Abstract
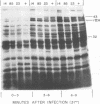
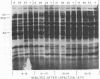
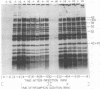
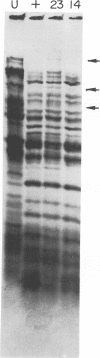
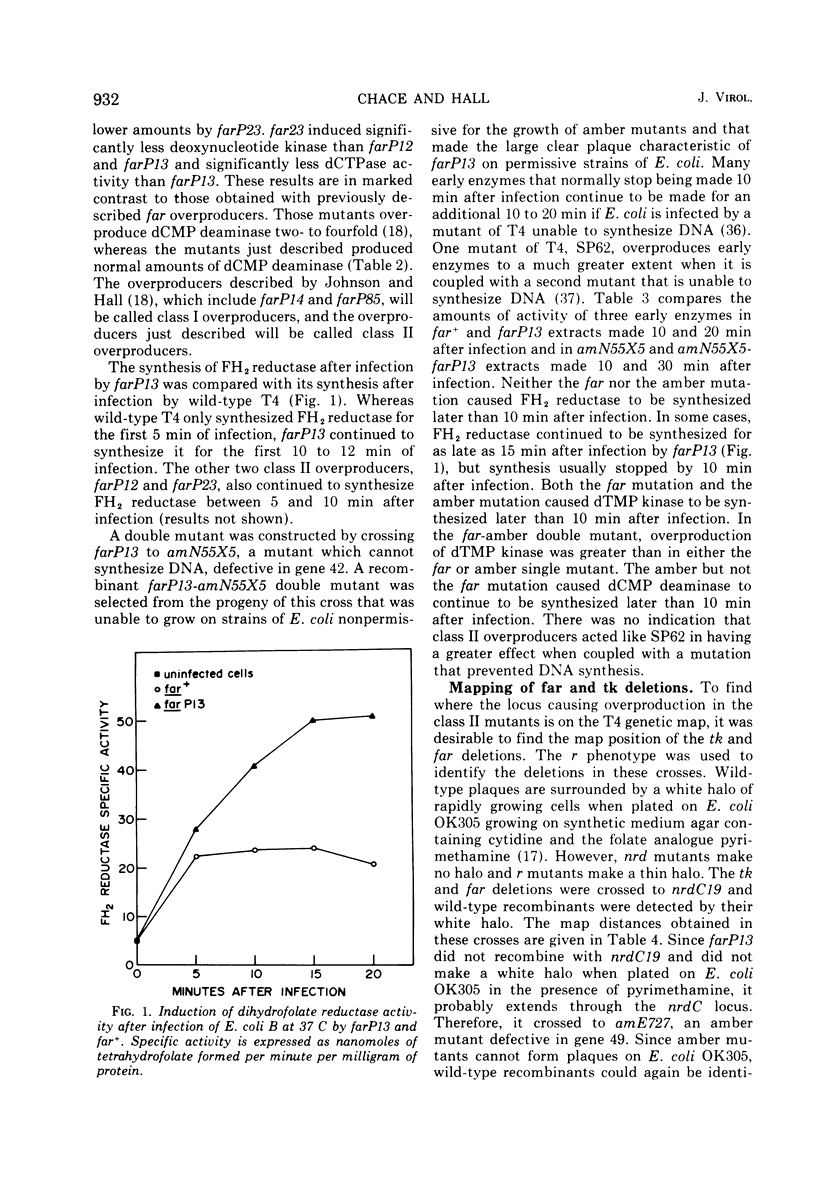
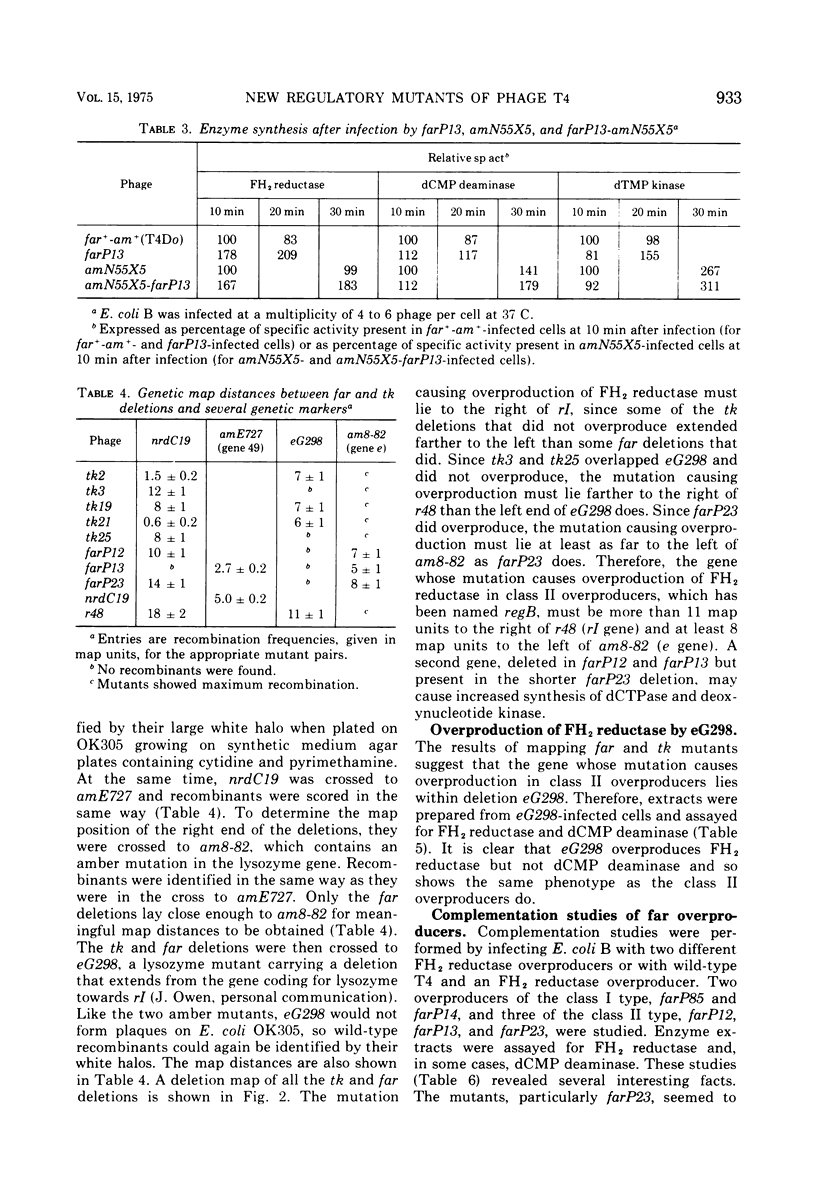
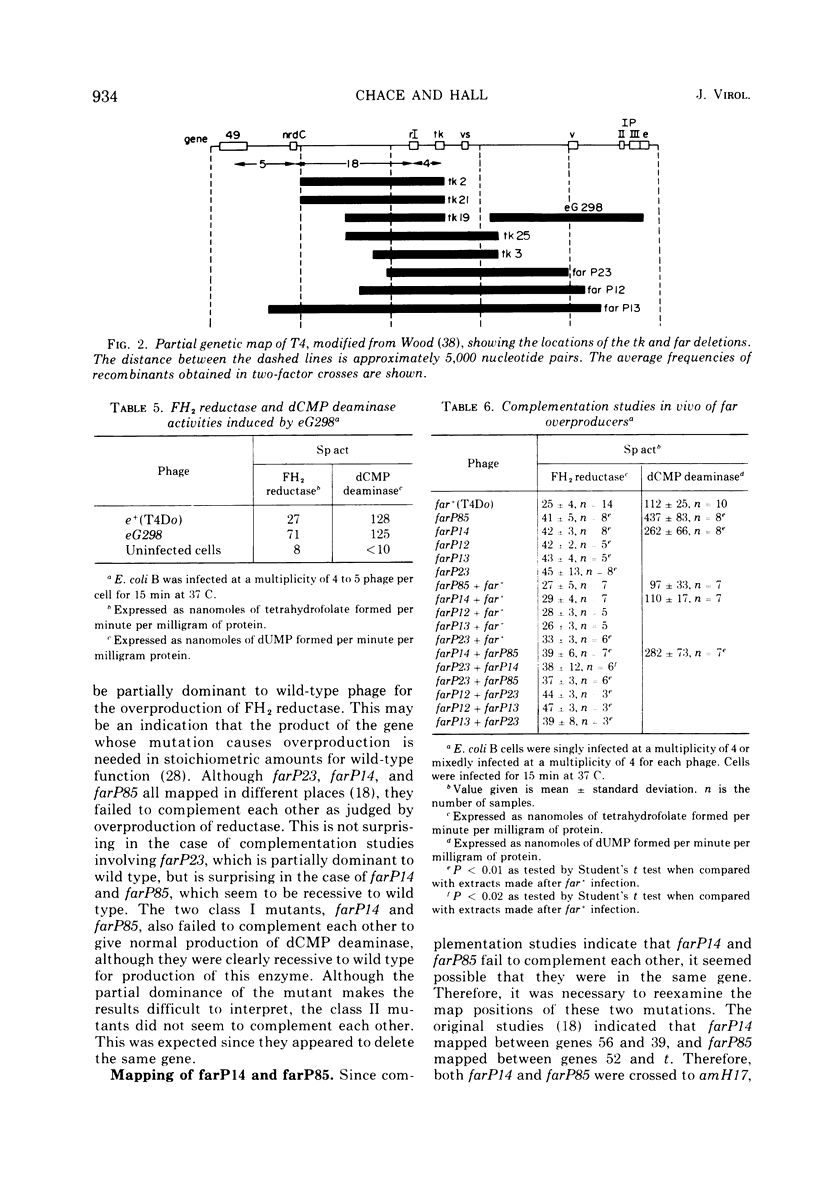
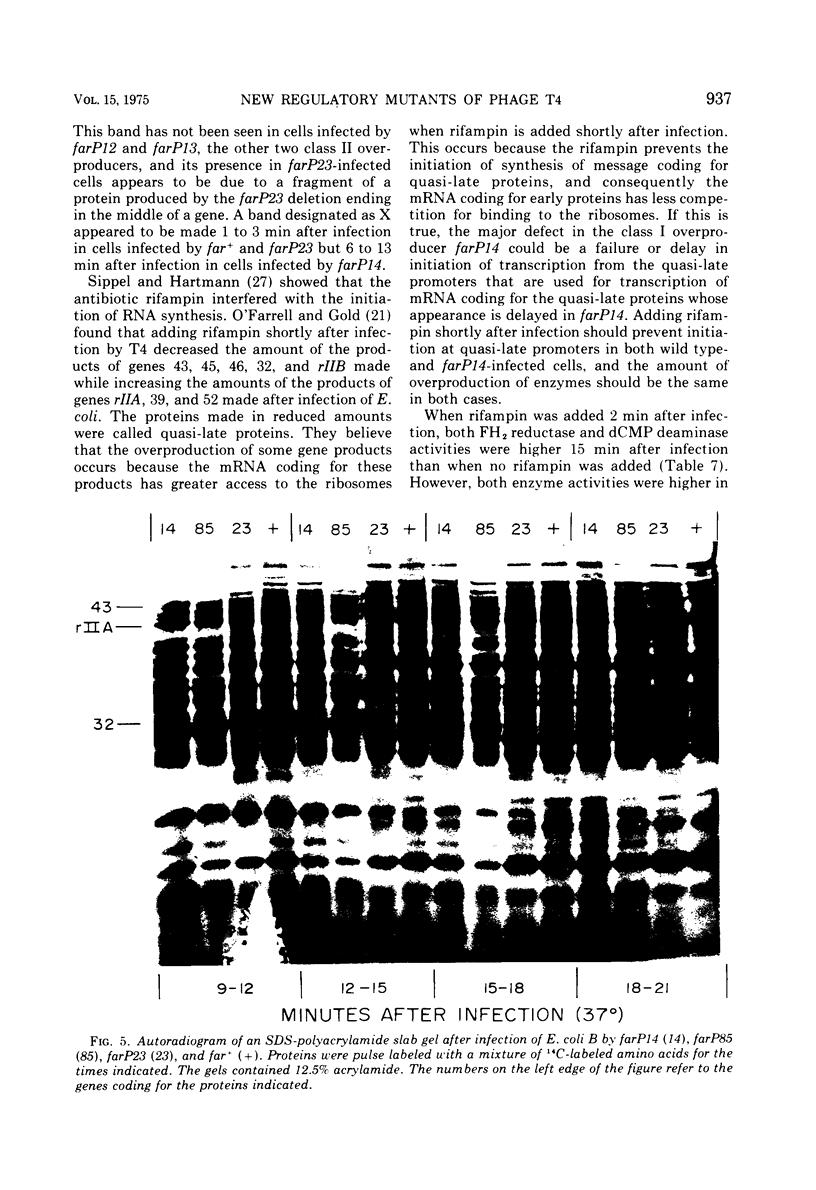
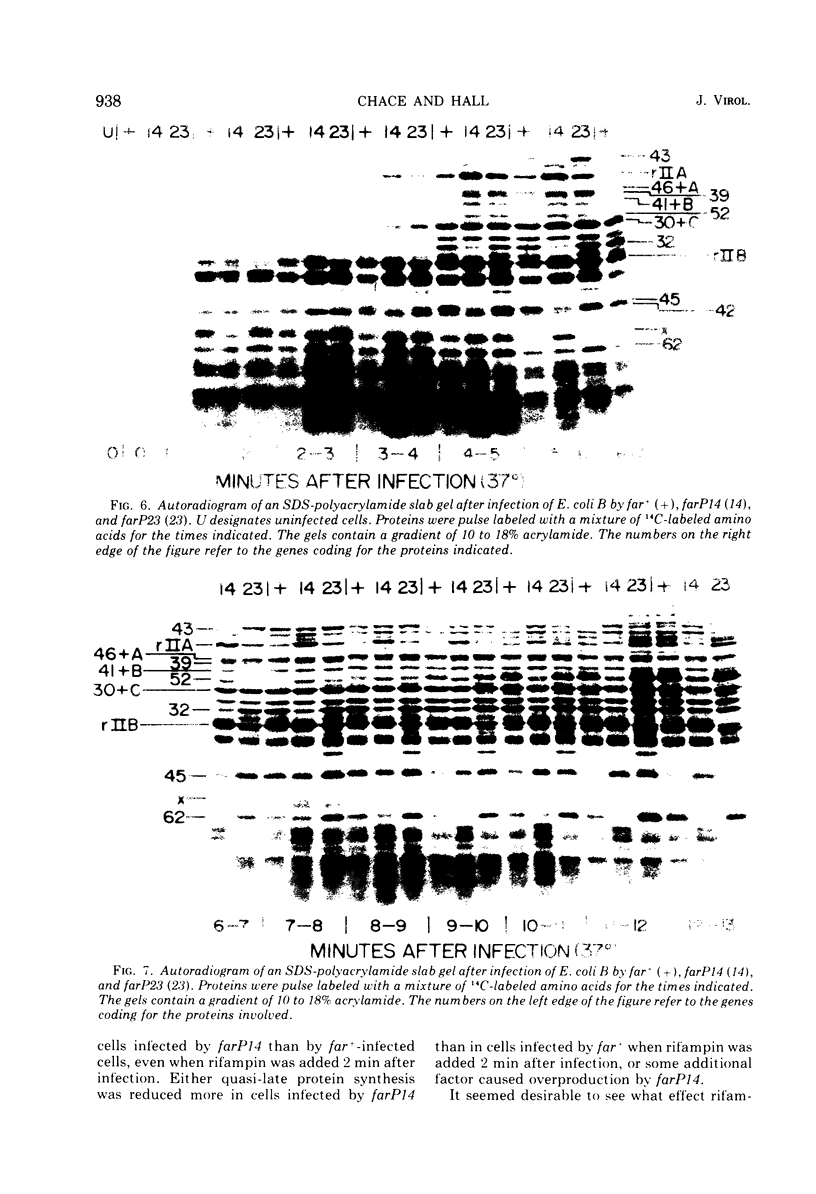
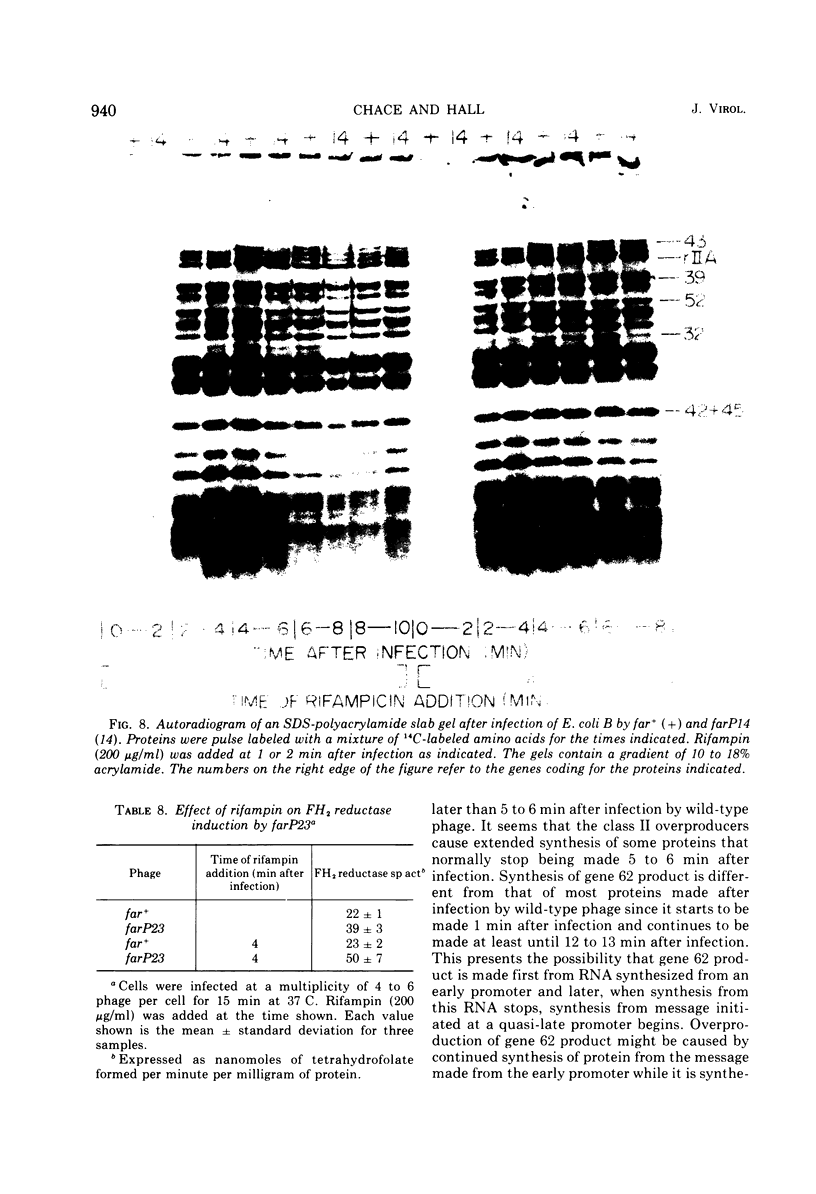
New mutants of bacteriophage T4 that overproduce the enzyme dihydrofolate reductase were investigated. Unlike previously described overproducers of this enzyme (Johnson and Hall, 1974), these mutants did not overproduce deoxycytidylate deaminase. Overproduction of dihydrofolate reductase by the new mutants occurred because enzymatic activity continued to increase for a longer period of time in cells infected by the mutants than in cells infected by wild-type phage. This continued increase occurred even in the presence of rifampin, indicating that the overproduction is probably due to a post-transcriptional event. Both these new overproducers and the previously described overproducers were studied by using polyacrylamide gel electrophoresis. The two types of overproducers appeared to be very different. The previously described overproducers showed a delay and/or reduction in the synthesis of several proteins that normally started to be made 4 to 6 min after infection. Several proteins could be seen to be overproduced on the gels. The new overproducers did not show the delay in the synthesis of some proteins and only overproduced a few proteins. The new gene defined by the new overproducers is between the gene coding for thymidine kinase and the gene coding for lysozyme.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzer S. ON THE TOPOGRAPHY OF THE GENETIC FINE STRUCTURE. Proc Natl Acad Sci U S A. 1961 Mar;47(3):403–415. doi: 10.1073/pnas.47.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner R., Souther A., Suggs S. Stability of cytosine-containing deoxyribonucleic acid after infection by certain T4 rII-D deletion mutants. J Virol. 1972 Jul;10(1):88–92. doi: 10.1128/jvi.10.1.88-92.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chace K. V., Hall D. H. Isolation of mutants of bacteriophage T4 unable to induce thymidine kinase activity. J Virol. 1973 Aug;12(2):343–348. doi: 10.1128/jvi.12.2.343-348.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doermann A H, Hill M B. Genetic Structure of Bacteriophage T4 as Described by Recombination Studies of Factors Influencing Plaque Morphology. Genetics. 1953 Jan;38(1):79–90. doi: 10.1093/genetics/38.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H., Bessman M. J. The enzymology of virus-infected bacteria. X. A biochemical-genetic study of the deoxynucleotide kinase induced by wild type and amber mutants of phage T4. J Biol Chem. 1967 Jun 25;242(12):2877–2885. [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- FUTTERMAN S. Enzymatic reduction of folic acid and dihydrofolic acid to tetrahydrofolic acid. J Biol Chem. 1957 Oct;228(2):1031–1038. [PubMed] [Google Scholar]
- Goscin L. A., Hall D. H. Hydroxyurea-sensitive mutants of bacteriophage T4. Virology. 1972 Oct;50(1):84–94. doi: 10.1016/0042-6822(72)90348-0. [DOI] [PubMed] [Google Scholar]
- Hall D. H. Mutants of bacteriophage T4 unable to induce dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1967 Aug;58(2):584–591. doi: 10.1073/pnas.58.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Tessman I., Karlström O. Linkage of T4 genes controlling a series of steps in pyrimidine biosynthesis. Virology. 1967 Mar;31(3):442–448. doi: 10.1016/0042-6822(67)90224-3. [DOI] [PubMed] [Google Scholar]
- Hall D. H., Tessman I. T4 mutants unable to induce deoxycytidylate deaminase activity. Virology. 1966 Jun;29(2):339–345. doi: 10.1016/0042-6822(66)90041-9. [DOI] [PubMed] [Google Scholar]
- Hercules K., Sauerbier W. Transcription units in bacteriophage T4. J Virol. 1973 Oct;12(4):872–881. doi: 10.1128/jvi.12.4.872-881.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules K., Sauerbier W. Two modes of in vivo transcription for genes 43 and 45 of phage T4. J Virol. 1974 Aug;14(2):341–348. doi: 10.1128/jvi.14.2.341-348.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda J., Levinthal C. Protein synthesis by Escherichia coli infected with bacteriophage T4D. Virology. 1968 Apr;34(4):709–727. doi: 10.1016/0042-6822(68)90092-5. [DOI] [PubMed] [Google Scholar]
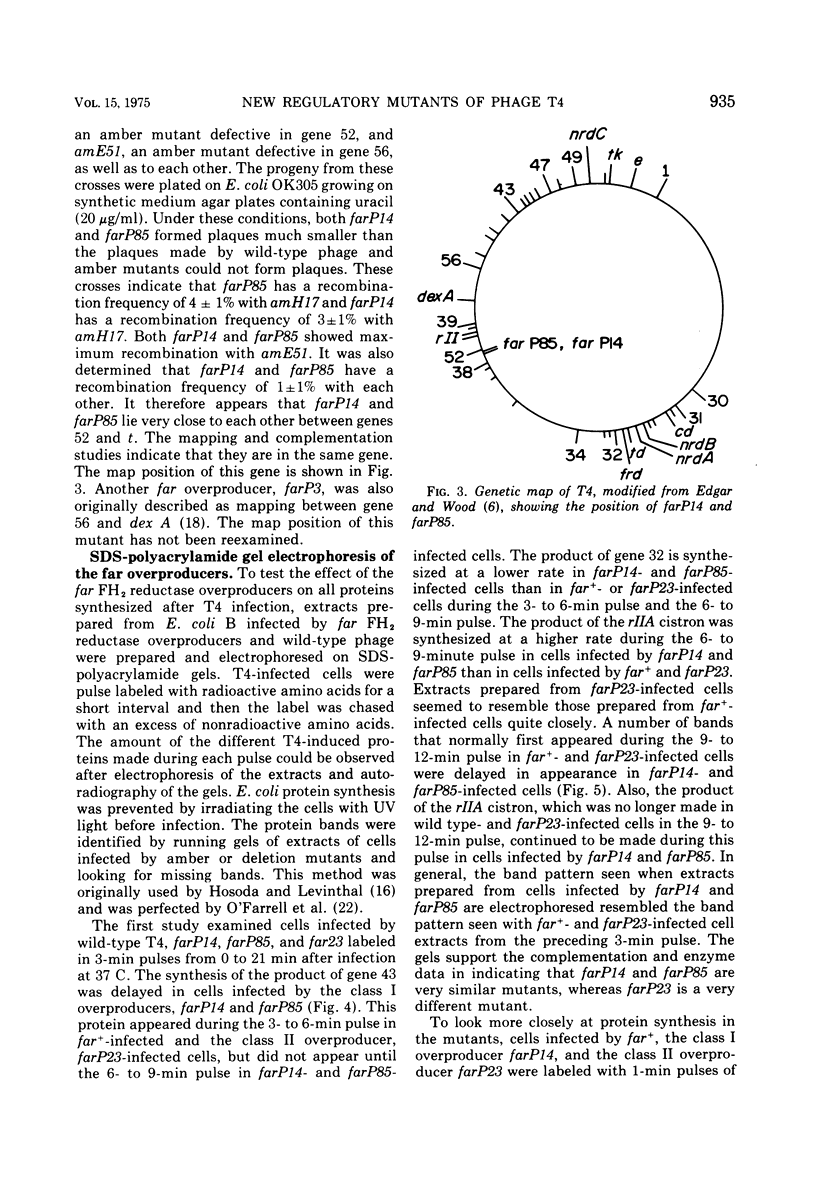
- Johnson J. R., Hall D. H. Characterization of new regulatory mutants of bacteriophage T4. J Virol. 1974 Mar;13(3):666–676. doi: 10.1128/jvi.13.3.666-676.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Hall D. H. Isolation and characterization of mutants of bacteriophage T4 resistant to folate analogs. Virology. 1973 Jun;53(2):413–426. doi: 10.1016/0042-6822(73)90221-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mattson T., Richardson J., Goodin D. Mutant of bacteriophage T4D affecting expression of many early genes. Nature. 1974 Jul 5;250(461):48–50. doi: 10.1038/250048a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Bacteriophage T4 gene expression. Evidence for two classes of prereplicative cistrons. J Biol Chem. 1973 Aug 10;248(15):5502–5511. [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- Price A. R., Warner H. R. A structural gene for bacteriophage T4-induced deoxycytidine triphosphate-deoxyuridine triphosphage nucleotidohydrolase. Virology. 1968 Nov;36(3):523–526. doi: 10.1016/0042-6822(68)90183-9. [DOI] [PubMed] [Google Scholar]
- Revel M., Pollack Y., Groner Y., Scheps R., Inouye H., Berissi H., Zeller H. IF3-interference factors: protein factors in Escherichia coli controlling initiation of mRNA translation. Biochimie. 1973;55(1):41–51. doi: 10.1016/s0300-9084(73)80235-4. [DOI] [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbier W., Hercules K. Control of gene function in bacteriophage T4. IV. Post-transcriptional shutoff of expression of early genes. J Virol. 1973 Sep;12(3):538–547. doi: 10.1128/jvi.12.3.538-547.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Snustad D. P. Dominance interactions in Escherichia coli cells mixedly infected with bacteriophage T4D wild-type and amber mutants and their possible implications as to type of gene-product function: catalytic vs. stoichiometric. Virology. 1968 Aug;35(4):550–563. doi: 10.1016/0042-6822(68)90285-7. [DOI] [PubMed] [Google Scholar]
- Stevens A. Deoxyribonucleic acid dependent ribonucleic acid polymerases from two T4 phage-infected systems. Biochemistry. 1974 Jan 29;13(3):493–503. doi: 10.1021/bi00700a015. [DOI] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Galivan J., Maley F. The temporal expression of T2r + bacteriophage genes in vivo and in vitro. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1659–1663. doi: 10.1073/pnas.69.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley G. F., Maley F. Relationship between Escherichia coli B titer and the level of deoxycytidylate deaminase activity induced on bacteriophage T2r + infection. J Virol. 1972 Mar;9(3):454–464. doi: 10.1128/jvi.9.3.454-464.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Lewis N. The synthesis of deoxycytidylate deaminase and dihydrofolate reductase and its control in Escherichia coli infected with bacteriophage T4 and T-4 amber mutants. Virology. 1966 May;29(1):172–175. doi: 10.1016/0042-6822(66)90208-x. [DOI] [PubMed] [Google Scholar]
- Wiberg J. S. Amber mutants of bacteriophage T4 defective in deoxycytidine diphosphatase and deoxycytidine triphosphatase. On the role of 5-hydroxymethylcytosine in bacteriophage deoxyribonucleic acid. J Biol Chem. 1967 Dec 25;242(24):5824–5829. [PubMed] [Google Scholar]
- Wiberg J. S., Mendelsohn S., Warner V., Hercules K., Aldrich C., Munro J. L. SP62, a viable mutant of bacteriophage T4D defective in regulation of phage enzyme synthesis. J Virol. 1973 Oct;12(4):775–792. doi: 10.1128/jvi.12.4.775-792.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y. C., Dubovi E. J., Tessman I. Control of pyrimidine biosynthesis by phage T4: mutants unable to catalyze the reduction of cytidine diphosphate. Virology. 1969 Apr;37(4):615–623. doi: 10.1016/0042-6822(69)90279-7. [DOI] [PubMed] [Google Scholar]