Abstract
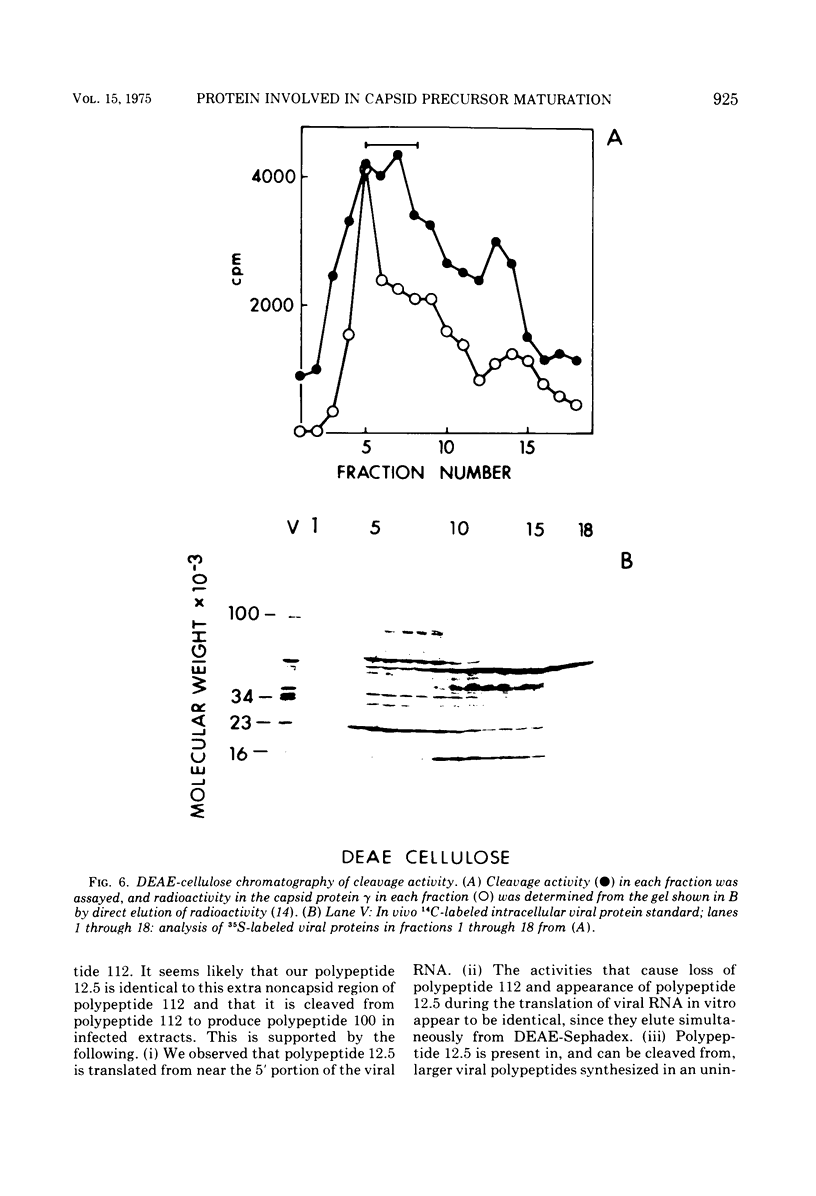
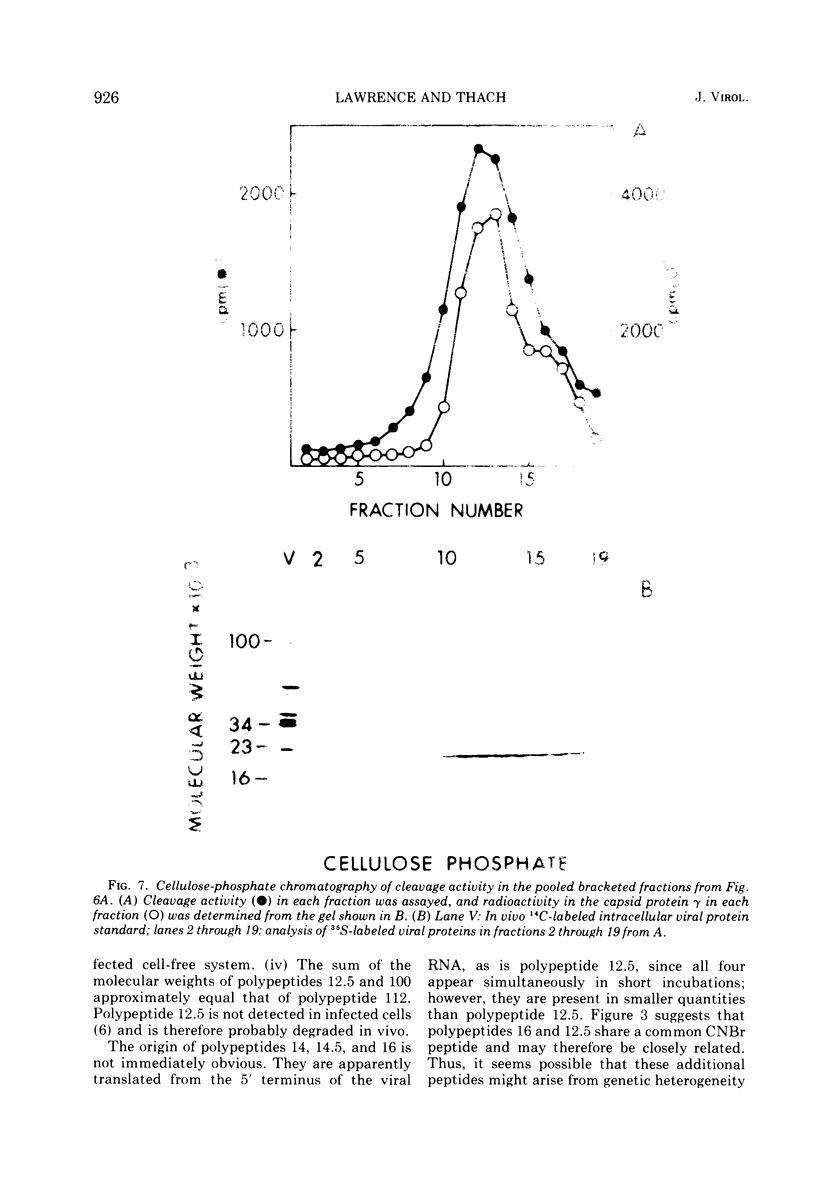
Translation of encephalomyocarditis virus RNA in a cell-free system from uninfected Krebs ascites cells results in the synthesis of a major polypeptide product with a molecular weight of approximately 112,000. In contrast, when the viral RNA is translated in a cell-free system from virus-infected cells, this polypeptide is absent and the largest polypeptide produced has a molecular weight of about 100,000. This latter polypeptide comigrates on sodium dodecyl sulfate-gels with in vivo virus capsid precursor A, and the two have identical patterns of CNBr-generated peptides. A polypeptide having a molecular weight of 12,500 is also a major translation product in the system from infected cells (but not from uninfected cells). This polypeptide appears to be generated by cleavage of the NH-2-terminal portion of the viral RNA-dependent polypeptides by a proteolytic activity present in the infected cell-free system. This proteolytic activity copurifies with the 23,000-molecular weight viral capsid protein gamma, found in infected cells, through chromatography on DEAE-cellulose and cellulose phosphate. This suggests that gamma is itself a proteolytic enzyme involved in maturation of the viral capsid precursor.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boime I., Aviv H., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA. II. The in vitro synthesis of high molecular weight proteins and elements of the viral capsid. Biochem Biophys Res Commun. 1971 Nov 5;45(3):788–795. doi: 10.1016/0006-291x(71)90486-4. [DOI] [PubMed] [Google Scholar]
- Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus mRNA. 3. Discrete polypeptides translated from a monocistronic messenger in vitro. Arch Biochem Biophys. 1972 Dec;153(2):706–713. doi: 10.1016/0003-9861(72)90389-x. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Korant B. D. Characterization of the large picornaviral polypeptides produced in the presence of zinc ion. J Virol. 1974 Aug;14(2):282–291. doi: 10.1128/jvi.14.2.282-291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Gene order of encephalomyocarditis virus as determined by studies with pactamycin. J Virol. 1972 May;9(5):823–828. doi: 10.1128/jvi.9.5.823-828.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Kinetics of synthesis and cleavage of encephalomyocarditis virus-specific proteins. Virology. 1972 Nov;50(2):535–549. doi: 10.1016/0042-6822(72)90405-9. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Swanson R., Schlesinger M. J. Viral proteins formed in a cell-free rabbit reticulocyte system programmed with RNA from a temperature-sensitive mutant of Sindbis virus. J Virol. 1974 Sep;14(3):664–671. doi: 10.1128/jvi.14.3.664-671.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P., Martin E. M. Virus-specific polypeptides in ascites cells infected with encephalomyocarditis virus. J Gen Virol. 1972 Nov;17(2):197–212. doi: 10.1099/0022-1317-17-2-197. [DOI] [PubMed] [Google Scholar]
- Esteban M., Kerr I. M. The synthesis of encephalomyocarditis virus polypeptides in infected L-cells and cell-free systems. Eur J Biochem. 1974 Jun 15;45(2):567–576. doi: 10.1111/j.1432-1033.1974.tb03583.x. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Tovell D. R. Characterization of the polypeptides formed in response to encephalomyocarditis virus ribonucleic acid in a cell-free system from mouse ascites tumor cells. J Virol. 1972 Jul;10(1):73–81. doi: 10.1128/jvi.10.1.73-81.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Encephalomyocarditis virus infection of mouse plasmacytoma cells. I. Inhibition of cellular protein synthesis. J Virol. 1974 Sep;14(3):598–610. doi: 10.1128/jvi.14.3.598-610.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- al-Janabi J., Hartsuck J. A., Tang J. Kinetics and mechanism of pepsinogen activation. J Biol Chem. 1972 Jul 25;247(14):4628–4632. [PubMed] [Google Scholar]