Abstract
Neurons in the nucleus accumbens (NAc) have been shown to participate in several behavioral states, including feeding and sleep. However, it is not known if the same neuron participates in both states and, if so, how similar are the responses. In addition, since the NAc contains several cell types, it is not known if each type participates in the transitions associated with feeding and sleep. Such knowledge is important for understanding the interaction between two different neural networks. For these reasons we recorded ensembles of NAc neurons while individual rats volitionally transitioned between the following states: awake and goal directed, feeding, quiet-awake, and sleeping. We found that during both feeding and sleep states, the same neurons could increase their activity (be activated) or decrease their activity (be inactivated) by feeding and/or during sleep, thus indicating that the vast majority of NAc neurons integrate sleep and feeding signals arising from spatially distinct neural networks. In contrast, a smaller population was modulated by only one of the states. For the majority of neurons in either state, we found that when one population was excited, the other was inhibited, suggesting that they act as a local circuit. Classification of neurons into putative interneurons [fast-spiking interneurons (pFSI) and choline acetyltransferase interneurons (pChAT)] and projection medium spiny neurons (pMSN) showed that all three types are modulated by transitions to and from feeding and sleep states. These results show, for the first time, that in the NAc, those putative inhibitory interneurons respond similarly to pMSN projection neurons and demonstrate interactions between NAc networks involved in sleep and feeding.
Keywords: nucleus accumbens, medium spiny neurons, fast-spiking interneurons, choline acetyltransferase, freely licking
despite the fact that feeding and sleeping cannot occur simultaneously, both behaviors are inextricably linked physiologically. For example, loss of sleep suppresses satiety and increases hunger signals (Beccuti and Pannain 2011), whereas manipulations of food intake may alter sleep patterns (Danguir and Nicolaidis 1979). Indeed, it has been shown that dysregulation in these behaviors may contribute to the development of obesity (Beccuti and Pannain 2011), indicating that sleep and feeding signals must be both integrated and coordinated. The lateral hypothalamus (LH) is one area involved in the regulation of sleep, metabolism, and feeding (Saper 2006; Yamanaka et al. 2003), as is the nucleus accumbens shell (NAc shell) (Sears et al. 2010; Stratford and Kelley 1999), a structure that reciprocally projects to the LH (Sano and Yokoi 2007). Hence, by virtue of its responses to reward and its projection to LH, the NAc plays an important role in goal-directed behaviors such as feeding (Baldo et al. 2004; Saper et al. 2002) and, on the opposite extreme, sleep (Braun et al. 1997). More importantly, NAc activity is influenced in both brain states. For example, in humans, sleep restriction leads to increased activation of the NAc to food stimuli (St-Onge et al. 2012), and pharmacological studies have found that a global inhibition of the NAc (which disinhibited LH neurons) permitted animals to feed (Baldo et al. 2004; Cota et al. 2006; Saper et al. 2002; Sears et al. 2010). Similar results were found from extracellular recordings of inhibitory NAc neuronal responses obtained while rats licked to obtain sucrose solutions (Krause et al. 2010; Nicola et al. 2004; Roop et al. 2002). In other studies it has been found that NAc neurons are also modulated during slow-wave sleep (SWS), but the recordings were not obtained over extended times nor were the responses compared with those that occur during feeding (Berke et al. 2004; Lansink et al. 2010; Mahon et al. 2006). To address the above issues, we recorded ensembles of neurons from rat NAc for several hours during which an animal was free to ingest several meals and between them exhibit bouts of SWS.
The NAc is composed of ∼95% GABAergic medium spiny neurons (MSNs) that integrate excitatory inputs from cortical and subcortical areas and outputs that information to several areas, including the ventral pallidum and LH (Humphries and Prescott 2010). The remaining ∼5% of NAc neurons [fast-spiking interneurons (FSIs), choline acetyltransferase interneurons (ChATs), and others] are interneurons that modulate the activity of the MSN population (Tepper et al. 2008; Witten et al. 2010). In the majority of studies involving the use of extracellular recordings, the cell type was not identified, although because the majority of NAc neurons are MSNs, it is commonly assumed that those are the ones being recorded (Frazier and Mrejeru 2010). To identify how the different cell types in the NAc are modulated in feeding and sleeping, we used a fuzzy clustering algorithm that permits us to putatively classify the NAc neurons as pMSNs, pFSIs, and pChATs (Kawaguchi et al. 1995; Lansink et al. 2010; Yarom and Cohen 2011). From this analysis we were able to show that all three types of NAc neurons can track transitions between active-awake and feeding and quiet-awake and sleep states and, importantly, to show that in each state, the activated and inactivated responses are completely out of phase. Moreover, the responses in one state may or may not parallel those in the other state. In addition, we identified novel responses that are modulated only in one of the two states. These results suggest that NAc is a node in the feeding circuit that is directly or indirectly coordinated by the sleep-awake circuitry.
MATERIAL AND METHODS
Subjects and Surgery
In these experiments we used eight male Sprague-Dawley rats weighing 300–350 g. Animals were housed individually in standard laboratory cages in a temperature-controlled (21 ± 1°C) room and maintained on a 12:12-h light-dark cycle (lights on 0600–1800). At the time of surgery, the animals were anesthetized using intraperitoneal injections of pentobarbital sodium (50 mg/kg) and 0.1 ml of atropine sulfate and were unilaterally-implanted with a movable electrode array consisting of 32 tungsten microwires (35-μm diameter) targeting NAc (centered at the following coordinates: AP = 1.5 mm, L = +1 mm, and DV = 6.5 mm from bregma). After the completion of experiments, the location of the electrodes was histologically verified using cresyl-stained brain sections. All procedures were approved by the CINVESTAV Institutional Animal Care and Use Committee.
Chocolate-Flavored Ensure
We decided to use this food, which contains a macronutrient mix of fat, proteins, sugar, carbohydrates, vitamins, and minerals, because it is a complete liquid meal that better resembles most properties of a solid palatable food (Archer et al. 2006; Davidson and Swithers 2005). In addition, trained animals will eat large amounts without water deprivation or the need for food.
Behavioral Procedures
All the experiments were performed at approximately the same time of day (11:00 AM) in an operant box enclosed in a ventilated and sound-attenuating cubicle. Each box contained a central drinking compartment, a lickometer (V shape, vertical slot) with a photo-beam sensor (MedAssociates) to register the times when the rat's tongue contacted the drinking tubing (Gutierrez et al. 2006). Briefly, 1 wk before surgery, the animals were habituated with the task. At least four training sessions were necessary to assess the animals habituated to the behavioral box and familiarized with Ensure. Access to the Ensure compartment was restricted by a sliding door; after 20 min, the door was opened and animals were allowed free access to Ensure until the end of the experiment. Importantly, the rats were neither food nor water deprived, but they were trained to ingest Ensure every day at about the same time of the day and could decide of their own volition when to begin and end a meal. On the experimental days, neural activity [single action potentials and local field potentials (LFPs)] was recorded and the animals remained in the operant box until they completed at least one meal and several awake-sleep cycles.
Measures of Behavior Associated with Ingestion of Ensure
Licking microstructure.
In this study, we characterized the neuronal responses of NAc neurons to several parameters of licking behavior (see below). For that reason, we performed a detailed analysis of the microstructure of licking. In this regard, rhythmic licking is punctuated by pauses ≥500 ms; these periods of licking are called “licking clusters” (see Fig. 1A, red; see also Davis and Smith 1992), and analysis of this behavior is referred to as the “microstructure of licking.” The cluster size and cluster duration refer to the time spanned by a cluster and the number of licks in that cluster, respectively (see Fig. 1A and Table 1; see also Davies and Smith 1992).
Fig. 1.
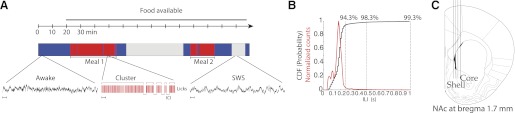
Awake, sleeping, and feeding States. A: behavioral protocol and associated local field potentials (LFPs) in awake and sleeping states as well as during feeding (licking). Top, after a 20-min habituation period, food (chocolate-flavored Ensure) was made available ad libitum. Middle, color-coded diagram shows the hypnogram and occurrence of various behavioral states the rat experienced during the experiment: blue, awake; red, feeding (licking); gray, slow-wave sleep (SWS). The awake state produces low-amplitude fast oscillations in the LFP (see left inset trace below). At the macrostructure level, it can be seen that the animal ate 2 large meals. Middle inset displays 20 s of the licking microstructure; shown are 5 licking clusters and the corresponding inter-cluster intervals (ICIs). As the SWS state deepens, delta waves in the LFP largely predominate (see right inset). In insets, bars = 1 s. B: normalized inter-lick interval (ILI) distribution (red line). For visualization purposes, the abscissa only shows up to 1-s interval. The cumulative density function (CDF) is shown as a black line. C: schematic of microarray in the medial ventral striatum where the electrodes were implanted. NAc, nucleus accumbens.
Table 1.
Micro- and macrostructure analysis of licking for Ensure
| Microstructure Analysis of Licking |
|||
|---|---|---|---|
| Cutoff Criteria | No. of clusters | Cluster size, s | No. of licks/cluster |
| ICI ≥0.5 s | 108 ± 10 | 14 ± 1 | 87 ± 8 |
| ICI ≥1 s | 48 ± 5 | 35 ± 3 | 202 ± 19 |
| ICI ≥5 s | 22 ± 2 | 85 ± 8 | 473 ± 48 |
| Macrostructure Analysis of Meals |
|||
|---|---|---|---|
| No. of meals | Meal size, min | Licks/meal | |
| IMI ≥5 min | 2.86 ± 0.2 | 12.43 ± 0.78 | 2936 ± 215 |
| IMI ≥10 min | 2.36 ± 0.12 | 16.18 ± 0.95 | 3269 ± 201 |
| IMI ≥15 min | 2.2 ± 0.1 | 19.63 ± 1.97 | 3503 ± 218 |
| IMI ≥20 min | 2.1 ± 0.97 | 21.9 ± 2.1 | 3631 ± 213 |
ICI, inter-cluster interval; IMI, inter-meal interval.
Meal-feeding analysis.
Meals were defined by inter-lick intervals (ILI) >10 min (Spector et al. 1998). The termination of each meal was taken as the onset of an ILI >10 min. Meal size and meal duration were measured from the first to the last lick of a meal. Meal size refers to the time elapsed from first to last lick, whereas meal duration refers to the number of licks occurring in the meal (see Table 1).
Electrophysiological recordings.
Procedures were basically the same as described previously (Gutierrez et al. 2010). That is, neural electrical activity was recorded from a movable 4 × 8 array of 32 tungsten microwires in the NAc using a multichannel acquisition processor (Plexon, Dallas, TX). Only single neurons with action potentials of signal-to-noise ratios >3:1 were analyzed. The action potentials were isolated online by means of voltage-time threshold windows and a three-principal components contour templates algorithm. Spikes were resorted using Offline Sorter software (Plexon). Only time stamps from offline-sorted waveforms were analyzed. LFPs were amplified 1,000 times, filtered at 0.7–300 Hz, and digitized at 1 kHz using a digital acquisition card (National Instruments, Austin, TX) and a multineuron acquisition processor (Plexon).
Neural Data Analysis
Lick-spike coherence analysis.
Multitaper spectral analysis and coherence were computed by segmenting two univariate binned point processes [licking and spike poststimulus time histograms (PSTHs)] into chunks (Gutierrez et al. 2010; Jarvis and Mitra 2001). The coherence “C” between licking and the spike trains was computed using the formula C(f) = Ixy/(IxxIyy), where Ixx represents the spectrum of licking behavior, Iyy is the spectrum of neuronal activity, and Ixy is the cross-spectrum of licking and spike trains. Note that the coherence is normalized to range between 0 and 1. A coherence of 0 or 1 means that two signals are completely uncorrelated or correlated in frequency and phase, respectively. The frequency band (f) is where coherence was computed (4–10 Hz) and corresponds to the normally observed freely licking behavior. The confidence interval of the coherence, C(f), and significance threshold (at α = 0.05%) were computed with a jackknife method and finite size corrections using the procedures developed by Jarvis and Mitra (2001). A neuron was classified as licking-coherent only if its lower confidence interval (95%) crossed the significance threshold (Gutierrez et al. 2010). Each chunk of data corresponded to the first lick in the lick cluster and to 2 s of activity. Only licking clusters ≥2 s were used for this analysis, to ensure that neural activity corresponded to moments when rats were feeding. The maximum coherence value and frequency were computed using the Chronux 2.0 software package (www.chronux.org).
Hypnograms.
Three behavioral states were defined: 1) awake, with relatively high theta (5–9 Hz) and gamma (30–55 Hz) power density (Winson 1974); in this state, the animals displayed little or no exploratory behavior, and indeed, for most of their waking time, they were quietly standing or sitting and sometimes grooming; 2) SWS, with sleep spindles (10–14 Hz) superimposed on delta waves (1–4 Hz); and 3) feeding, directly obtained from licking records. Awake and SWS states were assigned using the LFPs and the methodology outlined by Gervasoni et al. (2004). In brief, after elimination of segments with amplitude saturation, a sliding window Fourier-transform was applied to each LFP signal to calculate two spectral amplitude ratios (0.7–20/0.7–55 Hz and 0.7- 4.5/0.7–9 Hz for ratios 1 and 2, respectively). Principal component analysis (PCA) was then applied to these ratios obtained from all LFP channels, and the first PC was used as the overall ratios measure. These measures, obtained for each second of data, were further smoothed with a Hanning window (20-s length). Finally, these two first PCs of spectral ratios were plotted against each other to construct the two-dimensional (2-D) state space. In this 2-D state space, the density of points reflects the relative abundance of the different brain states. We note that in all our experiments, we did not record sufficient amounts of rapid-eye-movement (REM) sleep (see below). Thus REM sleep was not considered in any of our analyses.
Feeding-related neurons.
“Inactive” and “active” neurons were identified while the animal was feeding, following the criteria of Krause et al. (2010), who named them types 1 and 2, respectively. We extended these definitions, because these two types of neurons could also be activated or inactivated during sleep (SWS-on and off, respectively; see below). Briefly, we compared the spike rates in a 3-s prelick period (from −5 to −2 s before lick onset) with the spike rates during the first 3 s of licking using a Wilcoxon signed-rank test. We categorized inactive neurons as those that showed a significant firing rate decrease, and active neurons as those that showed a significant firing rate increase, during the licking period. For this analysis, we used all trials where a licking cluster had an inter-cluster interval (ICI) pause of at least 5 s, was at least 20 licks in length, and lasted for at least 4 s. These parameters matched the freely licking behavior with a similar feeding behavior observed in the lick task used by Krause et al. (2010).
SWS-off and SWS-on responses.
Briefly, using a Wilcoxon signed-rank test, at α < 0.05, we compared the spike rates in a 10-s pre-SWS window (from −15 to −5 s before SWS onset) with the spike rates during the first 10 s of SWS bout onset (from 0 to 10 s after SWS onset). We categorized SWS-off responses as those that showed a significant firing rate decrease, and SWS-on neurons as those that showed a significant increase, during the SWS bout. For this analysis, we used all sleep bouts where SWS had a minimum inter-SWS interval of at least 10 s and the SWS bout lasted for at least 10 s.
The Fano factor.
We used the Fano factor (FF), measured as the ratio of the variance to the mean response, to quantify spike count variability of the population activity (Truccolo et al. 2011).
Cell type classification.
Neurons were classified into groups according to four features: firing rate, coefficient of variation 2 (CV2), valley-to-amplitude ratio (VAR), and valley-to-peak width (V-P width). Firing rate was calculated as the number of recorded spikes divided by session duration. CV2 was calculated for each adjacent pair of inter-spike intervals (ISIs), and the average CV2_ISI for the entire session was used. In brief, the two-ISI coefficient of variation was computed as CV2_ISI = |2(ISI2 − ISI1)/(ISI 2 + ISI1)| (Holt et al. 1996). The VAR was calculated as the absolute value of the first valley in the waveform divided by the difference between its minimum value and the following maximum (see blue waveform in Fig. 7, inset, and Yarom and Cohen 2011). For computation of the V-P width, see black waveform in Fig. 7, inset. For each neuron recorded (m = rows), these four features (n = columns) were computed, which resulted in an m × n data matrix. Values in each column of the data matrix were normalized to range between 0 and 1 by using the following formula [n − min(n)]/[max(n) − min(n)], classified into groups with similar attributes by using the fuzzy cluster algorithm, and visualized by using PCA as seed followed by the fuzzy Sammon's mapping plot as described in the Fuzzy Clustering and Data Analysis Toolbox (http://www.fmt.vein.hu/softcomp/fclusttoolbox). For cluster number validation, we used the Dunn's index, which was maximized with four groups. We also evaluated three other features, which were shoulder-to-shoulder width, CV, and time to peak in the autocorrelogram histogram, which is an indicator of burstiness, but they did not improve classification (data not shown).
Fig. 7.
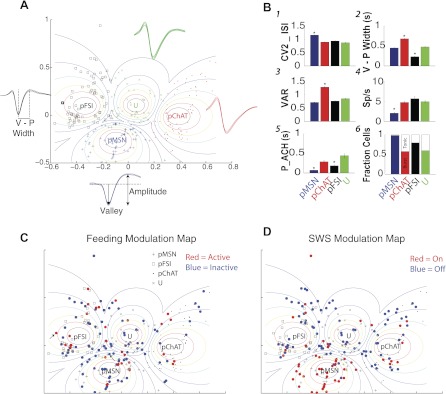
Cell types in the NAc. A: neurons were grouped into 4 categories: pMSNs (+), pFSIs (□), pChATs (●), and unidentified cells (U; ×). This grouping was accomplished by using a fuzzy clustering algorithm and the waveform valley-peak width (V-P width; distance between dotted lines in black waveform inset), the valley-to-amplitude ratio (VAR; see blue inset showing the waveform parameters used to calculate VAR), the firing rate (spikes/s), and the mean coefficient of variation 2 (CV2) of the inter-spike interval (ISI) distribution (CV2_ISI). For each group, the larger the ellipsoid, the smaller is the membership's certainty. Insets display mean waveforms and SE for each putative cell type. B: all panels display means and SE of 6 features as a function of cell type. B1–B4 show CV2_ISI, V-P width, VAR, and firing rate, respectively. B5 shows the time to peak (maximum value) in the autocorrelation histogram (P_ACH). B6 shows the fraction of cells with either irregular burst or tonic firing pattern (see materials and methods). *P > 0.05, significant difference among all groups. C: symbols shown in red and blue are responses that were either active or inactive, respectively, during feeding. Arrows indicate the location of neurons plotted in Fig. 3. D: symbols shown in red and blue are responses that were either on or off, respectively, during SWS.
RESULTS
Behavior: Awake, Sleeping, and Feeding States
During a typical experiment, rats mainly spent their time in three distinct states: awake but quiet (Fig. 1A, blue; 53.9 ± 0.02% of time in the session), feeding (Fig. 1A, red; 14.9 ± 0.01% of the session), or sleeping (Fig. 1A, gray; 30.1 ± 0.01% of the session). In addition to the quiet-awake state, there was another attentive awake state that was present at the onset of feeding. Transitions from SWS were followed by either a REM state that was observed <1% of the time or, more commonly, the quiet-awake state.
Feeding macrostructure (meals).
As noted, the feeding session involved the rat's licking for Ensure. As shown in Fig. 1A, feeding behavior consisted of relatively short bouts of continuous licking, called clusters, that were separated by pauses, the ICIs (Davis and Smith 1992). When the ICI was >10 min, the sum of these clusters constituted a meal (see Fig. 1A and Table 1). Subsequent meals had to have an inter-meal interval (IMI) of >10 min (see Fig. 1A, red, and Table 1). During the course of the average 2.6-h experimental session, rats usually (n = 41/46) ate two meals (Table 1). The average duration of the first meal was 24 ± 2 min (n = 41), whereas the duration of the second meal was significantly shorter at 9.4 ± 1 min (Wilcoxon signed-rank test, P < 0.0001). The difference in meal size may have arisen because the metabolic effects of the first meal had not yet returned to the original state (de Araujo et al. 2006). The latency between the first and second meal was 52.5 ± 5.6 min. In the other five sessions, the subjects ate only one meal.
Licking clusters.
For hedonically positive liquids, rats lick with a rhythmic and stereotypic protrusion and retraction of the tongue at frequencies of 5–7 Hz (Travers et al. 1997). The average ILI was 165 ms, or a licking frequency of 6.05 Hz. The majority of the licks were rapid and rhythmic, since 94.3% of contiguous licks have ILIs < 250 ms and only 4% of ILIs were ≥250 ms and <500 ms. A few remaining ILIs comprised pauses >500 ms (Fig. 1B). Details about “microstructure” and “macrostructure” of Ensure meals are shown in Table 1. The analysis of both the micro- and macrostructure of licking behavior is relevant because these analyses reflect the oromotor and palatability information as well as information regarding the initiation and termination of a meal (Spector et al. 1998). However, little is known about the neuronal correlates that result from animals ingesting liquid food of their own volition.
Electrophysiology.
From 8 rats in 46 experimental sessions, we recorded the activity of 356 neurons. Eighty-nine neurons were excluded from analysis either because the waveform abruptly drifted during the session or because the waveform had a complex shape (e.g., W shape; Gold et al. 2006); three additional neurons were excluded because no LFP was simultaneously recorded. Hence, we report the activity of 264 well-isolated single neurons located in the ventral striatum while the animals were in awake, feeding, and sleeping states (see Fig. 1C for the location of electrodes).
Neural Dynamics of Ventral Striatum Neurons During Ad Libitum Feeding
A small population of NAc neurons was entrained by rhythmic licking.
We first determined whether the firing activity of each of the 264 neurons was coherent with rhythmic licking. In this context, coherence is defined as a measure of the interdependence of licking and neural activity in the relevant frequency domain (e.g., 4–10 Hz; see materials and methods). This analysis revealed that only 3.7% (10/264) of NAc neurons were entrained by rhythmic licking in that they achieved a significant coherence (P < 0.05) with an average (±SE) coherence value of 0.23 ± 0.033. That is, only a small percentage of NAc neurons tracked the rhythm of licking behavior.
Neural activity during and around licking clusters.
A recent study by Krause et al. (2010) that involved rats performing a licking-related task to obtain sucrose solutions found that feeding was maintained when a subset of NAc neurons, named type 1, was inhibited. They also identified another type that was excitatory, named type 2. In their analyses, they used an ICI ≥5 s. Here we extended these observations by studying animals that took ad libitum food and also compared the responses in the wake and sleeping states. First, we examined the spiking rate modulations using two other time cutoffs commonly employed to define a licking cluster to determine whether they would be sufficient to analyze the data. Note that licking clusters necessarily have a cluster start (CS) and a cluster end (CE). Figure 2, A and B, shows the neural activity using 1-s and 0.5-s criteria, respectively, as the minimum ICI to group licks into clusters (see Fig. 2, times −5 to −1 s for CS, left, and times 1 to 5 s for CE, right). It is clear that the spiking modulation remains essentially unchanged, even though our data show that the smaller the ICI, the larger the number of clusters and the smaller their size (Table 1). However, because the analysis using larger ICIs to define a cluster extended the epoch in which the rat approached the sipper, with no contamination of licks preceding the onset of CS and to avoid confusion in the literature, we kept the ICI ≥5 s consistent with that of Krause et al. 2010. Hence, to align the spikes (time = 0 s) that define a trial, we used the first lick when the rat resumed licking behavior after a pause (ICIs) larger or equal to 5 s. We then evaluated the proportion of neurons that were significantly inhibited during the first 3 s of licking. In good agreement with that study, we identified two populations of neurons that modulated their firing rate during licking: one that we named “inactive” (36%; 94/264) and another named “active” (11%; 30/264; see Fig. 3, A and B, respectively). Inactive neurons were significantly more likely to be recorded than active ones [χ2 test(1) = 33.03, P < 0.0001]. These percentages are in good agreement with Krause et al. (2010), suggesting that freely licking for a liquid meal is a natural behavior that also can be used to identify neurons that they referred to as type 1 (present study: inactive) and type 2 (present study: active). For the remainder of this article, we refer to these neurons as active or inactive, because these terms intuitively indicate the directionality of firing rate modulation induced by feeding.
Fig. 2.
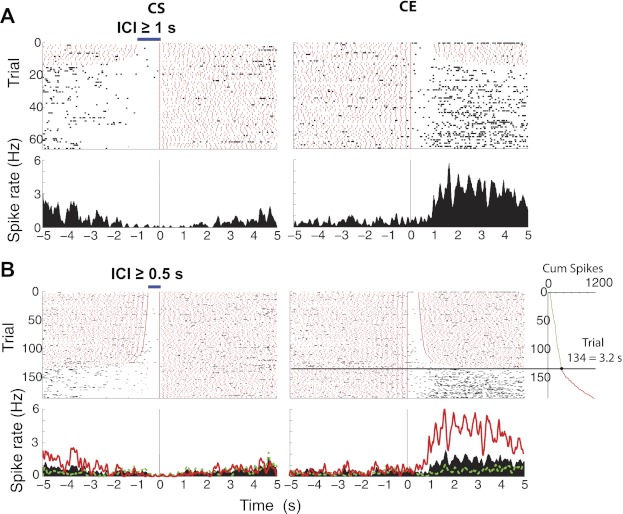
Representative example of a ventral striatum neuron whose activity is transiently inhibited upon feeding. For completeness, the neuronal activity of the same neuron plotted in Fig. 3A was analyzed at different ICIs. A: activity plotted for ICIs ≥1 s. B: activity plotted for ICIs ≥0.5 s. In A and B, red marks indicate licks and black marks indicate action potentials. The smaller the ICI, the greater the number of clusters (trial). Blue horizontal bar at top of the raster shows the minimum ICI for each criterion. Top panels show the raster for the number of clusters, and bottom panels show the corresponding poststimulus time histogram (PSTH) for spike rate (in Hz). Time = 0 s in left panels indicates cluster start (CS) and in right panels, cluster end (CE). In A and B, the trials are arranged according to the duration of the ICI with the smallest at top. In B, the inset shows the spike count cumulative sum (Cum spikes) during the epoch 0–5 s after CE. The horizontal line at trial 134 indicates the significant change point, where the neuron recovered from inhibition (Gallistel et al. 2004). The PSTH in red (also red in inset) shows the spiking rate during trials after the change point, whereas the PSTH in green shows the spiking in trials before the change point. It is clear that this inactive neuron tended to fire again after the rat paused licking for more than 3.2 s.
Fig. 3.
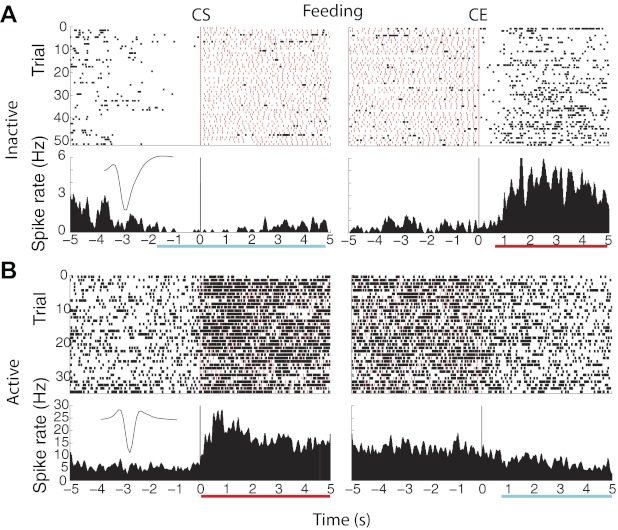
Representative examples of “inactive” and “active” neurons recorded from NAc during ad libitum intake of Ensure. The red marks indicate licks, and the black marks indicate action potentials. Top panels show the raster plots for the number of clusters (trial), and bottom panels show the corresponding PSTH (in Hz). The vertical lines shown in left panels indicate the licking CS, and those in right panels indicate the CE. Cyan horizontal bar indicates time of significant inhibition, and red horizontal bar indicates recovery of firing rate. A: inactive response. B: active response. For both neurons, the neuronal activity was analyzed at an ICI ≥5 s. The insets in PSTHs shown in A and B display the waveform of a putative medium spiny neuron (pMSN) and a putative fast-spiking interneuron (pFSI), respectively (see Fig. 7).
Figure 3A shows the raster and PSTH of an inactive neuronal response that is centered on CS and CE. This response revealed a 1.6-s inhibition before the onset of licking (CS) that was followed by a long-lasting inhibition (cyan horizontal bar) that returned to baseline 0.92 s after CE (red horizontal bar). To further substantiate the decision of choosing a pause of 5 s to define a licking cluster, we also computed the minimum pause in licking required to make an inactive neuron recover from its inhibition and return to baseline spiking. To accomplish this, we computed the cumulative spikes across trials (in the epoch from 0 to 5 s for CE) and identified the trial at which the slope of the cumulative sum significantly changed. Because trials are ordered according to the duration of ICI, the ICI of that trial directly indicates the minimum pause in which the cell fired again. The analysis revealed that this inactive response needed a pause in licking of at least 3.2 s (see Fig. 2B; black horizontal line). For all inactive neurons, rats needed to pause licking for at least 2.16 ± 0.024 s (n = 94) to return to baseline activity. This result suggests that the scale of the “microstructure of licking” that inactive NAc neurons encode is >2 s, and thus their activity cannot follow the details of the microstructure of licking that occurs during pauses (ICIs) smaller than that time scale. Hence, for all of the following analyses, we used ICIs ≥5 s.
Figure 3B shows the raster and PSTH of a representative active neuron that is centered between CS and CE. The response revealed that when food was obtained, there was a ramping up of excitation that reached a maximum and then decayed as the licking cluster progressed (red underline) and that returned to baseline 0.8 s after CE (cyan horizontal bar). We also computed the minimum pause in licking required to make an active neuron recover from its excitation and return to baseline spiking. For all active neurons, the mean ICI required to return to baseline spiking was 2.3 ± 0.07 s (n = 30). This result was not significantly different from that for inactive neurons [Wilcoxon rank test, P = 0.73, not significant (NS)], suggesting that active and inactive neurons act cooperatively in a local circuit.
Feeding and the sleep-wake cycle in ventral striatum.
Although others have shown that type 1 (inactive) neurons are modulated by feeding behavior associated with licking (Krause et al. 2010; Taha and Fields 2005), in this study we inquired whether their spiking activity could be modulated in other behavioral states. The LFP in the ventral striatum provided us with information about the quiet-awake–sleep transitions (QW-SWS and SWS-QW), allowing us to automatically compute hypnograms (Gervasoni et al. 2004). Interestingly, we found that like the feeding responses, the majority of NAc neurons were also inhibited by the wake-sleep cycle. That is, 33% (88/264) of the neurons were inactivated during SWS (SWS-off), whereas 20% (53/264) significantly increased their firing rate during the transition from quiet-awake and entered into the SWS state (SWS-on). In general, SWS-off responses were found in greater numbers than SWS-on responses [χ2 test(1) = 35.34, P < 0.0001; see Table 2].
Table 2.
Neurons with a significant firing rate modulation during feeding and sleep states as a function of cell types
| No. of Neurons |
|||||
|---|---|---|---|---|---|
| Response Type | All cell types | pMSN | pChAT | pFSI | Unidentified |
| Neurons modulated by feeding and/or SWS | |||||
| Inactive/SWS-off | 39/264 (14%) | 4 | 5 | 19* | 11 |
| Inactive/SWS-on | 24/264 (9%) | 15* | 1 | 5 | 3 |
| Inactive only | 31/264 (13%) | 11 | 7 | 8 | 5 |
| Subtotal | 94/264 (36%)# | 30/70 (43%)# | 13/71 (18%) | 32/73 (44%)# | 19/50 (38%)# |
| Active/SWS-off | 9/264 (3%) | 2 | 2 | 4 | 1 |
| Active/SWS-on | 6/264 (2%) | 2 | 2 | 1 | 1 |
| Active only | 15/264 (6%) | 5 | 2 | 5 | 3 |
| Subtotal | 30/264 (11%) | 9/70 (13%) | 6/71 (8%) | 10/73 (14%) | 5/50 (10%) |
| Neurons selectively modulated by SWS | |||||
| SWS-off only | 40/264 (15%) | 2 | 23* | 11 | 4 |
| SWS-on only | 23/264 (9%) | 16* | 4 | 1 | 2 |
Values are no. of neurons per indicated population with each response type, with percentages given in parentheses. Cell types include putative medium spiny neurons (pMSN; n = 70); putative choline acetyltransferase interneurons (pChAT; n = 71); putative fast-spiking interneurons (pFSI; n = 73); or unidentified (n = 50). SWS, slow-wave sleep.
P < 0.05, comparison between cell types (χ2 test). #P < 0.05, comparison of inactive vs. active subtotals for each cell type (χ2 test).
Figure 4 shows a representative response of an inactive neuron in both feeding and SWS sleep states. Figure 4A shows the response obtained from an inactive neuronal response that was inhibited just before feeding (CS) and that continued to be inhibited until, after a short pause, it resumed activity when the licking activity stopped (CE). Figure 4B shows that a similar (but not identical) inhibition occurred when the animal transitioned from a quiet-awake state into the SWS state. The neuron's spiking activity throughout several QW-SWS cycles and two meals is shown in Fig. 4C. It is clear that during both meals and during SWS, the activity was significantly depressed (see inset). These results indicate that at single neuronal level, a subpopulation of NAc neurons are modulated by both feeding and sleep signals.
Fig. 4.
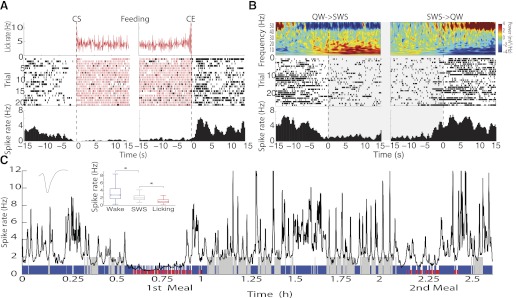
Responses of a pMSN whose activity was inhibited both by feeding and during SWS (inactive/SWS-off). A: top trace (red) shows that during CS and throughout the cluster to the CE, the licking rate (licks/s) remained approximately constant. Middle panel (trial) shows the raster plot, and bottom trace displays the PSTH showing that the activity decreased before CS and remained essentially depressed throughout the cluster. On the cession of licking, the activity once again increased. B: the same neuron as in A but during the quiet wake-SWS-quiet wake (QW→SWS→QW) transition. Top trace shows the spectrogram recorded during a “sleeping bout” when the animal went from QW to SWS and from SWS to QW states. The spectrogram shows that during the QW state, much of the power increased in the high gamma frequency centered at 50 Hz, which gradually decreased as the animal entered into the SWS state, where the power increased but in the low-frequency range (0.7–20 Hz). The raster and PSTH show that during SWS, the spike rate decreased and became active upon the animal waking. C: spike rate of the same neuron, shown during the 2.6 h when the animal ate 2 meals (red), was active (blue), and slept (gray). The activity was filtered at a 10-s Gaussian window and a 1-s steep moving window. Inset: median ± SE of the spike rate for each second throughout the 3 states. *P < 0.05. This neuronal response is the same as that plotted in Figs. 2 and 3A.
Figure 5 shows the spiking patterns of all eight different subcategories of responses of inactive and active ventral striatum neurons. Shown are the responses of all 187 neurons, each normalized to Z scores, that were either inhibited or excited during feeding (Fig. 5, left) or SWS (Fig. 5, right). The superimposed black lines represent the population PSTHs for each subcategory of neurons. The color-coded panel (far left) indicates the three putative types of NAc neurons, which are discussed separately below.
Fig. 5.
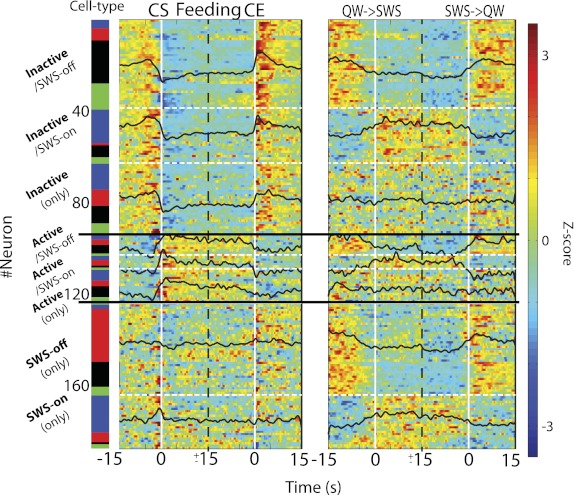
Population activity of NAc neurons during feeding (licking; left) and SWS (right). Shown are all the significant responses of 187 neurons, each normalized to Z scores (far right), centered around the onset (CE) and termination of licking clusters (CE) or the onset (QW→SWS) or termination (SWS→QW) of SWS bouts. Both of these onset times are indicated by vertical white lines (time = 0 s). The response types are referenced to the feeding (licking) state. The dashed white lines separate subcategories, and solid horizontal black lines identify the population of each category of inactive, active, and SWS-off and SWS-on neurons. It can be seen that inactive responsive cells can exhibit “on” or “off” responses in SWS or be nonresponsive (inactive only). The same is evident for active cells, whose response to SWS can be excitatory (on), inhibitory (off), or unresponsive. Finally, there are other populations of neurons that are unresponsive to feeding but can be inhibited (SWS-off only) or excited (SWS-on only) during SWS. For each subcategory, neurons were sorted as a function of their putative cell type (see Fig. 7 for more details). The color bar at far left indicates the putative cell type: blue, pMSN; red, putative choline acetyltransferase interneuron (pChAT); black, pFSI; green, unidentified cell type.
For inactive neurons, we found that neurons 1–39 were inhibited during both feeding and SWS (SWS-off), whereas neurons 40–63 were inhibited during feeding but excited during SWS (SWS-on). This, of course, raises the question of how to classify them as inactive or active. For both clarity and consistency with the literature, our classification scheme refers the response type to the feeding state. In this regard, neurons 64–94 represent a third category of inactive responses that are inhibited by feeding but unaffected in SWS.
For active neurons (also referred to the feeding state), we found that with respect to SWS, some were inhibited (neurons 95–103), some were excited (neurons 104–109), and some were unresponsive (neurons 110–124). In addition to these categories, there were also neurons that were unresponsive to licking (feeding) but inhibited in SWS (neurons 125–164; SWS-off only) or that were only excited in SWS (neurons 165–187; denoted SWS-on only). In summary, these results demonstrate that the increase or decrease of activity of NAc neurons during feeding is not predictive of whether the neuron will be active or suppressed during SWS.
Population variability during brain state transitions.
The population data shown in Fig. 6 for the transitions between states are best understood along with the information presented in Fig. 5 and Table 2. For example, the entire feeding-inhibited population, which represents 36% of the overall population and is presented in Fig. 6A, top, includes all transitions independent of the responses in the sleep transitions. The excited feeding population, shown in Fig. 6A, middle, comprises 11% of the total population. Similarly, Fig. 6B, top, gives the population responses of the 33% of neurons that were inhibited during SWS, which basically arose from neurons inhibited upon feeding and from the SWS-off population. In Fig. 6B, middle, the population responses of 20% of the neurons that were activated during SWS are shown.
Fig. 6.
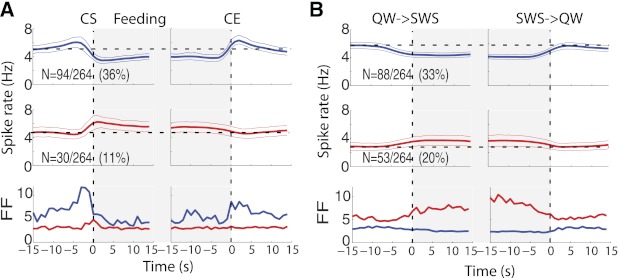
Spiking heterogeneity and population activity around feeding (A) and SWS (B). A, top: the population PSTH activity of all inactive neurons (blue, neurons 1–94 in Fig. 5 and Table 2) with responses aligned to time = 0 s for CS (left) and CE (right). Middle panels show the population activity of active neurons (neurons 95–124 in Fig. 5 and Table 2) around licking for Ensure. Bottom panels show the spiking heterogeneity measured by the Fano factor (FF) for inactive (blue) and active neurons (red). Note that during licking, the spiking patterns across inactive neurons showed greater variability preceding CS and at CE, whereas in contrast, both active responses show little spiking variability. B: the same as in A, but responses were aligned to the QW→SWS to SWS→QW transitions. Top panel (blue) plots all 88 neurons that showed a significant inhibition during SWS (SWS-off only, inactive/SWS-off, and active/SWS-off; see Table 2). Middle panel (red) plots all 53 neurons that were excited during onset SWS (SWS-on only, inactive/SWS-on, and active/SWS-on; see Table 2). Bottom panel plots the FF for each population. Note that in general, SWS-off neurons showed little if any spiking variability during SWS, suggesting that most neurons in this population basically follow the same temporal spiking patterns during SWS sleep. In contrast, SWS-on neurons had greater spike count variability during SWS.
The population responses of neurons modulated by feeding or SWS indicate some fundamental differences between these two brain states so that, in general, the responses in SWS do not simply recapitulate those of the feeding state. In addition, differences between neurons associated with feeding and sleeping states were also quantified using the Fano factor (FF), which indicates that low spike count variability yields low FF values, and vice versa. As shown in Fig. 6A, bottom, before CS, inactive cells (blue) exhibited a much greater variability before and after licking than active cells (red). In contrast, whereas during sleep the FF is relatively unchanged for the SWS-off neurons, it is much larger for the SWS-on neurons (Fig. 6B). Taken together, these data indicate that the responses before, during, and after SWS do not simply track the corresponding responses during feeding.
Cell types in ventral striatum and its sleep and feeding modulation map.
In the vast majority of electrophysiological recordings in behaving animals, the cell types either are not determined or, if determined, are not certain, and hence are called putative. Extracellular recordings in the striatum of awake, behaving animals have identified several types of neurons on the basis of their waveform width and firing rates (Berke et al. 2004; Kravitz et al. 2010). However, we could not find any pair of features that in 2-D or 3-D clearly separated neurons into different groups (data not shown). Therefore, we developed a novel method to classify extracellular waveforms from ventral striatum that is based on four main features: firing rate, CV2, VAR, and V-P width, which are combined in a fuzzy cluster classification algorithm (see materials and methods and Fig. 7). With the use of this analysis, all 264 NAc neurons could be classified into 4 major groups with fuzzy boundaries, and they then could be visualized by using a fuzzy Sammon's mapping plot (see materials and methods).
The first group corresponds to pMSNs and had the lowest firing rates and a larger inter-spike variability, measured as larger CV2 values. Furthermore, analysis of the autocorrelation histogram (ACH) revealed that most pMSNs had an irregular burst firing pattern with an early time to peak in the ACH (Fig. 7, B5 and B6). All these characteristics are attributed to pMSNs (Yarom and Cohen 2011).
The second subpopulation of neurons corresponded to pChAT interneurons, also known as tonically active neurons (TANs). The hallmark of pChATs is their large VAR values, which is indicative of broad action potentials due to a long afterhyperpolarization (Wilson and Goldberg 2006). These neurons discharged tonically at 3–10 Hz and had the largest valley-to-peak widths (Fig. 7B2). In addition, in our data set, slightly more than half of the pChATs showed bursting behavior (Kreitzer and Berke 2011).
The third subpopulation corresponded to pFSIs. This population had the smallest action potential width (Fig. 7B2) and discharged in an irregular bursting pattern (Fig. 7, B5 and B6). In the dorsolateral striatum, FSIs normally fire at high rates (40–100 Hz; Berke et al. 2004) and have a rapid repolarization. However, in our data set in the ventral striatum, only one neuron achieved 80-Hz spiking (see Fig. 7A, square outlier of the pFSI group), and the mean firing rates of pFSIs varied between 2 and 34 Hz (across the entire session), suggesting that pFSIs in ventral striatum tend to have smaller firing rates than those in the dorsal striatum (Cai et al. 2011). Finally, the fourth group (U, unidentified cell type) contained neurons that exhibited intermediate features and thus were difficult to assign to a cell type.
Feeding and sleep modulation maps.
One novel aspect of our classification method, described above, is that it provided a map of cell types located in ventral striatum that enabled us to determine whether pMSN neurons were the only cell type inhibited (or excited) during volitional feeding or during SWS. By plotting the neurons modulated during feeding into our classification map (Fig. 7C, food modulation map), we found a scattered distribution of inactive (and active) neurons comprising the neuronal subpopulations, suggestive of a broad recruitment of different cell types that likely encompasses all the cell types of the striatal microcircuit. A similar result was also found for SWS (Fig. 7D, SWS modulation map). The color-coded bar in Fig. 5, far left, indicates the putative neuron type associated with each type of response. The same data set is shown more explicitly in Table 2. One important point is that all three types of neurons were involved in each of the eight categories of responses. Other findings revealed that pFSIs were more likely to be inhibited by both feeding and SWS (inactive/SWS-off) than the other cell types [χ2 test(3) =14.78, P = 0.002]. In contrast, the pMSNs were more likely to be inhibited by feeding and excited during SWS sleep [inactive/SWS-on; χ2 test(3) = 17.25, P = 0.00062]. Either active- or inactive-type neurons that were selectively modulated by feeding were found in a similar proportion across cell types (inactive only and active only; P > 0.05, NS). With regard to neurons exhibiting a selective discharge rate modulation during sleep, the pMSNs were more frequently excited during SWS [SWS-on only; χ2 test(3) = 22.60, P < 0.0001], whereas the pChATs had a significantly larger proportion of neurons selectively inhibited during sleep [SWS-off only; χ2 test(3) = 22.63, P < 0.0001]. In summary, with respect to the types of neurons associated with feeding and sleep transitions, we found that for feeding, inactive populations that are involved in SWS, the major changes arose from changes in firing rate of pFSI and pMSNs. Similarly, for the population that was only modulated during SWS, we found that more pChATs and pMSNs were primarily involved. These results indicate that sleep and feeding exert a heterogeneous modulation on the different ventral striatum cell types.
To further evaluate differential responses among cell types and brain states, we plotted the population activity of inactive (active) and SWS-off (SWS-on) responses as a function of cell type and computed their population FF. The interpretation of the results presented in Fig. 8, A and B, follows those analyzed in Fig. 6, A and B. That is, in Fig. 8A, the top two rows represent the populations of all the inactive and active responses for each cell type associated with feeding, independent of their responses in SWS. For both inactive and active responses, the baseline and evoked firing rates were higher for the interneurons than for the pMSNs. It is also evident that all three neuronal classes types were modulated during feeding and that the modulation occurred at approximately the same times. For the QW-SWS transitions for the inactive responses, again, the interneurons had the greatest activity, and all three types showed a decrease in activity at the transition. For the SWS-on responses, a quite distinct pattern is shown. That is, whereas the pChATs and pMSNs were essentially unmodulated, the pFSIs exhibited a marked increase. The FF analysis (bottom 2 rows) also revealed striking differences with regard to the neuron type and feeding or SWS state. Specifically, in the feeding state, only pFSIs exhibited a marked modulation before CS, whereas in the SWS state, again, only pFSIs exhibited marked changes in the SWS state. Thus, with regard to variability, pFSIs have a specialized function in indicating the onset of feeding and SWS.
Fig. 8.
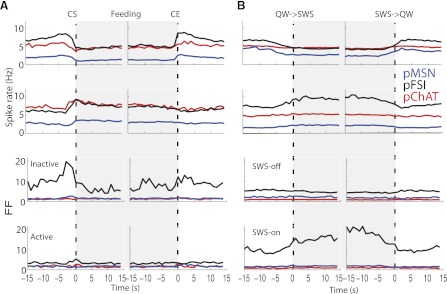
Population activity and spike count variability around feeding (A) and sleep (B) as a function of cell type. Conventions are the same as described in Fig. 6. Shown are population PSTHs plotted in Fig. 6 but now sorted as a function of cell type (indicated by color). A: top 2 rows show population activity of inactive (first row) and active neurons (second row) with responses aligned to time = 0 s for CS and CE. Bottom 2 rows display the FF for inactive (third row) and active neurons (fourth row). Note that pFSIs (black lines) had the largest population spike count variability preceding CS and at CE. B: the same as in A, but responses were aligned to the QW→SWS and SWS→QW transitions. In general, SWS-off neurons (third row) show little if any modulation of FF during SWS, suggesting that different cell types in this population basically follow the same spike count pattern during SWS sleep. In contrast, for SWS-on neurons (fourth row), mainly the pFSI subpopulation had a greater FF during SWS, suggesting that SWS-on/pFSI neurons have a heterogeneous population firing during this brain state.
DISCUSSION
In this study, we have sought to understand the relationship between networks involved in feeding and sleep by recording ensembles of neurons from the rat NAc for several hours in which an animal that was neither hungry nor thirsty was free to eat several hedonically positive meals and, between meals, exhibit several bouts of SWS. We have also developed an automatic classification method that permits us to classify the NAc neurons as pMSNs, pFSIs, and pChATs. We have first demonstrated that 1) only a few NAc neurons covary with rhythmic licking; 2) in both feeding and SWS states, the excited and inhibited populations are 180° out of phase; 3) all three putative types of NAc neurons track transitions between active awake and feeding and quiet awake and sleep states; and 4) a novel population of responses is present only in a single state. Our results suggest that NAc is an interacting node in the feeding and sleep networks and further support the idea that even at the level of single neurons, sleep and feeding are highly interconnected. For the above reasons, we propose that the NAc is a potential pharmacological target to modulate wakefulness and food appetite.
Rhythmic Licking and NAc Activity
We found, using a lick-spike coherence analysis, that only a small percentage of NAc neurons tracked the oromotor rhythm of licking behavior (also see Gutierrez et al. 2010). In another study, however, it was found that hedonic taste-related responses of NAc neurons occur in concert with the motor (EMG) responses (Roitman et al. 2005), suggesting that the NAc can encode some oromotor signals that accompany the delivery of tastants while animals freely lick. However, because we found that only 3.7% of NAc neurons covaried with licking, it is unlikely that the NAc markedly participates in the generation of rhythmic licking.
Feeding Behavior and Active and Inactive Responses
Our behavioral study is the first to be performed using freely moving animals in attending-awake, quiet-awake, feeding, or sleep states. For awake-feeding transitions, we found a greater number of inactive than active responses. Interestingly, both types of responses were evoked by a single, highly palatable tastant (Ensure). These results are consistent with reports in which only sucrose was delivered to animals in a dry-lick task (Krause et al. 2010) and with one where only a hedonically negative solution of 0.45 M NaCl was delivered via intraoral cannulas to salt-deprived rats (Loriaux et al. 2011). In this study we found that active consumption of a highly palatable food (Ensure) also modulated the ventral striatum activity toward a strong bias to inhibition (Roitman et al. 2010).
With regard to the classification of neuronal types based on extracellular waveforms, we acknowledge that our classification must be assigned the term putative (Gold et al. 2006). It has been noted that multiunit extracellular recordings will always be biased toward cells with large somas and/or high firing rates, characteristics that are normally present on interneurons (Henze et al. 2000; Shoham et al. 2006). In this regard, we report a higher percentage of putative striatal interneurons (e.g., pFSIs and pChATs) and a smaller percentage of pMSNs than are anatomically present in the NAc. The discrepancy between the physiological and anatomical estimates of interneurons and MSN is perhaps best explained by a large number of MSNs that do not fire a sufficient number of spikes during online sorting, to be identified as separate neurons (Henze et al. 2000; Yarom and Cohen 2011). In the absence of intracellular recordings, or optogenetic tagging (Kravitz et al. 2010), to definitively identify neuronal types, we used a fuzzy clustering algorithm that automatically classified NAc neurons into pMSNs, pFSIs, pChATs, and an unclassified group (Fig. 7). With regard to the awake-feeding transitions, one important aspect of this study is that all three putative neuronal types tracked the feeding task. That is, pMSNs, pFSIs, and pChATs all exhibited transient decreases (increases) before CS and remained inhibited (excited) until CE, when they returned to their respective baselines. In other words, during feeding, the entire striatal microcircuit can be active or inactive or even unresponsive (Table 2). Indeed, the population responses in Fig. 5, left, show that independently of cell type, all inactive neurons in the population respond in the same manner as do all active neurons but are 180° out of phase so that when one is excited, the other is inhibited. In addition, as discussed below, there are NAc neurons that have not been previously reported that are unresponsive upon feeding but are modulated during SWS (e.g., SWS-on only and SWS-off only). This neuronal population is unlikely to be in the circuit associated with feeding. Finally, one aspect of the inactive neurons is that they exhibit a transient increase in activity before the anticipation of a reward (see Fig. 4 in Roitman et al. 2005) that is followed by a long-lasting inhibition during consumption (Fig. 6). Such responses are typically seen in feedforward inhibition circuits (Pouille and Scanziani 2001; Tepper et al. 2008) and are not present when animals cannot anticipate the delivery of a tastant (see Fig. 3 in Loriaux et al. 2011). We found that the anticipatory population peak was also absent during QW-SWS transitions (Fig. 6B).
Sleep State and SWS-On and SWS-Off Responses
For studies in awake animals, Lansink et al. 2010 found the firing rate of pMSNs did not change when the rats were active, resting, or asleep, whereas pFSIs exhibited elevated firing rates when the animal was active compared with SWS or with reward consumption. Similarly, we found that pFSIs displayed reduced firing rates during both SWS and feeding (Table 2, inactive/SWS-off). In contrast, we found, for the first time, that many pMSNs displayed a slight, but significant, increase in firing rates during QW-SWS transitions (Fig. 7D, pMSN, red; SWS-on). This discrepancy can be rationalized as a consequence of the fact that we recorded substantial amounts of SWS, which enhanced our statistical power. We found that a large proportion of NAc neurons modulated their firing rate during QW-SWS transitions, including SWS-off and SWS-on responses. Thus the ventral striatum might play a more important role in sleep-wake regulation itself than previously considered (Braun et al. 1997). To this point, cell body-specific lesions of rat ventral striatum resulted in an increase in awake time and a decrease in the time of SWS (Qiu et al. 2010). In fact, we found that, just like in feeding, the large majority of neurons located in the NAc also displayed a strong inhibition during SWS (Fig. 6B). These results are consistent with a strong deactivation observed in human ventral striatum during QW-SWS transitions (Braun et al. 1997). Our results then raise the question whether, in the absence of food, a pharmacological treatment (e.g., muscimol) that inhibits NAc activity and promotes overfeeding, is also able to promote sleep. Although a direct confirmation of this hypothesis awaits experimental support, it has been shown that the drug modafinil produces a strong vigilance state, and increases dopamine, by decreasing GABA release in the NAc (Ferraro et al. 1996). Moreover, intra-NAc infusion of muscimol completely blocked modafinil-induced dopamine release, suggesting a potential role of NAc's inhibition in decreasing wakefulness. Furthermore, despite the fact that dopamine has been thought to play a minor role in sleep-wake regulation, both amphetamine-like compounds and modafinil induce similar increases in dopamine release in the NAc to equipotent wake-promoting doses, suggesting that dopamine in the NAc is important for increasing wakefulness (Wisor et al. 2001). Interestingly, both drugs also decrease food intake. On the basis of our results, we propose the NAc should be considered as a potential pharmacological target by which drugs could increase wakefulness and suppress food appetite.
Specialized Feeding and SWS Populations
Although most of the responses associated with feeding were also modulated during SWS, some populations of inactive and active neurons during feeding were unmodified during the QW-SWS transition (Fig. 5). Interestingly, this population contained all three NAc cell-types (Table 2, inactive and active only). Similarly, we identified a neuronal population, again containing all three neuron types, that were not modulated during feeding but during SWS were either inhibited (SWS-off only) or excited (SWS-on only). Obviously, this population does not recapitulate events happening during feeding. The question arises whether these state-selective populations are exclusively involved in generating the transitions between awake-feeding and QW-SWS states or whether they are simply part of ensembles that include the other modulated populations.
Other Differences Between Feeding and SWS Transitions
Although there are many similarities in the transitions of the feeding and SWS states, there are differences at the population level (Fig. 6) and in the types of neurons in the transitions (Table 2, Fig. 8). Differences would be expected for many reasons, not the least being the very different brain states of attending vs. quiet-awake and feeding vs. SWS. For the inactive population, the FF was greatest, especially for pFSIs, when the animals approached food (a reward), whereas in the QW-SWS transition, the FF of this population was essentially unchanged. This effect is understandable, because pFSIs exhibited a wide range of relatively high firing rates and also because a group of inactive neurons showed a gradual inhibition starting 1–1.5 s preceding CS, whereas another subset (not shown) had a ramp-up activity that peaked before CS that was followed by an inhibition during licking.
In contrast, the FF for the active population was unchanged for the awake-feeding transitions but increased at the QW-SWS transition (again for pFSIs). These data provide evidence that changes in FF related to the onset of feeding may be associated with the inactive/pFSI population and that changes associated with the onset of sleep are associated with the SWS-on/pFSI population.
Neuronal Coordination of Sleep and Feeding in Ventral Striatum
During the transitions to and from the various states of attending, quiet-awake, feeding, and sleeping, there are many neuromodulators that modify NAc activity that are selectively activated (deactivated) in each brain state and that could signal these transitions between states. Among them are orexin, melanin-concentrating hormone (MCH), and dopamine (Burdakov 2004). We propose that the different responses in local striatal circuits in the transitions between states arise from the temporal changes in activity of distributed circuits that project to the NAc in the different states. For example, orexin+ neurons in LH are active during arousal but not during SWS, whereas MCH+ neurons are maximally active during various states of sleep (Hassani et al. 2009). Whatever circuits are controlling the animals' behavior, it is evident that during either one of the brain states, the activities of the inactive and active neurons are completely out of phase, indicating that these circuits are coupled.
In summary, our results extend previous observations and indicate that ensemble activity of the NAc appears to be encoding more than just appetitive behavior, and thus the view of the NAc as a gating node for feeding must be directly or indirectly subordinated by the sleep-wake circuitry (Burdakov 2004). More importantly, our results highlight the intrinsic interconnection, at neuronal level, between naturally occurring feeding and sleep states.
GRANTS
This project was supported in part by National Institute of Deafness and Other Communications Disorders Grant DC-01065 (to S. A. Simon) and Consejo Nacional de Ciencia y Tecnologia de Mexico Grants 78879 and 179484, Salud2010-02-151001, ICYTDF-PICDS08-59, and Productos Medix 000652 (to R. Gutierrez).
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.A.T., S.A.S., and R.G. conception and design of research; L.A.T. and I.O.P. performed experiments; L.A.T. and R.G. analyzed data; L.A.T., S.A.S., and R.G. approved final version of manuscript; I.O.P. and R.G. prepared figures; S.A.S. and R.G. interpreted results of experiments; S.A.S. and R.G. drafted manuscript; S.A.S. and R.G. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Jim Meloy, Dayana Buendia, and Rosa E. Flores for invaluable technical support.
REFERENCES
- Archer ZA, Brown YA, Rayner DV, Stubbs RJ, Mercer JG. Effect of flavour of liquid Ensure diet supplement on energy intake in male SD rats. Physiol Behav 89: 414–419, 2006 [DOI] [PubMed] [Google Scholar]
- Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci 19: 376–386, 2004 [DOI] [PubMed] [Google Scholar]
- Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care 14: 402–412, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43: 883–896, 2004 [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H215O PET study. Brain 120: 1173–1197, 1997 [DOI] [PubMed] [Google Scholar]
- Burdakov D. Electrical signaling in central orexin/hypocretin circuits: tuning arousal and appetite to fit the environment. Neuroscientist 10: 286–291, 2004 [DOI] [PubMed] [Google Scholar]
- Cai X, Kim S, Lee D. Heterogeneous coding of temporally discounted values in the dorsal and ventral striatum during intertemporal choice. Neuron 69: 170–182, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Tschöp MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev 51: 85–107, 2006 [DOI] [PubMed] [Google Scholar]
- Danguir J, Nicolaidis S. Dependence of sleep on nutrients' availability. Physiol Behav 22: 735–740, 1979 [DOI] [PubMed] [Google Scholar]
- Davidson TL, Swithers SE. Food viscosity influences caloric intake compensation and body weight in rats. Obes Res 13: 537–544, 2005 [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci 106: 217–228, 1992 [PubMed] [Google Scholar]
- de Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A, Jr, Nicolelis MA, Simon SA. Neural ensemble coding of satiety states. Neuron 51: 483–494, 2006 [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tanganelli S, O'Connor WT, Antonelli T, Rambert F, Fuxe K. The vigilance promoting drug modafinil decreases GABA release in the medial preoptic area and in the posterior hypothalamus of the awake rat: possible involvement of the serotonergic 5-HT3 receptor. Neurosci Lett 220: 5–8, 1996 [DOI] [PubMed] [Google Scholar]
- Frazier CR, Mrejeru A. Predicted effects of a pause in D1 and D2 medium spiny neurons during feeding. J Neurosci 30: 9964–9966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR, Fairhurst S, Balsam P. The learning curve: implications of a quantitative analysis. Proc Natl Acad Sci USA 101: 13124–13131, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasoni D, Lin SC, Ribeiro S, Soares ES, Pantoja J, Nicolelis MA. Global forebrain dynamics predict rat behavioral states and their transitions. J Neurosci 24: 11137–11147, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold C, Henze DA, Koch C, Buzsaki G. On the origin of the extracellular action potential waveform: a modeling study. J Neurophysiol 95: 3113–3128, 2006 [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Carmena JM, Nicolelis MA, Simon SA. Orbitofrontal ensemble activity monitors licking and distinguishes among natural rewards. J Neurophysiol 95: 119–133, 2006 [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Simon SA, Nicolelis MA. Licking-induced synchrony in the taste-reward circuit improves cue discrimination during learning. J Neurosci 30: 287–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci USA 106: 2418–2422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsaki G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol 84: 390–400, 2000 [DOI] [PubMed] [Google Scholar]
- Holt GR, Softky WR, Koch C, Douglas RJ. Comparison of discharge variability in vitro and in vivo in cat visual cortex neurons. J Neurophysiol 75: 1806–1814, 1996 [DOI] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol 90: 385–417, 2010 [DOI] [PubMed] [Google Scholar]
- Jarvis MR, Mitra PP. Sampling properties of the spectrum and coherency of sequences of action potentials. Neural Comput 13: 717–749, 2001 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci 18: 527–535, 1995 [DOI] [PubMed] [Google Scholar]
- Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci 30: 4746–4756, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Berke JD. Investigating striatal function through cell-type-specific manipulations. Neuroscience 198: 19–26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, Pennartz CMA. Fast-spiking interneurons of the rat ventral striatum: temporal coordination of activity with principal cells and responsiveness to reward. Eur J Neurosci 32: 494–508, 2010 [DOI] [PubMed] [Google Scholar]
- Loriaux AL, Roitman JD, Roitman MF. Nucleus accumbens shell, but not core, tracks motivational value of salt. J Neurophysiol 106: 1537–1544, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon S, Vautrelle N, Pezard L, Slaght SJ, Deniau JM, Chouvet G, Charpier S. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. J Neurosci 26: 12587–12595, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Firing of nucleus accumbens neurons during the consummatory phase of a discriminative stimulus task depends on previous reward predictive cues. J Neurophysiol 91: 1866–1882, 2004 [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293: 1159–1163, 2001 [DOI] [PubMed] [Google Scholar]
- Qiu MH, Vetrivelan R, Fuller PM, Lu J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur J Neurosci 31: 499–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron 45: 587–597, 2005 [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Tiesinga PH, Roitman JD, Carelli RM. Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning. Learn Mem 17: 539–546, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop RG, Hollander JA, Carelli RM. Accumbens activity during a multiple schedule for water and sucrose reinforcement in rats. Synapse 43: 223–226, 2002 [DOI] [PubMed] [Google Scholar]
- Sano H, Yokoi M. Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons. J Neurosci 27: 6948–6955, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog Brain Res 153: 243–252, 2006 [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron 36: 199–211, 2002 [DOI] [PubMed] [Google Scholar]
- Sears RM, Liu RJ, Narayanan NS, Sharf R, Yeckel MF, Laubach M, Aghajanian GK, DiLeone RJ. Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J Neurosci 30: 8263–8273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S, O'Connor DH, Segev R. How silent is the brain: is there a “dark matter” problem in neuroscience? J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192: 777–784, 2006 [DOI] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci 112: 678–694, 1998 [DOI] [PubMed] [Google Scholar]
- St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr 95: 818–824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci 19: 11040–11048, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci 25: 1193–1202, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Wilson CJ, Koos T. Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res Rev 58: 272–281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Dinardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev 21: 631–647, 1997 [DOI] [PubMed] [Google Scholar]
- Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, Brown EN, Halgren E, Cash SS. Single-neuron dynamics in human focal epilepsy. Nat Neurosci 14: 635–641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Goldberg JA. Origin of the slow afterhyperpolarization and slow rhythmic bursting in striatal cholinergic interneurons. J Neurophysiol 95: 196–204, 2006 [DOI] [PubMed] [Google Scholar]
- Winson J. Patterns of hippocampal theta rhythm in the freely moving rat. Electroencephalogr Clin Neurophysiol 36: 291–301, 1974 [DOI] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci 21: 1787–1794, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330: 1677–1681, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38: 701–713, 2003 [DOI] [PubMed] [Google Scholar]
- Yarom O, Cohen D. Putative cholinergic interneurons in the ventral and dorsal regions of the striatum have distinct roles in a two choice alternative association task. Front Syst Neurosci 5: 36, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]


