Abstract
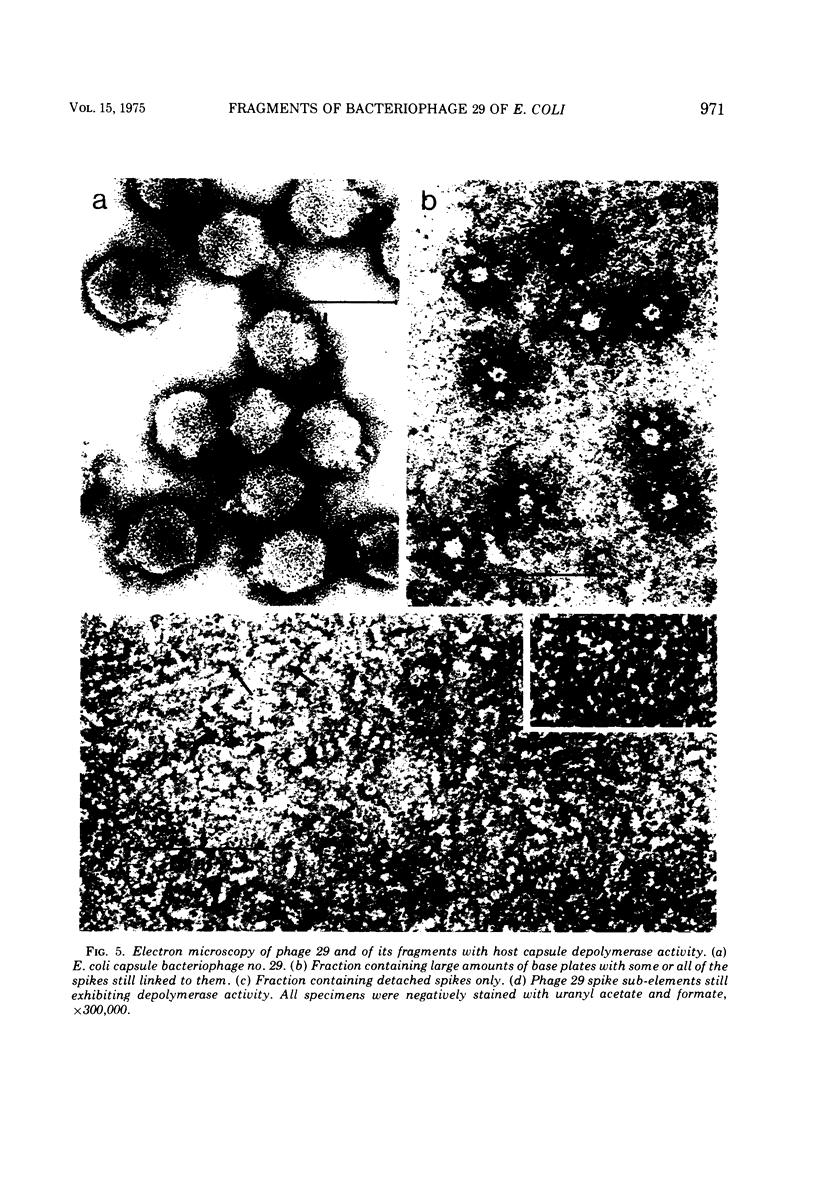
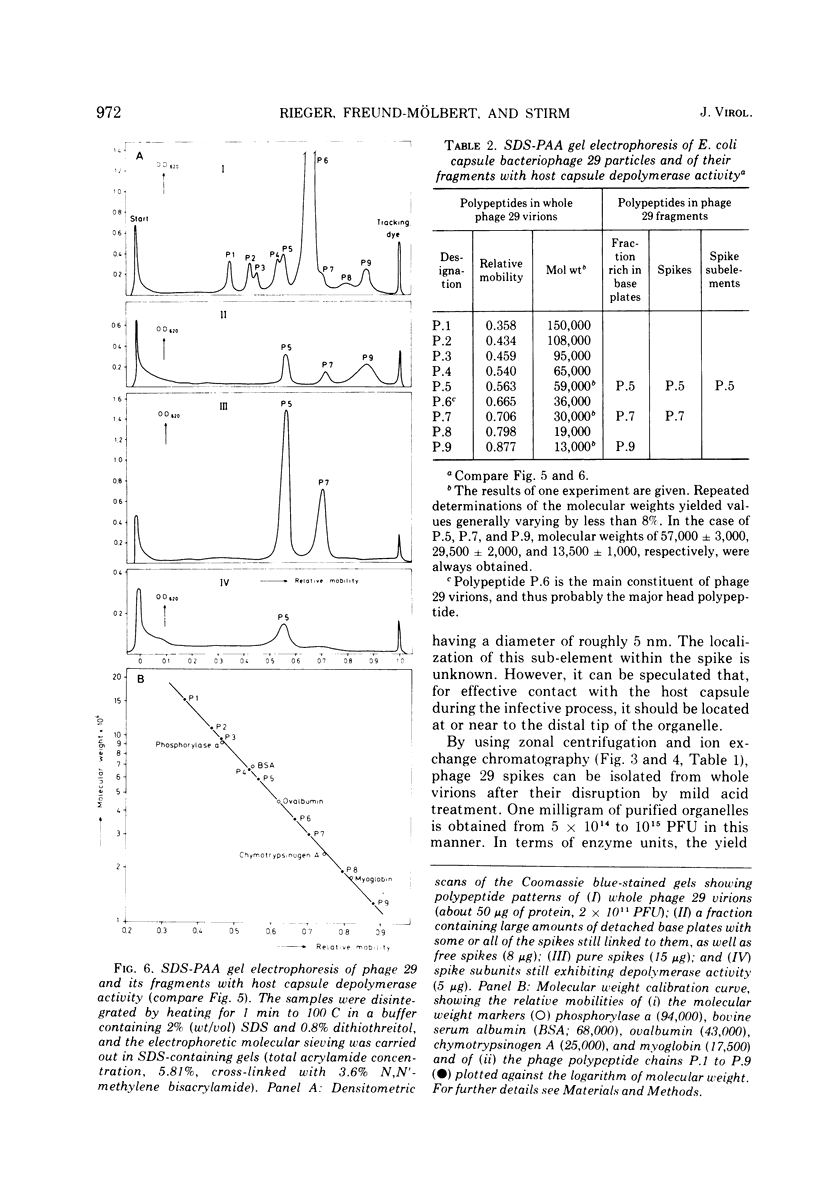
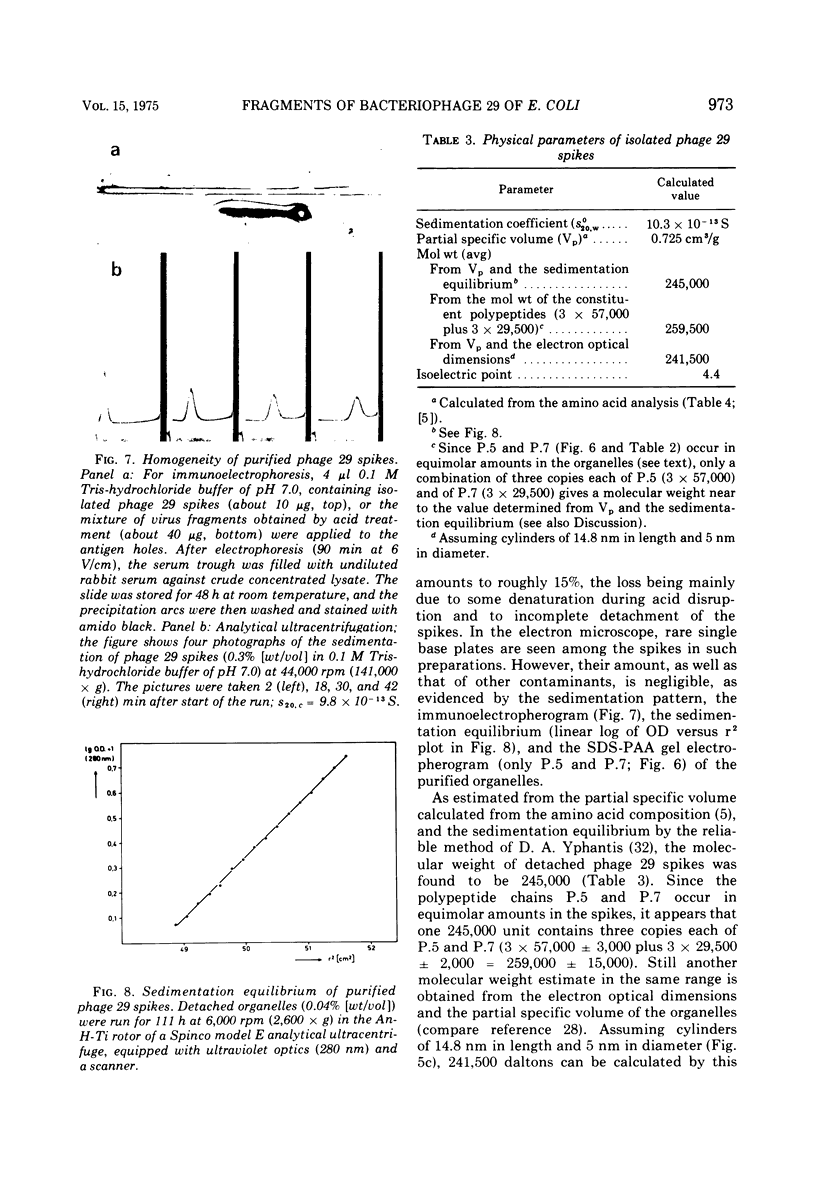
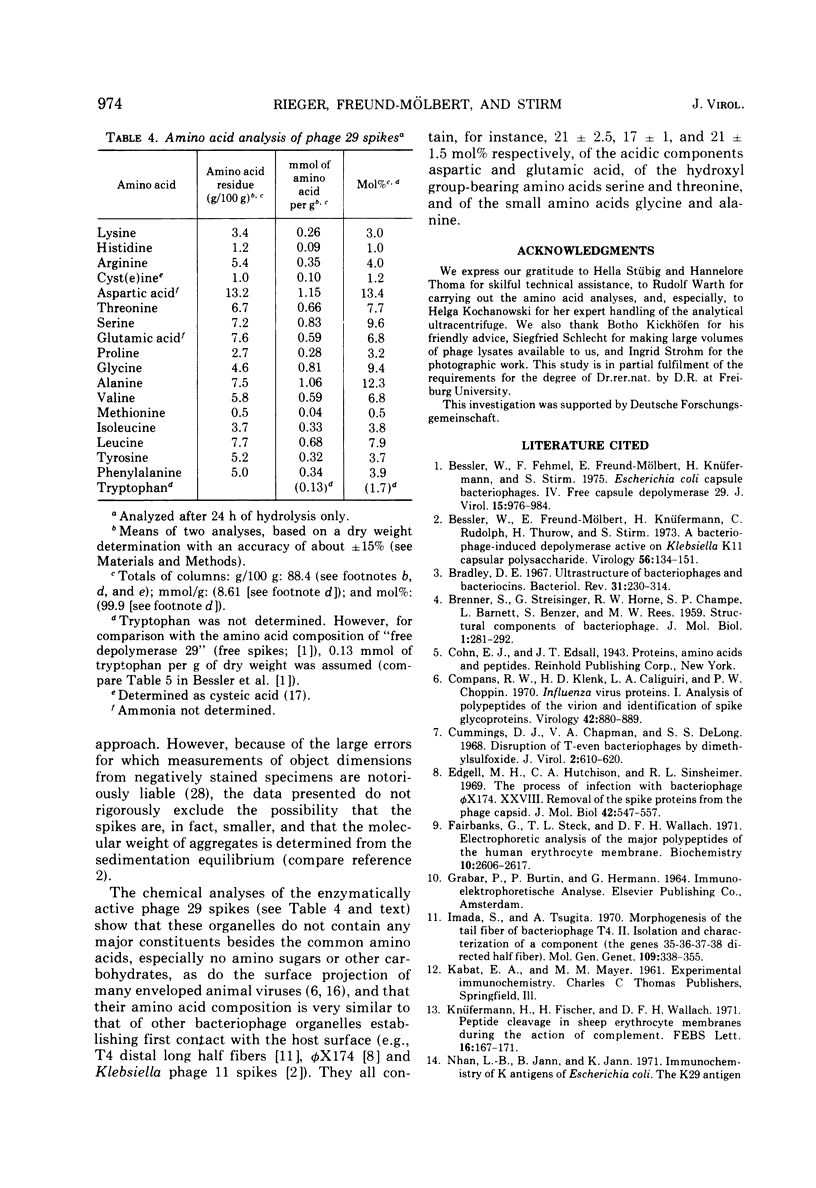
A glycanase activity, catalyzing the depolymerization of host capsular polysaccharide, is associated with Escherichia coli capsule bacteriophage no. 29, a small virus with an isometric head, carrying a base plate with a set of spikes. The bacteriophage particles were disrupted by mild acid treatment (5 to 8 min at pH 3.5 and 37 C), and the enzymatically active fragments were isolated and subjected to sodium dodecyl sulfate-gel electrophoresis as well as to electron microscopy. Of the at least nine different polypeptide chains found in the complete virion, three (of 57,000 plus or minus 3,000, 29,500 plus or minus 2,000 and 13,500 plus or minus 1,000 daltons) were detected in detached base plates. They had the appearance of six-pointed stars of about 14 nm in outer diameter, with a central hole or prop, carrying six (or, possibly, a multiple thereof) spikes. Two sizes of polypeptide chains (57,000 and 29,500) were found in pure spikes, cylindrical particles of about 14.5 to 15 nm in length and 5 nm in diameter, and one (57,000) in -- still capsule depolymerizing -- spike subunits of roughly 5 nm in diameter. Phage 29 spike preparations, homogeneous in analytical ultracentrifugation and immunoelectrophoresis, were found to have a molecular weight of 245,000, as determined from the sedimentation equilibrium, and to contain equimolar amounts of the two polypeptides, probably three copies of each per organelle. The amino acid analysis of the isolated spikes revealed that aspartic acid, alanine, serine, and glycine are their dominant constituents; no amino sugars or other carbohydrates were detected in the preparations.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessler W., Fehmel F., Freund-Mölbert E., Knüfermann H., Stirm S. Escherichia coli capsule bacteriophages. IV. Free capsule depolymerase 29. J Virol. 1975 Apr;15(4):976–984. doi: 10.1128/jvi.15.4.976-984.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessler W., Freund-Mölbert E., Knüfermann H., Rduolph C., Thurow H., Stirm S. A bacteriophage-induced depolymerase active on Klebsiella K11 capsular polysaccharide. Virology. 1973 Nov;56(1):134–151. doi: 10.1016/0042-6822(73)90293-6. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S. Disruption of T-even bacteriophages by dimethyl sulfoxide. J Virol. 1968 Jun;2(6):610–620. doi: 10.1128/jvi.2.6.610-620.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 28. Removal of the spike proteins from the phage capsid. J Mol Biol. 1969 Jun 28;42(3):547–557. doi: 10.1016/0022-2836(69)90242-3. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Imada S., Tsugita A. Morphogenesis of the tail fiber of bacteriophage T4. II. Isolation and characterization of a component (the genes 35-36-37-38 directing half fiber). Mol Gen Genet. 1970;109(4):338–355. doi: 10.1007/BF00267703. [DOI] [PubMed] [Google Scholar]
- Knüfermann H., Fischer H., Wallach D. F.H. Peptide cleavage in sheep erythrocyte membranes during the action of complement. FEBS Lett. 1971 Aug 15;16(3):167–171. doi: 10.1016/0014-5793(71)80123-0. [DOI] [PubMed] [Google Scholar]
- Le-Ba-Nhan, Jann B., Jann K. Immunochemistry of K antigens of Escherichia coli. The K29 antigen of E. coli 09:K29(A):H-. Eur J Biochem. 1971 Jul 29;21(2):226–234. doi: 10.1111/j.1432-1033.1971.tb01460.x. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Rifkin D., Compans R. W. Identification of the spike proteins of Rous sarcoma virus. Virology. 1971 Nov;46(2):485–489. doi: 10.1016/0042-6822(71)90049-3. [DOI] [PubMed] [Google Scholar]
- Spencer R. L., Wold F. A new convenient method for estimation of total cystine-cysteine in proteins. Anal Biochem. 1969 Oct 15;32(1):185–190. doi: 10.1016/0003-2697(69)90123-7. [DOI] [PubMed] [Google Scholar]
- Stirm S., Bessler W., Fehmel F., Freund-Mölbert E. Bacteriophage particles with endo-glycosidase activity. J Virol. 1971 Sep;8(3):343–346. doi: 10.1128/jvi.8.3.343-346.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirm S., Bessler W., Fehmel F., Freund-Mölbert E., Thurow H. Isolation of spike-formed particles from bacteriophage lysates. Virology. 1971 Jul;45(1):303–308. doi: 10.1016/0042-6822(71)90138-3. [DOI] [PubMed] [Google Scholar]
- Stirm S. Escherichia coli K bacteriophages. I. Isolation and introductory characterization of five Escherichia coli K bacteriophages. J Virol. 1968 Oct;2(10):1107–1114. doi: 10.1128/jvi.2.10.1107-1114.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirm S., Freund-Mölbert E. Escherichia coli capsule bacteriophages. II. Morphology. J Virol. 1971 Sep;8(3):330–342. doi: 10.1128/jvi.8.3.330-342.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirm S. Phage-receptor relationship reminiscent of the Vi II system. Nature. 1968 Aug 10;219(5154):637–639. doi: 10.1038/219637a0. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W., Wilkinson J. F. Depolymerases for bacterial exopolysaccharides obtained from phage-infected bacteria. J Gen Microbiol. 1965 Jun;39(3):373–383. doi: 10.1099/00221287-39-3-373. [DOI] [PubMed] [Google Scholar]
- To C. M., Kellenberger E., Eisenstark A. Disassembly of T-even bacteriophage into structural parts and subunits. J Mol Biol. 1969 Dec 28;46(3):493–511. doi: 10.1016/0022-2836(69)90192-2. [DOI] [PubMed] [Google Scholar]
- Ward S., Dickson R. C. Assembly of bacteriophage T4 tail fibers. 3. Genetic control of the major tail fiber polypeptides. J Mol Biol. 1971 Dec 28;62(3):479–492. doi: 10.1016/0022-2836(71)90149-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]