Abstract
Arylboronates capture aqueous 18F-fluoride in one step to afford a highly polar 18F-labeled aryltrifluoroborate anion (18F-ArBF3 -) that clears rapidly in vivo. To date however, there is little data to show that a ligand labeled with a prosthetic 18F-ArBF3 - will provide functional images. RGD, a high-affinity ligand for integrins that are present on the cell surface of numerous tumors, has been labeled in many formats with many different radionuclides, and as such represents a well-established ligand that can be used to evaluate new labeling methods. Herein we have labeled RGD with a prosthetic 18F-ArBF3 - via two approaches for the first time: 1) a RGD-boronate bioconjugate is directly labeled in one step and 2) an alkyne-modified arylborimidine is first converted to the corresponding 18F-ArBF3 - which is then conjugated to an RGD-azide via Cu+-mediated [2+3] dipolar cycloaddition in one pot over two steps. RGD-18F-ArBF3 - bionconjugates were produced in reasonable radiochemical yields using low amounts of 18F-fluoride anion (10-50 mCi). Despite relatively low specific activities, good tumor images are revealed in each case.
Keywords: One-step 18F-labeling, click labeling, RGD, PET imaging
Introduction
Integrins, a class of surface receptors, are the major receptors, by which cells attach themselves to the extracellular matrices, and some of which are involved in cell-cell adhesion events [1]. They have been found to participate in various biological processes including embryonic development, cell activity mediation, regulation of the balance between cell proliferation and cell death, and cancer development [2]. One of the most recognized peptide motifs that bind to the αvβ3 receptor, which is found to be overexpressed in many cancer cell lines [3], contains the amino acid sequence: Arg-Gly-Asp (RGD) [3,4], the discovery of which enabled the development of a wide range of RGD-based diagnostics and therapeutics [5-10]. Cyclopentapeptides with the RGD sequence have proven ideal ligands for molecular imaging to assist diagnosis, gauge the stage of cancers/tumor, and evaluate therapies and drugs. Accordingly, a great deal of beautiful imaging work has been reported recently by labeling RGD-peptides with a variety of radionuclides [11-13], fluorescent probes [14,15], and contrast agents to locate the in vivo distribution of the αvβ3 receptor [16]. Radiometals such as 64Cu, 68Ga, and 111In have also been used to radiolabel RGD-containing peptides [17-21].
Recognizing the importance of decreased lipophilicity of the 18F-bearing prosthetic, Haubner and coworkers prepared 18F-labeled galacto-c(RGDfK) by coupling 4-nitrophenyl 2-18F-fluoro-propionate to galacto-c(RGDfK) [22,23]. Towards similar ends, Hatano and co-workers reported a one-step electrophilic radiofluoridation to prepare 18F-c(RGDfMeV) with 18F-acetylhypofluorite (18F-AcOF), albeit at specific activities of <1 mCi/μmol [24]. Although several regioisomers were obtained during the radiosynthesis, the 18F-c(RGDfMeV) peptides showed high affinity and specificity for the targeted integrin. High tumor-to-blood/muscle ratios were obtained, however there was very high uptake of the radioactivity in the liver and kidney, and some in the bone. Chen et al. reported 18F-labeled RGD with N-succinimidyl 4-18Ffluorobenzoate to image brain tumor angiogenesis with an orthotopic U251T brain tumor model [25]. A high tumor-to-brain ratio was obtained with high receptor specificity for this radiotracer. Recently, O-(2-(2-18F-fluoroethoxy)-ethyl)-N-methylhydroxyl amine [26] and/or 4-18F-fluorobenzaldehyde [27] was regioselectively introduced onto the RGD-4C-derivatized analogues via either Michael addition or oxime formation. In each case, promising tumor uptake was reported. Taken together, these examples demonstrate that RGD-containing cyclic pentapeptides are robust diagnostic imaging agents. Over the past decade, numerous reports, as reviewed extensively [13,16,23,26,28-32], have featured many different approaches to label RGD. Indeed, new labeling methods with particular regards to facile one-step and one-pot reactions continue to be the subject of reports on labeled RGD [33-37]. Rapid and convenient labeling methods notwithstanding, concerns about prosthetic lipophilicity also continue to justify the development of modified RGDs through the incorporation of hydrophilic PEG linkers and glycosylated groups to decrease the tracer’s hydrophobicity to favour circulation and excretion.
Recently, we conceived of a novel method whereby arylboronates capture aqueous 18F-fluoride anion to afford an 18F-labeled aryltrifluoroborate anion (18F-ArBF3 -) [38-40]. Labeling proceeds rapidly at room temperature and at acidic pH 2-3 to afford a water-soluble, non-coordinating, highly polar ArBF3 - anion (log Pow < -4 for the ArBF3 - moiety). PET images of a biotinylated-18F-ArBF3 - demonstrated in vivo stability thereby highlighting their potential use in PET imaging [39]. Furthermore, the clinically trialed drug Marimastat was conjugated to a boronate via an amide bond and labeled directly at relatively low specific activities of 0.16-0.39 Ci/μmol to reveal tumor associated matrix metalloprotease activity in breast cancer xenografts. Others have labeled LymphoseekTM with pendant 18F-ArBF3 - groups and demonstrated excellent sentinel lymph node imaging at relatively low specific activity [41].
While PET images of tumors in animals [22,42] and humans [29,43,44] have been acquired using ligands with specific activities in the range of 0.5 Ci/μmol, and even as low as 0.08-0.25 Ci/μmol [45-48], in the case of the Marimastat-18F-ArBF3 - conjugate, tumor differentiation was poor and images were plagued by very high background [34,49]. Because RGD represents a powerful and reliable imaging agent that has been the subject of myriad reports, we hypothesized that an RGD-18F-ArBF3 - bioconjugate along with a visualization of functional images would validate the hypothesis that a highly polar, rapidly clearing 18F-ArBF3 -, when conjugated to a bona-fide ligand such as RGD, will provide excellent images. Moreover, because of the anionic character of the 18F-ArBF3 -, such a prosthetic greatly increases the hydrophilicity of the peptides to which it is attached, and therefore favors in vivo clearance and increases the chances for good tumour-to-blood ratios. To both simplify the synthesis and focus on verifying RGD-18F-ArBF3 - as a potential PET imaging agent, two monomeric RGD peptides (RGD-ArBF3 -s) were prepared and their 18F-labeling either in a single step or via a one-pot-two-step process to afford two different RGD-18F-ArBF3 - analogs is described herein. Preliminary tumor-specific images are presented to provide initial validation of this labeling method.
Materials and methods
Reagents and solvents were purchased from Fisher, Sigma-Aldrich, Alfa-Aesar, Novabiochem or Oakwood. All chemicals were used as supplied unless stated otherwise. The 18F Trap & Release column (HCO3 - form, ~ 10 mg) was purchased from ORTG, Inc. Deuterated solvents were obtained from Cambridge Isotope Laboratories. Analytical thin layer chromatography was undertaken on Silica Gel 60 F254 Glass TLC plates from EMD Chemicals and SiliaFlash F60 from Silicycle was used for flash chromatography. ESI-LRMS was performed on a Waters ZQ with a single quadrupole detector, attached to a Waters 2695 HPLC. ESI-HRMS were obtained on a Waters-Micromass LCT with a time-of-flight (TOF) detector. All NMR spectra were recorded at room temperature on a Bruker Avance 300 or 400 MHz spectrometer. Chemical shifts are reported using the δ scale in ppm and all coupling constants (J) are reported in hertz (Hz). Unless specified, 1H NMR spectra are referenced to the tetramethylsilane peak (δ = 0.00 ppm), 13C NMR spectra are referenced to the chloroform peak (δ = 77.23 ppm), and 19F NMR spectra are referenced to NEAT trifluoroacetic acid (δ = 0.00 ppm, -78.3 ppm relative to CFCl3). HPLC analysis was performed on the Agilent 1100 HPLC system equipped with an auto-injector, a fraction collector and a diode array detector (non-radiolabeling) or on Waters 515 binary HPLC pump with a Waters 486 UV detector and a Bioscan Flow-Count detector (radiolabeling). Phenomenex Jupiter 10μ C18 300Å 4.6 mm × 250 mm column (Column I) was used for analysis or purification of the radiolabeling reaction and Agilent Eclipse XDB-C18 5μm 9.4 mm × 250 mm column (Column II) was used for semi-preparative HPLC.
Peptide syntheses
In general, RGD peptides were synthesized as linear precursors where the lysine amino group was either protected with the Dde group or first converted to the azide. Linear peptides were then cyclized to provide the cyclo-RGD whereupon the lysine was acylated with boronate or where the lysine-azide was used for Cu+-mediated [2+3] cycloaddition. Briefly the following peptides and other components leading to the compounds were synthesized by a combination of standard solid phase and solution phase methods, purified either by standard flash silica chromatography or by HPLC when required, and characterized by 1H-NMR and 19F-NMR when possible and by ESI and HRMS: H-Asp(OtBu)-D-Phe-Lys(R)-Arg(Pbf)-Gly-OH (1a/b); Cyclo[Arg(Pbf)-Gly-Asp(OtBu)-D-Phe-Lys-(N3)] (2a); Cyclo[Arg(Pbf)-Gly-Asp(OtBu)-D-Phe-Lys(Dde)] (2b); Cyclo[Arg-Gly-Asp-D-Phe-Lys(N3)] (c[RGDfK(N3)]) (3a); Cyclo[Arg(Pbf)-Gly-Asp(OtBu)-D-Phe-Lys] (3b); Mono-tert-butyl succinate; Tert-butyl 4-oxo-4-(4-tritylpiperazin-1-yl)butanoate (4); Tert-butyl 4-oxo-4-(piperazin-1-yl)butanoate (5); Tert-butyl 4-oxo-4-(4-(2,4,6-trifluoro-3-(4,4,5,5-tetraphenyl-1,3,2-dioxaborolan-2-yl) benzoyl)piperazin-1-yl)butanoate (6); 4-Oxo-4-(4-(2,4,6-trifluoro-3-(4,4,5,5-tetraphenyl-1,3,2-dioxaborolan-2-yl)benzoyl) piperazin-1-yl)butanoic acid (7); Cyclo[Arg(pbf)-Gly-Asp(OtBu)-D-Phe-Lys(suc-piperazinyl-boronate)] (8); Cyclo-[Arg-Gly-Asp-D-Phe-Lys(suc-piperazinyl-boronate)] (9); Cyclo[Arg-Gly-Asp-D-Phe-Lys(suc-piperazinyl-ArBF3-)] (10, also referred to RGD-SuPi-ArBF3-); 2,4,6-Trifluoro-3-(1H-naphtho[1,8-de] [1,3,2]diazaborinin-2(3H)-yl)benzoic acid (11); Alkyneborimidine (12) and details of their synthesis are found in the supporting information.
Radiosyntheses
Both RGD-SuPi-18F-ArBF3 - and RGD-trAz-18F-ArBF3 - were radiolabeled using 1-5 mCi of 18F-fluoride anion that was supplemented with approximately 200-400 nmol of carrier 19F-fluoride anion. For RGD-trAz-18F-ArBF3 -, the alkyne-modified arylborimidine was first converted to the corresponding 18F-ArBF3 - according to the same method and then conjugated to the RGD-azide in the presence of copper ascorbate. Each labeling has been performed several times (n>4). Purification of each conjugate was achieved by HPLC. The same protocol was then applied for the production of both RGD species at relatively higher specific activity by using correspondingly higher levels of NCA 18F-fluoride anion. These detailed protocols are indicated below.
Radiosynthesis of RGD-SuPi-18F-ArBF3 - for animal imaging study
18F-Fluoride anion (163 mCi, t= 0 min.) in 18O-H2O (400 μL) was combined with 1 M NaHCO3 (6 μL) and CH3CN (600 μL) in a 2 mL glass V-vial and evaporated at 105 °C over Ar flow. Following evaporation, the 18F-fluoride anion was resuspended in a solution of 0.121 M KHF2 (3 μL) to give an 18F-fluoride anion cocktail. Of this cocktail, a volume of 2 μL was removed and transferred to a separate tube containing RGD-SuPi-boronate (100 nmol) that had been resuspended in CH3CN (4 μL) and conc. HCl (0.5 μL). This tube was counted and found to contain 50 mCi and was assumed to contain two-thirds of the added carrier 19F-fluoride. The resuspension of NCA 18F-fluoride anion that had been dried in small volumes, has been problematic at times and therefore after resuspension of dried-down 18F-fluoride anion, the tube into which the resuspended fluoride is always counted again to verify a) how much activity is actually resuspended and b) to calculate the specific activity of the 18F-fluoride used. In this case the specific activity is 50 mCi/484 nmol or ~0.1 Ci/μmol. The acid catalyzed labeling reaction was allowed to react for 1 hour at room temperature. After 67 min., the reaction was quenched by the addition of 5% NH4OH in 50% aqueous EtOH (200 μL). Approximately one third of this quenched solution, (70 μL, 12.4 mCi) was removed and injected into an analytical column for HPLC for purification (Waters), column: Dionex Acclaim 300, C18, 3 mm, 300Å, 4.6 × 150 mm; HPLC Program 4: solvent A: 0.04 M HCO2NH4, solvent B: CH3CN, 0 min. to 5 min., 0 % B to 5%, 5 min. to 10 min., 5% B to 20% B, 10 min. to 20 min., 20% B to 50% B, 20 min. to 25 min., 50% B to 100% B, 25 min. to 28 min., 100% B to 95% B, 28 min. to 30 min., 95% B to 5% B, 30 min. to 32 min., 5% B; flow rate: 1 mL/min.; and column temperature: room temperature). The fraction containing the desired product, which eluted between 13.5 min. and 15.0 min. was manually collected and found to contain 659 mCi at approximately 2 hours following the start of synthesis. This was then diluted with H2O (8 mL) and loaded on a preactivated C18 Sep-Pak cartridge (Waters, 1cc, 100 mg). The cartridge was then washed with H2O (8 mL) and EtOH was used to release the peptide in 50 μL per fraction. The major C18 fraction (338 μCi at 2.2 hours, SA= 0.164 Ci/μmol) was delivered for imaging. The final isolated yield, not corrected for decay, was 4% (because only one third of the reaction was purified, the yield is calculated as 0.65 mCi/16.33 mCi).
Preparation of RGD-trAz-18F-ArBF3 - for animal imaging study (14)-
To 13 mCi of NCA 18F-fluoride anion, that had been supplemented with 500 nmol 19F-fluoride anion (2 μL, 0.125 M KHF2), was added conc. HCl (0.5 μL) and the alkyneborimidine 12 in THF (4 μL). The reaction mixture was left for 77 min. at room temperature and quenched with 5% NH4OH in 50% aqueous EtOH (10 μL) that contained c[RGDfK(N3)] 3a (100 nmol). Then 0.6 M sodium ascorbate (6 μL) and 0.2 M CuSO4 (6 μL) was added successively. The click reaction was incubated for approximately 40 minutes. The crude reaction, which was counted and found to contain 5.14 mCi, was then injected to HPLC for purification with the same C18 column and HPLC program for RGD-SuPi-18F-ArBF3 -. The desired product (883 μCi) was manually collected and diluted with H2O (8 mL) for solid phase extraction. The fraction was then trapped on a pre-activated C18 sep-pak cartridge and the cartridge was washed with H2O (8 mL). The compound was then eluted with EtOH (50 μL per fraction). The major C18 fraction, 506 μCi at 3.7 hours following the start of synthesis, SA= 0.06 Ci/μmol was delivered for imaging. The final, isolated radiochemical yield, not corrected for decay, was 6.8%.
Specific activity calculations
The specific activity of an 18F-ArBF3 - is defined as Ci/μmol 18F-ArBF3 -. In order to determine the specific activity in this case we assumed that the amount of endogenous carrier 19F-fluoride anion present in NCA 18F-fluoride anion is negligible compared to the amount of carrier added. Hence, the specific activity of the carrier added 18F-fluoride anion used in labeling is Ci used divided by mmol total fluoride anion. Because 3 atoms of fluorine condense with one arylboronate, the specific activity of the 18F-ArBF3 - is triple that of the fluoride used in the reaction.
Octanol-water partition coefficient determination
RGD-SuPi-18F-ArBF3 - was prepared as noted above using 1 mCi 18F-fluoride. The product was isolated via HPLC chromatography, manually collected, desalted by solid phase extraction, eluted by EtOH flush, and concentrated by rotary evaporation. The product (approximately 20 μCi) was then redissolved in H2O (170 μL). Of this solution (50 μL) was removed to which was added n-octanol (50 μL) in an Eppendorf tube. The resulting mixture was thoroughly vortexed for 5 minutes and then centrifuged for 10 minutes at 13k rpm. The top and bottom layers were carefully removed and placed in new tubes. From each layer an aliquot was removed (0.5 μL) was spotted on a silica gel plate. The plate was then dried, covered with polyethylene film (saran wrap), and stored in a phosphorimager cassette for 5 minutes. The phosphorimager screen was then scanned using the Typhoon Phosphorimager and ImageQuant software to record relative radiographic density in each spot which reflects the octanol-water partion. The direct ratio of the activity in octanol over radioactivity in water was found to be 1.6·10-4 providing a logPOW of -3.8.
PET imaging
Cell culture and animal models
All animal studies were conducted in accordance with the principles and procedures outlined in the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Clinical Center, NIH. The human glioblastoma U87MG cell line was purchased from the American Type Culture Collection (ATCC) and grown in MEM medium supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) in a humidified atmosphere containing 5% CO2 at 37°C. The U87MG xenograft tumor models were developed in 5 to 6week old female athymic nude mice (Harlan Laboratories) by injection of 5×106 cells in the right shoulders. Tumor growth was monitored using caliper measurements of perpendicular axes of the tumor. The tumor volume was estimated by the formula: tumor volume = a× (b2) / 2, where a and b were the tumor length and width, respectively, in millimeters. The mice underwent small-animal PET studies when the tumor volume reached 100–300 mm3 (3–4 weeks after inoculation).
MicroPET imaging
PET scans and image analysis were performed using an Inveon microPET scanner (Siemens Medical Solutions). Approximately 100 μCi of RGD-SuPi-18F-ArBF3 - or RGD-trAz-18F-ArBF3 - in 100 μL of saline was administered via tail vein injection under isoflurane anesthesia. Five-minute static PET images were acquired at 30 and 60 min. post-injection (n = 3/group). The images were reconstructed using a two-dimensional ordered-subset expectation maximization (2D OSEM) algorithm without correction for attenuation or scattering. For each scan, regions of interest (ROIs) were drawn over the tumor and muscle region using vendor software (ASI Pro 5.2.4.0) on decay-corrected whole-body coronal images. The radioactivity concentrations (accumulation) within the tumors and muscle were obtained from mean pixel values within the multiple ROI volume and then converted to MBq/mL/min. using the calibration factor determined for the Inveon PET system. These values were then divided by the administered activity to obtain (assuming a tissue density of 1 g/mL) an image-ROI-derived percent injected dose per gram (%ID/g).
Results
This work was primarily undertaken to demonstrate both one-step and one-pot-two-step aqueous RGD labeling and to validate this method through in vivo images. We chose RGD because it is known to give excellent tumor images, even at low specific activity. To do this we synthesized two variations of RGD that would enable the synthesis and validation of 18F-labeling accordingly. These are shown in Figure 1; in the first case, labeling proceeds directly via acid catalyzed displacement of the tetraphenyl-pinacolate ester of boron 9 to give the corresponding RGD-SuPi-18F-ArBF3- 10 while in the second case, an alkyne-modified borimidine 12 is first converted to the corresponding alkyne-modified 18F-ArBF3 - 13 that is not isolated but which is then condensed with an RGD-azide by Cu+-catalyzed [2+3] cycloaddition to give the triazole linked RGD-trAz-18F-ArBF3 - 14. In each case there is only one 18F-fluorine atom on the 18F-ArBF3 - and this is noted for clarity. The three fluorine atoms on the aryl ring are not displaced and are present to stabilize the 18F-ArBF3 - against solvolysis.
Figure 1.
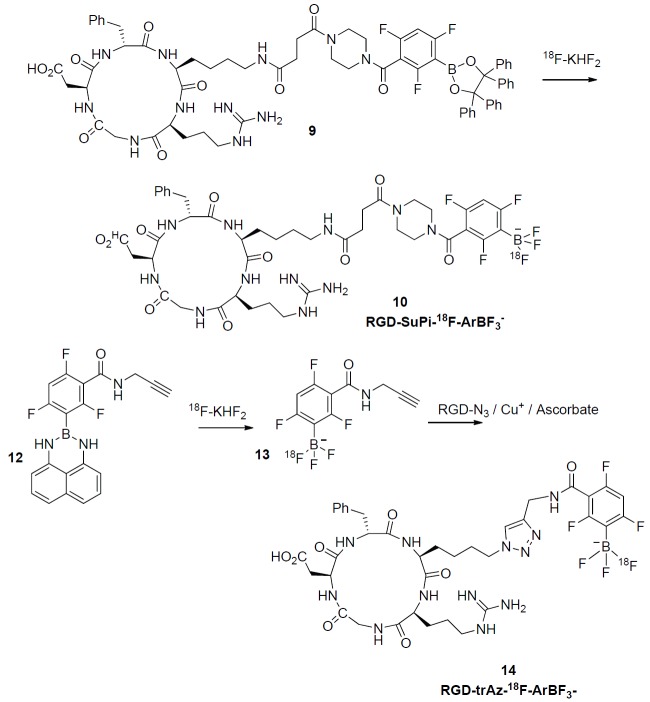
Synthetic scheme for the preparation of RGD-SuPi-18F-ArBF3 -and RGD-trAz-18F-ArBF3 -: Above a tetraphenyl-pinacolate boronate ester conjugate to RGD is directly converted to the RGD-SuPi-18F-ArBF3 -while below, the alkyne borimidine 12 is converted first to the corresponding 18F-ArBF3 -alkyne 13 in situ, which is then directly conjugated to the RGD-azide to provide RGD-trAz-18F-ArBF3 -.
Radiosynthesis prior to imaging
The fluoridation of RGD-boronate 9 was undertaken under the similar conditions as reported earlier [34]. HCO2NH4/CH3CN HPLC solvent system was found to be quite suitable for the HPLC analysis of the corresponding 18F-ArBF3-s. The unlabeled standard of RGD-SuPi-ArBF3- 10 was analyzed via ESI-LCMS with the desired masses of [M]-: 996.3 and [M-HF]-: 976.5 for RGD-SuPi-ArBF3- 10 (tR = 16.2 min., using Column I and HPLC Program 4 in the Agilent HPLC system). Because low levels of activity were used, the radiofluoridation of RGD-boronate 9 was carried out under carrier-added conditions that require approximately 200-300 nmol of total fluoride anion. Similarly, CH3CN was used as the aqueous cosolvent while HCl was used to acidify the system with 5 equivalents of carrier fluoride added to drive the fluoridation forward. Each reaction was undertaken at room temperature for one hour and then quenched with 5% NH4OH in 50% aqueous EtOH prior to the HPLC injection for analysis. The fluoridation was very reproducible (n ≥ 4), and an average radiochemical yield of 10-15% was achieved using 1-5 mCi 18F-fluoride anion as shown in Figure 2.
Figure 2.
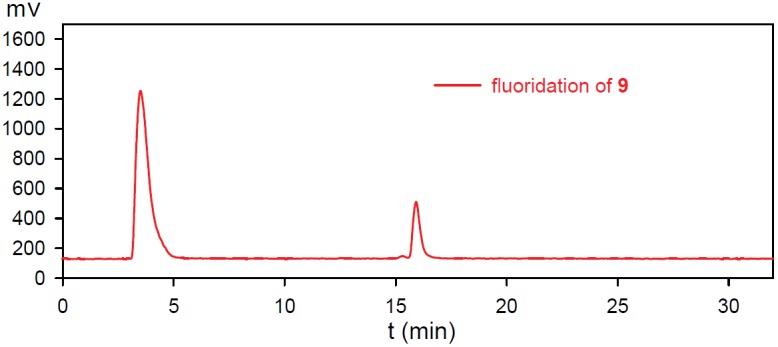
The radio-HPLC traces of the one-step 18F-fluoridation of RGD-boronates: The HPLC trace represented the 18F-fluoridation of RGD-SuPi-boronate 9.
Through this work, we appreciated that arylboronic acids, or arylboronates that are modified with acid-labile protecting groups, provide much faster fluoridation rates (data not shown). Hence, we produced a 1,8-diaminonaphthalene (dan) protected borimidine-alkyne that rapidly fluoridated with radiochemical yields of ~30% within ~ 30 min. at room temperature. The reaction was quenched with ammonium hydroxide and directly used for a subsequent copper (I) catalyzed [2+3] cycloaddition reaction, which proved very efficient for conjugating the 18F-ArBF3 - prosthetics into RGD. To compare this one-pot-two-step radiolabeling strategy for the incorporation of an 18F-ArBF3 - with the one-step labeling method, c[RGDfK(N3)] 3a was prepared and conjugated to the alkyne-18F-ArBF3 - 13 via the copper(I) catalysis for in vivo imaging. Briefly, the fluoridation of borimidine 12 was undertaken under the similar conditions for ~ 24 min. to provide the alkyne-18F-ArBF3 - intermediate 13 in a radiochemical yield of ~ 44%. The crude was then quenched with 5% NH4OH/50% aq. EtOH, to which was added the RGD-azide and CuSO4/sodium ascorbate to induce cycloaddition to give 18F-labeled 14. Following room temperature reaction for ~30 min., the crude reaction was injected into an analytical HPLC column. The HPLC results were shown in Figure 3. As observed below, the intermediate alkyne-18F-ArBF3 - was not completely converted to the final product in this case yet provided sufficiently promising yields for us to contemplate animal imaging.
Figure 3.
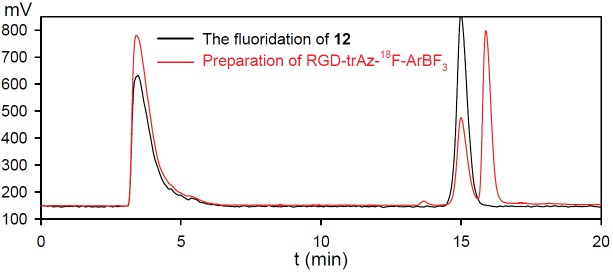
The HPLC traces of the one-pot two-step labeling reaction to prepare RGD-trAz-18F-ArBF3.The black trace was for the crude fluoridation reaction of 12 to prepare alkyne-18F-ArBF3 -and the red trace was for the click reaction between the crude alkyne-18F-ArBF3 - and c[RGDfK(N3)].
Radiosyntheses for animal imaging
For animal imaging, we used slightly higher levels of activity, 50 mCi and 13 mCi respectively in the radiosynthesis of RGD-SuPi-18F+-ArBF3 - or RGD-trAz-18F-ArBF3 - and consequently this enabled us to obtain radiotracers at respectable specific activities. Nevertheless, because much more radioactivity was loaded into an analytical column for purification, signal saturation was observed in the radiotraces as shown in Figure 4. Fortunately, free 18F-fluoride anion and the desired RGD-SuPi-18F-ArBF3 - 10 are readily separated by this method which affords a radiochemically pure desired product (the small amount of free 18F-fluoride anion observed in the analytical reinjection trace is attributed to residual free 18F-fluoride anion in the injector loop) as shown in Figure 4. Using a simple solid phase extraction, ammonium formate and CH3CN were successfully removed while a small amount of EtOH was used to release the product for formulation with saline buffer. The sample was then delivered for animal imaging.
Figure 4.

The HPLC traces for the preparation of RGD-SuPi-18F-ArBF3 -used in Imaging: (top – crude reaction trace: UV and radiotrace, bottom - analytical re-injection of purified material: UV and radiotrace.
In order to improve the yield of RGD-trAz-18F-ArBF3 - in the [2+3] cycloaddition step for use in animal imaging, concentrations of the reaction components were increased. As with the preparation of RGD-SuPi-18F-ArBF3 -, the entire crude reaction was applied to an analytical column and similarly the detector was saturated due to the large amount of radioactivity injected, as seen in Figure 5. Nevertheless, the desired product was easily isolated from free 18F-fluoride anion and carefully separated from the intermediate alkyne-18F-ArBF3 -. Following solid phase extraction, the product was reanalyzed for purity on the same column and then delivered for animal imaging. Again, while this time there appeared to be considerable amount of free fluoride in the analytical QC control injection (Figure 5), this was attributed to the presence of free 18F-fluoride anion in the injection loop or in the HPLC as otherwise there would have been considerable amount of bone signal in the images (vide infra). The overall isolated radiochemical yield in this step was approximately 6.8%.
Figure 5.
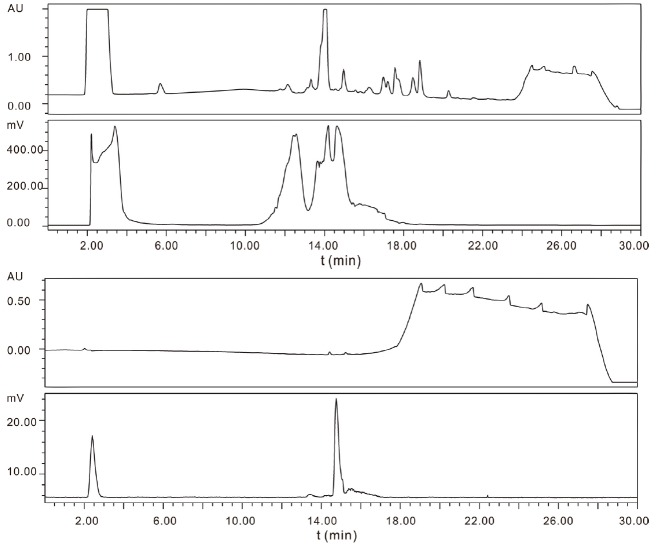
The HPLC traces for the preparation of RGD-trAz-18F-ArBF3 -. (top – crude reaction trace: UV and radiotrace, bottom - analytical re-injection of purified material: UV and radiotrace.
Animal imaging
Representative coronal microPET images of U87MG tumor bearing mice at different times after intravenous injection of 100 μCi of RGD-SuPi-18F-ArBF3 - or RGD-trAz-18F-ArBF3 - are shown in Figure 6. The U87MG tumors were clearly visible with both imaging RGD analogs. The tumor uptake was 2.17 ± 0.76 %ID/g with RGD-SuPi-18F-ArBF3 - and 2.04 ± 0.43 %ID/g with RGD-trAz-18F-ArBF3 - at 30 min. post-injection. At 60 min. post-injection, the tumor uptake decreased to 1.51 ± 0.27 %ID/g with RGD-SuPi-18F-ArBF3 - and 1.17 ± 0.20 %ID/g with RGD-trAz-18F-ArBF3 -. There is no significant difference as to the tumor uptake with both tracers at both time points (p > 0.05). However, images with RGD-trAz-18F-ArBF3 - showed lower background compared with that with RGD-SuPi-18F-ArBF3 -. The tumor/muscle ratios with RGD-SuPi-18F-ArBF3 - are significantly lower than that with RGD-trAz-18F-ArBF3 - (3.88 vs. 8.16 at 30 min. post-injection, p< 0.01; 4.08 vs. 7.80 at 60 min. post-injection, p< 0.01). Bone uptake appeared to be minimal in both scans and this is discussed below. Because the mice were to be used for other experiments, they were not sacrificed for post mortem biodistribution analysis.
Figure 6.
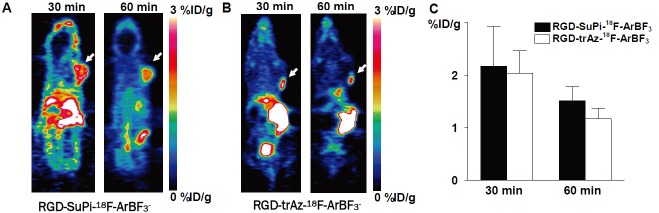
Representative coronal PET images of U87MG tumor bearing mice using RGD-SuPi-18F-ArBF3 -(A) and RGD-trAz-18F-ArBF3 - (B). 3.7 MBq of radioactivity was administered via tail vein injection. Five-minute static PET images were acquired at 30 and 60 min. post-injection. Tumors are indicated by arrows. (C) Uptake values at 30 and 60 min. time points in the U87MG tumor quantified from region of interest (ROI) analysis (n = 3).
Discussion
Two labeling strategies were investigated in this work to radiolabel cyclic pentapeptides containing the RGD sequence. The fluoridation was carried out under acidic aqueous conditions at room temperature. The acid labile 1,8-diaminonaphthalene protected borimidine demonstrated faster and more efficient fluoridation than the tetraphenylpinacolate ester. Whereas the one-step labeling of RGD-SuPi-boronate gave an isolated radiochemical yield of 4%, the preparation of RGD-trAz-18F-ArBF3 - gave an isolated radiochemical yield of 6.8%. These yields are reported for the syntheses that were performed on the day of imaging and these are found to be somewhat lower than we have seen in our hands. Notably, we performed preliminary labeling at the Triumf facility, while the radiosyntheses for animal imaging were performed at the NIH. While we do not have an immediate explanation for this, it is possible that differences in pH due to the presence carbonate from the anion exchange resin, or column differences could have resulted in lower yields.
The microPET images shown in Figure 6 clearly indicated tumor uptake of the radiotracers. For both radiotracers, the in vivo clearance was rapid as most of the radiotracer cleared from the body within 60 min. Nevertheless, the rather rapid clearance reflects the considerable polarity of the 18F-ArBF3 - prosthetic group, for which the overall polarity of RGD-trAz-18F-ArBF3 - was measured in terms of an octanol-water partition, log(POW), which was found to be -3.8. Nevertheless, there was also substantial uptake in the liver, spleen, and gall bladder immediately following injection. Reasonable tumor-to-muscle ratios (3.88 vs. 8.16 at 30 min. post-injection; 4.08 vs. 7.80 at 60 min. post-injection) [22,28,29] were obtained while the RGD-trAz-18F-ArBF3 - demonstrated slightly better tumor-to-muscle ratio despite of its lower specific activity. Bone uptake appeared to be minimal in both scans and this is consistent with previous reports on the in vivo stability of the 18F-ArBF3 - moiety. Indeed, recent work in our lab (unpublished) has observed signal in the bone at approximately 0.5%-1% ID/g. While this may be due to slow solvolytic defluorination and/or metabolism of the radiotracer to liberate 18F-fluoride anion, we contend that this level of bone uptake is not inconsistent with other radiotracers.
Because of the widespread acceptance of tumour-specific uptake of labeled RGDs we did not perform a blocking study in this preliminary work. Nevertheless, the lower tumour uptake in the images obtained with RGD-trAz-18F-ArBF3 -, that had been prepared at lower specific activity, serves to demonstrate tumor specificity. Interestingly however, despite the fact that RGD-trAz-18F-ArBF3 - was prepared at lower specific activity (we used less 18F-fluoride anion to start with), it appeared to have increased specificity for the tumor after 60 minutes. This suggests that linker arm chemistries may play subtle yet important roles in terms of tumor specificity [55].
Notably well-defined tumor images were obtained despite the rather low specific activity of labeling ca. 0.16 Ci/μmol and 0.06 Ci/μmol respectively. It is worth noting that 1 Ci at a specific activity of 2 Ci/μmol would provide 400 nmol total fluoride anion, or approximately the same quantity of total fluoride we used for the labeling. Hence had we used 1-2 Ci, that are commonly available to production labs, the use of added carrier 19F-fluoride could be avoided while specific activities would have increased substantially to >2 Ci/μmol. The ability to generate an 18F-ArBF3 - at much higher specific activity has been previously discussed [34,39] and recently we have synthesized an 18F-ArBF3 - at 15 Ci/μmol (submitted). Work towards higher yields and higher specific activity are underway.
While higher specific activity would likely provide greater contrast, this work was undertaken to validate the use of an 18F-ArBF3 - in the context of RGD. Moreover, we felt it was important to address the reason for which relatively poor images were obtained with Marimastat. Whereas molecular imaging is a complex phenomenon where all aspects of the radiotracer can affect image quality, here we labeled RGD via very similar radiosynthetic methods at roughly the same specific activity as we obtained for Marimastat. In contrast to poorly defined images with Marimastat, here we obtained quite well defined tumor images suggesting that for 18F-ArBF3 - bioconjugates, image quality is likely to be dominated by the targeting ligand and not the effect of the pendant 18F-ArBF3 - moiety. Based on the tumor specific images herein, one may surmise that the hydrophilicity of the 18F-ArBF3 - appendage that renders the tracer more polar may account for the relatively high tumor specific uptake despite low specific activity. Although in the absence of a blocking experiment we cannot be entirely certain to what extent the 18F-ArBF3 - moiety contributed to specific tumor uptake, it would be extraordinary if the tumor image were due to specific uptake of the 18F-ArBF3 - and not the RGD.
Conclusion
For the first time, RGD has been labeled with a prosthetic 18F-ArBF3 -. We featured both direct and indirect labeling that provided reasonable overall radiochemical chemical yields (10-30%) although somewhat lower yields on the day of functional imaging. In contrast to Marimastat, which has been similarly labeled at relatively low specific activity but which gave mediocre images of cancer-associated matrix metalloprotease activity [34,49], here we obtained relatively good animal images with two different RGD-18F-ArBF3 - compositions. Interestingly, RGD-trAz-18F-ArBF3 -, which was labeled in two steps, demonstrated somewhat better tumor-to-muscle ratios. Moreover, the radiosynthesis of RGD-trAz-18F-ArBF3 - also provided higher isolated radiochemical yield, which attributes to the application of an acid labile protection group 1,8-diaminonaphthalene. Hence, this method should provide a reliable means for radiolabeling biomolecules for PET imaging studies.
Acknowledgements
This work was supported by grant from the Canadian Cancer Society #20071.
Supporting Information
References
- 1.Hynes RO. Integrins - Versatility, Modulation, and Signaling in Cell-Adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. Integrins - Versatility, Modulation, and Signaling in Cell-Adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 3.Haubner R, Finsinger D, Kessler H. Stereoisomeric peptide libraries and peptidomimetics for designing selective inhibitors of the alpha(V)beta(3) integrin for a new cancer therapy. Angew Chem. 1997;36:1375–1389. [Google Scholar]
- 4.Aumailley M, Gurrath M, Muller G, Calvete J, Timpl R, Kessler H. Arg-Gly-Asp Constrained within Cyclic Pentapeptides - Strong and Selective Inhibitors of Cell-Adhesion to Vitronectin and Laminin Fragment-P1. Febs Lett. 1991;291:50–54. doi: 10.1016/0014-5793(91)81101-d. [DOI] [PubMed] [Google Scholar]
- 5.Pierschbacher MD, Ruoslahti E. Cell Attachment Activity of Fibronectin Can Be Duplicated by Small Synthetic Fragments of the Molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 6.Pierschbacher MD, Ruoslahti E. Variants of the Cell Recognition Site of Fibronectin That Retain Attachment-Promoting Activity. Proc Natl Acad Sci USA. 1984;81:5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruoslahti E, Pierschbacher MD. New Perspectives in Cell-Adhesion - Rgd and Integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 8.Albelda SM, Buck CA. Integrins and Other Cell-Adhesion Molecules. Faseb J. 1990;4:2868–2880. [PubMed] [Google Scholar]
- 9.Humphries MJ. The Molecular-Basis and Specificity of Integrin Ligand Interactions. J Cell Sci. 1990;97:585–592. doi: 10.1242/jcs.97.4.585. [DOI] [PubMed] [Google Scholar]
- 10.Ruoslahti E. Integrins. J Clin Investig. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haubner R. alpha(v)beta(3)-integrin imaging: a new approach to characterise angiogenesis? Eur J Nucl Med Mol Imaging. 2006;33:S54–S63. doi: 10.1007/s00259-006-0136-0. [DOI] [PubMed] [Google Scholar]
- 12.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin alpha(v)beta(3) targeted radiotracers for tumor imaging. Mol Pharma. 2006;3:472–487. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 13.Liu S. Radiolabeled Cyclic RGD Peptides as Integrin alpha(v)beta(3)-Targeted Radiotracers: Maximizing Binding Affinity via Bivalency. Bioconjug Chem. 2009;20:2199–2213. doi: 10.1021/bc900167c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Z, Wu Y, Xiong ZM, Gambhir SS, Chen XY. Near-infrared fluorescent RGD peptides for optical imaging of integrin alpha(v) beta 3 expression in living mice. Bioconjug Chem. 2005;16:1433–1441. doi: 10.1021/bc0501698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye YP, Bloch S, Xu BG, Achilefu S. Design, synthesis, and evaluation of near infrared fluorescent multimeric RGD peptides for targeting tumors. J Med Chem. 2006;49:2268–2275. doi: 10.1021/jm050947h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schottelius M, Laufer B, Kessler H, Wester HJ. Ligands for Mapping alpha(v)beta(3)-Integrin Expression in Vivo. Acc Chem Res. 2009;42:969–980. doi: 10.1021/ar800243b. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Zhang XZ, Xiong ZM, Cheng Z, Fisher DR, Liu S, Gambhir SS, Chen XY. microPET imaging of glioma integrin alpha(V)beta(3) expression using Cu-64-labeled tetrameric RGD peptide. J Nucl Med. 2005;46:1707–1718. [PubMed] [Google Scholar]
- 18.Li ZB, Cai WB, Cao QZ, Chen K, Wu ZH, He LN, Chen XY. 64Cu-Labeled “Tetrameric and octameric RGD peptides for small-animal PET of Tumor alpha(v)beta(3) integrin expression. J Nucl Med. 2007;48:1162–1171. doi: 10.2967/jnumed.107.039859. [DOI] [PubMed] [Google Scholar]
- 19.Li ZB, Chen K, Chen X. Ga-68-labeled multimeric RGD peptides for microPET imaging of integrin alpha(v)beta(3) expression. Eur J Nucl Med Mol Imaging. 2008;35:1100–1108. doi: 10.1007/s00259-007-0692-y. [DOI] [PubMed] [Google Scholar]
- 20.Dijkgraaf I, Rijnders AY, Soede A, Dechesne AC, van Esse GW, Brouwer AJ, Corstens FHM, Boerman OC, Rijkers DTS, Liskamp RMJ. Synthesis of DOTA-conjugated multivalent cyclic-RGD peptide dendrimers via 1,3-dipolar cycloaddition and their biological evaluation: implications for tumor targeting and tumor imaging purposes. Org Biomol Chem. 2007;5:935–944. doi: 10.1039/b615940k. [DOI] [PubMed] [Google Scholar]
- 21.Decristoforo C, Gonzalez IH, Carlsen J, Rupprich M, Huisman M, Virgolini I, Wester HJ, Haubner R. Ga-68- and In-111-labelled DOTA-RGD peptides for imaging of alpha v beta 3 integrin expression. Eur J Nucl Med Mol Imaging. 2008;35:1507–1515. doi: 10.1007/s00259-008-0757-6. [DOI] [PubMed] [Google Scholar]
- 22.Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, Senekowitsch-Schmidtke R, Kessler H, Schwaiger M. Noninvasive imaging of alpha(v)beta(3) integrin expression using F-18-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001;61:1781–1785. [PubMed] [Google Scholar]
- 23.Haubner R, Kuhnast B, Mang C, Weber WA, Kessler H, Wester HJ, Schwaiger M. [F-18] Galacto-RGD: Synthesis, radiolabeling, metabolic stability, and radiation dose estimates. Bioconjug Chem. 2004;15:61–69. doi: 10.1021/bc034170n. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa M, Hatano K, Oishi S, Kawasumi Y, Fujii N, Kawaguchi M, Doi R, Imamura M, Yamamoto M, Ajito K, Mukai T, Saji H, Ito K. Direct electrophilic radiofluorination of a cyclic RGD peptide for in vivo alpha(v)beta(3) integrin related tumor imaging. Nucl Med and Biol. 2003;30:1–9. doi: 10.1016/s0969-8051(02)00387-6. [DOI] [PubMed] [Google Scholar]
- 25.Chen XY, Park R, Shahinian AH, Tohme M, Khankaldyyan V, Bozorgzadeh MH, Bading JR, Moats R, Laug WE, Conti PS. F-18-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med and Biol. 2004;31:179–189. doi: 10.1016/j.nucmedbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Olberg DE, Cuthbertson A, Solbakken M, Arukwe JM, Qu H, Kristian A, Bruheim S, Hjelstuen OK. Radiosynthesis and Biodistribution of a Prosthetic Group ((18)F-FENMA) Conjugated to Cyclic RGD Peptides. Bioconjug Chem. 2010;21:2297–2304. doi: 10.1021/bc1003229. [DOI] [PubMed] [Google Scholar]
- 27.Battle MR, Goggi JL, Allen L, Barnett J, Morrison MS. Monitoring Tumor Response to Antiangiogenic Sunitinib Therapy with (18)F-Fluciclatide, an (18)F-Labeled alpha(v)beta(3)-Integrin and alpha(v)beta(5)-Integrin Imaging Agent. J Nucl Med. 2011;52:424–430. doi: 10.2967/jnumed.110.077479. [DOI] [PubMed] [Google Scholar]
- 28.Chen XY, Liu S, Hou YP, Tohme M, Park R, Bading JR, Conti PS. MicroPET imaging of breast cancer alpha(v)-integrin expression with Cu-64-labeled dimeric RGD peptides. Mol Imaging Biol. 2004;6:350–359. doi: 10.1016/j.mibio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Beer AJ, Niemeyer M, Carlsen J, Sarbia M, Nahrig J, Watzlowik P, Wester HJ, Harbeck N, Schwaiger M. Patterns of alpha(v)beta(3) expression in primary and metastatic human breast cancer as shown by F-18-galacto-RGD PET. J Nucl Med. 2008;49:255–259. doi: 10.2967/jnumed.107.045526. [DOI] [PubMed] [Google Scholar]
- 30.Dijkgraaf I, Rijnders AY, Soede A, Dechesne AC, van Esse GW, Brouwer AJ, Corstens FHM, Boerman OC, Rijkers DTS, Liskamp RMJ. Synthesis of DOTA-conjugated multivalent cyclic-RGD peptide dendrimers via 1,3-dipolar cycloaddition and their biological evaluation: implications for tumor targeting and tumor imaging purposes. Org Biomol Chem. 2007;5:935–944. doi: 10.1039/b615940k. [DOI] [PubMed] [Google Scholar]
- 31.Haubner R, Wester HJ, Burkhart F, Senekowitsch-Schmidtke R, Weber W, Goodman SL, Kessler H, Schwaiger M. Glycosylated RGD-containing peptides, tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J Nucl Med. 2001;42:326–336. [PubMed] [Google Scholar]
- 32.Haubner R, Wester HJ, Reuning U, Senekowitsch-Schmidtke R, Diefenbach B, Kessler H, Stocklin G, Schwaiger M. Radiolabeled alpha(v)beta(3) integrin antagonists: A new class of tracers for tumor targeting. J Nucl Med. 1999;40:1061–1071. [PubMed] [Google Scholar]
- 33.Liu SL, Liu ZF, Chen K, Yan YJ, Watzlowik P, Wester HJ, Chin FT, Chen XY. (18)F-Labeled Galacto and PEGylated RGD Dimers for PET Imaging of alpha(v)beta(3) Integrin Expression. Mol Imaging Biol. 2010;12:530–538. doi: 10.1007/s11307-009-0284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Ting R, Harwig CW, Keller UAD, Bellac CL, Lange PF, Inkster JAH, Schaffer P, Adam MJ, Ruth TJ, Overall CM, Perrin DM. Towards kit-like F-18-labeling of marimastat, a noncovalent inhibitor drug for in vivo PET imaging cancer associated matrix metalloproteases. Med Chem Commun. 2011;2:942–949. [Google Scholar]
- 35.Guo N, Lang LX, Li WH, Kiesewetter DO, Gao HK, Niu G, Xie QG, Chen XY. Quantitative Analysis and Comparison Study of F-18 AlF-NOTA-PRGD2, 18F FPPRGD2 and Ga-68 Ga-NOTA-PRGD2 Using a Reference Tissue Model. Plos One. 2012;7 doi: 10.1371/journal.pone.0037506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang LX, Li WH, Guo N, Ma Y, Zhu L, Kiesewetter DO, Shen BZ, Niu G, Chen XY. Comparison Study of F-18 FAI-NOTA-PRGD2, F-18 FPPRGD2, and Ga-68 Ga-NOTA-PRGD2 for PET Imaging of U87MG Tumors in Mice. Bioconjug Chem. 2011;22:2415–2422. doi: 10.1021/bc200197h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amigues E, Schulz J, Szlosek-Pinaud M, Fernandez P, Silvente-Poirot S, Brillouet S, Courbon F, Fouquet E. F-18 Si-RiboRGD: From Design and Synthesis to the Imaging of alpha(v)beta(3) Integrins in Melanoma Tumors. Chempluschem. 2012;77:345–349. [Google Scholar]
- 38.Ting R, Adam MJ, Ruth TJ, Perrin DM. Arylfluoroborates and alkylfluorosilicates as potential PET imaging agents: high-yielding aqueous biomolecular 18F-labeling. J Am Chem Soc. 2005;127:13094–13095. doi: 10.1021/ja053293a. [DOI] [PubMed] [Google Scholar]
- 39.Ting R, Harwig CW, auf dem Keller U, McCormick S, Austin P, Overall CM, Adam MJ, Ruth TJ, Perrin DM. Towards 18F-Labeled Aryltrifluoroborate Radiotracers - In Vivo PET Imaging of Stable Aryltrifluoroborate Clearance in Mice. J Am Chem Soc. 2008;130:12045–12055. doi: 10.1021/ja802734t. [DOI] [PubMed] [Google Scholar]
- 40.Ting R, Lo J, Adam MJ, Ruth TJ, Perrin DM. Capturing aqueous (18)F -fluoride with an arylboronic ester for PET: Synthesis and aqueous stability of a fluorescent (18)F -labeled aryltrifluoroborate. J Fluor Chem. 2008;129:349–358. [Google Scholar]
- 41.Ting R, Aguilera TA, Crisp JL, Hall DJ, Eckelman WC, Vera DR, Tsien RY. Fast F-18 Labeling of a Near-Infrared Fluorophore Enables Positron Emission Tomography and Optical Imaging of Sentinel Lymph Nodes. Bioconjug Chem. 2010;21:1811–1819. doi: 10.1021/bc1001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ceccarini G, Flavell RR, Butelman ER, Synan M, Willnow TE, Bar-Dagan M, Goldsmith SJ, Kreek MJ, Kothari P, Vallabhajosula S, Muir TW, Friedman JM. PET Imaging of Leptin Biodistribution and Metabolism in Rodents and Primates. Cell Metab. 2009;10:148–159. doi: 10.1016/j.cmet.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wester HJ, Schottelius M, Scheidhauer K, Meisetschlager G, Herz M, Rau FC, Reubi JC, Schwaiger M. PET imaging of somatostatin receptors: design, synthesis and preclinical evaluation of a novel F-18-labelled, carbohydrated analogue of octreotide. Eur J Nucl Med Mol Imaging. 2003;30:117–122. doi: 10.1007/s00259-002-1012-1. [DOI] [PubMed] [Google Scholar]
- 44.Anderson CJ, Dehdashti F, Cutler PD, Schwarz SW, Laforest R, Bass LA, Lewis JS, McCarthy DW. Cu-64-TETA-Octreotide as a PET imaging agent for patients with neuroendocrine tumors. J Nucl Med. 2001;42:213–221. [PubMed] [Google Scholar]
- 45.Ujula T, Huttunen M, Luoto P, Perkyl H, Simpura I, Wilson I, Bergman M, Roivainen A. Matrix Metalloproteinase 9 Targeting Peptides: Syntheses, 68Ga-labeling, and Preliminary Evaluation in a Rat Melanoma Xenograft Model. Bioconjug Chem. 2010;21:1612–1621. doi: 10.1021/bc1000643. [DOI] [PubMed] [Google Scholar]
- 46.Banerjee SR, Pullambhatla M, Byun Y, Nimmagadda S, Green G, Fox JJ, Horti A, Mease RC, Pomper MG. 68Ga-Labeled Inhibitors of Prostate-Specific Membrane Antigen (PSMA) for Imaging Prostate Cancer. J Med Chem. 2010;53:5333–5341. doi: 10.1021/jm100623e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Foss CA, Byun Y, Nimmagadda S, Pullambahatla M, Fox JJ, Castanares M, Lupold SE, Babich JW, Mease RC, Pomper MG. Radiohalogenated Prostate-Specific Membrane Antigen (PSMA)-Based Ureas as Imaging Agents for Prostate Cancer. J Med Chem. 2008;51:7933–7943. doi: 10.1021/jm801055h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flavell RR, Kothari P, Bar-Dagan M, Synan M, Vallabhajosula S, Friedman JM, Muir TW, Ceccarini G. Site-specific F-18-labeling of the protein hormone leptin using a general two-step ligation procedure. J Am Chem Soc. 2008;130:9106–9112. doi: 10.1021/ja801666z. [DOI] [PubMed] [Google Scholar]
- 49.Keller UAD, Bellac CL, Li Y, Lou YM, Lange PF, Ting R, Harwig C, Kappelhoff R, Dedhar S, Adam MJ, Ruth TJ, Benard F, Perrin DM, Overall CM. Novel Matrix Metalloproteinase Inhibitor F-18 Marimastat-Aryltrifluoroborate as a Probe for In vivo Positron Emission Tomography Imaging in Cancer. Cancer Res. 2010;70:7562–7569. doi: 10.1158/0008-5472.CAN-10-1584. [DOI] [PubMed] [Google Scholar]
- 50.Thumshirn G, Hersel U, Goodman SL, Kessler H. Multimeric cyclic RGD peptides as potential tools for tumor targeting: Solid-phase peptide synthesis and chemoselective oxime ligation. Chem Eur J. 2003;9:2717–2725. doi: 10.1002/chem.200204304. [DOI] [PubMed] [Google Scholar]
- 51.Thumshirn G, Hersel U, Poethko T, Rau F, Haubner R, Schwaiger M, Wester HJ, Kessler H. Multimeric cyclic RGD peptides with improved tumor uptake for tumor targeting. Peptide Revolution: Genomics, Proteomics & Therapeutics. 2004:693–694. [Google Scholar]
- 52.Liu ZF, Liu SL, Wang F, Liu S, Chen XY. Noninvasive imaging of tumor integrin expression using (18)F-labeled RGD dimer peptide with PEG(4) linkers. Eur J Nucl Med Mol Imaging. 2009;36:1296–1307. doi: 10.1007/s00259-009-1112-2. [DOI] [PubMed] [Google Scholar]
- 53.Auzzas L, Zanardi F, Battistini L, Burreddu P, Carta P, Rassu G, Curti C, Casiraghi G. Targeting alpha(v)beta(3) Integrin: Design and Applications of Mono- and Multifunctional RGD-Based Peptides and Semipeptides. Curr Med Chem. 2010;17:1255–1299. doi: 10.2174/092986710790936301. [DOI] [PubMed] [Google Scholar]
- 54.Dechantsreiter MA, Planker E, Matha B, Lohof E, Holzemann G, Jonczyk A, Goodman SL, Kessler H. N-methylated cyclic RGD peptides as highly active and selective alpha(v)beta(3) integrin antagonists. J Med Chem. 1999;42:3033–3040. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]
- 55.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


