the solution form of α-syn.
Parkinson’s disease (PD) is a common, age related neurodegenerative disorder that affects over one million individuals in the United States (1). PD results from the loss of dopaminergic neurons in the substantia nigra region of the brain and produces slowness of speech and movement, uncontrolled tremors and difficult breathing (2). While the exact cause of PD is unknown, the neurological protein α-synuclein (α-syn) has been clearly linked to the pathology of PD in both animal and human studies. α-Syn is the primary component of the cytosolic, filamentous inclusions known as Lewy Bodies (LB), a hallmark of the disease ((3, 4), reviewed here (5)).
The 140 residue α-syn protein is natively unfolded in solution, but its seven imperfect 11-residue repeats (approximately residues 1 – 100, Figure 1)) adopt an amphipathic helical structure when associated with cellular membranes (6, 7). The protein also possesses a highly amyloidogenic NAC (non-Abeta component) region and a flexible C terminal extension that is thought to interact with NAC and inhibit aggregation (Figure 1) (8). Although α-syn is found both inside and outside of the cell, it is localized primarily to the presynaptic terminals of dopaminergic neurons, an area of high vesicle traffic (9). Most α-syn research focuses on its role in the synucleinopathies, with emphasis on PD (2-4, 10-12). The normal physiologic function of α-syn is unknown, but recent work suggests a role in the formation of SNARE complexes that regulate vesicular-cell membrane fusion (13-15).
Figure 1.
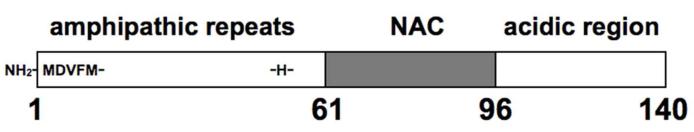
Features of the α-synuclein primary structure identifying the three consensus segments and the amino acids, at the N-terminus and His50, potentially involved in Cu2+ coordination. Residues 9-97, encompassing the amphipathic repeat region and the NAC, form an extended helix when associated with lipid membranes.
α-Syn takes up divalent copper ions with high affinity (Kd ≈ 0.1 nM) (16, 17). Although Cu2+ is normally found at micromolar concentrations in the cerebrospinal fluid (CSF), epidemiological studies identify a significant link between long-term environmental exposure to copper and an increased incidence of fatal PD (18-20). Copper enhances the in vitro aggregation rate of the solution form of α-syn, suggesting that elevated Cu2+ levels may contribute to PD by accelerating the formation of Lewy bodies (21-25). A BLAST sequence comparison shows that the specific residues required for Cu2+ binding, M1, D2 and H50, are highly conserved across species; the interaction between α-syn and Cu2+ may therefore play a role in the protein’s normal physiological function. Other neurodegenerative proteins, such as Aβ in Alzheimer’s disease and PrP in the prion diseases also take up copper (26-30). Unambiguous metalloprotein functions have not yet been identified in these cases, but disregulation of copper homeostasis and redox activity are emerging themes in neurodegenerative disease. α-Syn may serve as a sink for weakly complexed copper, with action localized to the membrane surface.
α-Syn is predominantly an intracellular species, however, the protein is exported to the extracellular space through exosomes in a calcium dependent manner, (31) and this process may be a factor in the pathogenesis of the PD and other synucleinopathies ((32, 33)). Extracellular α-syn is a component of the senile plaques of Alzheimer’s disease, where it contributes approximately 10% of the total protein (34, 35). Moreover, elevated levels of α-syn are found in the CSF of patients with PD and related neurological diseases (36-38), and studies of tissue grafts with α-syn lesions show that aggregates propagate in a prion like fashion ((39) reviewed here (40)).
Intensive work by a number of laboratories has identified Cu2+ coordination sites at several locations in the solution form of α-syn (16, 25, 41-49). (See recent reviews (50-52).) Our lab suggested a single high affinity Cu2+ complex (Kd ≈ 0.1 nM) arising primarily from the N-terminal amine, the backbone nitrogen and side chain carboxyl of Asp2. In addition, the imidazole of His50 simultaneously coordinates this N-terminally bound Cu2+ (Figure 2a), resulting in the formation of a large polypeptide loop. In a membrane environment, where most α-syn resides, this type of coordination environment must be altered since a helical polypeptide conformation would separate the N-terminus and His50 by approximately 75Å, as diagrammed in Figure 2b. Here, using small unilamellar vesicles (SUVs), we characterize the copper coordination environment and affinity in the membrane bound form of α-syn. Our work follows the initial findings of Lucas and Lee who provided the first insights into copper uptake by membrane bound α-syn and showed that helix content and copper affinity at the protein N-terminus are increased in the membrane environment (53). In our study here, there are three elements. First, using electron paramagnetic resonance (EPR) and circular dichroism (CD), we evaluate the region in α-syn that takes up Cu2+ and the consequence of this interaction on the helical structure of membrane bound α-syn. Next, using mutagenesis and EPR, we identify the residues responsible for the primary binding sites. Finally, competition studies are applied to evaluate affinity. We demonstrate that α-syn in its lipid membrane bound state remains helical and takes up a single equivalent of Cu2+ at its N-terminus, with affinity and coordination environment similar to that found for the solution form. However, His50 no longer contributes to equatorial coordination regardless of Cu2+ concentration, and copper does not influence the α-syn distribution between membrane and solution.
Figure 2.
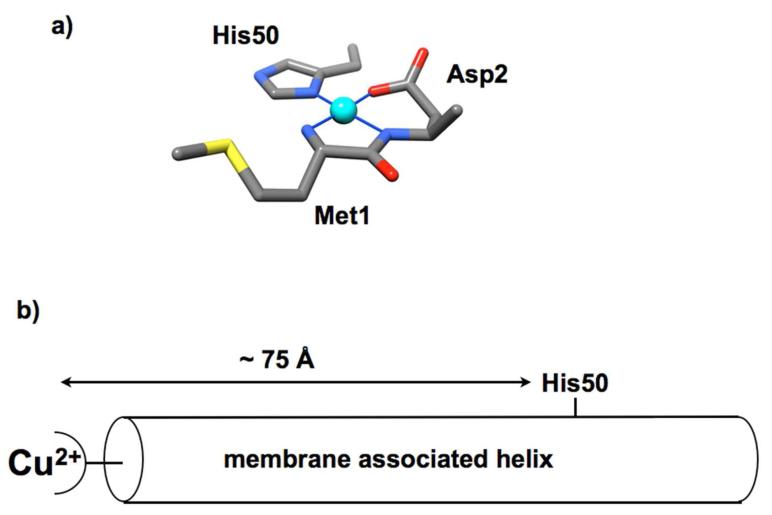
a) Coordination features of the primary solution Cu2+ site of α-syn. Competition studies show that this complex exhibits a dissociation constant of approximately 0.1 nM. b) α-Syn in its helical form, resulting from membrane association, would result in a separation of His50 from the N-terminus of approximately 75Å. Consequently, Cu2+ coordination details must be altered relative to that in solution.
Materials and Methods
Proteins and Reagents
The wt α-syn gene was cloned into pet21 plasmid vector manufactured by Genescript. The primers for the H50A mutation were obtained from Invitrogen. Mutations were performed using the GeneTailor™ Site Directed Mutagenesis System (Invitrogen Cat. Nos. 12397-014 and 12397-022). α-syn and α-syn(H50A) were recombinantly expressed in Escherichia coli BL21(DE3) competent cells (Invitrogen, Carlsbad, CA) using an auto induction procedure of Kim et al. described previously (54). Cells were harvested by centrifugation followed by sonication in lysis buffer (50mM NaCl, 20mM Tris, 0.2mM PMSF(phenylmethylsulfonylfluoride), 10%v/v Triton X100 (Sigma, Switzerland) pH=7.4). Purification was performed using ammonium sulfate precipitation followed by centrifugation, resuspension in 6M guanidine HCl and reverse phase HPLC (water and acetonitrile). The protein elutes between 63-60% acetonitrile on a C18 column (Grace Davidson Discovery Sciences, VYDAC HPLC column Cat.# 218TP101510).
Electron Paramagnetic Resonance
Samples were prepared in degassed buffer containing 25mM MOPS buffer and 25%v/v glycerol, where the glycerol served as a cryoprotectant. All continuous wave X-band spectra (ν = 9.44GHz, microwave power in the range of 0.6-5.0 mW, modulation amplitude of 5.0 G, and sweep width 1200G) were collected at approximately 125K, using a Bruker EleXsys spectrometer and an SHQ (Bruker) cavity equipped with a variable temperature controller. Competition assays were performed as described previously (16) and in the text here, and resultant composite spectra were analyzed using non negative least squares (NNLS) in the Matlab program suite. Three pulse ESEEM measurements were obtained at 20K on a Bruker E580 X band spectrometer using a dielectric resonator and an Oxford CF 935 cryostat. A π/2-τ-π-T-π/2-τ-echo sequence with pulse lengths of 12, 24, and 12ns was used. Initial value of τ = 136ns and T was lengthened in 799 steps of 12ns each with 100 samples per step.
Circular Dichroism
Far UV CD measurements were performed with an AVIV 60DS spectrophotometer, with a 0.1 cm path length cuvette. Solutions were 10.0 μM protein, 100 mM NaCl, and 20 mM MOPS buffer. Spectra were recorded from 250 to 200 nM with a step size of 1nm, a bandwidth of 1.5 nm and an averaging time of 4 s. An average of 20 scans was obtained for each spectrum. Separate buffer spectra were also collected as a reference.
Small Unilamellar Vesicle Preparation
Vesicles were prepared using standard procedures outlined by Langen et al. (7) from a 70% Phosphatidyl Choline (POPC), 30% Phosphatidyl Glycerol (POPG) mixture (Avanti Polar Lipids). The chloroform was evaporated off with nitrogen gas and the lipids were resolubilized in H2O and sonicated with a tip sonicator (Fisher Scientific model FB505) at 40% power, 30seconds on/40seconds rest for a total of 5 minutes on time. Lipids were deemed prepared when lipid/H2O solution appeared clear. Electron microscopy verified the formation of 20–50 nm diameter spheres, with no indication of tubes or other unusual structures (see Supplement).
Most phospholipids bind Cu2+, however, the specific head group plays a significant role in controlling affinity. Lipids with head groups that contain free amines, such as phosphatidylserine, bind Cu2+ quite strongly, and produce a dominant EPR spectrum (data not shown) (55-57). We sought a lipid composition that would allow for α-syn association but without strong Cu2+ coordination and found that a mixture of 70% phosphatidylcholine (neutral) and 30% phosphatidylglycerol (negative charge) produces these desired results.
Results
Membrane Bound α-syn Binds Cu2+
As discussed above, and emphasized in Figures 1 and 2, simultaneous coordination by the N-terminus and His50 would be incompatible with the lipid bound α-syn helical structure, which separates those polypeptide segments by approximately 75 Å. To evaluate Cu2+ uptake in membrane bound, helical α-syn, we used CD and X-band continuous wave EPR. Samples were prepared with lipid SUV and protein and incubated for ~15 minutes before flash freezing with liquid N2. Lipid composition for the POPC/POPG SUVs was chosen to minimize direct Cu2+ membrane interactions. All samples containing α-syn and SUV used for EPR experiments were found to have room temperature CD spectra corresponding to an alpha helix. The EPR spectra in Figure 3 show the typical Cu2+ hyperfine couplings in the parallel region (2700 – 3300 Gauss) observed for oxygen/nitrogen rich equatorial coordination and thereby demonstrate that α-syn binds Cu2+ in the presence of lipid SUV. Interestingly, the hyperfine lines in the EPR spectra for 100-200X lipids appear to be broadened in comparison to the α-syn in solution spectra, suggesting more than one coordination species at this lipid concentration. To quantify the relative species of protein bound we analyzed the EPR spectra in Figure 3 using a NNLS program with basis sets corresponding to α-syn-Cu2+ and α-syn(H50A)-Cu2+ spectra. The α-synH50A mutant lacks the His imidazole that contributes to the equatorial coordination observed in the solution α-syn Cu2+ complex, and thus takes up copper only at the N-terminus (16). As shown in Figure 3 (inset), at a lipid:protein ratio of 300:1 and greater, the EPR spectra appear to correspond solely to the α-synH50A spectrum, suggesting that Cu2+ is bound solely to the polypeptide N-terminus. To ensure that the EPR spectra are the result of Cu2+ binding α-syn and not another chelator, such as the bilayer phosphate head groups, spectra were taken with Cu2+ and SUV’s alone for comparison. The lipid-Cu2+ spectrum exhibits a Bo downfield shift of ~150G (compared to α-syn Cu2+ in the presence of lipids) and a different pattern of hyperfine splitting that suggests high O ligand character (see below). Furthermore, the addition of Cu2+ to SUV results in a spectrum that, when doubly integrated to determine the concentration of paramagnetic species bound, consistently shows less than the full amount of added Cu2+. This evidence suggests the POPC/POPG lipid SUV’s bind Cu2+ very weakly. Indeed when we add α-syn to the lipid-Cu2+ solution, the spectra revert to that obtained in the samples shown in Figure 3.
Figure 3.
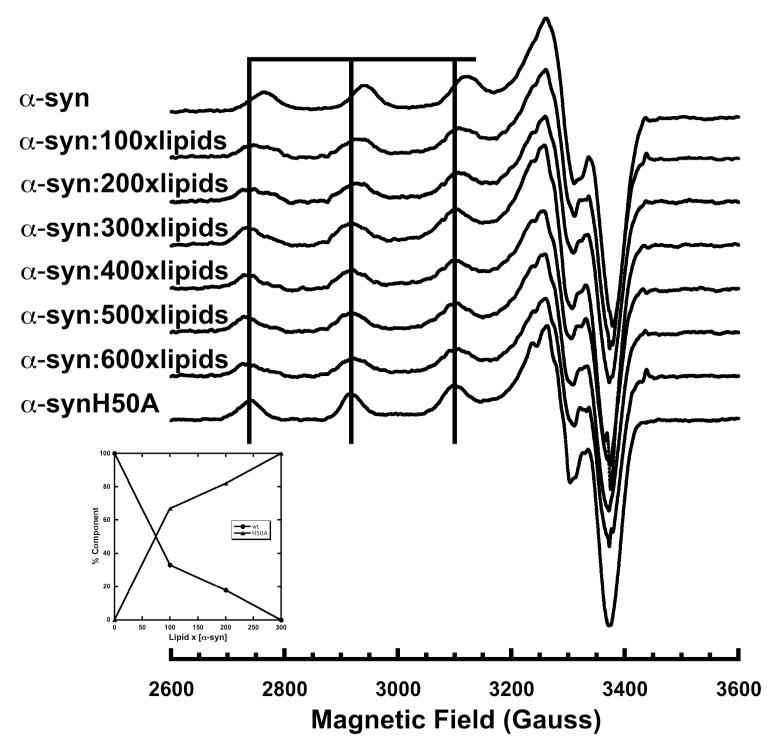
X-Band EPR spectra of α-syn (50μM) at pH 7.4 with 1 equivalent of Cu2+ in the presence of increasing molar equivalents of lipid molecules in the form of vesicles. The vertical lines correspond to the parallel hyperfine features of the α-synH50A mutant in solution. α-syn in the presence of lipids produces a similar if somewhat broadened spectra that has a downfield shift corresponding to an increase in lipid SUVs. At a lipid:protein ratio of 300:1 the α-syn spectra essentially overlaps the α-synH50A mutant spectra. Spectra were recorded at 111K, ν = 9.44 GHz, with a field sweep of 1200G.
Copper Does Not Affect the CD Spectra of Membrane Bound α-synuclein
In the presence of lipid bilayers residues ~10-90 of α-syn have been shown to form a single α-helix (7, 58). The lack of participation of His50 in Cu2+ coordination suggests that the α-syn helical structure in the SUV membrane environment should remain intact with copper bound. We tested this directly by evaluating the CD spectra as a function of lipid molecule to protein ratio. Dichroic peaks with negative intensity at wavelengths at 208nm and 222nm provide a measure of helical structure (59). As observed in Figure 4a, α-syn achieves it’s maximum alpha-helical signal intensity at a lipid:α-syn ratio of 300:1. The mean residue ellipticity (MRE) at 222 nm is approximately 24,000 deg cm2 dmol1, consistent with that previously reported (53). Using the 300:1 lipid:protein ratio we then conducted CD experiments with α-syn and lipids in the presence of Cu2+. Figure 4b shows that there is no change in the CD signal with the addition of 1.0× and 10.0× Cu2+, suggesting that α-syn copper binding has no detectable affect on α-syn helicity in the presence of SUVs. We note that excess Cu2+ results in an EPR spectrum showing the N-terminal site, along with a weak spectrum consistent with that observed for copper and lipid alone (Figure 6).
Figure 4.
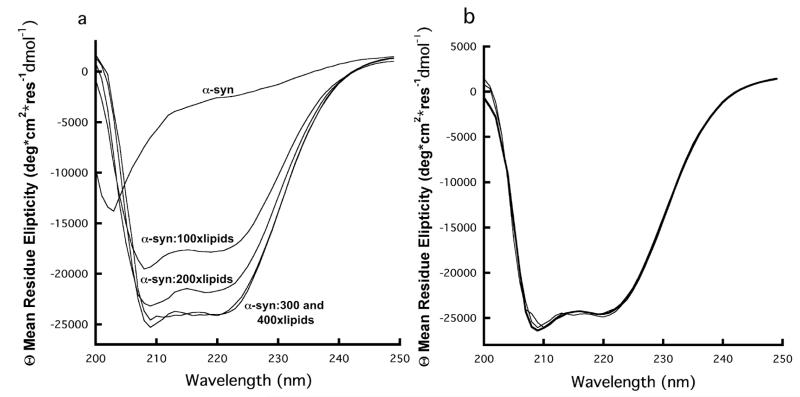
a) CD spectra of α-syn as a function of lipid addition in the form of SUVs. From top to bottom: 10 μM α-syn no lipids, 10 μM α-syn:1mM lipids, 10 μM α-syn:2mM lipids, 10 μM α-syn:3mM lipids, 10 μM α-syn:4mM lipids. CD signal intensity reaches a maximum at a lipid:α-syn molar ratio of 300:1. b) Cu2+ titration of 10 μM α-syn:3mM lipid complex. Cu2+ concentrations of 0, 10 and 100 μM give overlapping α-syn CD spectra.
Figure 6.
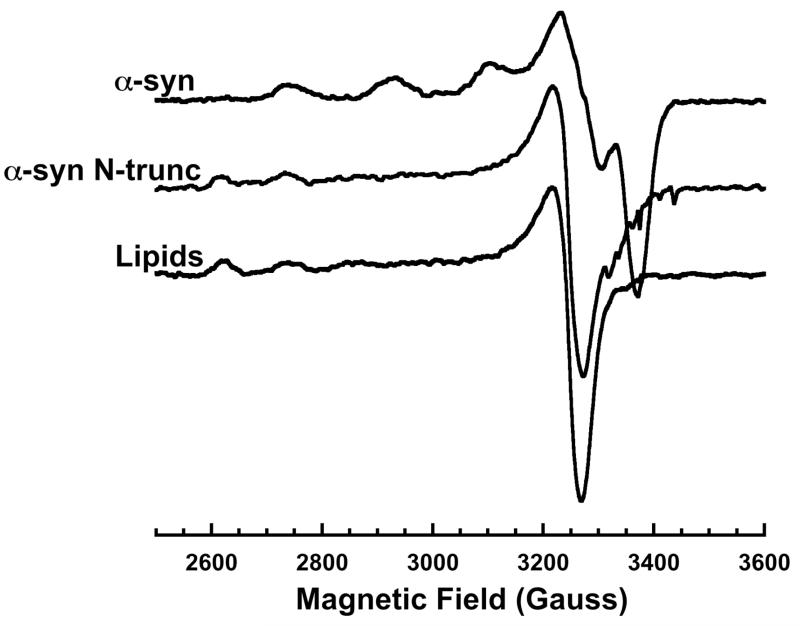
X-Band EPR spectra of α-syn and α-syn N-trunc (both at 50 μM) in the presence of lipid SUV with 1.0 equivalent Cu2+ and the lipid SUV alone with the same concentration of Cu2+. The α-syn N trunc spectra features qualitatively resemble the lipid SUV spectra, demonstrating N-terminal sequence involvement in the lipid-bound α-syn coordination sphere.
Identification of Cu2+Coordination Features
To evaluate the Cu2+ coordination environment in lipid bound α-syn, we developed mutations of the key copper binding α-syn residues previously identified for α-syn in solution. Figure 3 demonstrates using EPR that the greater the concentration of lipid SUV’s the more the α-syn copper binding spectrum shifts downfield to give a spectrum that is within experimental error of that obtained for the α-syn H50A mutant. This evidence, in addition to the aforementioned CD lipid titration spectra, suggests that His50 is not involved in lipid-bound α-syn copper coordination.
To further assess the potential involvement of His50 in α-syn-Cu2+ coordination, we used electron spin echo envelope modulation (ESEEM). ESEEM is a pulsed EPR technique with sensitivity to spin active nuclei within approximately 10Å from the paramagnetic copper center. At X-band frequencies, the distal 14N (I = 1) of a coordinated imidazole ring gives characteristic quadrupolar transitions and is diagnostic for interacting His side chains. The FT ESEEM of solution α-syn with 1.0 equivalent of Cu2+ shown in Figure 5a is typical for imidazole, with three low-frequency peaks that correspond to transitions among 14N quadropolar levels in exact cancellation, as well as the ≈ 4.0 MHz peak from the non cancelled electron spin manifold. Progressive addition of SUVs, measured by lipid concentration, produces a corresponding decrease in the 4.0 MHz peak (see Figure 5 inset). We also find that the lipid SUV alone and lipid SUV with α-syn H50A (not shown) fail to give an ESEEM spectrum with 1.0 equivalents of Cu2+. Together these assays unequivocally show that the H50 imidazole of α-syn does not coordinate Cu2+ when the protein is in its membrane bound, helical state.
Figure 5.
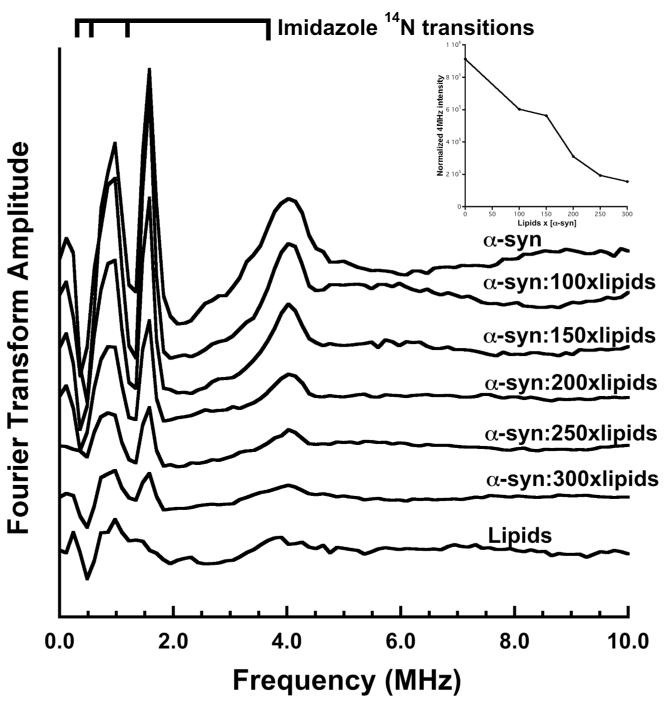
Three-pulse ESEEM spectra of α-syn (50 μM) with 1.0 equivalent of Cu2+ in the presence of increasing lipid SUV concentration. The spectra of the samples containing α-syn alone reveal the expected quadrupolar transitions associated with the imidazole remote nitrogen and demonstrate coordination by His50 whereas the lipids alone do not. The 4MHz peak is indicative of the non-canceled electron spin manifold from the Cu2+ -imidizole far nitrogen interaction. Those samples containing α-syn and lipid SUV demonstrate a decrease in intensity of the 4MHz peak with increasing lipid concentration relative to α-syn in solution. Inset shows normalized 4MHz peak intensity vs. sample lipid concentration.
Our laboratory previously demonstrated that the Met1 and Asp2 form the essential ligands of the Cu2+ coordination shell (Figure 2). In order to assess whether this H2N-M-D coordination motif remains intact in lipid bound α-syn, we created a 139 residue truncated D2A mutant protein (α-syn N trunc). Expression of this mutant in E. coli results in cleavage of the N-terminal methionine thereby changing the N-terminal sequence from NH2-MAVF- to NH2-AVF- (60) (MS verification in the Supplement). Figure 6 shows that the α-syn N trunc mutation gives a spectrum equivalent to that obtained in lipid alone, demonstrating that the N-terminal sequence NH2-M-D is necessary for high affinity copper coordination when α-syn is in its helical form.
We considered whether His50 might participate in Cu2+ coordination at higher metal concentrations. For example, copper could bridge between histidines on adjacent α-syns on the membrane surface. To test this hypothesis, we titrated up to 10 equivalents of Cu2+ and monitored by both EPR and CD. As seen in figure 4, the addition of extra equivalents of copper did not modify the CD spectra, suggesting that if there are higher order structures being formed, they do not disturb the protein’s α-helix. EPR of titrations up to 10 equivalents of Cu2+ show hyperfine broadening and the introduction of new hyperfines consistent with a mixture of the lipid bound α-syn:Cu2+ spectra and the lipid:Cu2+ spectra (Supplemental figure 1). We did not observe any additional EPR features consistent with a new species arising from His coordination. We also tested for His coordination at high Cu2+ concentrations using ESEEM. The resulting FT ESEEM spectra obtained from samples up to 10 equivalents of Cu2+ are low intensity relative to that of equal concentrations of solution α-syn and do not give a prominent 4.0 MHz signal indicative of multiple His coordination (supplemental Figure 2). Consistent with the continuous wave EPR, these data do not support the presence of a high affinity binding site involving His. Consequently, we find that copper partitions primarily between the high affinity site at the α-syn N-terminus and the membrane. Moreover, Figure 6 shows that with the removal of the α-syn N-terminal binding site, the resultant spectrum corresponds to the lipid only spectra, suggesting that if there is histidine involvement in an additional binding site, its affinity for Cu2+ is weaker than the lipids (Kd in the millimolar range). These data demonstrate that there is only one site at which copper binds to α-syn, and it is localized to the N-terminus of the protein without participation of His50.
Copper Binding Affinity
To evaluate the dissociation constant, Kd, of the α-syn Cu2+ complex in the presence of lipid SUV, we used an EPR competition technique previously developed in our lab (61). High affinity competitors that take up Cu2+ with a 1:1 stoichiometry are added to a Cu2+-α-syn lipid SUV solution and allowed to come to equilibrium. Both nitrilotriacetic acid (NTA) and triphosphate molecules are well-characterized chelators and give distinct Cu2+ EPR spectra that are readily separable from that of the Cu2+-α-syn lipid SUV complex. Spectral decomposition using non-linear least squares gives the ratio of copper bound to α-syn and specific competitor. Analysis using the known Kd of the competitor determines the α-syn dissociation constant. With this approach, the amount of competitor may be varied to ensure that both bound species give resolvable EPR spectra with similar signal strengths. Table 1 shows that lipid bound α-syn binds 1eq of Cu2+ with Kd values of .11nM and .095nM, determined by NTA and triphosphate, respectively. To further test for H50 coordination we also performed NTA and triphosphate competition experiments on the α-syn H50A mutant in the presence of lipid SUVs. As seen in Table 1 the resultant Kd is 0.19 nM, which differs only slightly from wildtype.
Table 1.
Dissociation Constants (nanomolar) Determined from Competition Studies
| protein | Nitrolotriacetic acid (Kd = 0.366nM) | Triphosphate (Kd = 2nM) |
|---|---|---|
| α-syn:lipids | 0.110 ± 0.005 | 0.095 ± 0.006 |
| α-syn(H50A):lipids | 0.194 ± 0.008 | 0.196 ± 0.007 |
Discussion
Our combined EPR and CD experiments demonstrate that helical, membrane bound α-syn is capable of chelating one equivalent of Cu2+ at its N-terminus, with a coordination shell composed of the Met1 amine and the backbone amide and carboxylate residue of Asp2. Furthermore, ESEEM rules out histidine imidazole coordination. Our studies do not identify a fourth atom in the coordination shell, but the downfield shift in the EPR spectrum observed upon addition of lipid is consistent with replacement of a nitrogen with an oxygen, likely from a water molecule. We find the dissociation constant at the maximum possible lipid bound saturation to be approximately 0.1 nM, similar to that previously reported for α-syn in solution. Together, our results demonstrate that the details of Cu2+ coordination depend on whether α-syn is in the solution or membrane bound form, as shown in Figure 7. (It is important to note that N-terminal Cu2+ uptake would be dramatically reduced or eliminated in acetylated α-syn (62, 63).)
Figure 7.
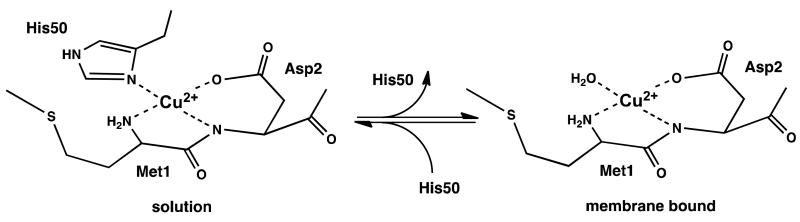
Models of the equilibrium of α-syn copper binding states. Availability of His50 involvement is dependent upon whether the protein is in solution (left) or in the membrane bound helical state (right).
α-Syn is found in both solution (inside and outside of the cell) and membrane associated states; it is important to identify cellular factors that influence this equilibrium. Given that His50 coordination to Cu2+ in incompatible with the helical, membrane bound form, we wondered whether copper might enhance release from the membrane surface. Our experiments find no evidence of this. Despite addition of up to 10x excess Cu2+, the EPR and CD show no variation pointing to an enhancement of the solution form concentration. Consequently, the energetics driving association between α-syn and the lipid bilayer are substantially stronger than that arising from additional stabilization arising from coordination by His50. However, it is possible that copper coordination to the solution form provides a kinetic barrier to helix formation and consequent membrane association. Our experiments did not address this. Beyond the dominant, fully helical structure encompassing approximately the first 100 residues, combined NMR and EPR experiments with micelles show that α-syn may also adopt a less ordered structure composed of two helices, separated by a flexible segment (residues 33 – 41) (64). Examination of this structure shows that even with additional conformations available in this partially ordered α-syn form, His50 would still not able to come in close contact to an N-terminally coordinated Cu2+.
The equilibrium we identify in Figure 7 suggests that membrane interactions may alter copper mediated redox activity. Aberrant redox reactions at α-syn-copper centers are likely to play an important role in the cellular toxicity associated with α-syn aggregates. Electrochemical properties of α-syn have been carefully evaluated using cyclic voltammetry, as well as reactivity in the presence of ascorbate, dopamine and other relevant species (65). Free copper in the presence of ascorbate releases peroxide. At high concentrations, peroxide can react at metal centers producing cytotoxic hydroxyl radicals, thereby contributing to the concentration of reactive oxygen species. The α-syn-copper complex is substantially less reactive than free copper, as measured by both peroxide production and cytotoxicity assays, suggesting that α-syn serves to absorb and modulate adventitious copper (65). Interestingly, parallel experiments show that α-syn(1-19), which lacks His50, gives a somewhat different redox profile than the full length protein. Under identical conditions in the presence of ascorbate, α-syn(1-19) produces 10 – 20% more peroxide. Both α-syn species, when complexed with copper, are cytotoxic against neuroblastoma cells, but α-syn(1-19) was more potent at reducing cell viability (65). These data suggest that the copper center in the membrane bound form of α-syn-copper may be more reactive than the solution form. But this is a tentative proposal since helical α-syn, stabilized by trifluoroethanol, produces lower levels of reactive oxygen species than the unstructured polypeptide (66).
As described in the Introduction, exposure to high levels of exogenous copper correlates with an increased incidence of PD. Our work here provides insight into the possible initial steps leading to cellular stress and LB formation. We find that copper does not alter the membrane-bound α-syn conformation or contribute to the protein’s release from the membrane to solution. However, once released from the bilayer, the simultaneous coordination of the protein N-terminus and His50 limits the distribution of α-syn’s random coil states. Although the resulting protein-Cu2+ complex is somewhat more protective against the production of reactive oxygen species than the membrane bound form, the loop arising from simultaneous copper coordination of the N-terminus and His50 might expose the amyloidogenic NAC region in a way that results in the observed enhancement of α-syn aggregates (24).
In conclusion, we find that membrane bound α-syn tightly binds one equivalent of Cu2+ at its N-terminus. This interaction does not affect the helical structure of α-syn or the energetics of membrane association. Indeed, lipid to protein ratio exerts a much stronger influence on α-syn conformation than copper coordination. Consistent with the literature described in the introduction, it is likely that copper uptake is part of α-syn’s natural function, with electrochemical properties dependent upon whether the protein is associated with cellular membranes. In PD arising from environmental copper exposure, the solution form of α-syn may be a precursor to LB formation. Future work will focus on how membrane association and copper uptake are affected by the known inherited mutations in PD, many of which are clustered around His50.
Supplementary Material
Acknowledgements
The authors thank Professor Ralf Langen, USC, for helpful advice in the preparation of membrane vesicles. A portion of this research was performed using EMSL, a national scientific user facility sponsored by the Department of Energy’s Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory.
Supported by NIH grants GM065790 (GLM) and GM072649 (MSJ), and a grant from the California Institute for Quantitative Biological Sciences (QB3)
Footnotes
Supporting Information
Additional EPR and ESEEM controls, along with mass spectra of the truncation mutant are provided. In addition, electron micrographs compare SUVs without and with added α-syn. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Shulman JM, De Jager PL, Feany MB. Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 3.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron. 2010;66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vila M, Przedborski S. Genetic clues to the pathogenesis of Parkinson’s disease. Nat Med. 2004;10(Suppl):S58–62. doi: 10.1038/nm1068. [DOI] [PubMed] [Google Scholar]
- 5.Devine MJ, Gwinn K, Singleton A, Hardy J. Parkinson’s disease and alpha-synuclein expression. Mov Disord. 2011;26:2160–2168. doi: 10.1002/mds.23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 7.Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement. Proc Natl Acad Sci U S A. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levitan K, Chereau D, Cohen SI, Knowles TP, Dobson CM, Fink AL, Anderson JP, Goldstein JM, Millhauser GL. Conserved C-terminal charge exerts a profound influence on the aggregation rate of alpha-synuclein. J Mol Biol. 2011;411:329–333. doi: 10.1016/j.jmb.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang ML, Hasadsri L, Woods WS, George JM. Dynamic transport and localization of alpha-synuclein in primary hippocampal neurons. Mol Neurodegener. 2010;5:9. doi: 10.1186/1750-1326-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 11.Chartier Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 12.Chua CE, Tang BL. Rabs, SNAREs and alpha-synuclein--membrane trafficking defects in synucleinopathies. Brain Res Rev. 2011;67:268–281. doi: 10.1016/j.brainresrev.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Darios F, Ruiperez V, Lopez I, Villanueva J, Gutierrez LM, Davletov B. Alpha-synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep. 2010;11:528–533. doi: 10.1038/embor.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonini NM, Giasson BI. Snaring the function of alpha-synuclein. Cell. 2005;123:359–361. doi: 10.1016/j.cell.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudzik CG, Walter ED, Millhauser GL. Coordination features and affinity of the Cu(2)+ site in the alpha-synuclein protein of Parkinson’s disease. Biochemistry. 2011;50:1771–1777. doi: 10.1021/bi101912q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong L, Simon JD. Binding of Cu(II) to human alpha-synucleins: comparison of wild type and the point mutations associated with the familial Parkinson’s disease. J Phys Chem B. 2009;113:9551–9561. doi: 10.1021/jp809773y. [DOI] [PubMed] [Google Scholar]
- 18.Fukushima T, Tan X, Luo Y, Kanda H. Serum Vitamins and Heavy Metals in Blood and Urine, and the Correlations among Them in Parkinson’s Disease Patients in China. Neuroepidemiology. 2011;36:240–244. doi: 10.1159/000328253. [DOI] [PubMed] [Google Scholar]
- 19.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposures to metals as risk factors for Parkinson’s disease. Neurology. 1997;48:650–658. doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- 20.Rybicki BA, Johnson CC, Uman J, Gorell JM. Parkinson’s disease mortality and the industrial use of heavy metals in Michigan. Mov Disord. 1993;8:87–92. doi: 10.1002/mds.870080116. [DOI] [PubMed] [Google Scholar]
- 21.Alimonti A, Bocca B, Pino A, Ruggieri F, Forte G, Sancesario G. Elemental profile of cerebrospinal fluid in patients with Parkinson’s disease. J Trace Elem Med Biol. 2007;21:234–241. doi: 10.1016/j.jtemb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Bush AI. Metals and neuroscience. Curr Opin Chem Biol. 2000;4:184–191. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 23.Desai V, Kaler SG. Role of copper in human neurological disorders. Am J Clin Nutr. 2008;88:855S–858S. doi: 10.1093/ajcn/88.3.855S. [DOI] [PubMed] [Google Scholar]
- 24.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 25.Binolfi A, Rasia RM, Bertoncini CW, Ceolin M, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO. Interaction of alpha-synuclein with divalent metal ions reveals key differences: a link between structure, binding specificity and fibrillation enhancement. J Am Chem Soc. 2006;128:9893–9901. doi: 10.1021/ja0618649. [DOI] [PubMed] [Google Scholar]
- 26.Feaga HA, Maduka RC, Foster MN, Szalai VA. Affinity of Cu+ for the copper-binding domain of the amyloid-beta peptide of Alzheimer’s disease. Inorg Chem. 2011;50:1614–1618. doi: 10.1021/ic100967s. [DOI] [PubMed] [Google Scholar]
- 27.Shin BK, Saxena S. Substantial contribution of the two imidazole rings of the His13-His14 dyad to Cu(II) binding in amyloid-beta(1-16) at physiological pH and its significance. J Phys Chem A. 2011;115:9590–9602. doi: 10.1021/jp200379m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens DJ, Walter ED, Rodriguez A, Draper D, Davies P, Brown DR, Millhauser GL. Early onset prion disease from octarepeat expansion correlates with copper binding properties. PLoS Pathog. 2009;5:e1000390. doi: 10.1371/journal.ppat.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walter ED, Stevens DJ, Visconte MP, Millhauser GL. The prion protein is a combined zinc and copper binding protein: Zn2+ alters the distribution of Cu2+ coordination modes. J Am Chem Soc. 2007;129:15440–15441. doi: 10.1021/ja077146j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millhauser GL. Copper and the prion protein: methods, structures, function, and disease. Annu Rev Phys Chem. 2007;58:299–320. doi: 10.1146/annurev.physchem.58.032806.104657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L. Pathological roles of alpha-synuclein in neurological disorders. Lancet Neurol. 2011;10:1015–1025. doi: 10.1016/S1474-4422(11)70213-7. [DOI] [PubMed] [Google Scholar]
- 33.Marques O. a. O., T.F. alpha-synuclein: from secretion to dysfunction and death. Cel Death and Disease. 2012;3 doi: 10.1038/cddis.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irizarry MC, Kim TW, McNamara M, Tanzi RE, George JM, Clayton DF, Hyman BT. Characterization of the precursor protein of the non-A beta component of senile plaques (NACP) in the human central nervous system. J Neuropathol Exp Neurol. 1996;55:889–895. doi: 10.1097/00005072-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Lucking CB, Brice A. Alpha-synuclein and Parkinson’s disease. Cell Mol Life Sci. 2000;57:1894–1908. doi: 10.1007/PL00000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ballard CG, Jones EL. CSF alpha-synuclein as a diagnostic biomarker for Parkinson disease and related dementias. Neurology. 2010;75:1760–1761. doi: 10.1212/WNL.0b013e3181fd6393. [DOI] [PubMed] [Google Scholar]
- 37.Park MJ, Cheon SM, Bae HR, Kim SH, Kim JW. Elevated Levels of alpha-synuclein Oligomer in the Cerebrospinal Fluid of Drug-Naive Patients with Parkinson’s Disease. J Clin Neurol. 2011;7:215–222. doi: 10.3988/jcn.2011.7.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Kim HM, Leverenz JB, Montine TJ, Ginghina C, Kang UJ, Cain KC, Wang Y, Aasly J, Goldstein D, Zhang J. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69:570–580. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. alpha-synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilker R, Brotchie JM, Chapman J. Pros and cons of a prion-like pathogenesis in Parkinson’s disease. BMC Neurol. 2011;11:74. doi: 10.1186/1471-2377-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson MS, Lee JC. Identification of the minimal copper(II)-binding alpha-synuclein sequence. Inorg Chem. 2009;48:9303–9307. doi: 10.1021/ic901157w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasia RM, Bertoncini CW, Marsh D, Hoyer W, Cherny D, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO. Structural characterization of copper(II)-binding to alpha-synuclein: Insights into the bioinorganic chemistry of Parkinson’s disease. Proc Natl Acad Sci U S A. 2005;102:4294–4299. doi: 10.1073/pnas.0407881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binolfi A, Lamberto GR, Duran R, Quintanar L, Bertoncini CW, Souza JM, Cervenansky C, Zweckstetter M, Griesinger C, Fernandez CO. Site-specific interactions of Cu(II) with alpha and beta-synuclein: bridging the molecular gap between metal binding and aggregation. J Am Chem Soc. 2008;130:11801–11812. doi: 10.1021/ja803494v. [DOI] [PubMed] [Google Scholar]
- 44.Binolfi A, Rodriguez EE, Valensin D, D’Amelio N, Ippoliti E, Obal G, Duran R, Magistrato A, Pritsch O, Zweckstetter M, Valensin G, Carloni P, Quintanar L, Griesinger C, Fernandez CO. Bioinorganic chemistry of Parkinson’s disease: structural determinants for the copper mediated amyloid formation of alpha-synuclein. Inorg Chem. 2010;49:10668–10679. doi: 10.1021/ic1016752. [DOI] [PubMed] [Google Scholar]
- 45.Bortolus M, Bisaglia M, Zoleo A, Fittipaldi M, Benfatto M, Bubacco L, Maniero AL. Structural characterization of a high affinity mononuclear site in the copper(II)-alpha-synuclein complex. J Am Chem Soc. 2010;132:18057–18066. doi: 10.1021/ja103338n. [DOI] [PubMed] [Google Scholar]
- 46.Davies P, Wang X, Sarell CJ, Drewett A, Marken F, Viles JH, Brown DR. The synucleins are a family of redox-active copper binding proteins. Biochemistry. 2011;50:37–47. doi: 10.1021/bi101582p. [DOI] [PubMed] [Google Scholar]
- 47.Drew SC, Leong SL, Pham CL, Tew DJ, Masters CL, Miles LA, Cappai R, Barnham KJ. Cu2+ binding modes of recombinant alpha-synuclein-insights from EPR spectroscopy. J Am Chem Soc. 2008;130:7766–7773. doi: 10.1021/ja800708x. [DOI] [PubMed] [Google Scholar]
- 48.Lee JC, Gray HB, Winkler JR. Copper(II) binding to alpha-synuclein, the Parkinson’s protein. J Am Chem Soc. 2008;130:6898–6899. doi: 10.1021/ja711415b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valensin D, Camponeschi F, Luczkowski M, Baratto MC, Remelli M, Valensin G, Kozlowski H. The role of His-50 of alpha-synuclein in binding Cu(II): pH dependence, speciation, thermodynamics and structure. Metallomics. 2011;3:292–302. doi: 10.1039/c0mt00068j. [DOI] [PubMed] [Google Scholar]
- 50.Binolfi A. s., Quintanar L, Bertoncini CW, Griesinger C, Fern√°ndez CO. Bioinorganic chemistry of copper coordination to alpha-synuclein: Relevance to Parkinson’s disease. Coordination Chemistry Reviews. 2012;256:2188–2201. [Google Scholar]
- 51.Kozlowski H, Luczkowski M, Remelli M, Valensin D. Copper, zinc and iron in neurodegenerative diseases (Alzheimer’s, Parkinson’s and prion diseases) Coordination Chemistry Reviews. 2012;256:2129–2141. [Google Scholar]
- 52.Zawisza I, R√≥zga MÇ, Bal W. Affinity of copper and zinc ions to proteins and peptides related to neurodegenerative conditions (AOE≤, APP, OE± synuclein, PrP) Coordination Chemistry Reviews. 2012;256:2297–2307. [Google Scholar]
- 53.Lucas HR, Lee JC. Copper(II) enhances membrane-bound alpha-synuclein helix formation. Metallomics. 2011;3:280–283. doi: 10.1039/c0mt00088d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim M, Elvin C, Brownlee A, Lyons R. High yield expression of recombinant pro-resilin: lactose-induced fermentation in E. coli and facile purification. Protein Expr Purif. 2007;52:230–236. doi: 10.1016/j.pep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Faraudo J, Travesset A. Phosphatidic acid domains in membranes: effect of divalent counterions. Biophys J. 2007;92:2806–2818. doi: 10.1529/biophysj.106.092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boettcher JM, Davis-Harrison RL, Clay MC, Nieuwkoop AJ, Ohkubo YZ, Tajkhorshid E, Morrissey JH, Rienstra CM. Atomic view of calcium-induced clustering of phosphatidylserine in mixed lipid bilayers. Biochemistry. 2011;50:2264–2273. doi: 10.1021/bi1013694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puskin JS. Divalent Cation Binding to Phospholipids: An EPR Study. Journal of Membrane Biology. 1977;35:39–55. doi: 10.1007/BF01869939. [DOI] [PubMed] [Google Scholar]
- 58.Jao CC, Der-Sarkissian A, Chen J, Langen R. Structure of membrane-bound alpha-synuclein studied by site-directed spin labeling. Proc Natl Acad Sci U S A. 2004;101:8331–8336. doi: 10.1073/pnas.0400553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Provencher SW, Glockner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981;20:33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- 60.Ben-Bassat A, Bauer K, Chang SY, Myambo K, Boosman A, Chang S. Processing of the initiation methionine from proteins: properties of the Escherichia coli methionine aminopeptidase and its gene structure. J Bacteriol. 1987;169:751–757. doi: 10.1128/jb.169.2.751-757.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walter ED, Chattopadhyay M, Millhauser GL. The affinity of copper binding to the prion protein octarepeat domain: evidence for negative cooperativity. Biochemistry. 2006;45:13083–13092. doi: 10.1021/bi060948r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson JP, Walker DE, Goldstein JM, de LR, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 63.Bartels T, Choi JG, Selkoe DJ. alpha-synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rao JN, Jao CC, Hegde BG, Langen R, Ulmer TS. A combinatorial NMR and EPR approach for evaluating the structural ensemble of partially folded proteins. J Am Chem Soc. 2010;132:8657–8668. doi: 10.1021/ja100646t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang C, Liu L, Zhang L, Peng Y, Zhou F. Redox reactions of the alpha-synuclein-Cu(2+) complex and their effects on neuronal cell viability. Biochemistry. 2010;49:8134–8142. doi: 10.1021/bi1010909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou B, Hao Y, Wang C, Li D, Liu YN, Zhou F. Conversion of natively unstructured alpha-synuclein to its alpha helical conformation significantly attenuates production of reactive oxygen species. J Inorg Biochem. 2013;118:68–73. doi: 10.1016/j.jinorgbio.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


