Abstract
The purpose of this study was to examine the cognitive control mechanisms in adult English speaking monolinguals compared to early sequential Spanish-English bilinguals during the initial stages of novel word learning. Functional magnetic resonance imaging during a lexico-semantic task after only two hours of exposure to novel German vocabulary flashcards showed that monolinguals activated a broader set of cortical control regions associated with higher-level cognitive processes, including the supplementary motor area (SMA), anterior cingulate (ACC), and dorsolateral prefrontal cortex (DLPFC), as well as the caudate, implicated in cognitive control of language. However, bilinguals recruited a more localized subcortical network that included the putamen, associated more with motor control of language. These results suggest that experience managing multiple languages may differentiate the learning strategy and subsequent neural mechanisms of cognitive control used by bilinguals compared to monolinguals in the early stages of novel word learning.
Keywords: cognitive control, bilingualism, fMRI, word learning
1.1 Introduction
The current study seeks to uncover how bilinguals and monolinguals differ in their ability to learn new vocabulary. Many researchers have sought to uncover the neural mechanisms required to learn both our first language as infants (Kuhl and Rivera-Gaxiola, 2008; Bates, 1999), and a second language later in life (Bosch et al., 2000; Kroll, 1994; Meschyan and Hernandez, 2006; Perani and Abutalebi, 2005; Hernandez et al., 2005). Very few studies, however, have specifically examined the cognitive control mechanisms needed during the earliest stages of second language vocabulary acquisition and how experience managing multiple languages may affect the brain regions employed. Current research suggests that bilinguals have an advantage in cognitive control related tasks (Bialystok, 2001; Bialystok and Shapero, 2005; Bialystok and DePape, 2009; Carlson and Meltzoff, 2008; Kaushanskaya and Marian, 2009; Waldie et al., 2009; Ye and Zhou, 2009). Bialystok and DePape (2009) propose that bilinguals train general and language specific control abilities on a daily basis through managing multiple languages, resulting in enhanced cognitive control across many cognitive domains. Recent neuroimaging studies support a bilingual cognitive advantage and have shown that this lifelong experience managing multiple languages increases both frontal white matter integrity and connectivity (Luk et al., 2011). Additionally, Abutalebi and colleagues (2012) recently found that specific brain areas implicated in both domain general and language specific cognitive control in bilinguals are activated more efficiently than in monolinguals. Specifically, the authors found that the anterior cingulate cortex (ACC), a domain-general and language specific control region, is activated less strongly in bilinguals compared to monolinguals, suggesting more efficient use of this brain area in monitoring conflict in verbal and nonverbal tasks. Hence, this developing line of research suggests that when learning novel vocabulary, bilinguals may utilize the cognitive control network differently or more efficiently than monolinguals, who do not have the same extensive experience managing multiple languages. To test differences in the use of control mechanisms between Spanish-English bilinguals and English monolinguals during early word learning, the current study scanned subjects using functional magnetic resonance imaging (fMRI) during a difficult lexico-semantic task after two hours of exposure to novel vocabulary flashcards. A set of targeted regions implicated by the literature in cognitive control of language was evaluated: dorsolateral prefrontal cortex (DLPFC), anterior cingulate (ACC), striatum (caudate and putamen), inferior parietal lobe, and supplementary motor area (SMA). The novel word stimuli were divided into cognates and noncognates to additionally examine if orthographic and phonological overlap modulates neural activity during this early period of learning and affects the activation of cognitive control regions. In the following sections, an overview of the neural mechanisms engaged in the early stages of novel word learning and how cognitive control significantly impacts a bilingual’s ability to manage multiple languages will be provided. The latter literature clearly shows that bilinguals may possess an advantage in cognitive control processes and suggests that the added experience managing multiple languages might allow them to learn a subsequent language differently than a monolingual.
1.1.1 Neural Activation in the First Stages of Word Learning
Whereas previous research has focused on novel word learning in infants (Kuhl and Rivera-Gaxiola, 2008) and word learning after extensive training (McCandliss et al., 1997), few studies have focused specifically on the first few hours of novel word learning (McLaughlin et al., 2004; Shtyrov et al., 2010) or the neural mechanisms that drive early second language vocabulary acquisition in adults (Raboyeau et al., 2010) who classically have greater difficulty learning a second language. McLaughlin et al. (2004) revealed that during the early stages of word learning in adults, subtle changes not yet evidenced in behavioral measures might be detectable in neural measures; event-related potential (ERP) differences suggested that individuals could distinguish words from pseudowords after 14 hours of total instruction, despite behavioral measures failing to show changes at greater than chance levels. Examining the neural changes that occurred within 14 minutes of passive perceptual exposure to novel spoken pseudowords, Shtyrov et al. (2010) discovered a neural response to learning, suggesting that neuronal circuits may be formed or altered for linguistic events very quickly.
McLaughlin et al. (2004) and Shtyrov et al. (2010) demonstrated the utility of ERP evidence in examining the immediate neural changes that occur in language learning. Other researchers have utilized alternative imaging techniques to identify the specific neural structures that underlie these rapid changes, which Catani et al. (2005) and Shtyrov et al (2010) suggest may involve areas that extend beyond traditional language processing regions such as Broca and Wernicke’s areas. Shtyrov et al. (2010) suggest that this human capability of developing large lexicons is facilitated by a network of brain regions that connect the left temporal and frontal perisylvian areas. In their examination of fiber tracts that connect classic language related regions, Catani et al. (2005) found that the arcuate fasciculus fiber distribution extends beyond what is traditionally thought of as Broca’s area to include middle frontal and inferior precentral gyri, which are known to be involved in cognitive control of language functions (Abutalebi and Green, 2007). Raboyeau et al. (2010) used event-related fMRI to examine the neural mechanisms involved in the early stages of word learning related to different word types in a group of French-English bilinguals. After exposure to picture-word pairs over five 20-minute training sessions, neural activation was seen in left inferior frontal regions associated with lexical retrieval and phonological processing, ACC, and the DLPFC in response to monitoring and control, suggesting that even after less than two hours of total instruction time, distinct neural patterns in response to learning can be identified. These regions extend beyond traditional temporal and frontal language related areas, and Abutalebi and Green (2007) theorize that these inferior frontal, middle frontal, and anterior cingulate regions are involved in cognitive control. Given this evidence, it is important to evaluate the selective activation of these cognitive control regions and others during the acquisition of novel vocabulary, as the networks that are involved in early word learning may be different between monolinguals and bilinguals, drawing upon areas associated with cognitive control. The current study expands on these and other studies that used longer periods of vocabulary instruction (Lee et al., 2003; McCandliss et al., 1997) by exploring how experience managing multiple languages affects the cognitive control network during early novel word learning.
1.1.2 Cognitive Control and the Bilingual Executive Advantage
Much of what is known about cognitive control in language comes from studies of bilinguals. Many studies have examined the neural mechanisms used by bilinguals to manage the control of multiple languages (Abutalebi and Green, 2007, 2008; Abutalebi and Costa, 2008; Hernandez, 2009; Wang et al., 2007). Bilingual research indicates that multiple languages share a common neural system rather than relying on separate neural representations for each language. The use of different languages is then managed by an intricate control system made up of cortical and subcortical regions. According to Abutalebi and Green (2007), the integration of the anterior cingulate cortex, basal ganglia, inferior parietal lobe, and prefrontal cortex is responsible for bilingual language control, which is “not concerned with the representation of language but the selection and temporal sequencing of such representations” (Abutalebi and Green, 2007, p. 249).
Abutalebi and colleagues (2007, 2008) introduced the exploration of cognitive control mechanisms in bilinguals; however, less research has focused on the development of these mechanisms in adult monolinguals learning a new language and if these pathways may differ from those individuals already fluent in two or more languages. In order to explore the bilingual executive advantage, the majority of current research focuses on comparisons of bilinguals and monolinguals during language and non-language related cognitive control tasks. Some studies suggest that this advantage is language specific, particularly in word learning (Kaushanskaya and Marian, 2009), but the advantage may extend to other more general executive domains (i.e. an executive advantage) as well (Kaushanskaya and Marian, 2009; Bialystok, 2001; Abutalebi and Costa, 2008; Abutalebi et al., 2012). Bilinguals across the lifespan show this advantage in language and non-language related control tasks that require conflict resolution, switching, and flexibility (Bialystok et al., 2004; Bialystok and Shapero, 2005). Abutalebi et al. (2012) recently addressed differences between monolinguals and bilinguals and found that the ACC, a region involved in both domain-general and language specific cognitive control, is used more efficiently by bilinguals in both verbal and nonverbal tasks of conflict resolution. Garbin et al. (2010) also examined the brain basis of the bilingual advantage in cognitive control by comparing the neural activity of monolinguals and bilinguals during a non-language switching task. Results indicated that monolinguals expressed activity in the right inferior frontal cortex and anterior cingulate, whereas bilinguals utilized the left inferior frontal cortex and striatum during task switching. All of these regions were implicated in linguistic cognitive control in bilinguals by Abutalebi and Green (2007), suggesting that monolinguals and bilinguals may utilize the components of this control network differently due to their experiences managing multiple languages.
Bialystok and DePape (2009) proposed that the advantage bilinguals have over monolinguals in cognitive control is the result of daily training of general executive abilities. That is, if these mechanisms are generalized, then training in a different domain may similarly contribute to greater development of cognitive control as well. Bialystok and DePape (2009) examined whether other intensive activities, such as musical training, could also bolster cognitive control. They hypothesized that intensive musical experience may contribute to enhanced general cognitive control mechanisms in musicians in much the same way that management of two language systems has strengthened cognitive control mechanisms in bilinguals. Bialystok and DePape (2009) concluded that much like bilingualism, musicians also showed heightened control on cognitive tasks requiring inhibition and restraint. These results suggest that regular experience with tasks that require inhibition, switching, and conflict monitoring is capable of altering, and possibly enhancing the development of certain general cognitive control abilities and support an association between bilingualism and better inhibitory and switching skills.
Although little is known about the earliest stages of learning a new language as an adult, Raboyeau et al. (2010) suggest that changes in brain activation are noted within the first few hours of exposure to novel words. Research in the area of cognitive control suggests that adult bilinguals may have an advantage over, and activate different areas than monolinguals when performing tasks requiring higher-order cognitive processes such as switching between languages and inhibiting the dominant language for a new language being learned. As such, we hypothesized that bilingual adults would utilize the components of the cognitive control network differently, possibly more efficiently, than monolinguals who do not have the same experience managing multiple languages. The current study compares activity after word learning in bilinguals and monolinguals in five pre-specified regions of interest in the cognitive control literature: DLPFC, ACC, SMA, striatum (caudate and putamen) and inferior parietal cortex. The SMA, which is not specifically mentioned by Abutalebi and Green (2007) as involved in the cognitive control circuit is included in these analyses, as this area has been referenced in regards to reading in a less proficient language (Meschyan and Hernandez, 2006) and articulation and motor control in language tasks (Hernandez, 2009). By isolating these regions, the specific control-related activity during the early stages of novel word learning during a difficult lexico-semantic task can be examined. It is hypothesized that bilinguals will excel at registering newly learned words and make semantic decisions quicker than monolinguals, due to their previous experience managing multiple languages. This study’s results will help in understanding how this control network is used during vocabulary acquisition in populations that have different life experiences that may influence the development of cognitive control abilities.
2.1 Materials and Methods
2.1.1 Participants
This study compared two groups of right-handed adults living in the United States at the time of the assessment. The first group consisted of 20 early sequential Spanish-English bilinguals (mean age=21.75, SD=2.38, 13 females). For these participants, Spanish was the native language, with an average English age of acquisition of 4.55 years (SD=1.22). The second group consisted of 20 English monolinguals (mean age= 23.25, SD=4.82, 8 females). All participants (n=40) were assessed to be healthy and denied use of psychiatric medications. Language history was assessed through a questionnaire to ensure that within groups, participants had similar linguistic backgrounds and did not have extensive experience with other languages outside the English or Spanish proficiency requirements. Language proficiency was assessed using a modified administration of the Boston Naming Test (BNT) (Kaplan et al., 1983) and by a self-rated proficiency scale ranging from one to seven (1=poor, 7=like a native) that assessed oral expression, listening comprehension, reading, and writing skills. The monolingual group completed both assessments in reference to their English language skills, while the bilinguals completed the BNT and the self-rating scale in Spanish and English. All participants provided written informed consent on a protocol approved by the University of Houston’s Committee for the Protection of Human Subjects.
2.1.2 Stimuli and Procedures
All participants were given a set of 100 German vocabulary flashcards and asked to learn the “new words” at their own pace. Auditory presentations of the words were not given. Participants had approximately two hours to learn the words, and had to score 90 percent or higher on a paper/pencil matching vocabulary pre-test before completing the fMRI portion of the study. The 100 German nouns were split into two equal categories, cognates and noncognates, where cognates were orthographically and phonologically similar to their English translations. As a control for the experimental condition, 100 English nouns were also chosen, with all words matched on frequency, word length, number of syllables, age of acquisition, and imageability (CRL International Picture- Naming Project, Bates et al., 2000). No German word and its English translation were presented in the same session.
The purpose of the behavioral task performed in the scanner was to ensure that the participants attempted to access the semantic representation of each word. Participants were asked to decide if the word presented was living or nonliving. By asking this question, participants would have to access the semantic representation to make an informed decision about animacy. Using an event-related paradigm, the 100 German words learned from the flashcards and 100 matched English controls were presented one at a time on a computer screen. Stimuli were presented using NEMO (Network Experiment Management Objects) software. In accordance with the event-related design, the German and English words were randomized, with each word presented for 500ms followed by a blank screen, at a mean of one every seven seconds. Participants were instructed to silently read each word and decide if it was something that was living or nonliving; participants had four seconds to make their decision. Their responses were recorded on button boxes held in each hand, and the designation of each box was counterbalanced across participants. An event-related presentation was chosen in order to prevent the grouping of words into categories (cognates, noncognates) to reduce the chances of predisposing participants to recognize the phonological and orthographical overlap of German cognates with their English translations. Additionally, this design was chosen to create a more naturalistic representation of how novel vocabulary are integrated into the lexicon, which is not always in an orderly and categorical (cognate, noncognate) way during the very early stages of foreign word learning.
2.1.3 fMRI Data Acquisition
Imaging data was collected at Baylor College of Medicine’s Human Neuroimaging Laboratory. All subjects signed the Human Neuroimaging Laboratory’s consent form and were screened for claustrophobia, health conditions, and presence of metal in the body. Prior to the scan, participants were given sound dampening headphones to reduce scanner noise, a squeeze ball that could signal the technician in an emergency, and a set of button boxes with assigned living/nonliving designations. A mirror attached to the head coil allowed the participants to see the computer screen on which the words were presented.
Imaging data was collected using a 3.0 Tesla head-only Siemens Magnetom Allegra imager. A localizer scan assessed each participants head position prior to data acquisition with the following parameters: voxel size 2.2×1.1×10 mm, TR = 20ms, TE = 5ms. High-resolution T1-weighted anatomical images were collected using a Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence with voxel size 1.0×1.0×1.0 mm, TR = 1200ms, and TE = 2.93ms reconstructed into 192 slices. Functional images were acquired with a gradient recalled, echo-planar imaging (EPI) sequence with voxel size 3.4×3.4×4.0 mm, TR = 2000ms, TE = 40ms, flip angle = 90°, and a 64×64 matrix. During the functional run, 26 axial slices per volume were obtained parallel to the anteroposterior commissural line (ACPC).
2.1.4 Image Processing and Analysis
Data processing was done using SPM8 software (Wellcome Trust Centre for Neuroimaging, London, UK) running on a Matlab10 (The MathWorks, Inc., Natick, MA, USA) platform. During preprocessing, images were realigned for motion correction, resliced, and slice time corrected. The functional images were coregistered to align the mean functional image with the structural image, segmented, and normalized to a standard MNI (Montreal Neurological Institute) template. Functional data was spatially smoothed using a 8mm full-width half maximum (FWHM) Gaussian kernel to compensate for any additional variability after normalization.
In first level processing, the stimulus presentation onsets for each condition, German cognate (GC), German noncognate (GN), and English (E) were implicitly modeled against rest in both the monolingual and bilingual groups. The motion estimates from preprocessing were included in the individual subject GLM as covariates of no interest to further control for motion artifact, which has been shown to produce results more robustly controlled for motion in event-related designs (Johnstone et al., 2006). Using a full factorial design, all pairwise contrasts of interest were assessed (GC > E; GN > E; E > GC; E > GN) at the group level for both monolinguals and bilinguals. German cognate and noncognate conditions were collapsed together to create a total German word group (G) in the group analyses so pairwise contrasts comparing German activation to English (G > E; E > G) could be evaluated. For these analyses, a threshold of p < .001, uncorrected was used, with a minimum of 5 voxels per cluster. Activation coordinates (MNI) were provided by SPM, with anatomical labeling obtained from Anatomy Toolbox (Eickhoff et al., 2005).
For between group comparisons, small volume correction (SVC) was done on the results obtained from a full-factorial ANOVA, with group (monolingual vs. bilingual) as one factor, and word type (German cognate, German noncognate, and English) as the second factor. Five regions of interest were pre-specified from the cognitive control literature: DLPFC, ACC, SMA, striatum (caudate and putamen), and inferior parietal cortex. For the SVC analysis, a threshold of p, uncorrected .05, was initially used, and statistics were only reported when the peak-level threshold was familywise error (FWE) corrected to be equal to or less than .05. A sphere of 15mm was used to localize activity around the five specified regions of interest. Coordinates for the DLPFC were acquired from Hernandez’s (2009) switching study. The Talairach coordinates (+/−40, 22, 30) were converted to MNI (+/−45, 28, 25) using GingerALE software (Eickhoff et al., 2009). The coordinates for the SMA were also obtained from a combination of the results of Hernandez (2009) and De Bleser et al. (2003). The switching study by Garbin et al. (2010) comparing monolinguals and bilinguals in addition to general anatomical knowledge were the source of the MNI coordinates for ACC (+/−14, 30, 30), caudate (+/−16, 10, 2), putamen (+/−16, −10, 2) and inferior parietal cortex (+/−50, −52, 54). Final peaks of activation were confirmed by anatomical labeling obtained from Anatomy Toolbox (Eickhoff et al., 2005) within SPM.
3.1 Results
3.1.1 Behavioral Data
Language proficiency was determined from scores on the BNT. The monolinguals had an average English BNT score of 54.05 (SD=2.93), while the bilinguals had an average score of 48.3 (SD=4.53) in English and 33.53 (SD=7.09) in Spanish. These English scores fell near the expected norms of Kaplan et al. (1983) (monolingual English mean score of 55.71) and Kohnert et al. (1998) (bilingual English mean score of 46.66). Similarly, the bilingual Spanish scores were also near the expected norms from Kohnert et al. (1998) (bilingual Spanish mean score of 32). A comparison of the groups showed no significant difference in age of the participants F (1, 38)= 1.56, p = .2199; however, the groups were significantly different in their English BNT scores, F (1, 38)= 22.71, p < .0001, suggesting that monolinguals were more proficient in English than bilinguals.
Accuracy and reaction time (RT) data were recorded for the semantic decision task performed in the scanner. The final word stimuli used in the analysis were determined based on consistency in response and rate of non-response across all 40 participants. Words were eliminated from both the behavioral and imaging analyses if the rate of non-response was greater than or equal to 30-percent. Additionally, stimuli were removed from both types of analyses when percent accuracy (right or wrong responses) fell between .4 and .7. Words within this range consistently received both living and nonliving responses and were cut due to ambiguity. In total, 25 of the original 200 words were cut (10 English controls, 8 German cognates, and 7 German noncognates). Given that all participants scored at least 90 percent on the vocabulary test before scanning, and all trials included in the analyses had strong consistency in living/nonliving responses, we can be confidant that participants were activating semantic representations of words included in the analyses.
Percent accuracy data placed into a 2×3 repeated-measures ANOVA revealed no significant main effect of group, F (1, 38) = .096, p = .758, suggesting that monolinguals and bilinguals did not differ on overall accuracy in the semantic decision task. There was also no significant interaction between group (monolingual and bilingual) and word type (English, German cognate, German noncognate), F (2, 76) = .703, p = .498; however there was a significant main effect of word type, F (2, 76) = 33.31, p < .0001. Follow-up comparisons, Bonferroni corrected, showed significant differences (p < .05) in percent accuracy between English and German cognates, English and German noncognates, and German cognates and noncognates. In the semantic decision task, English words had the highest percent accuracy, with 87.58 percent of words correctly labeled as living or nonliving (SD = 1.29). German cognates and noncognates followed with 78.10 percent accuracy (SD = 2.09) and 71.11 percent accuracy (SD = 2.92) respectively. Therefore, in general, it was easiest for participants to decide animacy for words in a proficiency language. Additionally, it was easier for all subjects to decide animacy for words that shared similar orthographic and phonological overlap with words in their most proficient language.
The pattern of reaction times according to word type was also consistent with previous literature examining the cognate facilitation effect (Lotto and de Groot, 1998; de Groot and Keijzer, 2000; Raboyeau et al., 2010), in that English words were processed fastest (Average RT for monolinguals = 1472.86 ms; bilinguals = 1394.83 ms), followed by German cognates (Average RT monolinguals = 1977.49 ms; bilinguals = 1588.89 ms), and German noncognates (Average RT monolinguals = 2306.70 ms; bilinguals = 1691.42 ms) for both groups (Figure 1A). Reaction time data placed into a 2×3 repeated-measures ANOVA (Table 1) revealed a main effect of word type, F (2, 114) = 23.52, p < .0001 and of group, F (1, 114) = 28.21, p < .0001. There was also an interaction between group and word type, F (2, 114) = 5.26, p < .0065. Follow-up simple effects of word type were significant for monolinguals (p < .0001) but only approached significance in bilinguals (p = .0412). For monolinguals, only differences between English words and German cognates or noncognates were significant (p < .025, αEW = .05); comparisons of German cognates to noncognates were not significant. Follow-up simple effects for group showed that bilinguals were faster in their responses than monolinguals to German cognates and noncognates (p < .017, αEW = .05), but not to English words (p = .5084). Despite being more proficient in English as indicated by their higher BNT scores, monolinguals did not make semantic decisions about English words faster than bilinguals.
Figure 1.
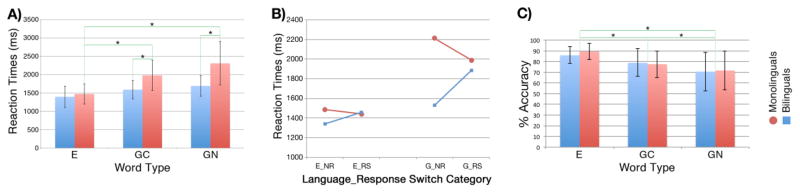
A) Average Reaction Times (RT)
Simple effects of word class showed monolinguals were significantly faster at processing English than German cognates or noncognates (p < .025). Simple effects of group showed that despite being more proficient in English, monolinguals were not significantly faster than bilinguals on English trials. Bilinguals were significantly faster than monolinguals on both German cognates and noncognates (p < .017).
B) Three-way Interaction of Group*Language*Response Switch on RT
Graphical representation of the significant three-way interaction of group, language, and response switch category on reaction time. E = English, G = German, NR = no response switch, RS = response switch.
C) Percent Accuracy
There was no main effect of group or an interaction effect. The main effect of word type was significant, such that percent accuracy on English trials was different from German cognate trials and German noncognate trials.
Table 1.
Reaction Time Statistics
| Effect | F | p |
|---|---|---|
| Main Effect of Group | F (1, 114) = 28.21 | < .0001 |
| Main Effect of Word Type | F (2, 114) = 23.52 | < .0001 |
| Interaction (Group*Word Type) | F (2, 114) = 5.26 | < .0065 |
Both main effects and the interaction were significant (p < .05). Follow-up simple effects of word type were significant for monolinguals (p < .0001) but only approached significance in bilinguals (p = .0412). For monolinguals, differences between English and German cognates or noncognates were significant (p < .025, αEW = .05). Follow-up simple effects for group showed bilinguals were faster on German cognate and noncognate trials (p < .017, αEW = .05) only.
To further elucidate why the full cognate facilitation effect was not significant, in addition to explaining subcortical neural activation differences between groups, a post-hoc 2 × 2 × 2 × 2 ANOVA was done to examine the effects of group (monolingual/bilingual), language (total German and English), language switch (English to German and German to English), and semantic response switch (living to nonliving and nonliving to living) on reaction time. There was a significant two-way interaction between group and language, F (1, 38) = 13.389, p = .001. Follow-up contrasts, Bonferroni corrected, showed that the difference between reaction times on German words compared to English words was significant for bilinguals compared to monolinguals, suggesting that bilinguals were overall quicker at responding to German words than monolinguals. There was also a significant two-way interaction between group and semantic response switch, F (1, 38) = 10.515, p = .002. Follow up contrasts, Bonferroni corrected, showed that the difference between response switch trials and no-switch trials was significant for bilinguals compared to monolinguals, suggesting that bilinguals were slower at managing the semantic response switch, as their reaction times increased on trials where the response switched, compared to monolinguals, whose reaction times decreased on switch trials. Lastly, the three-way interaction between group, language, and semantic response switch was significant, F (1, 38) = 11.721, p = .001. Figure 1B shows the graphical representation of this three-way interaction. It is apparent that on semantic switch trials in the newly learned language, the monolinguals actually speed up their response times, whereas bilinguals are slowed; this may suggest that the two groups are using different processing mechanisms to manage newly learned words.
3.1.2 fMRI Within-Group Analyses
A one-way ANOVA modeled pairwise comparisons of all three word-class conditions (GC, GN, E) in each group (bilinguals, monolinguals). An additional pairwise contrast was created to compare activation across both German conditions, total German activity (G), to English (E) and vice versa. In the monolingual group only two pairwise contrasts showed significant activity (E > G and G > E) at a threshold of p < .001, uncorrected. In the English > German contrast, modest activation was seen in only one area at the border of the left inferior parietal and temporal lobes, corresponding to the angular gyrus (left peak Z = 3.29, 5 voxels). There was considerably more activity overall in the German > English condition. Significant activity was present in a left superior parietal region (precuneus) extending bilaterally (left peak Z = 6.3, 523 voxels) and in bilateral inferior frontal cortex (left peak Z = 4.92, 388 voxels; right peak Z = 4.85, 95 voxels). There was also significant activation of the left caudate (left peak Z = 4.34, 330 voxels) extending bilaterally and left posterior cingulate (left peak Z = 3.81, 9 voxels). Additionally, there was activity in the right inferior parietal lobe (BA 39) (right peak Z = 4.28, 41 voxels) and the left dorsal medial frontal gyrus at the location of the SMA extending bilaterally (left peak Z = 4.63, 180 voxels). Coordinates and statistics can be seen for all regions of activation in Table 2 and images in Figure 2.
Table 2.
Within-group Imaging Results
| Group | Condition | Region | x | y | z | Z | k | Side |
|---|---|---|---|---|---|---|---|---|
| Monolinguals | E v G | Angular gyrus (BA 39) | −48 | −58 | 22 | 3.29 | 5 | Left |
| G v E | Superior parietal cortex (precuneus) | −30 | −64 | 37 | 6.3 | 523 | Left | |
| −36 | −55 | 43 | 5.68 | |||||
| −12 | −67 | 34 | 5.26 | |||||
| Inferior frontal cortex (BA 44 and 45) | −39 | 2 | 31 | 4.92 | 388 | Left | ||
| −39 | 20 | 25 | 4.82 | |||||
| Caudate | 9 | −1 | 7 | 4.34 | 330 | Bilateral | ||
| 12 | 8 | 7 | 4.13 | |||||
| Dorsal posterior cingulate | 0 | −28 | 28 | 5.73 | 195 | Left | ||
| −9 | −46 | 16 | 4.21 | |||||
| SMA (BA 6) | −9 | 14 | 49 | 4.63 | 180 | Left | ||
| Inferior frontal cortex/Insula (BA 45) | 36 | 23 | 10 | 4.85 | 95 | Right | ||
| Angular gyrus (BA 39) | 33 | −58 | 37 | 4.28 | 41 | Right | ||
| Posterior cingulate (BA 29) | 9 | −43 | 13 | 3.81 | 9 | Right | ||
| Bilinguals | G v E | Inferior frontal cortex | −36 | 23 | 22 | 4.09 | 75 | Left |
| Midbrain | −6 | −25 | − 14 | 3.65 | 23 | Left | ||
| Angular gyrus | −30 | −55 | 37 | 3.78 | 20 | Left | ||
| Posterior cingulate | −3 | −43 | 13 | 3.70 | 10 | Left | ||
| Dorsal posterior cingulate | 0 | −22 | 28 | 3.44 | 8 | Bilateral |
Coordinates are listed in MNI space. Within-group results used a threshold of p, uncorrected .001, with a minimum of 5 voxels per cluster (k). Significant sub-peaks included if peak pFWE-corr < .05.
Figure 2. Monolingual Group Activation (G > E).
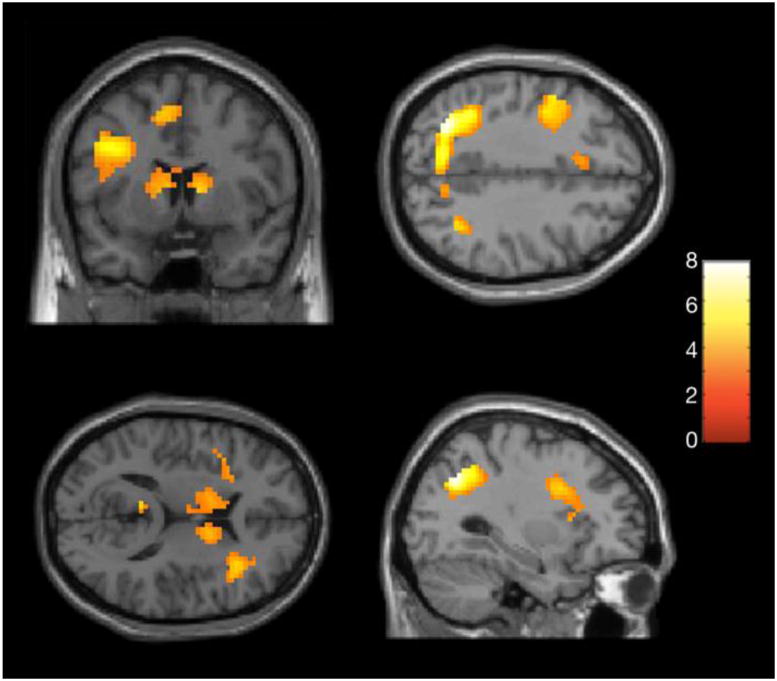
Within-group results used a p threshold, uncorrected .001, with a minimum of 5 voxels per cluster.
In the bilingual group, at a threshold of p < .001, uncorrected, there was no activity in the English > German condition, or either contrast comparing English to German cognates or noncognates alone. However, there was robust activation in the overall German > English condition. Activity in the bilingual group for this contrast (G > E) was more localized compared to the monolingual group, which had bilateral activation of most areas (i.e. inferior frontal, parietal, posterior cingulate, SMA, and caudate). The bilingual group showed left sided activity in both inferior frontal (left peak Z = 4.09, 75 voxels) and inferior parietal (left peak Z = 3.78, 20 voxels) regions. There was modest bilateral activity in posterior cingulate regions (see Table 2 and Figure 3).
Figure 3. Bilingual Group Activation (G > E).
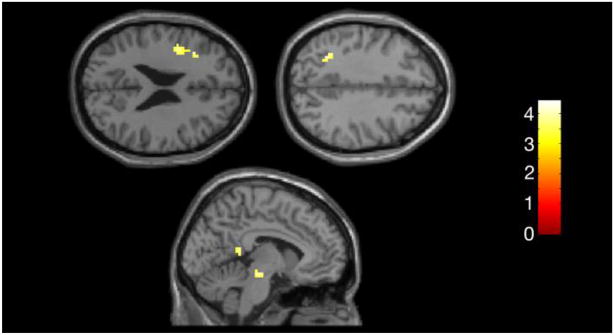
Within-group results used a p threshold, uncorrected .001, with a minimum of 5 voxels per cluster.
3.1.3 fMRI Between-Groups Analyses
Small volume correction was used to do a region of interest analysis on the results obtained from a full-factorial 2×3 ANOVA, with language (monolingual and bilingual) as one factor, and word type (German cognate, German noncognate, and English) as the second factor in order to evaluate five brain regions within the cognitive control literature (DLPFC, SMA, ACC, striatum, and inferior parietal cortex). When comparing monolinguals to bilinguals in response to all learned German words (M > B, G) activity was seen in right DLPFC (right peak t = 3.64, 38 voxels, pfwe = .01) and right SMA (right peak t = 3.32, 30 voxels, pfwe = .035). Left-sided activity was also seen for this contrast in the ACC (left peak t = 3.24, 175 voxels, pfwe = .001) and striatum, specifically the caudate (left peak t = 3.23, 86 voxels, pfwe = .046). The reverse of this condition, which compared bilinguals to monolinguals for all German words (B > M, G), showed activity that was significantly more localized. Out of the five regions of interest evaluated, only the left striatum, specifically the putamen, was active for bilinguals in response to German words (left peak t = 3.89, 96 voxels, pfwe = .006), whereas four of the regions of interest were active in monolinguals in response to German words (DLPFC, SMA, anterior cingulate, and caudate).
When all learned words were separated into cognates and noncognates, monolinguals showed activity in the right DLPFC in response to German cognates (M > B, GC), whereas bilinguals showed no activity in any of the five pre-specified regions in the same contrast (B > M, GC). However, bilinguals showed specific activity in response to German noncognates where monolinguals did not show any activity. In the bilingual versus monolingual German noncognate condition (B > M, GN), there was localized activity in the left putamen. These between-subjects results can be seen in Table 3 and Figure 4.
Table 3.
Between-groups Imaging Results: Small Volume Correction
| Condition | Region | Side | x | y | z | t | k | Peak-level pFWE |
|---|---|---|---|---|---|---|---|---|
| M > B (G) | DLPFC | Right | 42 | 23 | 16 | 3.64 | 38 | .01 |
| SMA | Right | 15 | −1 | 49 | 3.32 | 30 | .035 | |
| Anterior cingulate | Left | −21 | 35 | 19 | 3.24 | 175 | .001 | |
| Striatum (caudate) | Left | −15 | 23 | 10 | 3.23 | 86 | .046 | |
| M > B (GC) | DLPFC | Right | 42 | 23 | 16 | 3.28 | 38 | .05 |
| B > M (G) | Striatum (putamen) | Left | −27 | −7 | −2 | 3.89 | 96 | .006 |
| B > M (GN) | Striatum (putamen) | Left | −27 | −7 | −2 | 3.64 | 135 | .013 |
Coordinates are listed in MNI space. Small volume correction was used to isolate activity in five pre-specified regions of interest (DLPFC, SMA, Anterior Cingulate, Striatum, and Inferior Parietal Cortex). Statistics were only reported when the peak-level threshold, FWE controlled, was less than or equal to .05.
Figure 4. Between-groups SVC Results.
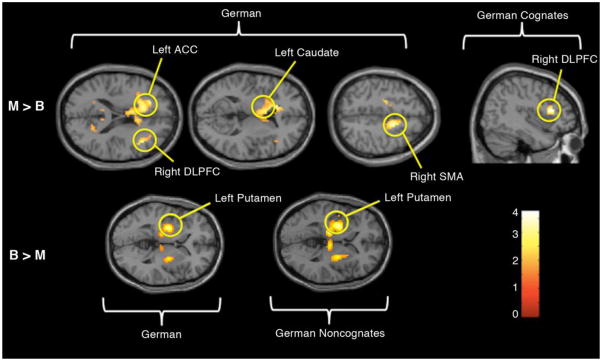
The top row of images shows results from the monolingual > bilingual contrasts of German words and German cognates (M > B G; M > B GC). The bottom row of images displays activation for the bilingual > monolingual German and German noncognate contrasts (B > M G; B > M GN).
4.1 Discussion
The purpose of this study was to test whether bilingual adults activate components of the cognitive control network differently or more efficiently when learning new vocabulary than monolinguals, who do not have the experience of managing multiple languages. Results of both the imaging and behavioral data obtained shed light on the differences between monolinguals and bilinguals in the processing of newly learned vocabulary. Between-group differences revealed monolinguals showed activation of right DLPFC, right SMA, left ACC, and left caudate (Table 3), in response to newly learned German words. However, bilinguals showed activity more localized to the left striatum, specifically the putamen, in response to newly learned words. This between-group analysis confirms that monolinguals activate a more broadly distributed, bilateral set of regions implicated in the cognitive control of language compared to bilinguals, whose activity is more subcortically localized in a motor control region.
The monolinguals’ more distributed activation in cognitive control regions (DLPFC, SMA, ACC, caudate) suggests that this group worked harder to access lexico-semantic information during the scanner task; however, due to the design of this study, we cannot be sure at this point if this difference results from difficulty in language retrieval or inhibition and monitoring. Abutalebi and Green (2007) propose that the prefrontal cortex is recruited in situations where language retrieval is not automatic and requires greater cognitive control. Specifically, the DLPFC is required for response selection and inhibition of competing responses, while the ACC is active during error detection and conflict monitoring (Abutalebi and Green, 2007); both the DLPFC and the ACC were activated in the M > B, G condition; therefore, it cannot be definitively concluded what is driving monolinguals’ increased activation of the cognitive control network. Abutalebi and colleagues (2012) recently found that the ACC specifically, is differentially activated and recruited in bilinguals compared to monolinguals. They found that bilinguals more efficiently activated this region, resulting in decreased activity in the area compared to monolinguals, who more strongly recruited this area in domain-general and language specific control tasks. This supports out finding that monolinguals did in fact require stronger activation of this area in a cognitive control heavy task.
The conclusion that monolinguals and bilinguals differ in their control of language is further supported by the behavioral reaction time data. Given that the monolinguals were more proficient in English according to BNT scores (see section 3.1.1), in theory, this group should have produced faster responses overall to English words in the semantic task. However, this hypothesis was not supported. Simple effects analyses of group did not show monolinguals were faster than bilinguals at responding to English words (Table 1). The bilingual group actually trended towards being significantly faster than monolinguals at responding to English stimuli. Further simple effects analyses also showed that bilinguals were faster than monolinguals at responding to all newly learned words, both cognates and noncognates. Additional post-hoc analyses used to determine the influence of other factors within the event-related design on reaction time showed that there was a three-way interaction between group, language, and semantic response switch. Investigation of this interaction revealed that the difference in reaction time on semantic response switch trials compared to no switch trials between German and English words was significant for bilinguals compared to monolinguals. In Figure 1B, it is apparent that monolinguals’ reaction times significantly increased from English to German trials; however, their responses to newly learned German words were actually quickened when a semantic response switch was required. This differed from the pattern evident in bilinguals’ reaction times. The bilingual group was mildly slowed in response to newly learned German words compared to English words; however, their reaction times did not significantly increase until a response switch was required. This difference in reaction to the semantic response switch can be taken as an indication that monolinguals may be using a higher-level, more explicit semantic strategy during the task relative to bilinguals. It is possible that by paying very close attention to the semantic category in which each word falls, monolinguals may be anticipating the semantic switch; therefore, when the semantic category does switch, there could be a release of inhibition so that their response time is actually faster. The bilinguals may not be relying as much on this more explicit, higher-level semantic strategy to guide their responses. They may have made more direct links between the newly learned German words and known English words, which is a more motor controlled paired-associate type of process, possibly mediated by phonological or articulatory processes. Hence, a semantic response switch leads to slowing by bilinguals due to the interference of attending specifically to the semantic category. As the design of this study was not to specifically examine the effects of semantic response switch and language switch, this interpretation should be viewed conservatively. These are preliminary findings, and future studies are needed to more closely tease apart the influences of semantic response switch and language switch on cognitive control mechanisms activated in the early stages of foreign word learning.
The more distributed activation pattern displayed by monolinguals, as well as slower behavioral reaction times suggests that monolinguals’ processing of newly learned vocabulary information is more effortful than bilinguals’. Additionally, the bilingual group showed activation of the left putamen, whereas monolinguals activated the left caudate in addition to other cognitive control regions. Abutalebi and Green (2007) propose that the basal ganglia are vital to the cognitive control of language. These subtle differences in striatal and prefrontal activation may further suggest that monolinguals and bilinguals utilize different mechanisms of language control during the early stages of word learning. In the language literature, the putamen is often associated with motor processing (Menon et al., 2000; Wildgruber et al., 2001; Wise et al., 1999), particularly for late learned words in bilinguals (Hernandez & Fiebach, 2006). Abutalebi and colleagues (in press) found that trilinguals in particular have increased grey matter density in the left putamen, which suggests greater plasticity in this region for enhanced articulatory processing. Similar to Hernandez and Fiebach’s (2006) finding of increased putamen activity for late learned words, Abutalebi et al. (in press) also found this increased putamen activation only occurred in multilinguals’ later learned, less proficient language, as there is a greater articulatory burden for producing words just introduced into the lexicon, which requires greater motor control. From these studies, it is clear that the putamen plays a significant role in the motor control of language, possibly through phonological and articulatory processes. Therefore, in the current study, bilinguals’ greater activation of the putamen in response to newly learned German words in addition to their pattern of reaction times related to German words and semantic response switches suggests that this group may be relying more heavily on a motor control system to aid the mapping of a new word to a known word in English. It appears that the direct connection between the newly learned German word and an English word, which is already in their lexicon, relies heavily on an articulatory motor process. The semantic category may be more directly linked to the known English word for this group, so that access to category information is less effortful, even for newly learned words. However, when the semantic category changes, the switch may interfere with the bilinguals’ motor control strategy, resulting in slower reaction times on semantic response switch trials. This may confirm why bilinguals fail to respond significantly slower to German words on trials without a semantic response switch (Figure 1B). The influence of semantics plays a different role in monolinguals. Specifically, they do not experience this interference because they may already be using a higher-level cognitive strategy in which the semantic category is actively attended to. The monolinguals’ lack of putamen activity and their intense activation of other cognitive control regions, including the caudate, DLPFC, ACC, and SMA may suggest this group might be using a different, higher-level method of language control for newly learned words.
This theory that monolinguals rely on a higher-level cognitive control of language compared to bilinguals, who utilize a more articulatory-based motor control can be closely linked to the work of Ashby and Crossley’s (2012) discussion of automaticity and memory systems, where activation of the caudate versus the putamen signifies a difference in the neural mechanism of learning. In Ashby and Crossley’s (2012) study, participants learn to either explicitly or implicitly map a motor response to a particular stimulus in a category discrimination task. Their assertion that the putamen mediates procedural learning, while the caudate is involved in more explicitly learned tasks, parallels findings within the bilingual literature. Abutalebi and Green (2007) conclude that the putamen is vital to bilingual language management, specifically during phonological and articulatory tasks, processes that are generally learned through daily use of language and not bound by explicitly outlined rules. Alternatively, the caudate has been found to be involved in more cognitive tasks involving working memory and explicit, rule-based learning (Ashby and Crossley, 2012; Chee, 2006). Chee (2006) suggests that the caudate is involved in classifying linguistic stimuli to ensure contextually meaningful responses, which supports the current study’s findings that monolinguals utilize this region, in addition to other cognitive control regions (DLPFC, ACC, SMA) to cognitively map a new German word onto a known English word in an effort to retrieve the correct semantic response. Bilinguals however, utilized a more motor-control based processing strategy that might allow for a more direct link to be made between a newly learned word and a known word. It may be that the bilinguals’ life experience managing multiple languages made their use of a more motor-based processing strategy more familiar, and thus more automatic to retrieve semantic category information, while the monolinguals were less equipped to handle the difficulty of the semantic task after having had such little time to learn the new German words. Their more cognitively based strategy and increased attention to semantic category may have been less efficient in the early stages of word learning.
While it is clear from the imaging and behavioral data that the groups showed differences in their activation of the cognitive control network, the within-group analyses also showed similarities in the pattern of activation both groups displayed in response to newly learned words, suggesting that regardless of language experience, learning novel vocabulary is difficult as an adult. In the within-group analyses, both monolinguals and bilinguals showed increased activity for newly learned German words relative to English words (G > E conditions). In the German > English conditions (Table 2), for both monolinguals and bilinguals, there was significant activation in language related inferior frontal, inferior parietal, and cingulate regions. However, in the English > German contrasts, only monolinguals showed activity in the angular gyrus (BA 39), while the bilingual group showed no activation. This is consistent with literature showing that the brain engages more broadly distributed neural mechanisms for later acquired languages (Vingerhoets et al., 2003) or in response to a less proficient language (Meschyan and Hernandez, 2006; Perani and Abutalebi, 2005). De Bleser et al. (2003) concluded in their review that there is generally less activation of language related areas in the left temporal lobe and more widespread contribution of other areas in response to low proficiency in a language. Abutalebi and Green (2007) propose that the prefrontal cortex is recruited in these situations where language retrieval is less automatic and requires greater cognitive control. This supports our results showing activation of the angular gyrus, near the superior temporal sulcus, in response to accessing lexico-semantic information related to English words, the most proficient language for monolinguals, in comparison to the inferior frontal, parietal, and cingulate regions activated in response to German words, the newly introduced language.
The behavioral reaction time data also display patterns consistent with greater difficulty processing newly learned vocabulary information. As expected, there was a general pattern in the reaction time data consistent with the cognate facilitation effect identified in previous research (Dijkstra et al., 2010; de Groot and Nas, 1991; Lotto and de Groot, 1998; de Groot and Keijzer, 2000; Tonzar et al., 2009; Costa et al., 2000; Raboyeau et al., 2010) in which newly learned cognates were processed faster or presumably more easily than noncognates (Figure 1A), but slower than the native language. However, further simple effects analyses of the significant interaction between group and word type showed that the cognate facilitation effect was not entirely present. Pairwise comparisons of German cognates to English words were significant (p < .025, αEW = .05) for monolinguals (p < .0001) and only approached significance in bilinguals (p < .0412), but there was no significant difference between noncognates and cognates in either group. This may be a result of the short learning period, or the undirected flashcard learning approach where the auditory presentation of words was not given. It is possible that if word pronunciations were provided, learners may have been more attuned to the phonological similarities between stimuli. Regardless, there was a significant effect of word type in monolinguals in which English was processed faster than either German cognates or noncognates, with this same pattern approaching significance in bilinguals. Therefore, it can be concluded that learning novel vocabulary is difficult for both groups, as they both showed distributed activation in inferior frontal, parietal, and cingulate regions and had slower reaction times for newly learned words compared to English, the native language for monolinguals, and the second language for bilinguals.
The above discussion of the imaging and behavioral results suggests that the bilingual brain is more prepared through experience managing multiple languages to cope with the demands of interpreting newly learned words by accessing the most efficient neural pathways to control the process, allowing bilinguals easier access to motor control regions to process lexico-semantic information. Monolinguals required a more cognitively based network that included areas of the prefrontal cortex and the caudate. The areas of activation in this study further support that over the entire task, monolinguals experienced greater difficulty with the task, which required recruitment of cognitive control regions, whereas bilinguals activated regions related to more direct phonological and articulatory motor processes, which may have aided in their quicker response times. These results suggest that learning novel vocabulary is difficult for both groups as evidenced by the more distributed brain network that included subcortical control regions in both groups; however, the difference between groups in these prefrontal and subcortical networks suggests that this difficulty in processing early lexico-semantic information may be approached in different ways, either at a higher cognitive level, or at a lower, more automatic motor level, based on the experience one has managing multiple languages.
5.1 Conclusion
In conclusion, this study supports the idea that adults learning novel vocabulary may employ different regions within the control network as a result of their experience managing previously learned languages in which they are proficient. Our results showed that adult monolinguals activated a set of prefrontal cortical control regions in addition to the caudate, suggesting a higher-level cognitive mode of control. Bilinguals demonstrated more localized activity within the subcortical striatum, specifically the putamen, which is responsible for phonological and articulatory processes, suggesting a more motor-based mode of control. These differences in activation suggest that individuals who have experience managing multiple languages may more efficiently access specific cognitive control regions associated with more direct motor processing to pair a newly learned word with one already in the lexicon, as opposed to struggling before activating the semantic category. While activation patterns suggested that processing the newly learned German words was difficult for both groups, the bilinguals seemed better prepared to recall the newly learned vocabulary in a difficult task by utilizing a more automatic, direct processing route until a semantic switch requiring higher-level cognitive processes interfered with this processing. The monolinguals’ higher-level processing actually sped up their response on semantic category switch trials, further supporting the idea that the two groups utilize different types of control.
More investigation is required given the paucity of research examining the development of cognitive control mechanisms in language acquisition. This study provides the foundational information that monolinguals and bilinguals activate different components of the control network during the early stages of novel word learning, possibly due to their different lifelong experiences managing just one or multiple languages. Future studies will be required to examine the specific contributions of language switch and response switch that might have influenced the activation of control regions in these groups. Additionally, it will be important to evaluate the connectivity of these regions and how activation of this control system develops as proficiency in a new language improves.
Highlights.
Experience managing multiple languages influenced control regions activated
Monolinguals activated prefrontal brain regions
Monolinguals utilized a higher-level cognitive-based control mechanism
Bilinguals activated the left putamen, associated with motor control of language
Acknowledgments
This research was supported under the grant Neural correlates of lexical processing in child L2 learners from NIH/NICHD 1R21HD059103. Additional thanks are given to the Baylor Human Neuroimaging Laboratory for the use of scanning equipment, to Kenia Velasquez for recruitment and data collection, and to Pilar Archila-Suerte for assisting in the proofreading and editing process.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kailyn A. L. Bradley, Email: kailyn.bradley@gmail.com.
Kelly E. King, Email: kingx780@umn.edu.
Arturo E. Hernandez, Email: aehernandez@uh.edu.
References
- Abutalebi J, Costa A. Acquisition, processing, and loss of L2: Functional, cognitive and neural perspectives. Journal of Neurolinguistics. 2008;21(6):473–476. [Google Scholar]
- Abutalebi J, Della Rosa PA, Gonzanga AKC, Keim R, Costa A, Perani D. The role of the left putamen in multilingual language production. Brain & Language. doi: 10.1016/j.bandl.2012.03.009. (in press) http://dx.doi.org/10.1016/j.bandl.2012.03.009. [DOI] [PubMed]
- Abutalebi J, Della Rosa PA, Green DW, Hernandez M, Scifo P, Keim R, Cappa SF, Costa A. Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cerebral Cortex. 2012;22(9):2076–2086. doi: 10.1093/cercor/bhr287. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Green D. Bilingual language production: The neurocognition of language representation and control. Journal of Neurolinguistics. 2007;20(3):242–275. [Google Scholar]
- Abutalebi J, Green D. Control mechanisms in bilingual language production: Neural evidence from language switching studies. Language and Cognitive Processes. 2008;23(4):557–582. [Google Scholar]
- Ashby FG, Crossley MJ. Automaticity and multiple memory systems. Wires Cognitive Science. 2012;3:363–376. doi: 10.1002/wcs.1172. [DOI] [PubMed] [Google Scholar]
- Bates E. Plasticity, localization and language development. In: Broman S, Fletcher JM, editors. The changing nervous system: Neurobehavioral consequences of early brain disorders. New York: Oxford University Press; 1999. pp. 214–253. [Google Scholar]
- Bates E, Andonova E, D’Amico S, Jacobsen T, Kohnert K, Lu CC, Székely A, Wicha N, Federmeier K, Herron D, et al. Center for Research in Language Newsletter. 1. Vol. 12. La Jolla: University of California San Diego; 2000. Introducing the CRL International Picture-Naming Project (CRL-IPNP) [Google Scholar]
- Bialystok E. Bilingualism in development: Language, literacy, and cognition. New York: Cambridge University Press; 2001. [Google Scholar]
- Bialystok E, Klein R, Craik FIM, Viswanathan M. Bilingualism, aging, and cognitive control: Evidence from the Simon Task. Psychology and Aging. 2004;19(2):290–303. doi: 10.1037/0882-7974.19.2.290. [DOI] [PubMed] [Google Scholar]
- Bialystok E, DePape A. Musical expertise, bilingualism, and executive functioning. Journal of Experimental Psychology. 2009;35(2):565–574. doi: 10.1037/a0012735. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Shapero D. Ambiguous benefits: The effect of bilingualism on reversing ambiguous figures. Developmental Science. 2005;8(6):595–604. doi: 10.1111/j.1467-7687.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- Bosch L, Costa A, Sebastian-Galles N. First and second language vowel perception in early bilinguals. European Journal of Cognitive Psychology. 2000;12(2):189–221. [Google Scholar]
- Carlson S, Meltzoff A. Bilingual experience and executive functioning in young children. Developmental Science. 2008;11(2):282–298. doi: 10.1111/j.1467-7687.2008.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chee MWL. Dissociating language and word meaning in the bilingual brain. TRENDS in Cognitive Sciences. 2006;10(12):527–529. doi: 10.1016/j.tics.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Costa A, Caramazza A, Sebastian-Galles N. The cognate facilitation effect: Implications for models of lexical access. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(5):1283–1296. doi: 10.1037//0278-7393.26.5.1283. [DOI] [PubMed] [Google Scholar]
- De Bleser R, Dupont P, Postler J, Bormans G, Speelman D, Mortelmans L, Debrock M. The organization of the bilingual lexicon: a PET study. Journal of Neurolinguistics. 2003;16(4–5):439–456. [Google Scholar]
- de Groot AMB, Keijzer R. What is hard to learn is easy to forget: The roles of word concreteness, cognate status, and word frequency in foreign-language vocabulary learning and forgetting. Language Learning. 2000;50(1):1–56. [Google Scholar]
- de Groot AMB, Nas GL. Lexical representation of cognates and noncognates in compound bilinguals. Journal of Memory and Language. 1991;30(1):90–123. [Google Scholar]
- Dijkstra T, Miwa K, Brummelhuis B, Sappelli M, Baayen H. How cross-language similarity and task demands affect cognate recognition. Journal of Memory and Language. 2010;62(3):284–301. [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Garbin G, Sanjuan A, Forn C, Bustamante JC, Rodriguez-Pujadas A, Belloch V, Hernandez M, Costa A, Avila C. Bridging language and attention: Brain basis of the impact of bilingualism on cognitive control. NeuroImage. 2010;53(4):1272–1278. doi: 10.1016/j.neuroimage.2010.05.078. [DOI] [PubMed] [Google Scholar]
- Hernandez AE. Language switching in the bilingual brain: What’s next? Brain & Language. 2009;109(2–3):133–140. doi: 10.1016/j.bandl.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Fiebach CJ. The brain bases of reading late learned words: Evidence from functional MRI. Visual Cognition. 2006;13(7–8):1027–1043. [Google Scholar]
- Hernandez A, Li P, MacWhinney B. The emergence of competing modules in bilingualism. TRENDS in Cognitive Sciences. 2005;9(5):220–225. doi: 10.1016/j.tics.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davisdon RJ, Oakes TR. Motion correction and the use of motion covariates in multiple-subject fMRI. Human Brain Mapping. 2006;27:779–788. doi: 10.1002/hbm.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lee & Febiger; 1983. [Google Scholar]
- Kohnert KJ, Hernandez AE, Bates E. Bilingual performance on the Boston Naming Test: Preliminary Norms in Spanish and English. Brain and Language. 1998;65(3):422–440. doi: 10.1006/brln.1998.2001. [DOI] [PubMed] [Google Scholar]
- Kroll JF. Accessing conceptual representations for words in a second language. In: Schreuder R, Weltens B, editors. The bilingual lexicon. Amsterdam: Benjamins; 1994. [Google Scholar]
- Kuhl P, Rivera-Gaxiola M. Neural substrates of language acquisition. Annual review of neuroscience. 2008;31(1):511–534. doi: 10.1146/annurev.neuro.30.051606.094321. [DOI] [PubMed] [Google Scholar]
- Kaushanskaya M, Marian V. The bilingual advantage in novel word learning. Psychonomic Bulletin & Review. 2009;16(4):705–710. doi: 10.3758/PBR.16.4.705. [DOI] [PubMed] [Google Scholar]
- Lee H, Fujii T, Okuda J, Tsukiura T, Umetsu A, Suzuki M, Nagasaka T, Takahashi S, Yamadori A. Changes in brain activation patterns associated with learning of Korean words by Japanese: An fMRI study. NeuroImage. 2003;20(1):1–11. doi: 10.1016/s1053-8119(03)00254-4. [DOI] [PubMed] [Google Scholar]
- Lotto L, de Groot AMB. Effects of learning method and word type on acquiring vocabulary in an unfamiliar language. Language Learning. 1998;48(1):31–69. [Google Scholar]
- Luk G, Bialystok E, Craik FIM, Grady CL. Lifelong bilingualism maintains white matter integrity in older adults. The Journal of Neuroscience. 2011;31(46):16808–16813. doi: 10.1523/JNEUROSCI.4563-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss B, Posner M, Givon T. Brain plasticity in learning visual words. Cognitive Psychology. 1997;33(1):88–110. [Google Scholar]
- McLaughlin J, Osterhout L, Kim A. Neural correlates of second-language word learning: Minimal instruction produces rapid change. Nature Neuroscience. 2004;7(7):703–704. doi: 10.1038/nn1264. [DOI] [PubMed] [Google Scholar]
- Menon V, Anagnoson RT, Glover GH, Pfefferbaum A. Basal ganglia involvement in memory-guided movement sequencing. Neuroreport. 2000;11:3641–3645. doi: 10.1097/00001756-200011090-00048. [DOI] [PubMed] [Google Scholar]
- Meschyan G, Hernandez A. Impact of language proficiency and orthographic transparency on bilingual word reading: An fMRI investigation. NeuroImage. 2005;29(4):1135–1140. doi: 10.1016/j.neuroimage.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Perani D, Abutalebi J. The neural basis of first and second language processing. Current Opinion in Neurobiology. 2005;15(2):202–206. doi: 10.1016/j.conb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Raboyeau G, Marcotte K, Adrover-Roig D, Ansaldo AI. Brain activation and lexical learning: The impact of learning phase and word type. NeuroImage. 2010;49(3):2850–2861. doi: 10.1016/j.neuroimage.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Shtyrov Y, Nikulin VV, Pulvermuller F. Rapid cortical plasticity underlying novel word learning. The Journal of Neuroscience. 2010;30(50):16864–16867. doi: 10.1523/JNEUROSCI.1376-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonzar C, Lotto L, Job R. L2 vocabulary acquisition in children: Effects of learning method and cognate status. Language Learning. 2009;59:623–646. [Google Scholar]
- Vingerhoets G, Borsel J, Tesink C, van den Noort M, Deblaere K, Seurinck R, Vandemaele P, Achten E. Multilingualism: An fMRI study. NeuroImage. 2003;20(4):2181–2196. doi: 10.1016/j.neuroimage.2003.07.029. [DOI] [PubMed] [Google Scholar]
- Waldie KE, Badzakova-Trajkov G, Miliivojevic B, Kirk IJ. Neural activity during Stroop colour-word task performance in late proficient bilinguals: a functional magnetic resonance imaging study. Psychology & Neuroscience. 2009;2(2):125–136. [Google Scholar]
- Wang Y, Xue G, Chen C, Xue F, Dong Q. Neural bases of asymmetric language switching in second-language learners: An ER-fMRI study. NeuroImage. 2007;35(2):862–870. doi: 10.1016/j.neuroimage.2006.09.054. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Ackermann H, Grodd W. Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: Effects of syllable repetition rate evaluated by fMRI. NeuroImage. 2001;13:101–109. doi: 10.1006/nimg.2000.0672. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Greene J, Buchel C, Scott SK. Brain regions involved in articulation. Lancet. 1999;353:1057–1061. doi: 10.1016/s0140-6736(98)07491-1. [DOI] [PubMed] [Google Scholar]
- Ye Z, Zhou X. Executive control in language processing. Neuroscience and Biobehavioral Reviews. 2009;33(8):1168–1177. doi: 10.1016/j.neubiorev.2009.03.003. [DOI] [PubMed] [Google Scholar]


