Abstract
Mrp8 and Mrp14 are endogenous alarmins amplifying inflammation via Toll-like receptor-4 (TLR-4) activation. Due to their pro-inflammatory properties, alarmins are supposed to enhance adaptive immunity via activation of dendritic cells (DCs). In contrast, analysing a model of allergic contact dermatitis (ACD) we observed a more severe disease outcome in Mrp8/14-deficient compared to wild-type mice. This unexpected phenotype was associated with an enhanced T-cell response due to an accelerated maturation of DCs in Mrp8/14-deficient mice. Accordingly, Mrp8, the active component of the heterocomplex, inhibits early DC maturation and antigen presentation in a TLR-4-dependent manner. Transfer of DCs purified from the local lymph nodes of sensitized Mrp8/14-deficient to wild-type mice determined the outcome of ACD. Our results link a pro-inflammatory role of the endogenous TLR-4 ligand Mrp8/14 to a regulatory function in adaptive immunity, which shows some similarities with the ‘hygiene hypothesis’ regarding continuous TLR-4 stimulation and decreased risk of allergy.
Keywords: adaptive immunity, alarmins, calprotectin, DC, Mrp8/14
Introduction
In industrialized countries, allergic contact dermatitis (ACD) represents one of the most common inflammatory skin diseases (Uter et al, 1998). The underlying mechanism is a contact hypersensitivity reaction depending on the generation of an allergen-specific T lymphocyte response (Martin et al, 2000; Bour et al, 2005; Vocanson et al, 2006). The resulting T-cell response is critically dependent on the state of differentiation and activation of dendritic cells (DCs) but the molecular mechanisms involved in the process of skin sensitization are still not fully understood (Banchereau and Steinman, 1998; Gutcher and Becher, 2007). Recently, so-called danger-associated molecular pattern proteins (DAMPs) or alarmins attracted attention. They represent endogenous danger signals inducing inflammatory responses after being released from activated or necrotic cells. Several receptors including the multiligand receptor for advanced glycation end products (RAGE), Toll-like receptor (TLR)-2, TLR-4, and TLR-9 appear to mediate effects of different DAMPs (Tian et al, 2007; Vogl et al, 2007; Zhang and Mosser, 2008; Ichikawa et al, 2011). Some DAMPs like High-mobility group nucleosome-binding protein 1 (HMGN1), High-mobility group box 1 protein (HMGB1), and eosinophil-derived neurotoxin are able to enhance antigen-specific immune responses via activation of DCs (Rovere-Querini et al, 2004; Yang et al, 2006, 2008, 2010, 2011, 48). Moreover, a crucial role of TLR-4 has been shown recently in allergic contact hypersensitivity indicating that not only the environmental microbial flora but also endogenous ligands or metal ions as Ni2+ may contribute to the progress of the disease (Martin et al, 2008; Schmidt et al, 2010).
We recently demonstrated that Mrp8 (myeloid related protein 8, S100A8) and Mrp14 (S100A9) show typical characteristics of endogenous TLR-4 ligands and alarmins. Complexes of Mrp8 and Mrp14, also known as calprotectin, are released by activated phagocytes at sites of inflammation (Strupat et al, 2000; Foell et al, 2007; Korndörfer et al, 2007; Vogl et al, 2007; van Lent et al, 2008a; Ehrchen et al, 2009) and exhibit pro-inflammatory effects on endothelial cells, phagocytes, or lymphocytes (Viemann et al, 2005; Vogl et al, 2007; Frosch et al, 2009; Loser et al, 2010; Grevers et al, 2011). Mrp8 specifically interacts with TLR-4, thus representing an endogenous activator of this receptor (Vogl et al, 2007). In addition to TLR-4, RAGE has also been described as a receptor for Mrp8 and Mrp14 (Björk et al, 2009; Ichikawa et al, 2011). In animal models, we could show that Mrp8 and Mrp14 are able to promote arthritis (van Lent et al, 2008a, 2008b) as well as the induction of autoreactive CD8+ T cells in systemic autoimmunity in a TLR-4-dependent manner (Loser et al, 2010). In models of tumour development, Mrp14−/− mice show a shift between mature DCs and immature so-called myeloid-derived suppressor cells (MDSCs) (Cheng et al, 2008; Gabrilovich and Nagaraj, 2009).
In the present study, we demonstrate that Mrp8 and Mrp14 directly modulate adaptive immunity during allergic reactions. Remarkably, we found that mice lacking these proteins show a stronger inflammatory response during ACD. We deciphered a novel molecular mechanism by which prolonged exposure of myeloid progenitor cells to Mrp8 and Mrp14 blocks DC differentiation and antigen presentation resulting in a diminished T-cell response. Our data link the pro-inflammatory role of the danger molecule Mrp8/14 on innate immune mechanisms to a regulatory function in adaptive immunity, which may be an important mechanism to avoid tissue damage due to overwhelming immune responses.
Results
Mrp8 and Mrp14 activate bone marrow-derived DCs via TLR-4
To determine the effect of Mrp8 and Mrp14 on DC activation, we generated immature (i) bone marrow-derived DCs (BMDCs) from wild-type (wt) mice in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) for 6 days and incubated them thereafter for 48 h with Mrp8, Mrp14, or Mrp8/14 heterocomplex. Flow cytometric analyses revealed that the expression of activation markers (CD86 and MHC class II molecules, MHC-II) is upregulated on iBMDCs by Mrp8 and Mrp8/14 (P<0.05) but not by Mrp14 alone (Figure 1A). In addition, DCs stimulated by Mrp8 and Mrp8/14 induced allogenic T-cell proliferation to a significant greater extent than controls and comparable to LPS-treated BMDCs (Figure 1B and C). No induction of DC activation occurred in TLR-4−/− BMDCs whereas RAGE−/− BMDCs showed expression levels comparable to wt BMDCs (Figure 1D). These data indicate that DC activation by Mrp8 is mediated via TLR-4, while RAGE is not involved.
Figure 1.
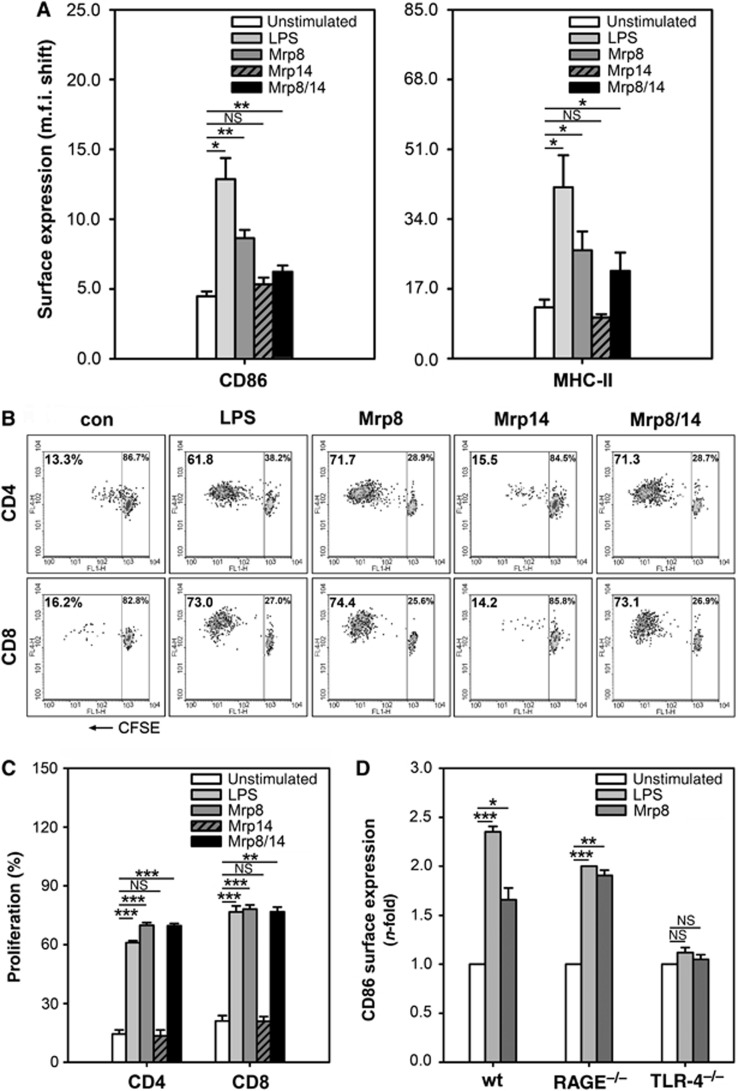
Mrp8/14-induced TLR-4-mediated activation of iBMDCs. (A) Expression of activation markers CD86 and MHC-II on in vitro generated iBMDCs from C57BL/6 mice. iBMDCs were activated for 48 h in the presence of Mrp8 or Mrp14 (8 μg/ml), Mrp8/14 (20 μg/ml), or LPS (300 ng/ml). Unstimulated iBMDCs served as control. M.f.i. shifts were determined by flow cytometry (three independent experiments; mean±s.e.m.). (B, C) Proliferation of CFSE-labelled CD4+ and CD8+ allogenic spleen T cells from BALB/c mice after 5 days of culture with unstimulated, Mrp8-, Mrp14-, Mrp8/14-, or LPS-stimulated C57BL/6 BMDCs (DC:T-cell ratio was 1:5). Panel (B) shows results from one of three independent experiments which are summarized in (C) (mean±s.e.m.). (D) Comparison of CD86 expression of unstimulated, LPS- (300 ng/ml) and Mrp8- (8 μg/ml) stimulated BMDCs from C57BL/6 wt, RAGE−/−, and TLR-4−/− mice. Following 48 h of stimulation, m.f.i. shifts were measured by flow cytometry and normalized to unstimulated control cells (three independent experiments; mean±s.e.m.). *P<0.05, **P<0.01, ***P<0.001, and NS=not significant.
More severe disease outcome of ACD in Mrp14−/− mice
To investigate the biological relevance of the Mrp8-mediated effects on DC activation, we elicited ACD in C57BL/6 wt and Mrp14−/− mice as a model for a DC-mediated T-cell response. It is important to note that Mrp14−/− mice also lack Mrp8 on the protein level, probably due to high metabolic turnover of Mrp8 in the absence of its complex partner. Therefore, the Mrp14−/− strain represents a functional Mrp8/14 double knockout mouse (Hobbs et al, 2003; Manitz et al, 2003). Surprisingly, ear swelling during ACD was significantly more pronounced in Mrp14−/− mice in comparison to wt mice (Figure 2A). Accordingly, ear sections of Mrp14−/− mice showed an increased inflammatory infiltration by CD11b+ myeloid cells, Gr-1+ granulocytes, and F4/80+ macrophages (Figure 2B). No differences regarding the amounts of CD11b+, Gr-1+, and F4/80+ cells were found in untreated ear tissue of wt and Mrp14−/− mice (Supplementary Figure S1). As expected, ears of Mrp14−/− mice were completely negative for both, Mrp8 and Mrp14, whereas high levels of these proteins could be observed in wt mice upon challenge (Figure 2C). Our data clearly demonstrate an enhanced allergic response in Mrp14−/− mice compared to wt controls, which somehow contradicts our data regarding the activation of BMDCs by Mrp8 in vitro. This finding is not due to an anti-inflammatory effect of Mrp8 or Mrp14 since Mrp8/14−/− mice do not show higher inflammatory activity in a T cell-independent model of irritant contact dermatitis (ICD) (Figure 2D).
Figure 2.
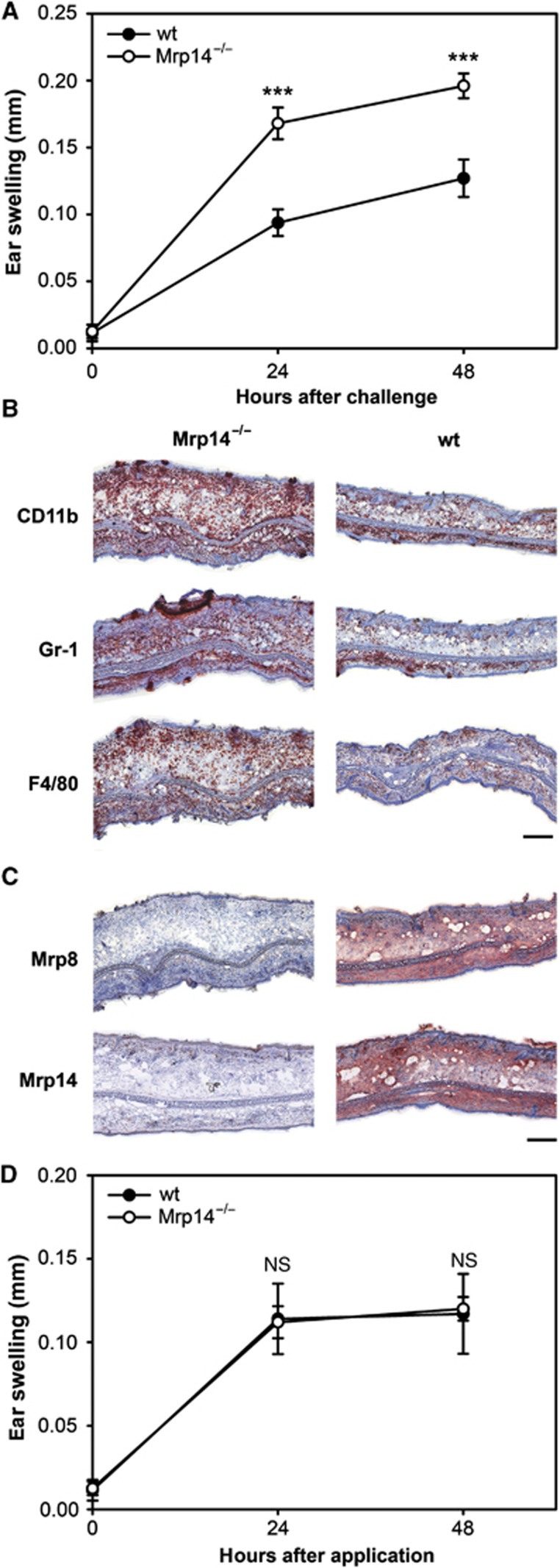
(A–C) DNFB-induced ACD response in ears of C57BL/6 wt and Mrp14−/− mice. (A) Ear swelling of wt and Mrp14−/− mice after elicitation of ACD. Mice were sensitized by painting 0.4% DNFB in acetone/olive oil on the shaved abdomen. After 6 days, the right ears were challenged with 0.4% DNFB in acetone/olive oil. The left ears were treated with acetone/olive oil only and served as controls. Ear thickness was measured 24 and 48 h after challenge. Swelling was determined by calculating the difference between the control ear and the challenged ear (four independent experiments with each 8–15 mice per group; mean±s.e.m.) (***P<0.001). (B, C) Histological analyses of ear sections from DNFB-treated wt and Mrp14−/− mice 48 h after challenge. Sections were stained for (B) immigrated immune cell (CD11b+ myeloid cells, Gr-1+ granulocytes, F4/80+ macrophages) and (C) expression of Mrp8 and Mrp14. (B, C) scale bar, 250 μm. (D) Croton oil-induced ICD response in ears of C57BL/6 wt and Mrp14−/− mice. Mice were painted with 10 μl 3% croton oil in acetone/olive oil (4:1) onto the right ears. The left ears served as controls and were treated with acetone/olive oil only. Ear thickness was measured 24 and 48 h after the treatment. Ear swelling was determined by calculating the difference between the untreated ear and the treated ear (three independent experiments with five mice per group each; mean±s.e.m.).
Mrp8 inhibits early differentiation of bone marrow progenitors to mature immunogenic BMDCs
In order to unravel the unexpected outcome of ACD in Mrp14−/− mice, we investigated the influence of the active component Mrp8 on early differentiation of bone marrow (BM) cells to iBMDCs (days 1–6 of GM-CSF and IL-4 treatment of BM cells) in vitro. Subsequently, cells were activated by LPS for another 48 h and the state of maturation was compared between Mrp8-pretreated [Mrp8/LPS] and untreated [-/LPS] BMDCs. In comparison to conventionally LPS-activated DCs [-/LPS], a significant reduction in expression of CD11c, CD86, and MHC-II could be observed in both mouse strains after pretreatment with Mrp8 (Figure 3A). Moreover, inhibition of BMDC differentiation by Mrp8 resulted in a markedly reduced capability of stimulating T cells (Figure 3B). These data indicate that Mrp8 impairs early BMDC differentiation.
Figure 3.
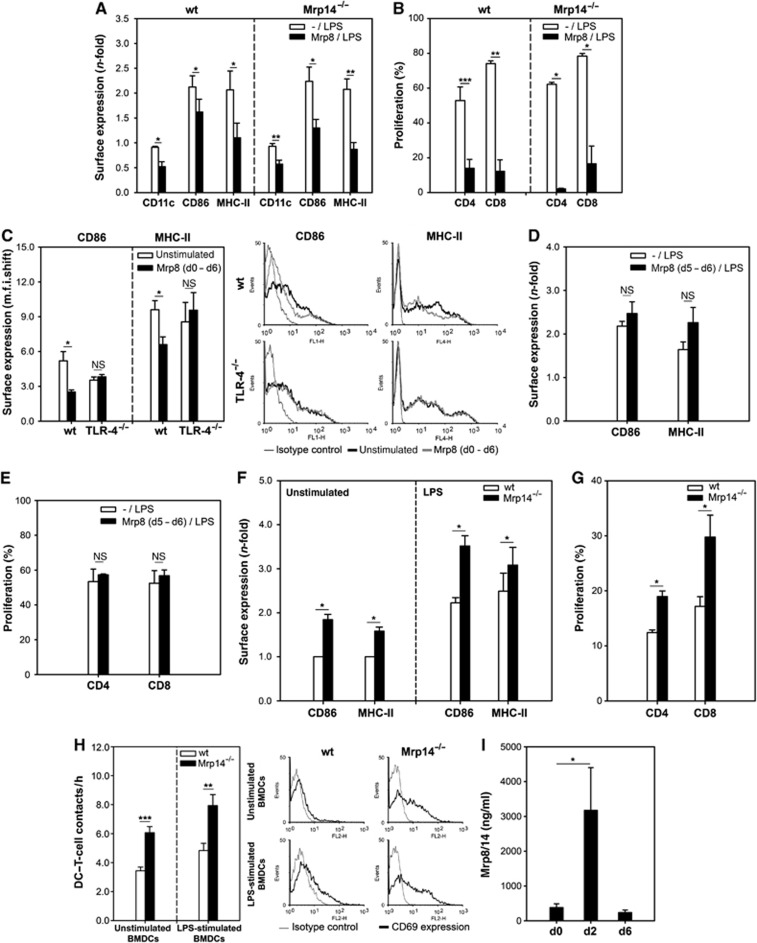
Extracellular Mrp8 impairs DC differentiation. (A) C57BL/6 wt and Mrp14−/−BMDCs generated in the presence of 8 μg/ml Mrp8 [Mrp8/LPS] were stimulated with 300 ng/ml LPS for 48 h on day 6. CD11c, CD86, and MHC-II surface expression was compared to conventionally LPS-stimulated BMDCs [-/LPS] (four independent experiments; mean±s.e.m.). (B) Proliferation of allogenic T cells (BALB/c) induced by C57BL/6 BMDCs generated as described in (A) (DC:T cells, 1:5) (four independent experiments; mean±s.e.m.). (C) CD86 and MHC-II expression on C57BL/6 wt and TLR-4−/− BMDCs generated in the absence or presence of 8 μg/ml Mrp8 [Mrp8 (d0–d6)]. The right part shows one of three independent experiments that are summarized in the left part (mean±s.e.m.). (D) Phenotype of C57BL/6 wt BMDCs stimulated with 8 μg/ml Mrp8 on day 5 for 24 h [Mrp8 (d5–d6/LPS)]. On day 6, 300 ng/ml LPS was added for further 48 h. CD86 and MHC-II expression was compared to conventionally LPS-stimulated BMDCs [-/LPS] (three independent experiments; mean±s.e.m.). (E) Proliferation of allogenic T cells (BALB/c) induced by C57BL/6 wt BMDCs generated as described in (D) (DC:T cells, 1:5) (three independent experiments; mean±s.e.m.). (F) Accelerated BMDC differentiation due to the lack of Mrp8/14. CD86 and MHC-II expression on wt and Mrp14−/− BMDCs stimulated for 48 h with LPS (300 ng/ml) (right) or left unstimulated (left). M.f.i. shifts were normalized to unstimulated wt cells (four independent experiments; mean±s.e.m.). (G) Proliferation of allogenic spleen T cells (BALB/c) induced by C57BL/6 wt or Mrp14−/− BMDCs (DC:T cells, 1:5) (four independent experiments; mean±s.e.m.). (H) Improved ability of Mrp14−/− BMDCs to contact T cells. Allogenic T cells (BALB/c) were embedded with unstimulated or LPS-stimulated wt and Mrp14−/− BMDCs (C57BL/6) in 3-D collagen gels. Interactions were monitored by time-lapse microscopy. Left part: Number of DC–T-cell contacts per hour (two independent experiments; 40 DCs evaluated per condition). Right part: Embedded T cells were analysed for CD69 surface expression. (I) Mrp8/14 serum levels of DNFB-sensitized wt mice. Sera of 10 mice were analysed (two independent experiments, five mice each, mean±s.e.m.) *P<0.05, **P<0.01, ***P<0.001, and NS, not significant.
With regard to our finding that TLR-4 is the relevant receptor for Mrp8-induced effects on activation of differentiated DCs (Figure 1D) we also investigated the influence of Mrp8 on early BMDC differentiation in TLR-4−/− BMDCs. In contrast to wt, no reduction of CD86 and MHC-II expression could be observed on TLR-4−/− BMDCs after differentiation in the presence of Mrp8 [Mrp8 (d0–d6)] (Figure 3C).
To further test whether the dampened immune response in the presence of Mrp8 may be the result of a classical desensitization of TLR-4, we incubated iBMDCs with Mrp8 for 24 h prior to LPS activation [Mrp8 (d5–d6)]. No impaired expression of DC activation markers as well as no impaired stimulation of allogenic T-cell proliferation could be observed (Figure 3D and E). Hence, Mrp8 does not induce a classical desensitization of TLR-4.
Since Mrp8 impairs BMDC differentiation we asked whether this effect contributes to the enhanced ACD in Mrp14−/− mice. Comparing the maturation state of wt and Mrp14−/− iBMDCs after 6 days of differentiation (GM-CSF/IL-4), we found substantially higher expression levels of maturation markers (CD86 and MHC-II) expressed on BMDCs obtained from Mrp14−/− mice compared to controls (Figure 3F, left part). Additionally, LPS-activated Mrp14−/− BMDCs expressed maturation markers to a greater extent and induced a stronger allogenic T-cell proliferation than wt BMDCs (Figure 3F, right part and G) indicating an accelerated BMDC differentiation in Mrp14−/− mice. In addition, we analysed the physical contacts of C57BL/6 wt and Mrp14−/− BMDCs with allogenic BALB/c T cells. Therefore, time-lapse video microscopy of cells embedded in a 3-D collagen matrix was used. We found that the ability to establish contacts with T cells was improved in Mrp14−/− BMDCs compared to wt BMDCs (Figure 3H, left part). The embedded T cells were isolated from the collagen gels after 8 h and further analysed for the expression of the activation marker CD69. We observed a clearly higher CD69 expression on T cells incubated with Mrp14−/− BMDCs than with wt BMDCs (Figure 3H, right part). These data indicate that Mrp8 does not simply inhibit the expression of co-stimulatory molecules but induces a more complex modulation of DC differentiation. Furthermore, by quantifying serum concentrations in wt mice before and after sensitization we observed a strong increase in Mrp8/14 release (about ten-fold) 2 days after 2,4-dinitrofluorobenzene (DNFB) application (Figure 3I). These findings suggest that release of Mrp8/14 during sensitization may result in impaired DC differentiation and restricted antigen-presenting capacity.
Enhanced DC activation leads to an amplified T-cell response in Mrp14−/− mice during ACD
Since DCs of Mrp14−/− mice showed an enhanced maturation phenotype, we examined Mrp-induced effects on DC-mediated T-cell proliferation during ACD in vivo. Forty-eight hours after challenge with DNFB, spleen T cells were isolated and incubated with either BMDCs obtained from naive mice, loaded with 100 μM dinitrobenzene-sulfonate (DNBS), the water soluble analogue of DNFB, or in vivo-primed epithelial cell adhesion molecule negative (EpCAM−) dermal DCs (dDCs) isolated from ear-draining lymph nodes (LNs) 48 h after ear challenge. We found that both BMDCs and dDCs from Mrp14−/− mice induced a significantly higher T-cell proliferation than the corresponding wt cells (Figure 4A). Moreover, we observed an enhanced secretion of interferon-γ (IFN-γ) and IL-17 by Mrp14−/− T cells cultured with Mrp14−/− dDCs compared to their wt counterparts (Figure 4B).
Figure 4.
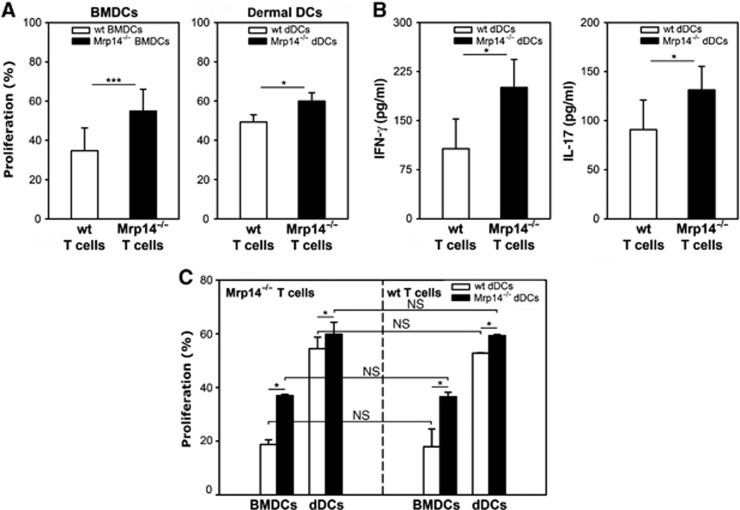
Increased DC-mediated T-cell response in Mrp14−/− mice during ACD. (A) Proliferation of wt and Mrp14−/− T cells during ACD. Wt and Mrp14−/− spleen T cells isolated from DNFB-challenged C57BL/6 mice were cultured with DNBS-loaded wt or Mrp14−/− BMDCs of naive C57BL/6 mice (left) or in vivo-primed EpCAM-negative dDCs from ear-draining LNs of the allergic wt or Mrp14−/− C57BL/6 mice (right) (DC:T-cell ratio was 1:5). Proliferation was measured after 5 days of co-culture (four independent experiments; mean±s.e.m.). (B) Expression of pro-inflammatory cytokines IFN-γ and IL-17 by spleen T cells of DNFB-challenged wt and Mrp14−/− C57BL/6 mice in response to stimulation with wt or Mrp14−/− DNFB-primed EpCAM-negative dDCs from the same ACD mice, respectively (three independent experiments; mean±s.e.m.). (C) Proliferation of Mrp14−/− T cells (left) or wt T cells (right) from spleens of DNFB-challenged C57BL/6 mice co-cultured with either DNBS-charged BMDCs of naive C57BL/6 mice or in vivo-primed EpCAM-negative dDCs from ear-draining LNs of the allergic wt or Mrp14−/− C57BL/6 mice. T-cell proliferation was measured after 5 days of co-culture (three independent experiments; mean±s.e.m.). *P<0.05, ***P<0.001, and NS, not significant.
Next, T cells of DNFB-challenged Mrp14−/− mice were incubated with either wt or Mrp14−/− DCs. We found that DNBS-loaded BMDCs as well as dDCs from DNFB-sensitized Mrp14−/− mice induced a stronger T-cell response compared to wt DCs (Figure 4C, left part). Similar results were observed when using wt T cells in proliferation assays (Figure 4C, right part) indicating that the T-cell source does not affect proliferation rates. Of note, T cells do not express Mrp8/14, therefore T-cell proliferation rates cannot be influenced by missing intracellular Mrp8/14. These findings demonstrate that the more pronounced T-cell response in Mrp14−/− mice is a consequence of higher stimulatory capacities of Mrp14−/− DCs as compared to wt controls.
Reduced numbers of MDSCs partially contribute to the enhanced DC-mediated T-cell response of Mrp14−/− mice during ACD
Blocking the differentiation of mature professional antigen-presenting cells (APCs) may not only result in a decrease of mature DCs but could also induce a shift to more immature myeloid cell populations with known suppressive effects on adaptive immunity, like CD11b+Ly-6C+ so-called monocytic MDSCs which are known to be associated with autoimmunity, inflammation, and cancer (Cheng et al, 2008; Gabrilovich and Nagaraj, 2009). Therefore, we quantified MDSC fractions in ear-draining LNs 48 h after elicitation of ACD. We observed decreased numbers of CD11b+Ly-6C+ ‘double positive’ MDSCs in allergic Mrp14−/− mice compared to wt controls (Figure 5A, P=0.04). We next investigated whether the quantitative difference of MDSCs affects the T-cell response during ACD. We isolated dDCs from ear-draining LNs of DNFB-challenged wt and Mrp14−/− mice and removed MDSCs by positive selection (Ly-6C). The MDSC-depleted dDC fractions were used for co-culture experiments with spleen-derived T cells of the same animals. We found that in the absence of MDSCs the wt T-cell response increases moderately but still significantly (P=0.017), whereas the increase in Mrp14−/− T-cell proliferation was less pronounced, as expected (Figure 5B and C) indicating that the reduced number of MDSCs have some impact on the enhanced T-cell response in Mrp14−/− mice. However, the different proliferation rates of wt and Mrp14−/− T cells in the absence of MDSCs again confirm an enhanced antigen-presenting capacity of Mrp14−/− DCs compared to wt controls. The T-cell proliferation data are in accordance with the increased secretion of the pro-inflammatory cytokine IL-17 in the absence of MDSCs by wt T cells as well as by Mrp14−/− T cells (Figure 5D). Taken together, these data indicate that Mrp8 induces a shift from mature, immunogenic DCs to rather immature myeloid cells, which finally results in a minor but still significant inhibitory effect on adaptive immunity.
Figure 5.
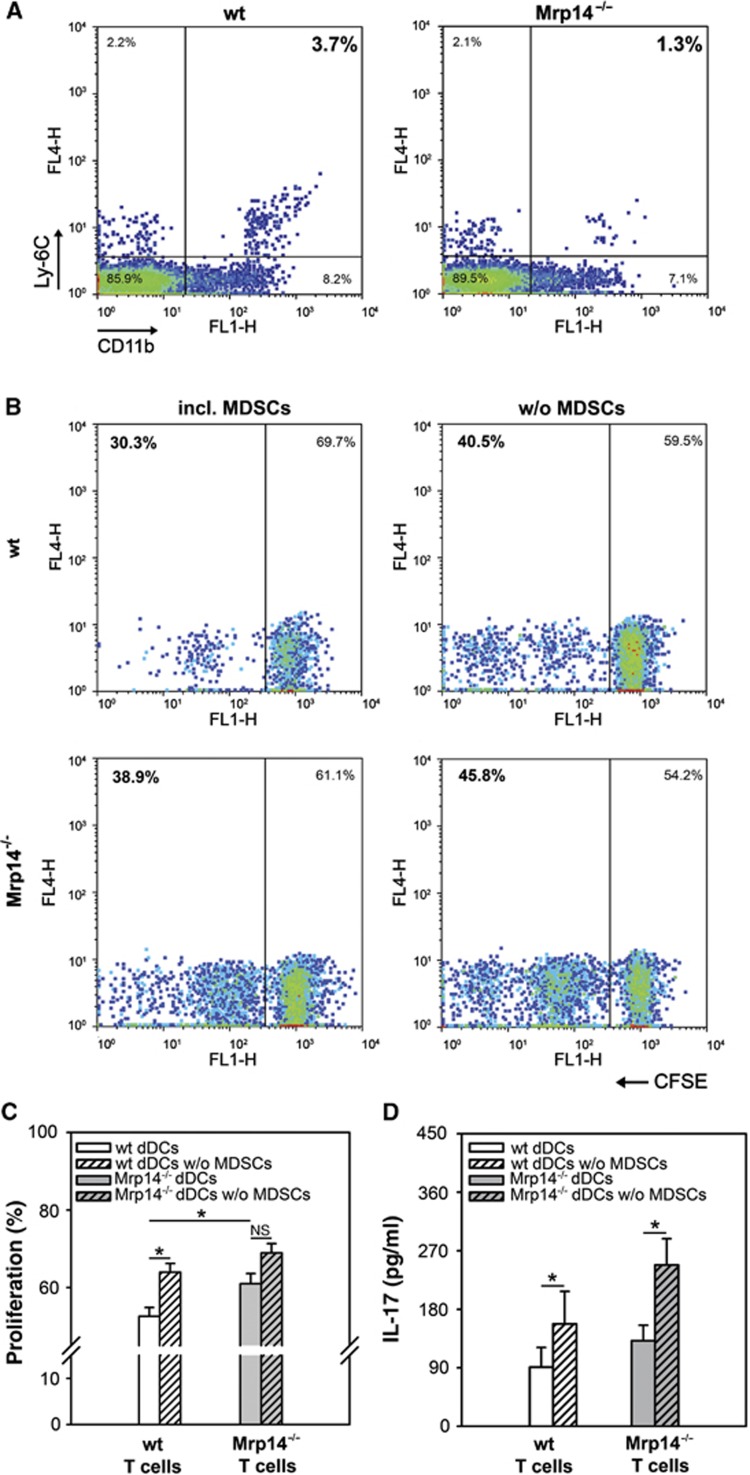
Increased T-cell response during ACD is partly dependent on the number of MDSCs. (A) Quantitative analysis of CD11b+Ly-6C+ MDSCs in LN suspensions from wt and Mrp14−/− mice 48 h after ACD elicitation with DNFB (*P=0.04). Results from one of four independent experiments are shown. (B, C) Proliferation of T cells from allergic wt or Mrp14−/− mice (C57BL/6) induced by MDSC-depleted EpCAM-negative dDC fractions (B, right and C white or grey hatched bars) in comparison to untreated EpCAM-negative dDCs (B, left and C white or grey bars) from the same DNFB-challenged wt and Mrp14−/− mice. Proliferation was determined by CFSE dilution (four independent experiments). *P<0.05 and NS, not significant. (D) Release of the pro-inflammatory cytokine IL-17 by spleen T cells from DNFB-treated wt and Mrp14−/− mice (C57BL/6) in response to MDSC-depleted EpCAM-negative dDCs or untreated EpCAM-negative dDCs of the same mice (three independent experiments; mean±s.e.m.). *P<0.05.
Antigen-presenting capability of DCs is crucial for the enhanced ACD outcome in Mrp14−/− mice in vivo
Finally, we aimed to analyse whether the Mrp14−/− phenotype during ACD can be induced in wt mice by transferring Mrp14−/− DCs. dDCs from ear-draining LNs of DNFB-challenged wt and Mrp14−/− mice 48 h after elicitation of ACD were transferred intravenously into two identical groups of DNFB-sensitized wt mice. Subsequently, ear swelling was measured 24 and 48 h after challenge with DNFB (Figure 6). After transferring Mrp14−/− DCs to wt animals we observed an increase in ear swelling comparable to Mrp14−/− mice in ACD. The ear swelling of wt mice was not affected by transfer of wt dDCs. To make sure that these differences were not due to differences in cellular homing of the injected dDCs from wt and Mrp14−/− mice, isolated DNFB-primed dDCs were stained with cell tracker orange (CTO) and injected into sensitized wt mice prior to challenge. After 48 h, the ear-draining LNs were analysed for the number of immigrated CTO-positive dDCs. Identical numbers of CTO-positive cells were found in LNs of mice injected with wt and Mrp14−/− DCs (Supplementary Figure S2A). In a second independent approach to test whether cellular homing of wt and Mrp14−/− dDCs is identical FITC was applied to the ears of wt and Mrp14−/− mice. After 18 h, the draining LNs were isolated and analysed for FITC-positive DCs migrated from the skin to regional LNs. No differences in the number of FITC-positive immigrated cells could be observed in wt and Mrp14−/− mice (Supplementary Figure S2B). Thus, the observed differences in wt and Mrp14−/− mice are not due to differences in cellular homing of DCs. Taken together, these results clearly illustrate that DCs are mainly responsible for the more pronounced inflammatory response of Mrp14−/− mice during ACD and show that altered DC migration rates could not account for the observed effects.
Figure 6.
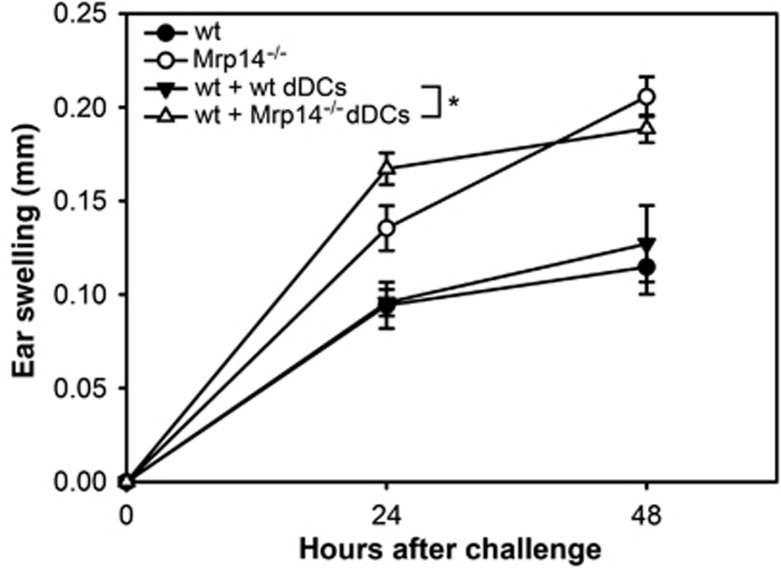
Wt mice develop an increased ear swelling after adoptive transfer of DNFB-primed Mrp14−/− dDCs. EpCAM-negative dDCs were isolated from wt and Mrp14−/− mice 48 h after challenge with DNFB. Subsequently, two identical groups of sensitized wt mice were injected intravenously with these freshly isolated wt or Mrp14−/− dDCs (1.6 × 106 dDCs per mouse) immediately before challenge with 0.4% DNFB. Ear swelling of both groups was evaluated 24 and 48 h after challenge. Data were compared to ear swelling of DNFB-challenged wt and Mrp14−/− mice. Data are from five mice per group (mean±s.e.m.). *P<0.05.
Discussion
In the present study, we demonstrate that loss of Mrp8/14 leads to accelerated DC differentiation. This effect results in increased T-cell activation, a mechanism promoting the initiation and intensity of allergen-specific immune responses. Previously, we have shown that Mrp8/14 plays an important activating role in innate immunity. Extracellular Mrp8/14 amplifies phagocyte activation via binding to TLR-4 (Rammes et al, 1997; Vogl et al, 2007; Frosch et al, 2009). Stimulation of this receptor is known to induce maturation of iBMDCs, which is prerequisite for effective antigen presentation to T lymphocytes (Rescigno et al, 1999). Accordingly, we found that Mrp8/14 promotes the activation of iBMDCs in a TLR-4-dependent manner inducing the expression of MHC and co-stimulatory molecules. Mrp8 is the active component of the Mrp8/14 complex inducing iBMDC activation. The role of TLR-4 in the pathogenesis of ACD is complex. The most common contact allergen Ni2+ has been shown to trigger pro-inflammatory signalling via direct interaction with TLR-4 (Schmidt et al, 2010). However, mice deficient in TLR-4 develop normal responses in contact hypersensitivity. On the other hand, several organic contact allergens indirectly activate TLR-4 via release of endogenous ligands like breakdown products of hyaluronic acid and simultaneous absence of IL-12 and TLR-4. This prevents development of ACD indicating that beside TLR activation additional mechanisms are necessary for effective sensitization (Martin et al, 2008; Kaplan et al, 2012). These results are in line with earlier reports demonstrating stimulatory effects of other DAMPs, for example, HMGB1 (Rovere-Querini et al, 2004; Yang et al, 2006, 2010), HMGN1 (Yang et al, 2011), or uric acid (Shi et al, 2003; Martinon et al, 2006) on DCs leading to the general conclusion that alarmins are capable to activate APCs (Yang et al, 2009; Nace et al, 2012). However, we obtained the unexpected result that Mrp14−/− mice, that represent functional ‘double knockout’ mice for Mrp8 and Mrp14 (Hobbs et al, 2003; Manitz et al, 2003; Vogl et al, 2007), show a more severe inflammatory response in ACD compared to wt mice, which is not due to a general anti-inflammatory function but rather specific for antigen-driven ACD. T cell-independent inflammation in ICD is not amplified in the absence of these proteins. The Mrp14−/− phenotype during ACD relies on the suppression of early differentiation of myeloid progenitors to (BM)DCs resulting in a diminished capacity of T-cell stimulation. Our data clearly show that the maturation state of DCs is the critical factor for the more severe course of ACD, rather than an intrinsic abnormality of T-cell function in Mrp14−/− mice. Thus, the alarmin Mrp8 has ambivalent effects on DC functions depending on the time and the duration of stimulation in vivo.
We show for the first time for members of the alarmin family that Mrp8 and Mrp14 are released during the sensitization phase of ACD. Since the functional state of DCs may depend on the local microenvironment, we analysed whether lack of Mrp8 and Mrp14 influences the antigen-presenting capacity of phagocytes at the site of inflammation in contrast to earlier reports focusing on spleen-derived DCs (Cheng et al, 2008). In accordance to our in vitro studies, we found a higher ratio of mature DCs to immature MDSCs in local ear-draining LNs of Mrp14−/− mice compared to wt mice during ACD in vivo.
We could confirm the biological relevance of changes in this local cell composition since transfer of DNFB-primed myeloid cells obtained from local ear-draining LNs of Mrp14−/− mice to wt mice increased the immune response to DNFB comparable to Mrp14−/− mice without cell transfer. In addition, the effect of these transfer experiments confirms the more potent antigen-presenting capacity of Mrp14−/− DCs compared to wt controls and cannot be explained by the lower number of MDSCs in Mrp14−/− mice as described earlier in a model of tumour immunology (Cheng et al, 2008).
The regulatory function of DCs in inflammatory processes is dichotomous, including induction of specific immune responses as well as maintenance of self-tolerance depending on the expression of stimulatory or regulatory co-factors (Hume, 2008; Geissmann et al, 2010; Nace et al, 2012). Mononuclear phagocytes represent a continuum of mature APCs to immature suppressors of T-cell activation (Geissmann et al, 2010). Furthermore, resolution of inflammation is a crucial step in maintaining tissue homeostasis. Particularly, permanently occurring micro-traumatization and subclinical infections lead to the release of autoantigens, which are then presented by APCs. Therefore, elicitation of an adaptive immune response needs to be strictly controlled (Sallusto et al, 1999) to avoid allergic or autoimmune reactions and subsequent tissue damage. During development of an allergic reaction in otherwise healthy mice blocking of early differentiation of myeloid progenitors to i(BM)DCs by Mrp8 obviously hampers induction of an antigen-specific immune response.
Despite the phenotype presented here for ACD, our data may also have impact for a wider field of DC biology. Intestinal mononuclear phagocytes, including DCs, are crucial for maintaining intestinal homeostasis allowing tolerance against the high number of commensal microorganisms, which is generally of mutual benefit (Sansonetti and Medzhitov, 2009; Varol et al, 2010). Maintenance of this homeostatic immune tolerance may involve active downregulation of immune responses by the host during contact with commensal bacteria to a state of ‘physiological inflammation’ (Sansonetti and Medzhitov, 2009). This is assured via readily killing of invading bacteria by macrophages, which do not elicit an immunological response, ensuring that the systemic immune system is essentially ignorant of these microorganisms (Macpherson and Harris, 2004). In this context, Mrp8 and Mrp14 may be reasonable candidates for maintaining ‘physiological inflammation’ since both proteins are highly upregulated in the intestine during infection or tissue damage (Foell et al, 2009), promote effector mechanisms of innate immunity, and show a moderate antimicrobial activity (Vogl et al, 2007; Ehrchen et al, 2009). On the other hand, Mrp8 and Mrp14 may limit induction of adaptive immune responses against harmless commensal microorganisms by blocking the development of pro-inflammatory DCs. Moreover, our finding of an endogenous TLR-4 activator suppressing ACD shows an interesting parallel to the so-called ‘hygiene hypothesis’. Albeit short-term activation of TLR-4 by microbial products triggers innate immune mechanisms, this theory claims that continuous stimulation of TLRs by microbial products, especially LPS-mediated stimulation of TLR-4, is one major molecular mechanism preventing manifestation of allergic diseases (Macpherson and Harris, 2004; Okada et al, 2010; Ege et al, 2011). Our data point to the possibility that continuous exposure of APC progenitors to endogenous TLR-4 ligands may be involved in this process as well.
Breaking these control mechanisms on the other hand may be a possible cause promoting autoimmune disease. In an experimental model of autoimmunity driven by epidermal expression of CD40 ligand, we have previously shown that continuous pathologic co-stimulation of DCs obviously overruns inhibitory effects of Mrp8 on early DC differentiation (Loser et al, 2010). In this model, TLR-4-dependent inflammatory effects of Mrp8 on mature DCs dominate and promote the inflammatory process. Thus, the function of Mrp8, or potentially also of other alarmins, is strictly dependent on the time and duration of expression. Identification of triggers breaking those innate mechanisms of immunological self-control may be of relevance for future therapeutic strategies, since expression of Mrp8 and Mrp14 is prominently upregulated in a couple of clinically relevant autoimmune diseases like rheumatoid arthritis, vasculitis, systemic lupus erythematosus, or Crohn’s disease (Foell and Roth, 2004; Ehrchen et al, 2009).
Taken together, we describe a new mechanism of immune regulation mediated by Mrp8, which has not been shown for another alarmin so far. Our data show for the first time that a member of this protein family is an important factor in orchestrating coordinated immune reactions. On the one hand, it allows elicitation of an appropriate innate inflammatory response by acting as a classical DAMP protein, but on the other hand it prevents hyper-activation of the adaptive immune system most likely in order to avoid tissue damage due to overwhelming and inadequate immune responses.
Materials and methods
Mice
Adult C57BL/6 and BALB/c mice were obtained from Janvier (France) and Harlan (Germany) laboratories. Mrp14−/− (Manitz et al, 2003) and RAGE−/− mice were generated by targeted gene disruption in our laboratory and backcrossed to C57BL/6 for at least 10 generations. TLR-4−/− mice were provided by P van Lent (Department of Rheumatology, Radboud University Medical Centre, Nijmegen, The Netherlands) or CJ Kirschning (Institute of Medical Microbiology, University of Duisburg-Essen, Essen, Germany). We performed all experiments according to institutional regulations (licence 8.87-50.10.37.09.244, District Government and District Veterinary Office, Münster, Germany).
Purification of Mrp8, Mrp14, and Mrp8/14
Mrp8, Mrp14, and Mrp8/14 were purified as described earlier (Vogl et al, 2007). All preparations revealed a purity of >98%. Endotoxin contaminations were excluded by limulus amebocyte lysate (LAL) assay or were below 2 pg LPS/μg protein and addition of polymyxin B did not affect the activities of the Mrp proteins.
Reagents
DNFB, DNBS, 3-amino-9-ethylcarbazol (AEC), LPS from E. coli 055:B5, periodic acid, albumin from bovine serum (BSA), FITC, polymyxin B, and croton oil were obtained from Sigma-Aldrich (Munich, Germany). LAL assay was from BioWhittaker (Walkersville, USA). RPMI-1640, L-Glutamine, Gentamycin, non-essential amino acids (NEAA), and PBS Dulbecco were from Biochrom (Berlin, Germany). Sodium azide (NaN3), hydrogen peroxide (H2O2), and Mayer’s hemalum solution were from Merck (Darmstadt, Germany). Normal goat serum (NGS) and 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFDA, SE) were obtained from Invitrogen (Darmstadt, Germany). FlowCytomix Multiple Analyte Detection System was purchased from eBioscience (Frankfurt, Germany) and fetal bovine serum (FBS) from Biowest (Renningen, Germany). CTO was obtained from Molecular Probes (Darmstadt, Germany).
Monoclonal anti-mouse antibodies
Purified CD4 (H129.19), CD8a (53-6.7), CD19 (ID3), CD11b (M1/70), CD16/CD32 (2.4G2), CD45R/B220 (RA3-6B2), Ly-6G/Ly-6C (RB6-8C5), TER-119 (TER-119), CD49b (DX5), as well as biotin-conjugated CD24 (M1/69) and CD11c (HL3) Abs were purchased from Becton-Dickson (Heidelberg, Germany). Purified F4/80 (CI:A3-1) Ab was obtained from AbD Serotec (Düsseldorf, Germany). Purified EpCAM (G8.8) and biotin-conjugated-γδ TCR (GL-3), as well as CD86 FITC (GL1), MHC class II FITC (M5/114), CD11c APC (N418), F4/80 FITC (BM8), CD4 APC (RM4-5), and CD8a APC (53-6.7) Abs were from eBioscience. CD69 PE (310106) Ab was from R&D Systems (Wiesbaden, Germany). Purified Ly-6C (HK1.4) and CD11b FITC (M1/70) Abs were obtained from BioLegend (Fell, Germany) and Ly-6C APC (1G7.G10) Ab from Miltenyi Biotech (Bergisch Gladbach, Germany). Peroxidase-conjugated affin pure F’(ab)2 fragment goat anti rabbit IgG (H+L) was purchased from Dianova (Hamburg, Germany). Rabbit antisera against recombinant murine Mrp8 and Mrp14 were produced in our laboratory as described previously (van Lent et al, 2008a).
Allergic contact dermatitis
At day zero, C57BL/6 wt and Mrp14−/− mice were sensitized by painting 100 μl of 0.4% DNFB in acetone/olive oil (4:1) onto the shaved abdominal skin. Six days later, mice were challenged by applying 10 μl 0.4% DNFB in acetone/olive oil on the dorsal and ventral surface of the right ears, respectively. The left ears served as controls and were treated with acetone/olive oil only. Ear thickness was measured 24 and 48 h after challenge using a digital calliper (Roth, Karlsruhe, Germany). Ear swelling was determined by calculating the difference between the unchallenged ear (left) and the challenged ear (right).
Irritant contact dermatitis
C57BL/6 wt and Mrp14−/− mice were painted with 10 μl 3% croton oil in acetone/olive oil (4:1) onto the right ears. The left ears served as controls and were treated with acetone/olive oil only. Ear thickness was measured 24 and 48 h after the treatment using a digital calliper. Ear swelling was determined by calculating the difference between the unchallenged ear and the challenged ear.
DC isolation from ear-draining LNs
For isolation of immigrated skin-derived DCs from ear-draining LNs, mice were sacrificed 48 h after challenge. Ear-draining LNs were extracted and single cell suspensions were produced. Cells were washed in cold PBS containing 1% FBS. For depletion of lymphocytes and erythrocytes, cells were incubated with rat anti-mouse CD4, CD8a, CD19, and TER-119 mAbs (0.5 μg/1 × 106 cells, respectively) and magnetic labelled goat anti-rat IgG microbeads (Miltenyi Biotech). After washing with cold PBS/FBS, antibody-coated cells were removed on MACS columns (Miltenyi Biotech). To further enrich dDCs, the cells were depleted of EpCAM-positive epidermal Langerhans cells as described above, using a rat anti-mouse EpCAM mAb. In some experiments, EpCAM-negative dDC fractions were additionally depleted of MDSCs by positive selection using an anti-mouse Ly-6C mAb. In all experiments, enriched DC fractions were checked for macrophage contamination by CD11c-F4/80 double staining using flow cytometry. The amount of F4/80 single positive macrophages was always <7%.
Measurement of Mrp8/14 concentrations
Mrp8/14 concentrations were determined in sera of sensitized wt mice by ELISA as described previously (Vogl et al, 2007).
Flow cytometry
Immunofluorescence staining was performed after washing the cells with PBS/1% FBS. In all experiments, 1–2 × 105 cells were incubated for 20 min at 4°C with optimal dilution of fluorescently labelled mAbs in PBS/FBS. After washing, the cells were analysed by flow cytometry (FACS Calibur and CellQuestPro software; Becton-Dickson, Heidelberg, Germany). Mean fluorescence intensity (m.f.i.) shifts were calculated by dividing the geometric mean fluorescence of cells stained with antigen-specific mAb by the geometric mean fluorescence for the same cells stained with isotype control Ab.
Analysis of cell–cell interactions by time-lapse microscopy
Interactions of C57BL/6 wt and Mrp14−/− BMDCs with allogenic BALB/c T cells were analysed using 3-D collagen gels. DCs and T cells (ratio 1:10) were embedded in bovine collagen type I at a total cell number of 11 × 106 cells/ml. Interactions were monitored for 8 h by time-lapse microscopy using an Olympus BX61 microscope (Olympus, Hamburg, Germany) with an UAPO lens (20 × ) and a FView camera with CellP software (Olympus, Münster, Germany).
Spleen T-cell purification and CFSE labelling
For purification of T cells, spleen cell suspensions were passed through a nylon wool matrix column. Further enrichment of T cells was achieved by depletion of monocytes, macrophages, granulocytes, B cells, erythrocytes, γδT cells, platelets, and DCs using a cocktail of rat-CD11b, rat-CD19, rat-CD16/CD32, rat-CD45R/B220, rat-Ly-6G/Ly-6C, rat-CD49b, rat-TER-119, rat-biotin-CD24, hamster-biotin-γδ TCR, and hamster-biotin-CD11c mAbs. Antibody-coated cells were removed using anti-rat IgG- and anti-biotin coated magnetic beads according to manufacturer’s instructions (Miltenyi Biotech). For T-cell proliferation experiments, T cells were stained with CFSE (Invitrogen) for 4 min at 37°C. Following washing in PBS/1% FBS, cells were resuspended in complete medium (RPMI 1640 containing 1% NEAA, 1% L-Glutamine, 10% FBS, and 10 μg/ml Gentamycin) at 1 × 106/ml.
T-cell proliferation assay
CFSE-labelled T cells (1 × 105) were co-cultured with DCs (2 × 104) in 200 μl complete medium in 96-well round-bottomed plates (Greiner bio-one, Frickenhausen, Germany) for 5 days (DC:T-cell ratio was 1:5). T-cell proliferation was assessed by CFSE dilution on a FACS Calibur. Supernatants were collected for detection of cytokines.
Measurement of cytokine production
Cell culture supernatants were subjected to cytokine analysis (IFN-γ, IL-17) by FlowCytomix Multiple Analyte Detection System (eBioscience) according to manufacturer’s protocol.
In vitro generation of BMDCs
BM cells were isolated from murine femur and tibia. Cells were cultured in complete medium supplemented with 100 U/ml rmIL-4 and 100 U/ml rmGM-CSF (Immunotools, Friesoythe, Germany) for 6 days at 37°C and 5% CO2. On days 3 and 6, half of the supernatant was replaced with fresh cytokine-containing medium. On day 6, immature DCs were cultured for further 48 h in the presence of different stimulants as indicated in the figures. On day 8, mature DCs were harvested. In some experiments, BM cells were cultured in the presence of 8 μg/ml Mrp8 throughout the course of the complete experiment.
Immunohistochemistry of ear sections
Tissue sections from DNFB-treated and untreated ears were fixed in acetone. For blockage of endogenous peroxidase, slides were incubated in PBS/NaN3/H2O2. To avoid unspecific binding, Fc receptors were blocked by incubating sections in PBS/1% BSA including 50% NGS. Afterwards, slides were treated with appropriate dilution of primary antibody or isotype control. After washing, the samples were incubated with a horseradish peroxidase-conjugated secondary antibody for 45 min. To visualize peroxidase activity, AEC was used as chromogen. To completely destroy endogenous peroxidase, slides were incubated in 14.5 mM periodic acid. Counterstaining of tissue sections was performed with Mayer’s hemalum solution. Images were acquired by using an upright microscope (Axioskop, Zeiss, Jena, Germany) and imported into Photoshop version 9.0 (Adobe Systems) for production of the final figures.
Statistical analysis
Statistical significance of the data was calculated using Student’s t-test for paired samples. Probability (P-value) of <0.05 was considered to be significant.
Supplementary Material
Acknowledgments
We thank C Solé-Amell, E Nattkemper, and H Hater for excellent technical support; M Goebeler as well as C Sunderkötter for critical reading of the manuscript; and CJ Kirschning for providing TLR-4−/− mice. This work was supported by the Interdisciplinary Center of Clinical Research grants Vo2/014/09 and Ro2/004/10, by the Federal Ministry of Education and Research, Germany, project AID-Net, and the German Research Foundation (DFG) CRC Transregio 128-A2, CRC 1009 B8 and B9 to JR and TV.
Author contributions: TV, JR, and CS supervised the project. TV, JR, and BP designed experiments and wrote the paper. BP, JA, and MA performed experiments. BP and TV analysed data. MW, BP, KL, and VK performed microscopy experiments. PvL and DF performed experiments with TLR-4−/− and RAGE−/− mice, respectively.
Footnotes
The authors declare that they have no conflict of interest.
References
- Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392: 245–252 [DOI] [PubMed] [Google Scholar]
- Björk P, Björk A, Vogl T, Stenström M, Liberg D, Olsson A, Roth J, Ivars F, Leanderson T (2009) Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol 7: e97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour H, Peyron E, Gaucherand M, Garrigue J, Desvignes C, Kaiserlian D, Revillard J, Nicolas J (2005) Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol 25: 3006–3010 [DOI] [PubMed] [Google Scholar]
- Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 205: 2235–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ege MJ, Mayer M, Normand A, Genuneit J, Cookson W, Braun-Fahrländer C, Heederik D, Piarroux R, von Mutius E (2011) Exposure to environmental microorganisms and childhood asthma. N Engl J Med 364: 701–709 [DOI] [PubMed] [Google Scholar]
- Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J (2009) The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol 86: 557–566 [DOI] [PubMed] [Google Scholar]
- Foell D, Roth J (2004) Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum 50: 3762–3771 [DOI] [PubMed] [Google Scholar]
- Foell D, Wittkowski H, Vogl T, Roth J (2007) S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 81: 28–37 [DOI] [PubMed] [Google Scholar]
- Foell D, Wittkowski H, Roth J (2009) Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut 58: 859–868 [DOI] [PubMed] [Google Scholar]
- Frosch M, Ahlmann M, Vogl T, Wittkowski H, Wulffraat N, Foell D, Roth J (2009) The myeloid-related proteins 8 and 14 complex, a novel ligand of Toll-like receptor 4, and interleukin-1beta form a positive feedback mechanism in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 60: 883–891 [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9: 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K (2010) Development of monocytes, macrophages and dendritic cells. Science 327: 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevers LC, de Vries TJ, Vogl T, Abdollahi-Roodsaz S, Sloetjes AW, Leenen PJ, Roth J, Everts V, van den Berg WB, van Lent PL (2011) S100A8 enhances osteoclastic bone resorption in vitro through activation of Toll-like receptor 4: implications for bone destruction in murine antigen-induced arthritis. Arthritis Rheum 63: 1365–1375 [DOI] [PubMed] [Google Scholar]
- Gutcher I, Becher B (2007) APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest 117: 1119–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume DA (2008) Macrophages as APC and the dendritic cell myth. J Immunol 181: 5829–5835 [DOI] [PubMed] [Google Scholar]
- Hobbs JA, May R, Tanousis K, McNeill E, Mathies M, Gebhardt C, Henderson R, Robinson MJ, Hogg N (2003) Myeloid cell function in MRP-14 (S100A9) null mice. Mol Cell Biol 23: 2564–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G (2011) S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res 9: 133–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Igyártó BZ, Gaspari AA (2012) Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol 12: 114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korndörfer IP, Brueckner F, Skerra A (2007) The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J Mol Biol 370: 887–898 [DOI] [PubMed] [Google Scholar]
- Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, Klenner L, Kuhn A, Foell D, Sorokin L, Luger TA, Roth J, Beissert S (2010) The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med 16: 713–717 [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL (2004) Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol 4: 478–485 [DOI] [PubMed] [Google Scholar]
- Manitz MP, Horst B, Seeliger S, Strey A, Skryabin BV, Gunzer M, Frings W, Schönlau F, Roth J, Sorg C, Nacken W (2003) Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol 23: 1034–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Lappin MB, Kohler J, Delattre V, Leicht C, Preckel T, Simon JC, Weltzien HU (2000) Peptide immunization indicates that CD8+ T cells are the dominant effector cells in trinitrophenyl-specific contact hypersensitivity. J Invest Dermatol 115: 260–266 [DOI] [PubMed] [Google Scholar]
- Martin SF, Dudda JC, Bachtanian E, Lembo A, Liller S, Dürr C, Heimesaat MM, Bereswill S, Fejer G, Vassileva R, Jakob T, Freudenberg N, Termeer CC, Johner C, Galanos C, Freudenberg MA (2008) Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J Exp Med 205: 2151–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241 [DOI] [PubMed] [Google Scholar]
- Nace G, Evankovich J, Eid R, Tsung A (2012) Dendritic cells and damage-associated molecular patterns: endogenous danger signals linking innate and adaptive immunity. J Innate Immun 4: 6–15 [DOI] [PubMed] [Google Scholar]
- Okada H, Kuhn C, Feillet H, Bach JF (2010) The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin Exp Immunol 160: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C (1997) Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem 272: 9496–9502 [DOI] [PubMed] [Google Scholar]
- Rescigno M, Granucci F, Citterio S, Foti M, Ricciardi-Castagnoli P (1999) Coordinated events during bacteria-induced DC maturation. Immunol Today 20: 200–203 [DOI] [PubMed] [Google Scholar]
- Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Müller S, Iannacone M, Traversari C, Bianchi ME, Manfredi AA (2004) HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 5: 825–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Palermo B, Lenig D, Miettinen M, Matikainen S, Julkunen I, Forster R, Burgstahler R, Lipp M, Lanzavecchia A (1999) Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol 29: 1617–1625 [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ, Medzhitov R (2009) Learning tolerance while fighting ignorance. Cell 7: 416–420 [DOI] [PubMed] [Google Scholar]
- Schmidt M, Raghavan B, Müller V, Vogl T, Fejer G, Tchaptchet S, Keck S, Kalis C, Nielsen PJ, Galanos C, Roth J, Skerra A, Martin SF, Freudenberg MA, Goebeler M (2010) Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol 11: 814–819 [DOI] [PubMed] [Google Scholar]
- Shi Y, Evans JE, Rock KL (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425: 516–521 [DOI] [PubMed] [Google Scholar]
- Strupat K, Rogniaux H, Van Dorsselaer A, Roth J, Vogl T (2000) Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 are confirmed by electrospray ionization-mass analysis. J Am Soc Mass Spectrom 11: 780–788 [DOI] [PubMed] [Google Scholar]
- Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E et al. (2007) Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8: 487–496 [DOI] [PubMed] [Google Scholar]
- Uter W, Schnuch A, Geier J, Frosch PJ (1998) Epidemiology of contact dermatitis. The information network of departments of dermatology (IVDK) in Germany. Eur J Dermatol 8: 36–40 [PubMed] [Google Scholar]
- van Lent PL, Grevers L, Blom AB, Sloetjes A, Mort JS, Vogl T, Nacken W, van den Berg WB, Roth J (2008a) Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann Rheum Dis 67: 1750–1758 [DOI] [PubMed] [Google Scholar]
- van Lent PL, Grevers LC, Blom AB, Arntz OJ, van de Loo FA, van der Kraan P, Abdollahi-Roodsaz S, Srikrishna G, Freeze H, Sloetjes A, Nacken W, Vogl T, Roth J, van den Berg WB (2008b) Stimulation of chondrocyte-mediated cartilage destruction by S100A8 in experimental murine arthritis. Arthritis Rheum 58: 3776–3787 [DOI] [PubMed] [Google Scholar]
- Varol C, Zigmond E, Jung S (2010) Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat Rev Immunol 10: 415–426 [DOI] [PubMed] [Google Scholar]
- Viemann D, Strey A, Janning A, Jurk K, Klimmek K, Vogl T, Hirono K, Ichida F, Foell D, Kehrel B, Gerke V, Sorg C, Roth J (2005) Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 105: 2955–2962 [DOI] [PubMed] [Google Scholar]
- Vocanson M, Hennino A, Cluzel-Tailhardat M, Saint-Mezard P, Benetiere J, Chavagnac C, Berard F, Kaiserlian D, Nicolas JF (2006) CD8+ T cells are effector cells of contact dermatitis to common skin allergens in mice. J Invest Dermatol 126: 815–820 [DOI] [PubMed] [Google Scholar]
- Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J (2007) Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 13: 1042–1049 [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ (2006) High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol 81: 59–66 [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ (2008) Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med 205: 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, de la Rosa G, Tewary P, Oppenheim JJ (2009) Alarmins link neutrophils and dendritic cells. Trends Immunol 30: 531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Tewary P, de la Rosa G, Wei F, Oppenheim JJ (2010) The alarmin functions of high-mobility group proteins. Biochim Biophys Acta 1799: 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Postnikov YV, Li Y, Tewary P, de la Rosa G, Wei F, Klinman D, Gioannini T, Weiss JP, Furusawa T, Bustin M, Oppenheim JJ (2011) High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses. J Exp Med 209: 157–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mosser DM (2008) Macrophage activation by endogenous danger signals. J Pathol 214: 161–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


