Abstract
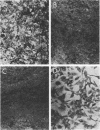
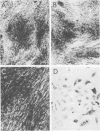
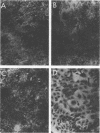
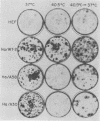
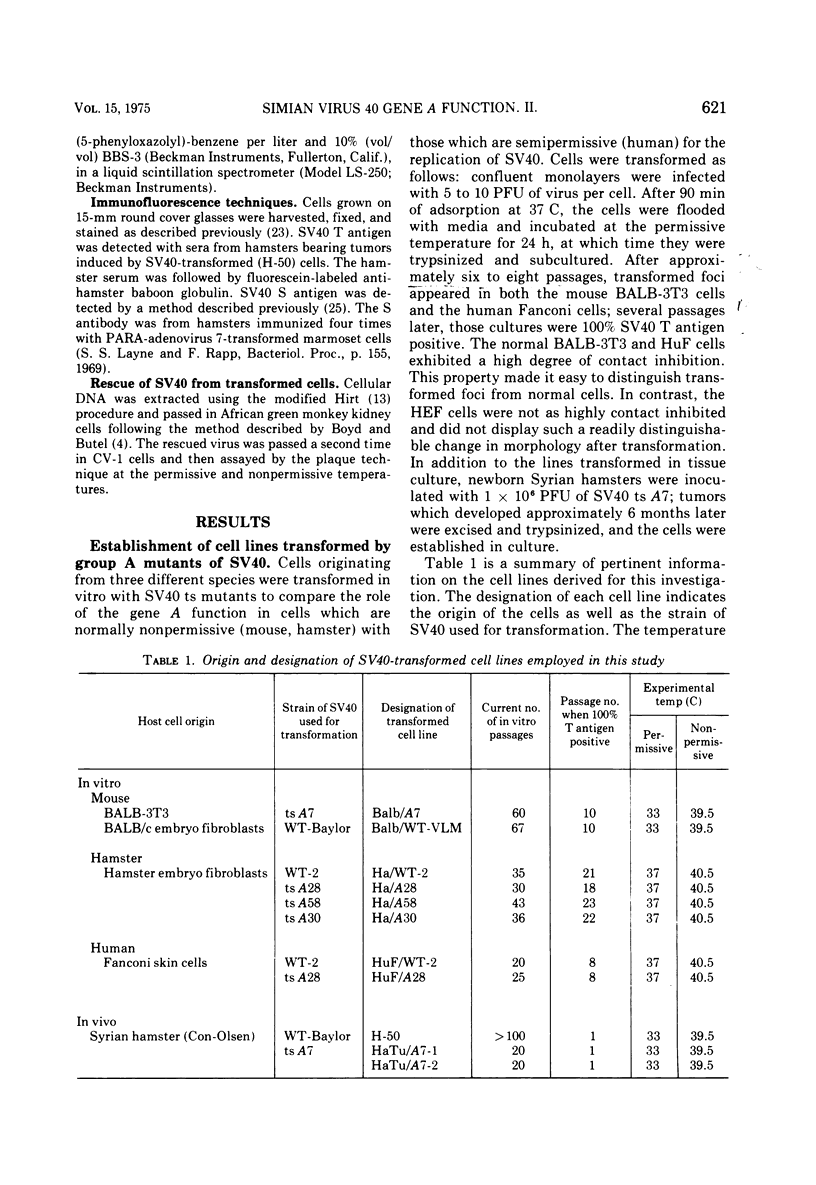
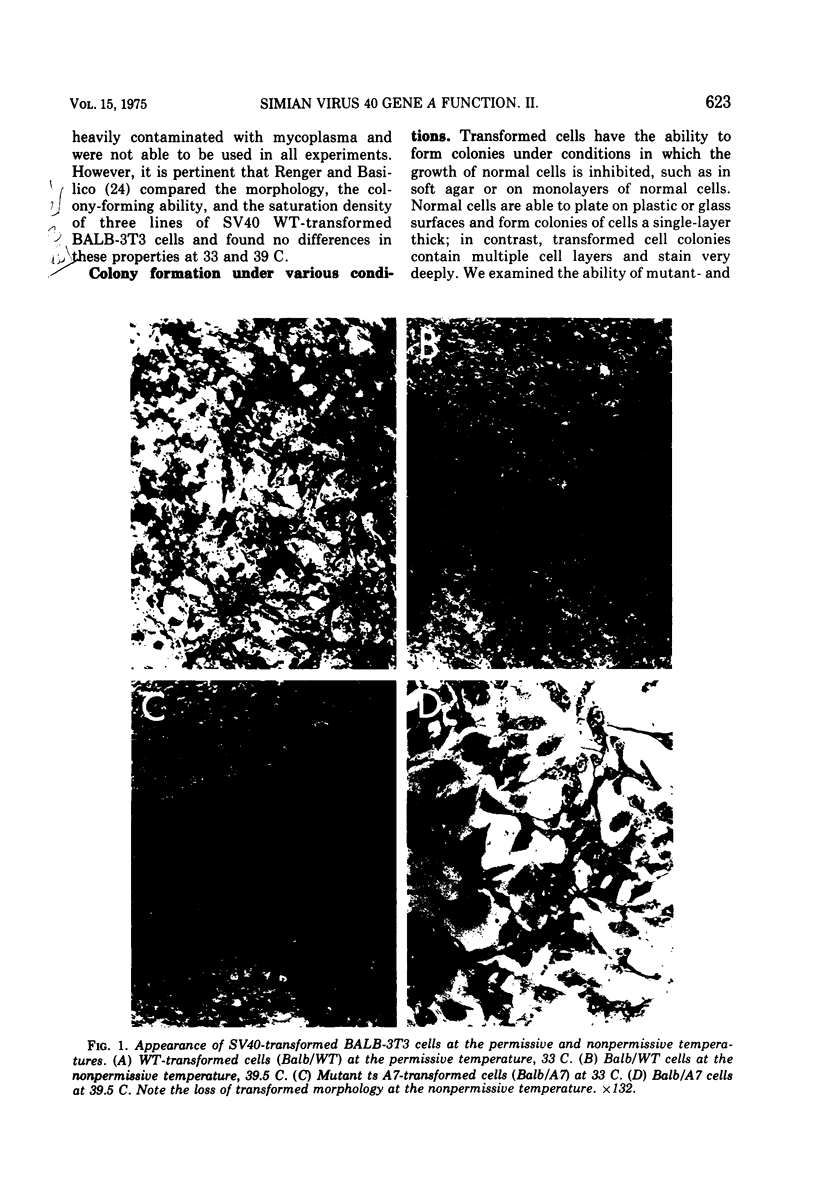
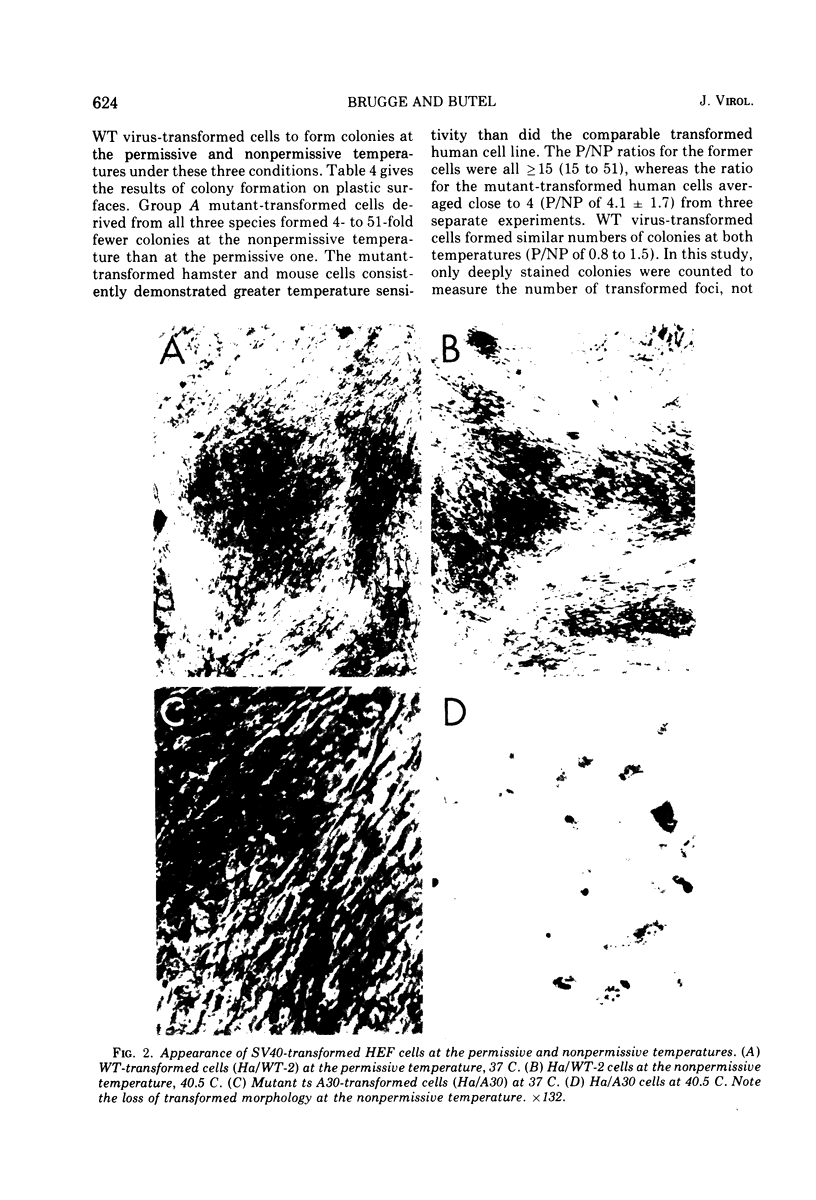
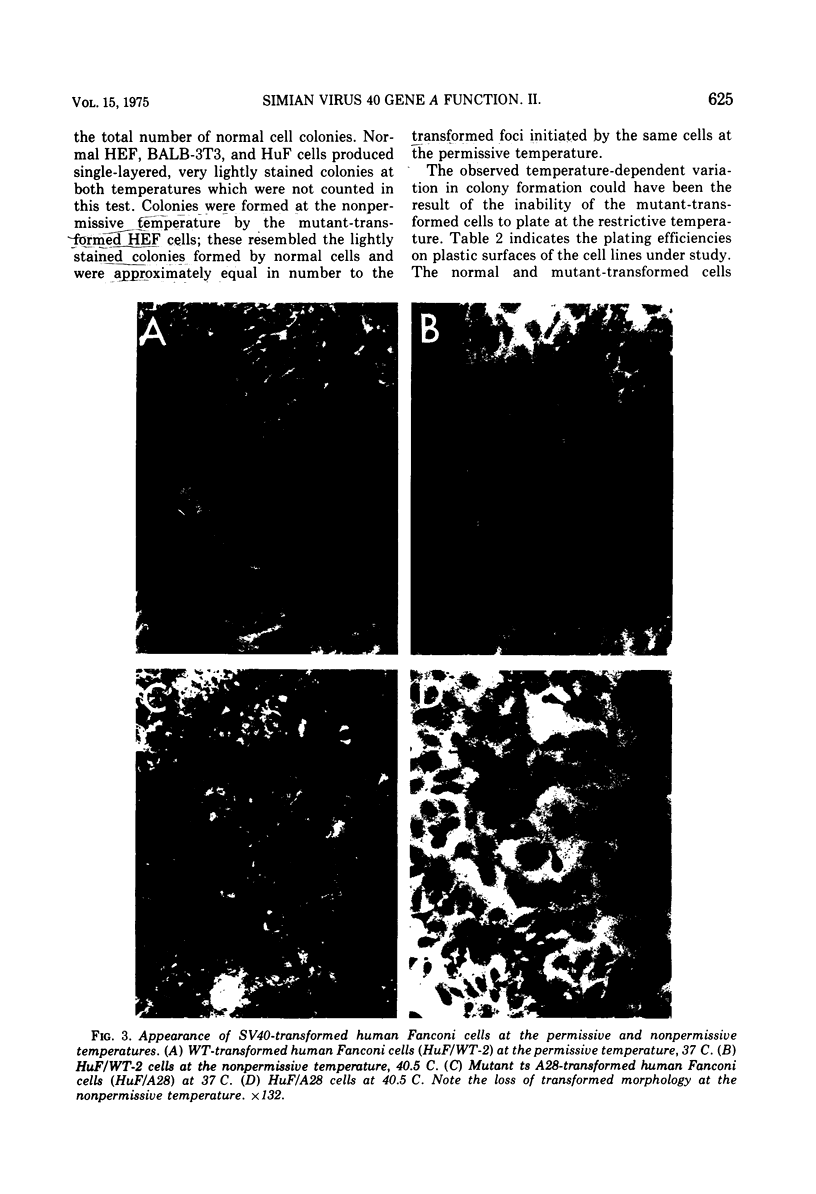
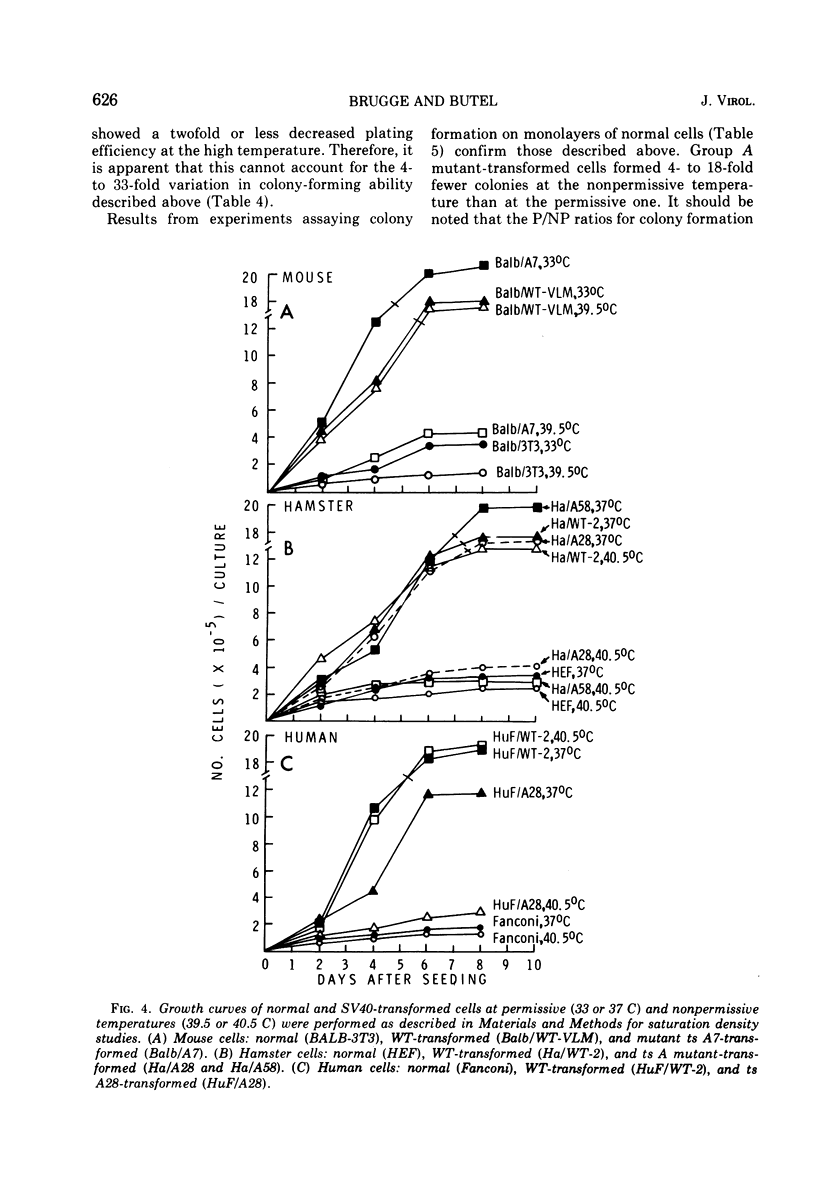
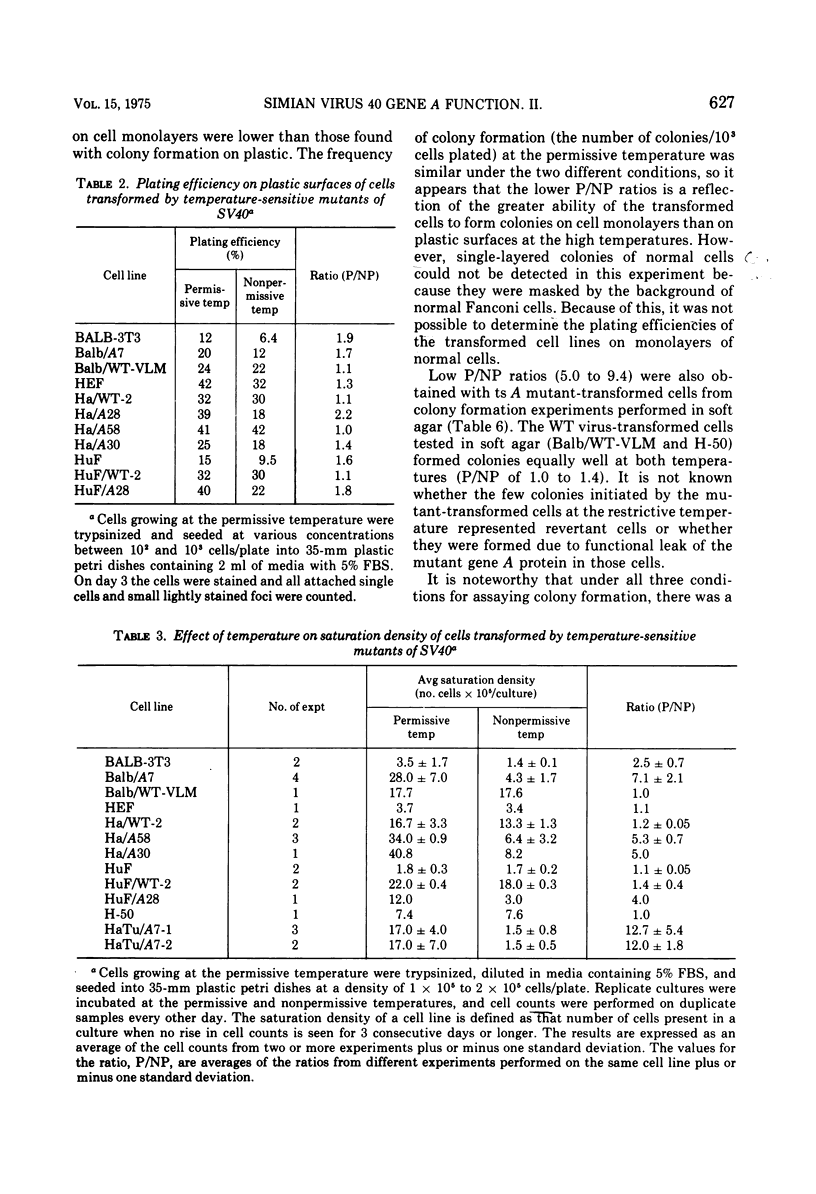
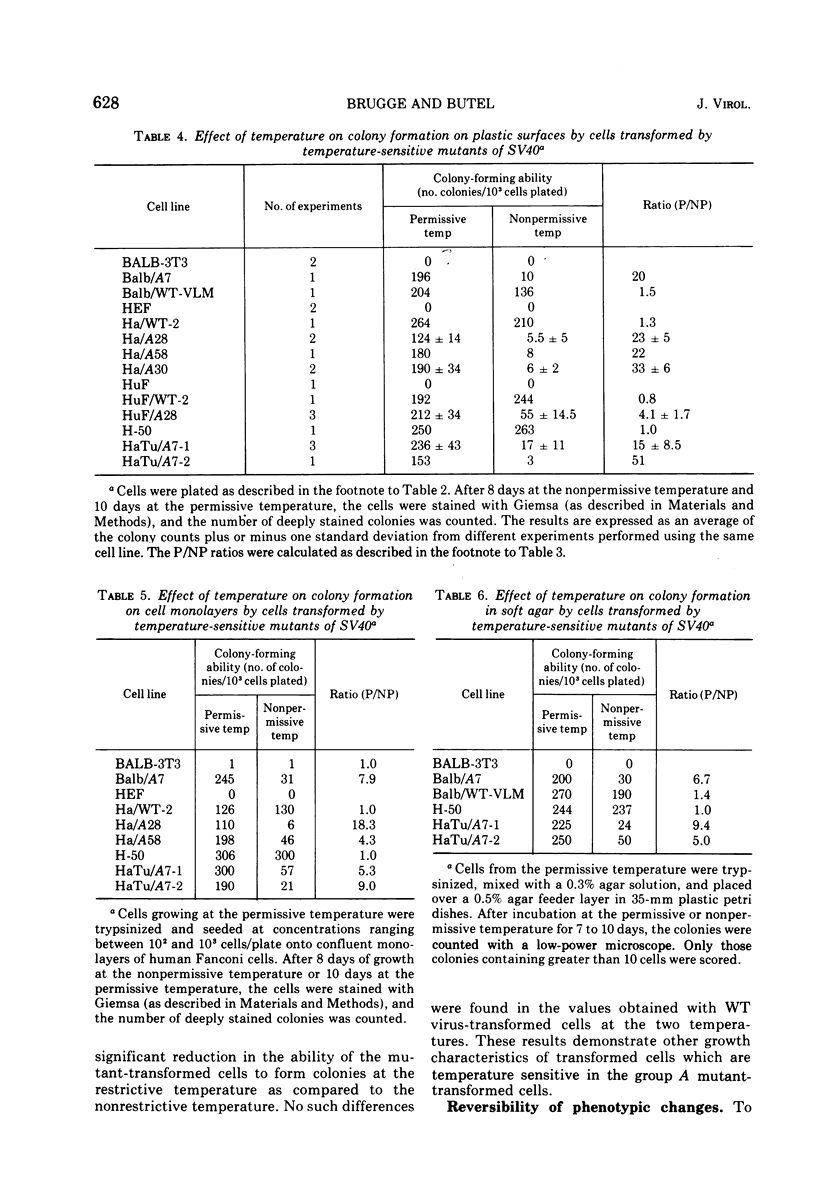
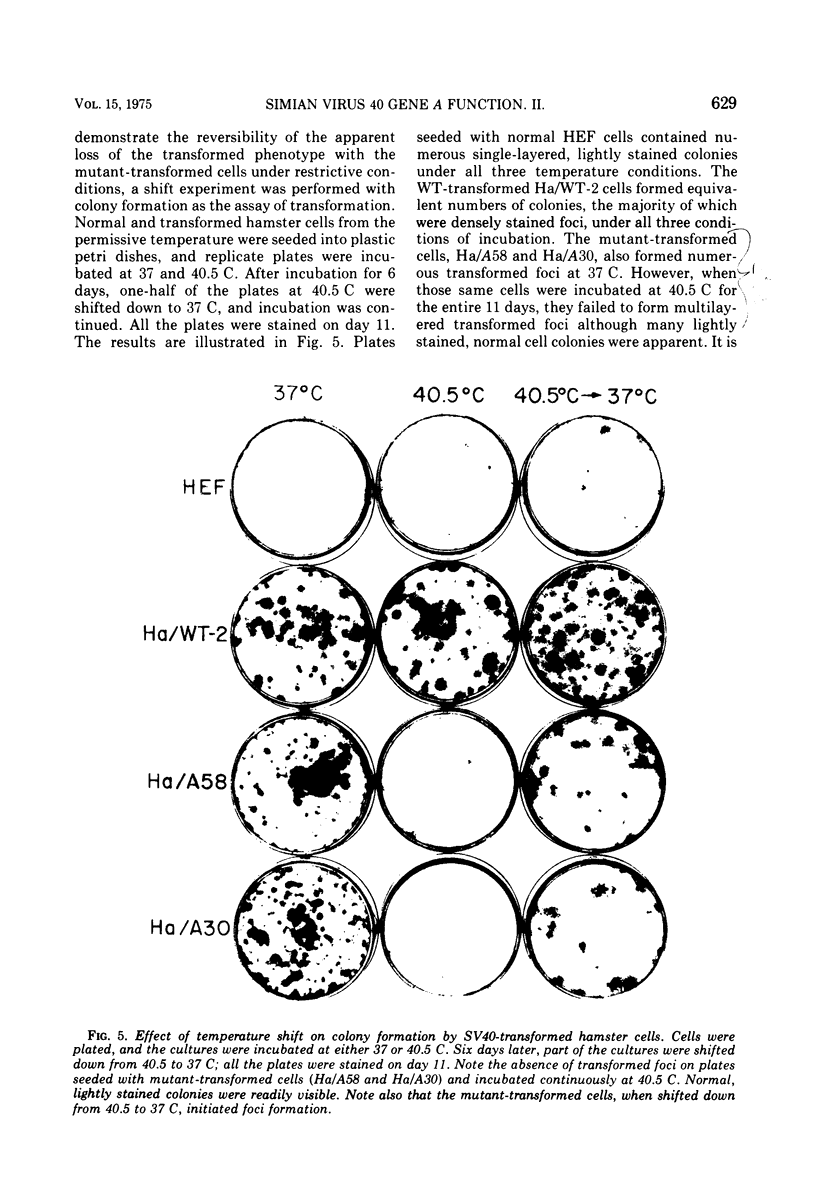
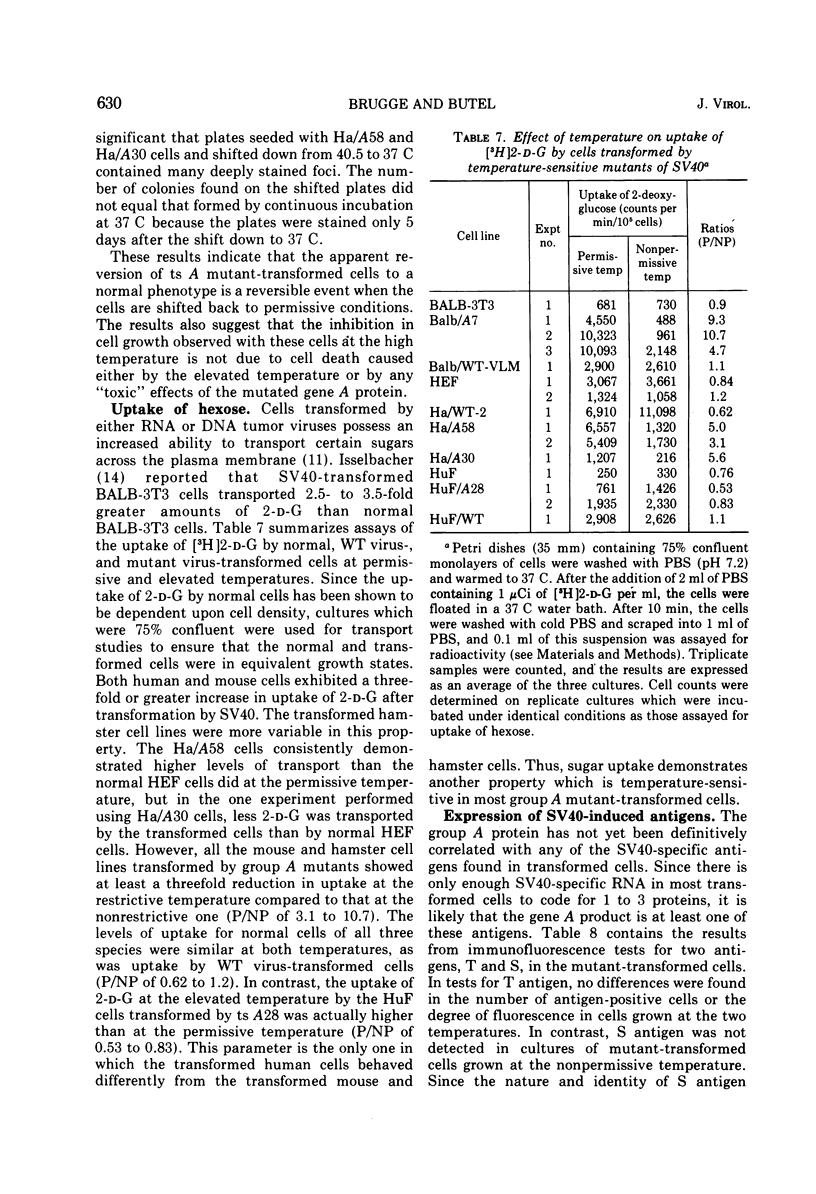
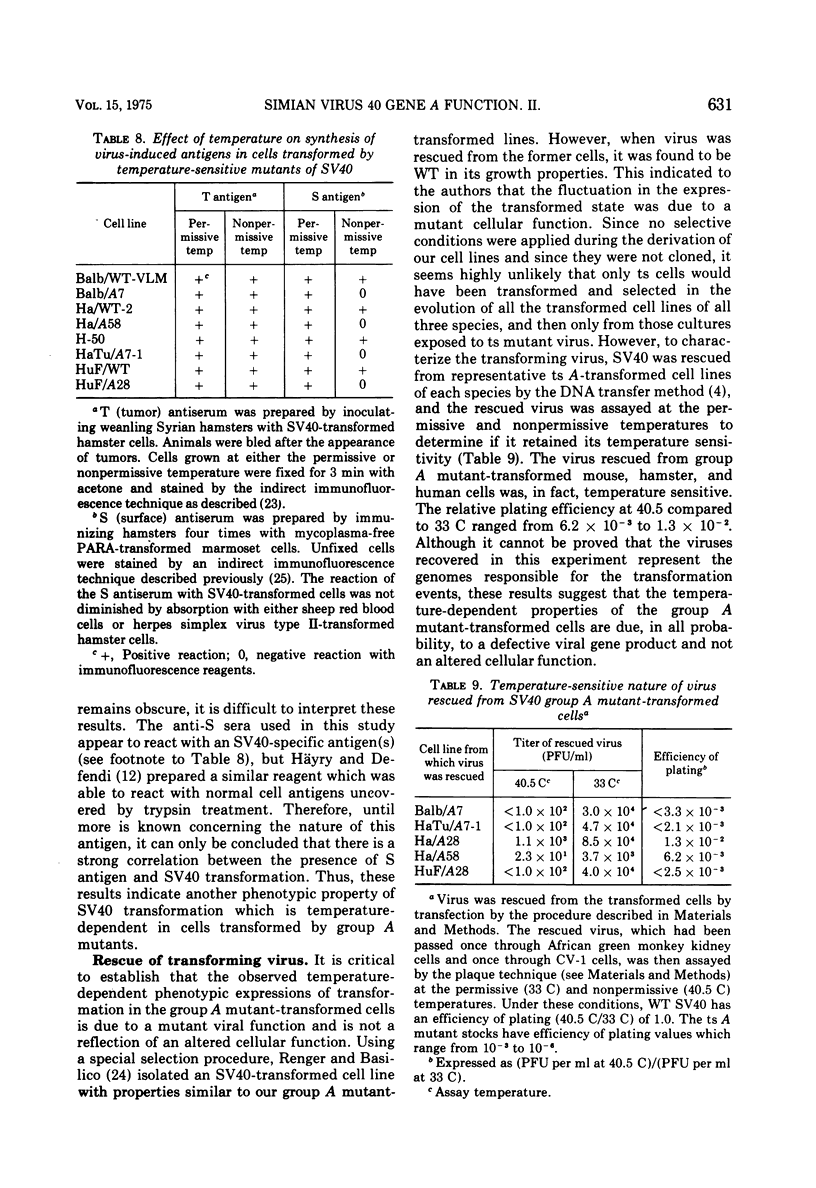
Mouse, hamster, and human cells were transformed at the permissive temperature by mutants from simian virus 40 (SV40) complementation group A in order to ascertain the role of the gene A function in transformation. The following parameters of transformation were monitored with the transformed cells under permissive and nonpermissive conditions: morphology; saturation density; colony formation on plastic, on cell monolayers, and in soft agar; uptake of hexose; and the expression of SV40 tumor (T) and surface (S) antigens. Cells transformed by the temperature-sensitive (ts) mutants exhibited the phenotype of transformed cells at the nonrestrictive temperature for all of the parameters studied. However, when grown at the restrictive temperature, they were phenotypically similar to normal, untransformed cells. Growth curves showed that the (ts) A mutant-transformed cells exhibited the growth characteristics of wild-type virus-transformed cells at the permissive temperature and resembled normal cells when placed under restrictive conditions. There were 3-to 51-fold reductions in the levels of saturation density, colony formation, and uptake of hexose when the mutant-transformed cells were the elevated temperature as compared to when they were grown at the permissive temperature. Mutant-transformed cells from the nonpermissive temperature were able to produce transformed foci when shifted down to permissive conditions, indicating that the phenotypically reverted cells were still viable and that the reversion was a reversible event. SV40 T antigen was present in the cells at both temperatures, but S antigen was not detected in cells maintained at the nonpremissive temperature. All of the wild-type virus-transformed cells exhbited a transformed cells exhibited a transformed phenotype when grown under either restrictive or nonrestrictive conditions. Thers results indicate that the SV40 group A mutant-transformed cells are temperature sensitive for the maintenance of growth properties characteristics of transformation. Virus rescued from the mutant-transformed cells by the transfection method was ts, suggesting that the SV40 gene A function, rather than a cellular one, is responsible for the ts behavior of the cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHKENAZI A., MELNICK J. L. Tumorigencity of simian papovavirus SV40 and of virus-transformed cells. J Natl Cancer Inst. 1963 Jun;30:1227–1265. [PubMed] [Google Scholar]
- Biquard J. M., Vigier P. Isolement et étude d'un mutant conditionnel du virus de Rous à capacité transformante thermosensible. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2430–2433. [PubMed] [Google Scholar]
- Boyd V. A., Butel J. S. Demonstration of infectious deoxyribonucleic acid in transformed cells. I. Recovery of simian virus 40 from yielder and nonyielder transformed cells. J Virol. 1972 Sep;10(3):399–409. doi: 10.1128/jvi.10.3.399-409.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Tevethia S. S., Melnick J. L. Oncogenicity and cell transformation by papovavirus SV40: the role of the viral genome. Adv Cancer Res. 1972;15:1–55. doi: 10.1016/s0065-230x(08)60371-1. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Blangy D., Rouget P. Activité endonucléasique de préparations purifiées du virus du Polyome. C R Acad Sci Hebd Seances Acad Sci D. 1971 Dec 20;273(25):2650–2653. [PubMed] [Google Scholar]
- Dulbecco R., Eckhart W. Temperature-dependent properties of cells transformed by a thermosensitive mutant of polyoma virus. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1775–1781. doi: 10.1073/pnas.67.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W., Dulbecco R., Burger M. M. Temperature-dependent surface changes in cells infected or transformed by a thermosensitive mutant of polyoma virus. Proc Natl Acad Sci U S A. 1971 Feb;68(2):283–286. doi: 10.1073/pnas.68.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973 Sep;55(1):127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- Graf T., Friis R. R. Differential expression of transformation in rat and chicken cells infected with an avian sarcoma virus ts mutant. Virology. 1973 Nov;56(1):369–374. doi: 10.1016/0042-6822(73)90314-0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974 Apr 29;355(1):77–104. doi: 10.1016/0304-419x(74)90008-0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Häyry P., Defendi V. Surface antigen(s) of SV40-transformed tumor cells. Virology. 1970 May;41(1):22–29. doi: 10.1016/0042-6822(70)90050-4. [DOI] [PubMed] [Google Scholar]
- Isselbacher K. J. Increased uptake of amino acids and 2-deoxy-D-glucose by virus-transformed cells in culture. Proc Natl Acad Sci U S A. 1972 Mar;69(3):585–589. doi: 10.1073/pnas.69.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachani Z. F., Sabin A. B. Reproductive capacity and viability at higher temperatures of various transformed hamster cell lines. J Natl Cancer Inst. 1969 Aug;43(2):469–480. [PubMed] [Google Scholar]
- Kimura G., Dulbecco R. A temperature-sensitive mutant of simian virus 40 affecting transforming ability. Virology. 1973 Apr;52(2):529–534. doi: 10.1016/0042-6822(73)90348-6. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Pauluzzi S., Butel J. S. Variation in properties of plaque progeny of PARA (defective simian papovavirus 40)-adenovirus 7. J Virol. 1969 Nov;4(5):626–631. doi: 10.1128/jvi.4.5.626-631.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger H. C., Basilico C. Mutation causing temperature-sensitive expression of cell transformation by a tumor virus (SV40-3T3 mouse cells-growth control). Proc Natl Acad Sci U S A. 1972 Jan;69(1):109–114. doi: 10.1073/pnas.69.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson L. S., Butel J. S. Properties of transformed hamster cells containing SV40 tumor antigen in the cytoplasm. Int J Cancer. 1971 Jan 15;7(1):75–85. doi: 10.1002/ijc.2910070109. [DOI] [PubMed] [Google Scholar]
- Ritzi E. M., Levine A. J. The fragmentation of cellular DNA and the formation of pseudovirions during SV 40 infection of African green monkey kidney cells. J Gen Virol. 1973 Sep;20(3):353–367. doi: 10.1099/0022-1317-20-3-353. [DOI] [PubMed] [Google Scholar]
- Sambrook J. Transformation by polyoma virus and simian virus 40. Adv Cancer Res. 1972;16:141–180. [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Huebner R. J. N.A.S. symposium: new evidence as the basis for increased efforts in cancer research. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1009–1015. doi: 10.1073/pnas.69.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke J. A., Linial M. Temperature-sensitive avian sarcoma viruses: a physiological comparison of twenty mutants. Virology. 1973 May;53(1):152–161. doi: 10.1016/0042-6822(73)90474-1. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Tevethia S. S. Transplantation immunity to simian virus 40-transformed cells in tumor-bearing mice. I. Development of cellular immunity to simian virus 40 tumor-specific transplantation antigens during tumorigenesis by transplanted cells. J Natl Cancer Inst. 1973 Jan;50(1):137–147. doi: 10.1093/jnci/50.1.137. [DOI] [PubMed] [Google Scholar]