Abstract
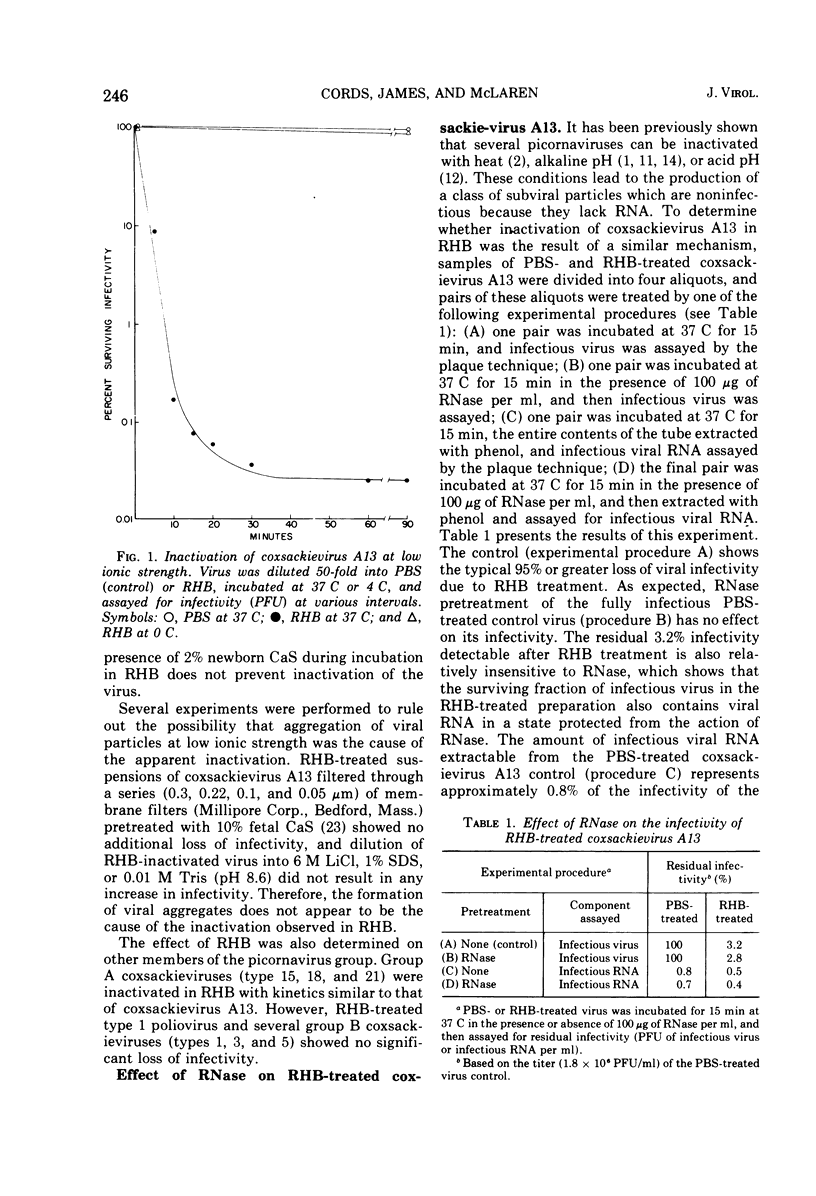
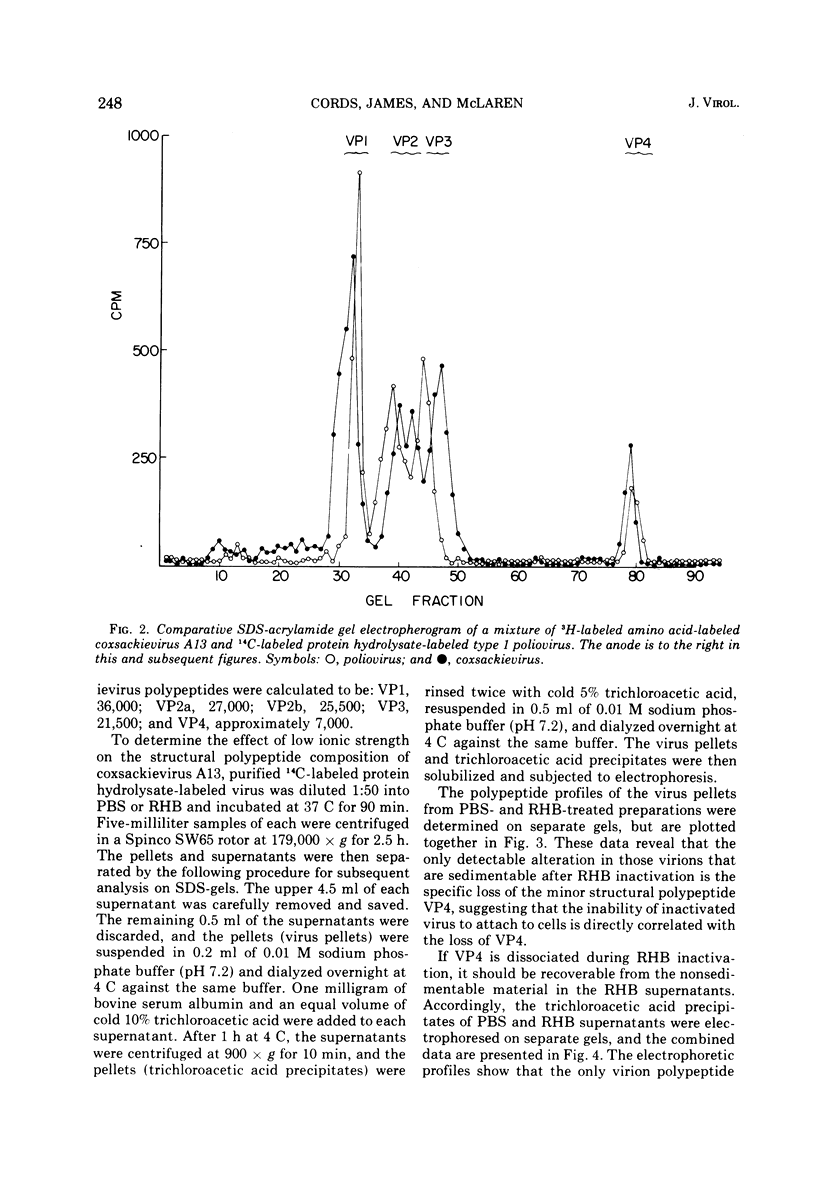
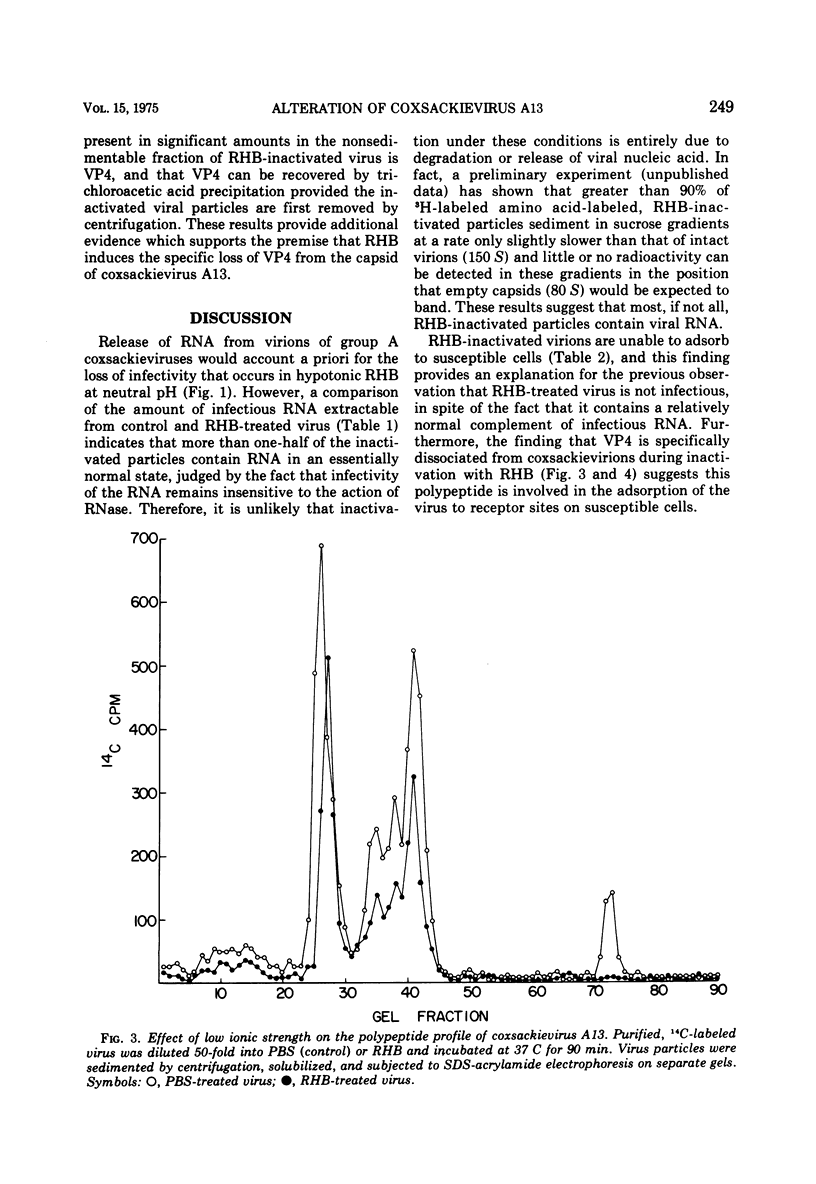
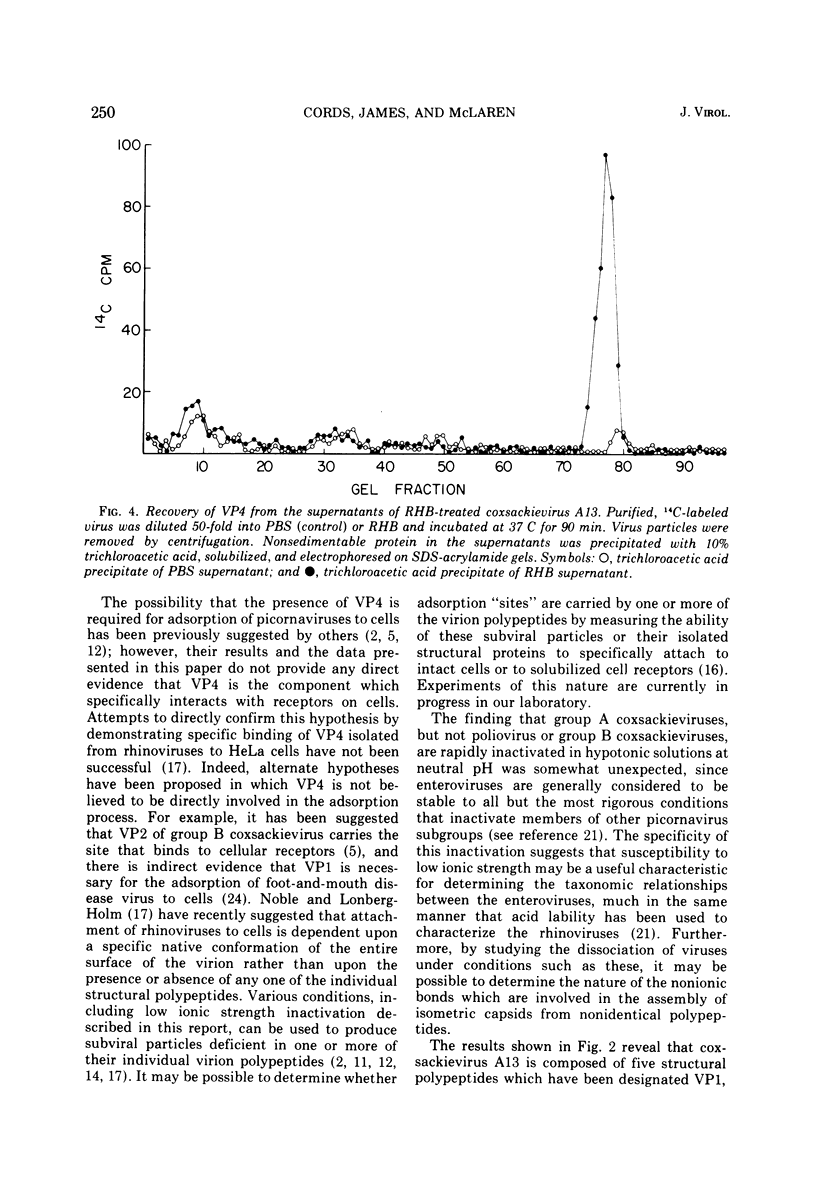
Several group A coxsackieviruses (A13, 15, 18, and 21), but not polioviruses or group B coxsackieviruses, are rapidly inactivated in low ionic strength solutions at neutral pH. The extent of inactivation is dependent upon temperature and molarity. Virions inactivated in this manner contain a normal complement of infectious RNA which remains in a state resistant to the action of ribonuclease. However, more than 95% of the virus particles are unable to attach to susceptible cells. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis reveals that coxsackievirus A13 virions contain five structural polypeptides (VP1, VP2a, VP2b, VP3, and VP4). Electrophoretic analysis indicates that inactivation of coxsackievirus A13 in low ionic strength solutions is due to the specific loss of the smallest polypeptide VP4 from the virus particle. These results suggest that adsorption of coxsackievirus A13 to receptors on susceptible cells is dependent upon the presence of the capsid protein VP4.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeyé A., Van Elsen A. Alkaline disruption of poliovirus: kinetics and purification of RNA-free particles. Virology. 1967 Oct;33(2):335–343. doi: 10.1016/0042-6822(67)90152-3. [DOI] [PubMed] [Google Scholar]
- Breindl M., Koch G. Competence of suspended HeLa cells for infection by inactivated poliovirus particles and by isolated viral RNA. Virology. 1972 Apr;48(1):136–144. doi: 10.1016/0042-6822(72)90121-3. [DOI] [PubMed] [Google Scholar]
- Breindl M. The structure of heated poliovirus particles. J Gen Virol. 1971 Jun;11(3):147–156. doi: 10.1099/0022-1317-11-3-147. [DOI] [PubMed] [Google Scholar]
- Cooper P. D., Summers D. F., Maizel J. V. Evidence for ambiguity in the posttranslational cleavage of poliovirus proteins. Virology. 1970 Jul;41(3):408–418. doi: 10.1016/0042-6822(70)90161-3. [DOI] [PubMed] [Google Scholar]
- Crowell R. L., Philipson L. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J Virol. 1971 Oct;8(4):509–515. doi: 10.1128/jvi.8.4.509-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Fragments generated by pH dissociation of ME-virus and their relation to the structure of the virion. J Mol Biol. 1971 May 28;58(1):217–235. doi: 10.1016/0022-2836(71)90242-7. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. Improved method for staining cell monolayers for virus plaque counts. J Bacteriol. 1959 Oct;78:596–597. doi: 10.1128/jb.78.4.596-597.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Katagiri S., Aikawa S., Hinuma Y. Stepwise degradation of poliovirus capsid by alkaline treatment. J Gen Virol. 1971 Oct;13(1):101–109. doi: 10.1099/0022-1317-13-1-101. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K., Noble J., Stasny J. T. Naturally occurring and artificially produced components of three rhinoviruses. Virology. 1972 Apr;48(1):71–86. doi: 10.1016/0042-6822(72)90115-8. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Summers D. F. Evidence for differences in size and composition of the poliovirus-specific polypeptides in infected HeLa cells. Virology. 1968 Sep;36(1):48–54. doi: 10.1016/0042-6822(68)90115-3. [DOI] [PubMed] [Google Scholar]
- McLaren L. C., Scaletti J. V., James C. G. Isolation and properties of enterovirus receptors. Wistar Inst Symp Monogr. 1968;8:123–135. [PubMed] [Google Scholar]
- Noble J., Lonberg-Holm K. Interactions of components of human rhinovirus type 2 with Hela cells. Virology. 1973 Feb;51(2):270–278. doi: 10.1016/0042-6822(73)90427-3. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., McCutchan J. H., Vaheri A. Factors influencing the enhancement of the infectivity of poliovirus ribonucleic acid by diethylaminoethyl-dextran. J Virol. 1967 Oct;1(5):891–897. doi: 10.1128/jvi.1.5.891-897.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K., Newman J. F., Rueckert R. R. Synthesis of Maus-Elberfeld viral RNA in ascites tumor cells. J Mol Biol. 1966 Jan;15(1):92–101. doi: 10.1016/s0022-2836(66)80211-5. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe D., Boeyé A. New polypeptides in poliovirus. Virology. 1972 May;48(2):604–606. doi: 10.1016/0042-6822(72)90073-6. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Virus aggregation as the cause of the non-neutralizable persistent fraction. J Virol. 1967 Jun;1(3):478–488. doi: 10.1128/jvi.1.3.478-488.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild T. F., Burroughs J. N., Brown F. Surface structure of foot-and-mouth disease virus. J Gen Virol. 1969 Apr;4(3):313–320. doi: 10.1099/0022-1317-4-3-313. [DOI] [PubMed] [Google Scholar]


