Abstract
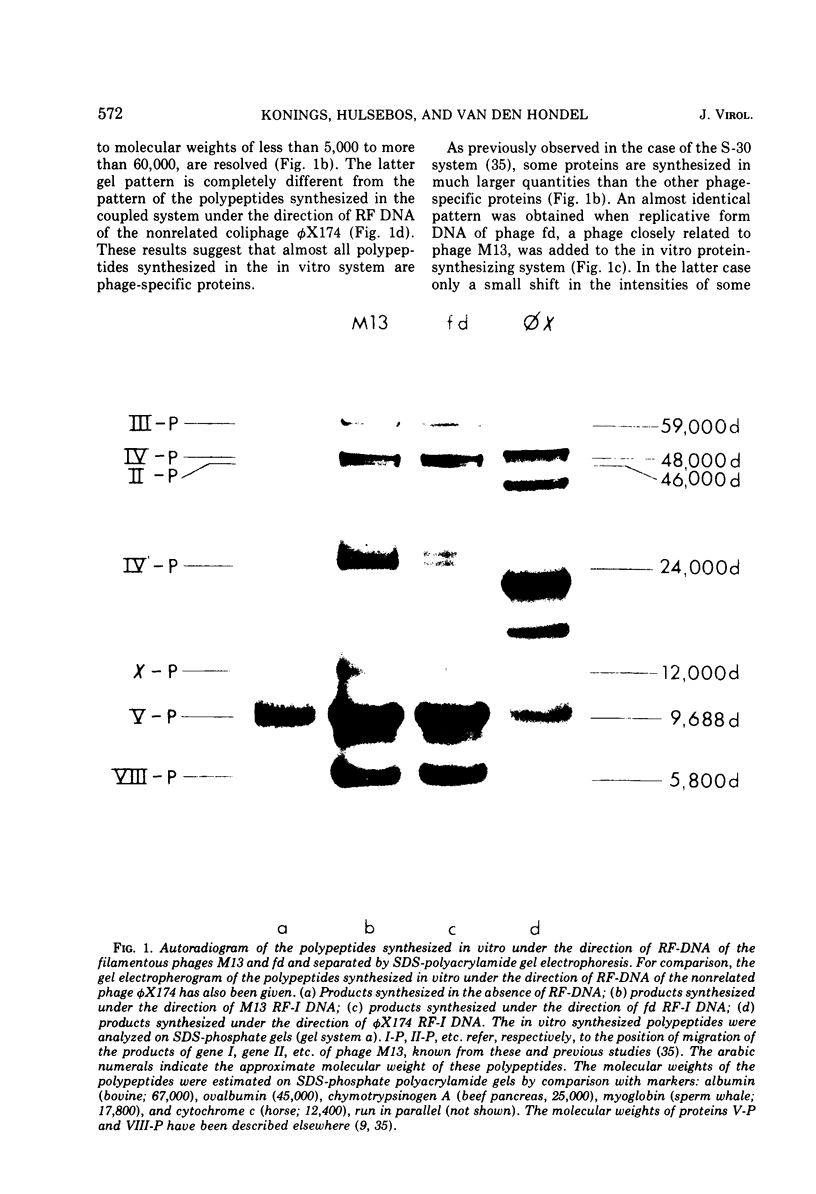
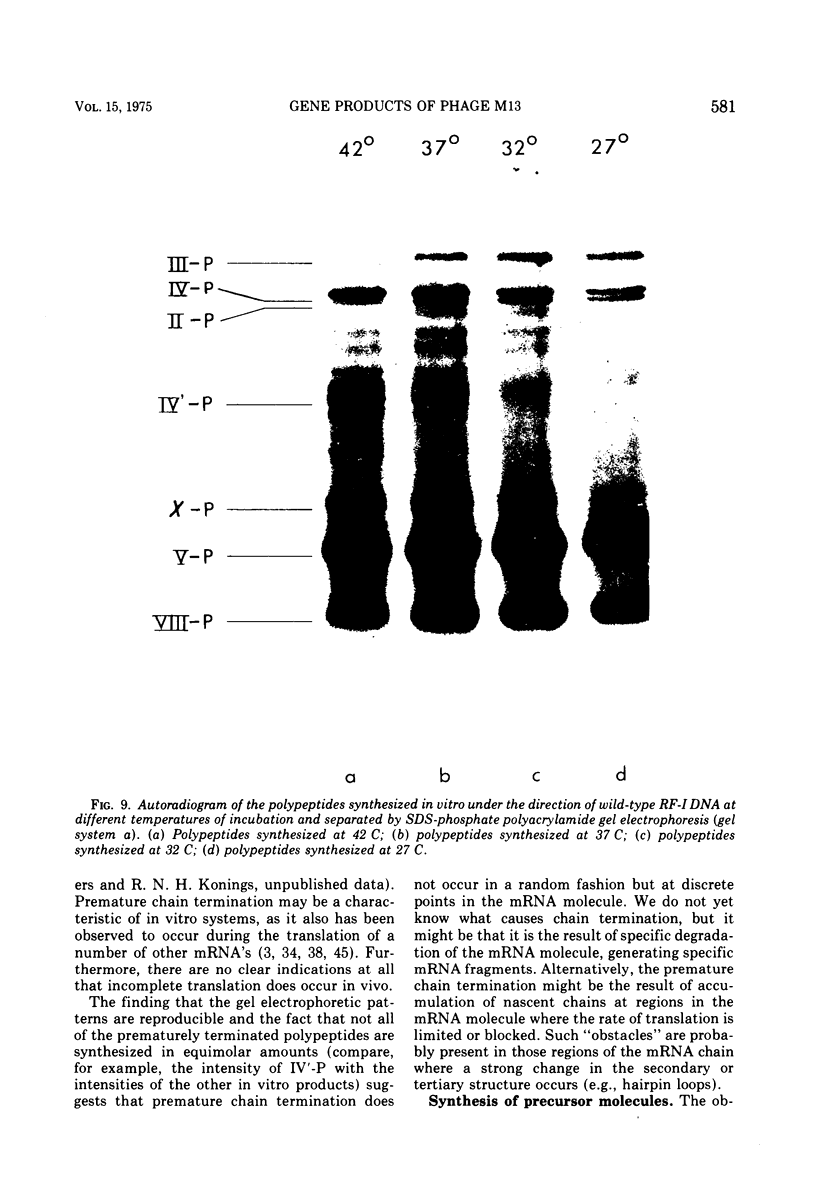
Bacteriophage M13 replicative form (RF) DNA was used to direct coupled transcription and translation in cell-free extracts prepared from Escherichia coli. By using RF DNA, isolated from cells infected with appropriate amber mutants of this phage, it has been possible to identify the products of genes I through IV. By using the same methods no gene-product relationship could be demonstrated for genes VI and VII. Coupled in vitro protein synthesis studies on RF-III DNA, a linear double-stranded DNA molecule, obtained after cleavage of either RF-I or RF-II DNA with the restriction endonuclease R.Hin11 from Haemophilus influenzae, indicated that the cleavage site for this enzyme is located in gene II. The in vitro products of both gene III and gene VIII are about 30 and six amino acids longer, respectively, than their native counterparts present within the virion. These results suggest that the latter proteins arise in vivo by cleavage of precursor molecules. Coupled transcription and translation studies on a DNA fragment which only contained the genetic information coding for gene IV protein, obtained after cleavage of RF DNA with the restriction endonuclease R.Hap11 from Haemophilus aphirophilus, indicated that a large number of the in vitro synthesized polypeptides are the result of premature chain termination.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus mRNA. 3. Discrete polypeptides translated from a monocistronic messenger in vitro. Arch Biochem Biophys. 1972 Dec;153(2):706–713. doi: 10.1016/0003-9861(72)90389-x. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Ehrlich H. P., Wyke A. W. Procollagen: conversion of the precursor to collagen by a neutral protease. Science. 1972 Feb 4;175(4021):544–546. doi: 10.1126/science.175.4021.544. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Harrison T. M., Mathews M. B., Milstein C. Translation of messenger RNA for immunoglobulin light chains in a cell-free system from Krebs II ascites cells. FEBS Lett. 1972 Jun 15;23(2):244–248. doi: 10.1016/0014-5793(72)80352-1. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. Cell-free protein synthesis programmed with R17 RNA: identification of two phage proteins. J Mol Biol. 1966 Oct 28;21(1):173–193. doi: 10.1016/0022-2836(66)90086-6. [DOI] [PubMed] [Google Scholar]
- Cuypers T., van der Ouderaa F. J., de Jong W. W. The amino acid sequence of gene 5 protein of bacteriophage M 13. Biochem Biophys Res Commun. 1974 Jul 24;59(2):557–563. doi: 10.1016/s0006-291x(74)80016-1. [DOI] [PubMed] [Google Scholar]
- DAVIE E. W., NEURATH H. Identification of a peptide released during autocatalytic activation of trypsinogen. J Biol Chem. 1955 Feb;212(2):515–529. [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Fidanián H. M., Ray D. S. Replication of bacteriophage M13. VII. Requirement of the gene 2 protein for the accumulation of a specific RFII species. J Mol Biol. 1972 Dec 14;72(1):51–63. doi: 10.1016/0022-2836(72)90067-8. [DOI] [PubMed] [Google Scholar]
- Files J. G., Weber K., Miller J. H. Translational reinitiation: reinitiation of lac repressor fragments at three internal sites early in the lac i gene of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Mar;71(3):667–670. doi: 10.1073/pnas.71.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Cis-limited action of the gene-A product of bacteriophage phiX174 and the essential bacterial site (E. coli-electron microscopy-cis-acting protein-specifically-nicked RF). Proc Natl Acad Sci U S A. 1972 Feb;69(2):475–479. doi: 10.1073/pnas.69.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Formation of the parental replicative form DNA of bacteriophage phi-X174 and initial events in its replication. J Mol Biol. 1971 Nov 14;61(3):565–586. doi: 10.1016/0022-2836(71)90065-9. [DOI] [PubMed] [Google Scholar]
- Garen A., Garen S., Wilhelm R. C. Suppressor genes for nonsense mutations. I. The Su-1, Su-2 and Su-3 genes of Escherichia coli. J Mol Biol. 1965 Nov;14(1):167–178. doi: 10.1016/s0022-2836(65)80238-8. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F., Kahn C. Synthesis of bacteriophage lambda proteins in vitro. Nat New Biol. 1972 Nov 1;240(96):3–6. doi: 10.1038/newbio240003a0. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Gold L. M., Schweiger M. Synthesis of phage-specific alpha- and beta-glucosyl transferases directed by T-even DNA in vitro. Proc Natl Acad Sci U S A. 1969 Mar;62(3):892–898. doi: 10.1073/pnas.62.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. T., Coombs T. L. Proinsulin, a biosynthetic precursor of insulin. Essays Biochem. 1970;6:69–92. [PubMed] [Google Scholar]
- Griffith J., Kornberg A. Mini M13 bacteriophage: circular fragments of M13 DNA are replicated and packaged during normal infections. Virology. 1974 May;59(1):139–152. doi: 10.1016/0042-6822(74)90211-6. [DOI] [PubMed] [Google Scholar]
- HOFFMANN BERLING H., MAZE R. RELEASE OF MALE-SPECIFIC BACTERIOPHAGES FROM SURVIVING HOST BACTERIA. Virology. 1964 Mar;22:305–313. doi: 10.1016/0042-6822(64)90021-2. [DOI] [PubMed] [Google Scholar]
- HOFSCHNEIDER P. H., PREUSS A. M 13 BACTERIOPHAGE LIBERATION FROM INTACT BACTERIA AS REVEALED BY ELECTRON MICROSCOPY. J Mol Biol. 1963 Oct;7:450–451. doi: 10.1016/s0022-2836(63)80038-8. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Hayashi M. Template activities of the phi X-174 replicative allomorphic deoxyribonucleic acids. Biochemistry. 1971 Nov;10(23):4212–4218. doi: 10.1021/bi00799a009. [DOI] [PubMed] [Google Scholar]
- Henry T. J., Knippers R. Isolation and function of the gene A initiator of bacteriophage phi-chi 174, a highly specific DNA endonuclease. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1549–1553. doi: 10.1073/pnas.71.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T. J., Pratt D. The proteins of bacteriophage M13. Proc Natl Acad Sci U S A. 1969 Mar;62(3):800–807. doi: 10.1073/pnas.62.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., von Schütz H., Marvin D. A. Filamentous bacterial viruses. II. Killing of bacteria by abortive infection with fd. J Mol Biol. 1971 Feb 28;56(1):155–165. doi: 10.1016/0022-2836(71)90091-x. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Cone R. Analysis of T4 phage proteins. I. Conversion of precursor proteins into lower molecular weight peptides during normal capsid formation. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1275–1281. doi: 10.1073/pnas.66.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M., Marco R., Kornberg A. A coat protein of the bacteriophage M13 virion participates in membrane-oriented synthesis of DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):205–209. doi: 10.1073/pnas.70.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. J., Waite M. R., Bose H. R. Cleavage of a viral envelope precursor during the morphogenesis of Sindbis virus. J Virol. 1974 Apr;13(4):809–817. doi: 10.1128/jvi.13.4.809-817.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger E., Der Kamp C. K.-V. On a modification of the gene product P23 according to its use as subunit of either normal capsids of phage T4 or of polyheads. FEBS Lett. 1970 Jun 1;8(3):140–144. doi: 10.1016/0014-5793(70)80247-2. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Tovell D. R. Characterization of the polypeptides formed in response to encephalomyocarditis virus ribonucleic acid in a cell-free system from mouse ascites tumor cells. J Virol. 1972 Jul;10(1):73–81. doi: 10.1128/jvi.10.1.73-81.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings R. N., Jansen J., Cuypers T., Schoenmakers J. G. Synthesis of bacteriophage M13-specific proteins in a DNA-dependent cell-free system. II. In vitro synthesis of biologically active gene 5 protein. J Virol. 1973 Dec;12(6):1466–1472. doi: 10.1128/jvi.12.6.1466-1472.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings R. N., Schoenmakers J. G. Bacteriophage M13 DNA-directed in vitro synthesis of gene 5 protein. Mol Biol Rep. 1974 Feb;1(5):251–256. doi: 10.1007/BF00417579. [DOI] [PubMed] [Google Scholar]
- Konings R. N. Synthesis of phage M13 specific proteins in a DNA-dependent cell-free system. FEBS Lett. 1973 Sep 1;35(1):155–160. doi: 10.1016/0014-5793(73)80600-3. [DOI] [PubMed] [Google Scholar]
- Konings R. N., Ward R., Francke B., Hofschneider P. H. Gene order of RNA bacteriophage M 12. Nature. 1970 May 16;226(5246):604–607. doi: 10.1038/226604a0. [DOI] [PubMed] [Google Scholar]
- Korant B. D. Cleavage of viral precursor proteins in vivo and in vitro. J Virol. 1972 Oct;10(4):751–759. doi: 10.1128/jvi.10.4.751-759.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin N. S., Pratt D. Role of bacteriophage M13 gene 2 in viral DNA replication. J Mol Biol. 1972 Dec 14;72(1):37–49. doi: 10.1016/0022-2836(72)90066-6. [DOI] [PubMed] [Google Scholar]
- Linney E. A., Hayashi M. N., Hayashi M. Gene A of X174. 1. Isolation and identification of its products. Virology. 1972 Nov;50(2):381–387. doi: 10.1016/0042-6822(72)90389-3. [DOI] [PubMed] [Google Scholar]
- Linney E., Hayashi M. Two proteins of gene A of psiX174. Nat New Biol. 1973 Sep 5;245(140):6–8. doi: 10.1038/newbio245006a0. [DOI] [PubMed] [Google Scholar]
- Mach B., Faust C., Vassalli P. Purification of 14S messenger RNA of immunoglobulin light chain that codes for a possible light-chain precursor. Proc Natl Acad Sci U S A. 1973 Feb;70(2):451–455. doi: 10.1073/pnas.70.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Osborn M. The rate of polypeptide chain elongation in a cell-free system from Krebs II ascites cells. Biochim Biophys Acta. 1974 Mar 8;340(2):147–152. doi: 10.1016/0005-2787(74)90107-5. [DOI] [PubMed] [Google Scholar]
- Model P., Zinder N. D. In vitro synthesis of bacteriophage f1 proteins. J Mol Biol. 1974 Feb 25;83(2):231–251. doi: 10.1016/0022-2836(74)90389-1. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Dunker A. K., Marvin D. A., Konigsberg W. The amino acid sequence of a DNA binding protein, the gene 5 product of fd filamentous bacteriophage. FEBS Lett. 1974 Apr 1;40(2):290–292. doi: 10.1016/0014-5793(74)80246-2. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Transcription and translation of prereplicative bacteriophage T4 genes in vitro. J Biol Chem. 1973 Aug 10;248(15):5512–5519. [PubMed] [Google Scholar]
- Oey J. L., Knippers R. Properties of the isolated gene 5 protein of bacteriophage fd. J Mol Biol. 1972 Jul 14;68(1):125–138. doi: 10.1016/0022-2836(72)90268-9. [DOI] [PubMed] [Google Scholar]
- Pratt D., Erdahl W. S. Genetic control of bacteriophage M13 DNA synthesis. J Mol Biol. 1968 Oct 14;37(1):181–200. doi: 10.1016/0022-2836(68)90082-x. [DOI] [PubMed] [Google Scholar]
- Pratt D., Laws P., Griffith J. Complex of bacteriophage M13 single-stranded DNA and gene 5 protein. J Mol Biol. 1974 Feb 5;82(4):425–439. doi: 10.1016/0022-2836(74)90239-3. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Beaudoin J. Conditional lethal mutants of the small filamentous coliphage M13. II. Two genes for coat proteins. Virology. 1969 Sep;39(1):42–53. doi: 10.1016/0042-6822(69)90346-8. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Erdahl W. S. Conditional lethal mutants of the small filamentous coliphage M13. I. Isolation, complementation, cell killing, time of cistron action. Virology. 1966 Nov;30(3):397–410. doi: 10.1016/0042-6822(66)90118-8. [DOI] [PubMed] [Google Scholar]
- Ray D. S. Replication of bacteriophage M13. II. The role of replicative forms in single-strand synthesis. J Mol Biol. 1969 Aug 14;43(3):631–643. doi: 10.1016/0022-2836(69)90364-7. [DOI] [PubMed] [Google Scholar]
- Ray D. S., Schekman R. W. Replication of bacteriophage M13. 3. Identification of the intracellular single-straned DNA. J Mol Biol. 1969 Aug 14;43(3):645–647. doi: 10.1016/0022-2836(69)90365-9. [DOI] [PubMed] [Google Scholar]
- Ray D. S., Schekman R. W. Replication of bacteriophage M13. I. Sedimentation analysis of crude lysates of M13-infected bacteria. Biochim Biophys Acta. 1969 Apr 22;179(2):398–407. [PubMed] [Google Scholar]
- Russell J. H., Geller D. M. Rat serum albumin biosynthesis: evidence for a precursor. Biochem Biophys Res Commun. 1973 Nov 1;55(1):239–245. doi: 10.1016/s0006-291x(73)80085-3. [DOI] [PubMed] [Google Scholar]
- SALIVAR W. O., TZAGOLOFF H., PRATT D. SOME PHYSICAL-CHEMICAL AND BIOLOGICAL PROPERTIES OF THE ROD-SHAPED COLIPHAGE M13. Virology. 1964 Nov;24:359–371. doi: 10.1016/0042-6822(64)90173-4. [DOI] [PubMed] [Google Scholar]
- Salstrom J. S., Pratt D. Role of coliphage M13 gene 5 in single-stranded DNA production. J Mol Biol. 1971 Nov 14;61(3):489–501. doi: 10.1016/0022-2836(71)90061-1. [DOI] [PubMed] [Google Scholar]
- Schweiger M., Herrlich P. DNA-directed enzyme synthesis in vitro. Curr Top Microbiol Immunol. 1974;65:59–132. doi: 10.1007/978-3-642-65875-4_3. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Hofschneider P. H. Replication of bacteriophage M-13. Positive role of gene-5 protein in single-strand-DNA synthesis. Eur J Biochem. 1973 May 2;34(3):569–576. doi: 10.1111/j.1432-1033.1973.tb02797.x. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Taketo A. Sensitivity of Escherichia coli to viral nucleic acid. V. Competence of calcium-treated cells. J Biochem. 1972 Oct;72(4):973–979. doi: 10.1093/oxfordjournals.jbchem.a129988. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Baldi I. Electrophoretically homogeneous myeloma light chain mRNA and its translation in vitro. Biochem Biophys Res Commun. 1973 Mar 5;51(1):81–87. doi: 10.1016/0006-291x(73)90510-x. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Marvin D. A. Filamentous bacterial viruses. VI. Role of fd gene 2 in deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):384–391. doi: 10.1128/jvi.10.3.384-391.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorma H. O., Benne R., den Hertog T. J. Binding of aminoacyl-tRNA to ribosomes programmed with bacteriophage MS2-RNA. Eur J Biochem. 1971 Feb;18(4):451–462. doi: 10.1111/j.1432-1033.1971.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Cashman J. S. Abortive infection of Escherichia coli with the bacteriophage f1: cytoplasmic membrane proteins and the f1 DNA-gene 5 protein complex. Virology. 1973 Sep;55(1):20–38. doi: 10.1016/s0042-6822(73)81005-0. [DOI] [PubMed] [Google Scholar]