Abstract
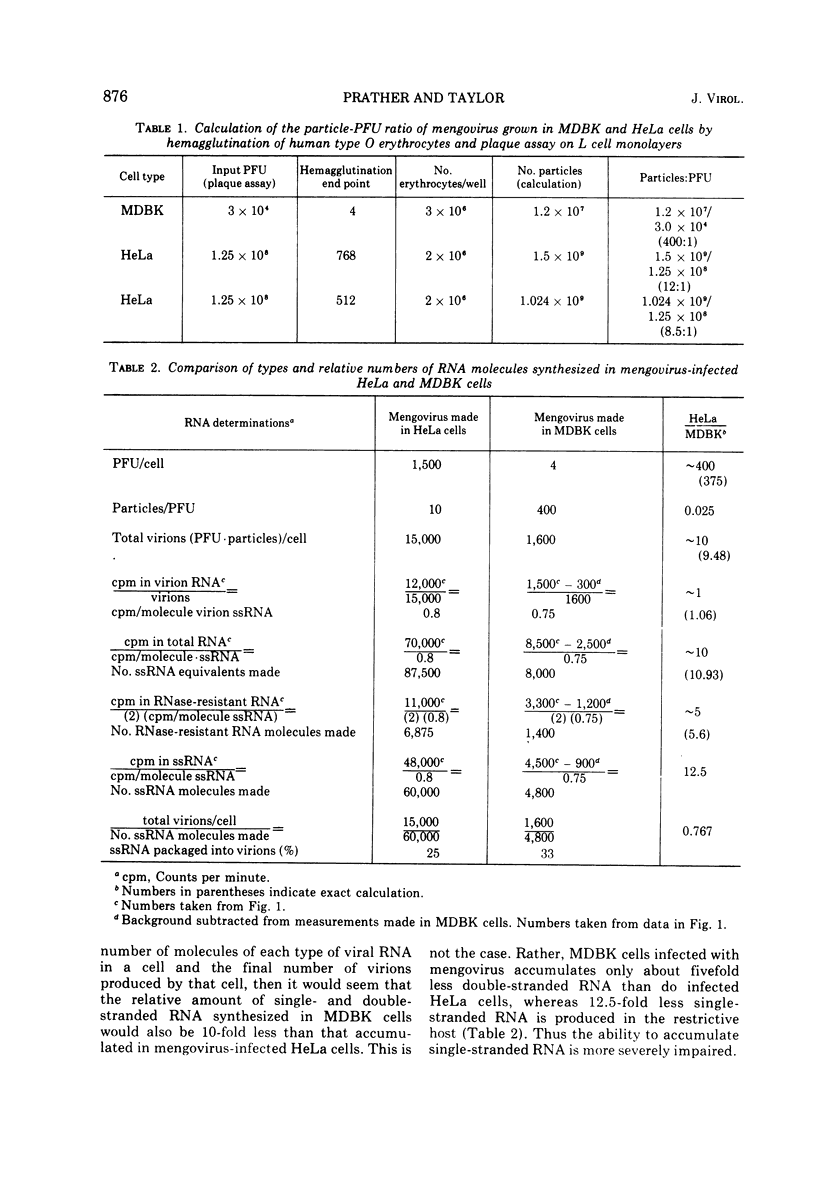
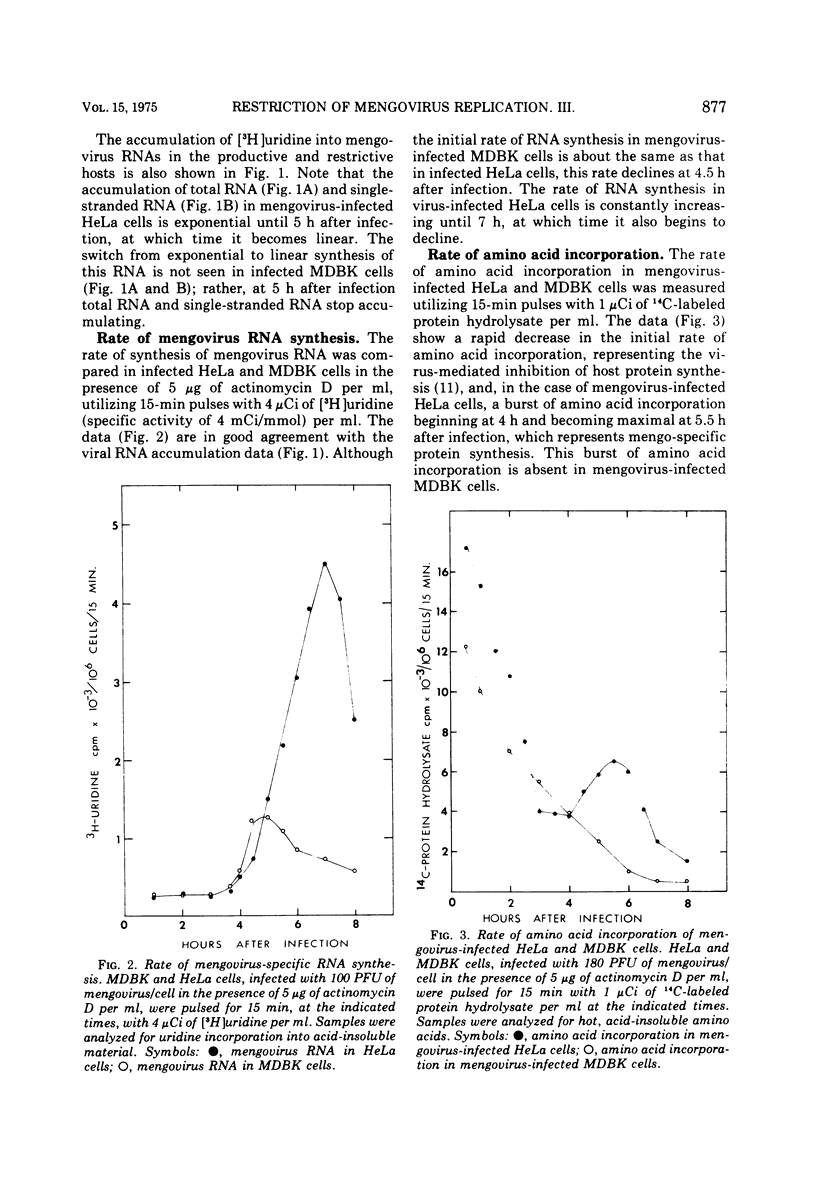
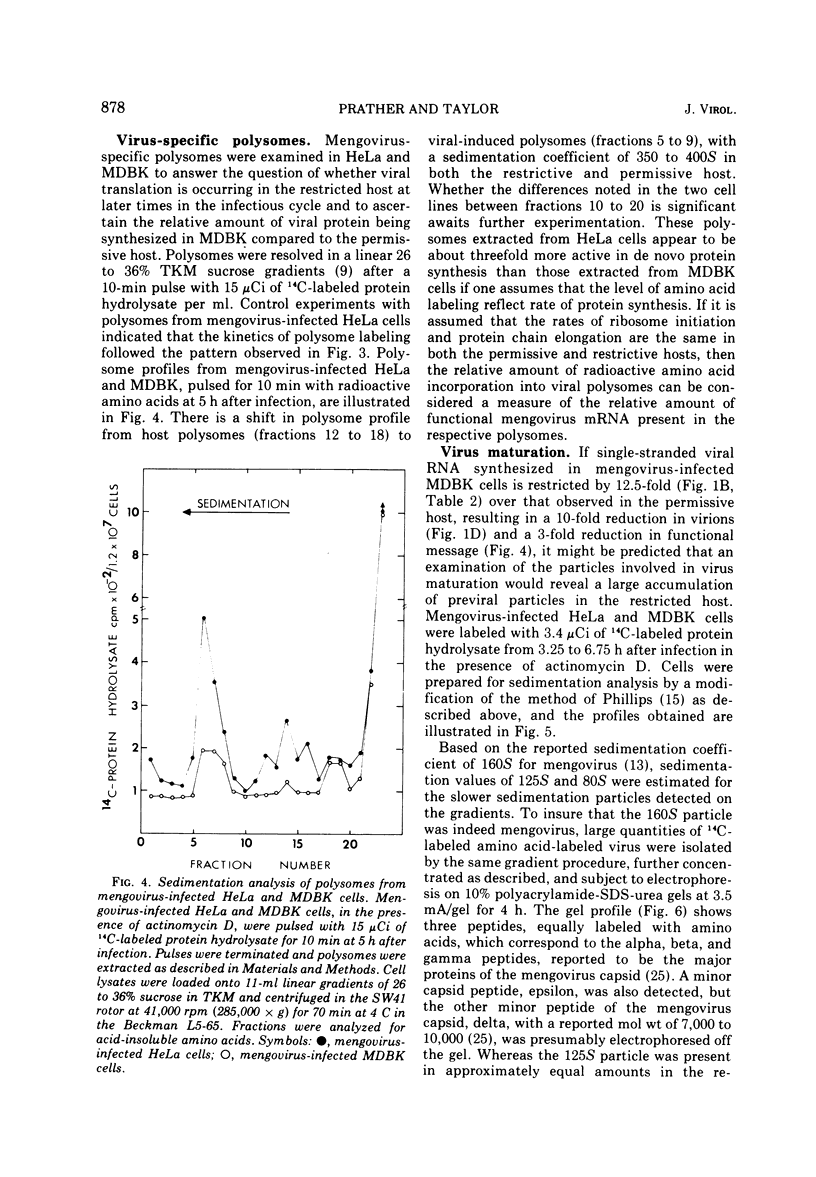
Restricted mengovirus replication in Mandin-Darby bovine kidney (MDBK) cells is characterized by a 400-fold reduction in infectious virus yield and a 40-fold increase in the production of noninfectious virus. Using conditions which insure that all MDBK cells are infected, virus-specific RNA and protein synthesis were measured in the restrictive host and in a permissive host for mengovirus, HeLa cells. Labeling kinetics and sucrose gradient analysis of mengovirus-specific RNA from MDBK cells show a reduction of 10-fold in virion RNA, 5-fold in double-stranded RNA, and 12.5-fold in single-stranded RNA. The viral RNA biosynthetic processes which occur late in the replicative cycle and result in the production of 90% of the single-stranded viral RNA that is packaged into capsid proteins in the permissive host are absent in restrictive MDBK cells. Viral protein synthesis as measured by labeled viral-specific polysome is decreased, and there is an accumulation of 80S subviral particles in the restricted host. It is suggested that restriction may act at a number of stages of viral replication and maturation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlinghaus R. B., Syrewicz J. J., Loesch W. T., Jr RNA polymerase complexes from mengovirus infected cells. Arch Gesamte Virusforsch. 1972;38(1):17–28. doi: 10.1007/BF01241352. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Girard M., Darnell J. E. Aspects of the synthesis of poliovirus RNA and the formation of virus particles. Virology. 1966 Jun;29(2):179–189. doi: 10.1016/0042-6822(66)90024-9. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Granger G. A., Taylor M. W., Holland J. J. Efficient, inefficient, and abortive infection of different mammalian cells by small RNA viruses. Virology. 1967 Sep;33(1):36–46. doi: 10.1016/0042-6822(67)90091-8. [DOI] [PubMed] [Google Scholar]
- CRAIGHEAD J. E., SHELOKOV A. Encephalomyocarditis virus hemagglutination-inhibition test using antigens prepared in HeLa cell cultures. Proc Soc Exp Biol Med. 1961 Dec;108:823–826. doi: 10.3181/00379727-108-27080. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A. Analysis of RNA associated with the poliovirus RNA replication complexes. Virology. 1974 Apr;58(2):526–535. doi: 10.1016/0042-6822(74)90086-5. [DOI] [PubMed] [Google Scholar]
- DARNELL J. E., Jr, SAWYER T. K. The basis for variation in susceptibility to poliovirus in HeLa cells. Virology. 1960 Aug;11:665–675. doi: 10.1016/0042-6822(60)90113-6. [DOI] [PubMed] [Google Scholar]
- Fernandez-Tomas C. B., Baltimore D. Morphogenesis of poliovirus. II. Demonstration of a new intermediate, the proviron. J Virol. 1973 Nov;12(5):1122–1130. doi: 10.1128/jvi.12.5.1122-1130.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. Irreversible eclipse of poliovirus by HeLa cells. Virology. 1962 Feb;16:163–176. doi: 10.1016/0042-6822(62)90292-1. [DOI] [PubMed] [Google Scholar]
- McCormick W., Penman S. Inhibition of RNA synthesis in HeLa and L cells by Mengovirus. Virology. 1967 Jan;31(1):135–141. doi: 10.1016/0042-6822(67)90017-7. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Plagemann P. G. Effect of ultraviolet light on mengovirus: formation of uracil dimers, instability and degradation of capsid, and covalent linkage of protein to viral RNA. J Virol. 1974 Mar;13(3):729–739. doi: 10.1128/jvi.13.3.729-739.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J. F., Rowlands D. J., Brown F. A physico-chemical sub-grouping of the mammalian picornaviruses. J Gen Virol. 1973 Feb;18(2):171–180. doi: 10.1099/0022-1317-18-2-171. [DOI] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B. A. In vitro assembly of poliovirus. II. Evidence for the self-assembly of 14 S particles into empty capsids. Virology. 1971 May;44(2):307–316. doi: 10.1016/0042-6822(71)90262-5. [DOI] [PubMed] [Google Scholar]
- Phillips B. A. In vitro assembly of polioviruses. I. Kinetics of the assembly of empty capsids and the role of extracts from infected cells. Virology. 1969 Dec;39(4):811–821. doi: 10.1016/0042-6822(69)90018-x. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Reversible inhibition of induction of mengovirus RNA polymerase and of virus maturation in Novikoff rat hepatoma cells by phenethyl alcohol. Virology. 1968 Feb;34(2):319–330. doi: 10.1016/0042-6822(68)90242-0. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Replication of mengovirus. I. Effect on synthesis of macromolecules by host cell. J Bacteriol. 1966 Jun;91(6):2317–2326. doi: 10.1128/jb.91.6.2317-2326.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff R. A., Greenhouse G., Gross K. W., Gross P. R. Synthesis and storage of microtubule proteins by sea urchin embryos. J Cell Biol. 1971 Aug;50(2):516–527. doi: 10.1083/jcb.50.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff R. A., Kaumeyer J. F. Soluble microtubule proteins of the sea urchin embryo: partial characterization of the proteins and behavior of the pool in early development. Dev Biol. 1973 Jun;32(2):309–320. doi: 10.1016/0012-1606(73)90243-1. [DOI] [PubMed] [Google Scholar]
- SCHWERDT C. E., FOGH J. The ratio of physical particles per infectious unit observed for poliomyelitis viruses. Virology. 1957 Aug;4(1):41–52. doi: 10.1016/0042-6822(57)90042-9. [DOI] [PubMed] [Google Scholar]
- Sturman L. S., Tamm I. Formation of viral ribonucleic acid and virus in cells that are permissive or nonpermissive for murine encephalomyelitis virus (GDVII). J Virol. 1969 Jan;3(1):8–16. doi: 10.1128/jvi.3.1.8-16.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Taylor M. W. Host-dependent restriction of mengovirus replication. J Virol. 1969 Nov;4(5):681–687. doi: 10.1128/jvi.4.5.681-687.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Taylor M. W. Mengovirus RNA synthesis in productive and restrictive cell lines. Virology. 1970 Sep;42(1):78–86. doi: 10.1016/0042-6822(70)90240-0. [DOI] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Structure of the Mengo virion. I. Polypeptide and ribonucleate components of the virus particle. Virology. 1974 Feb;57(2):531–542. doi: 10.1016/0042-6822(74)90192-5. [DOI] [PubMed] [Google Scholar]


