Abstract
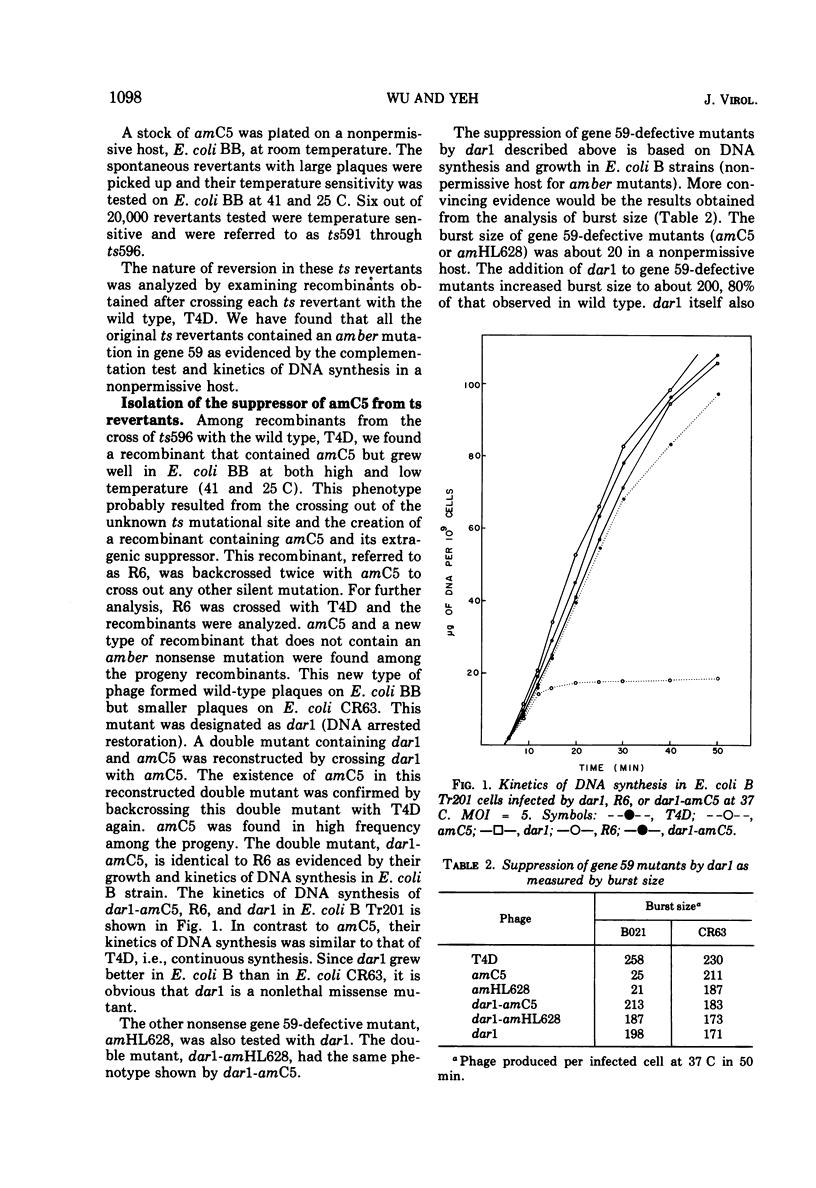
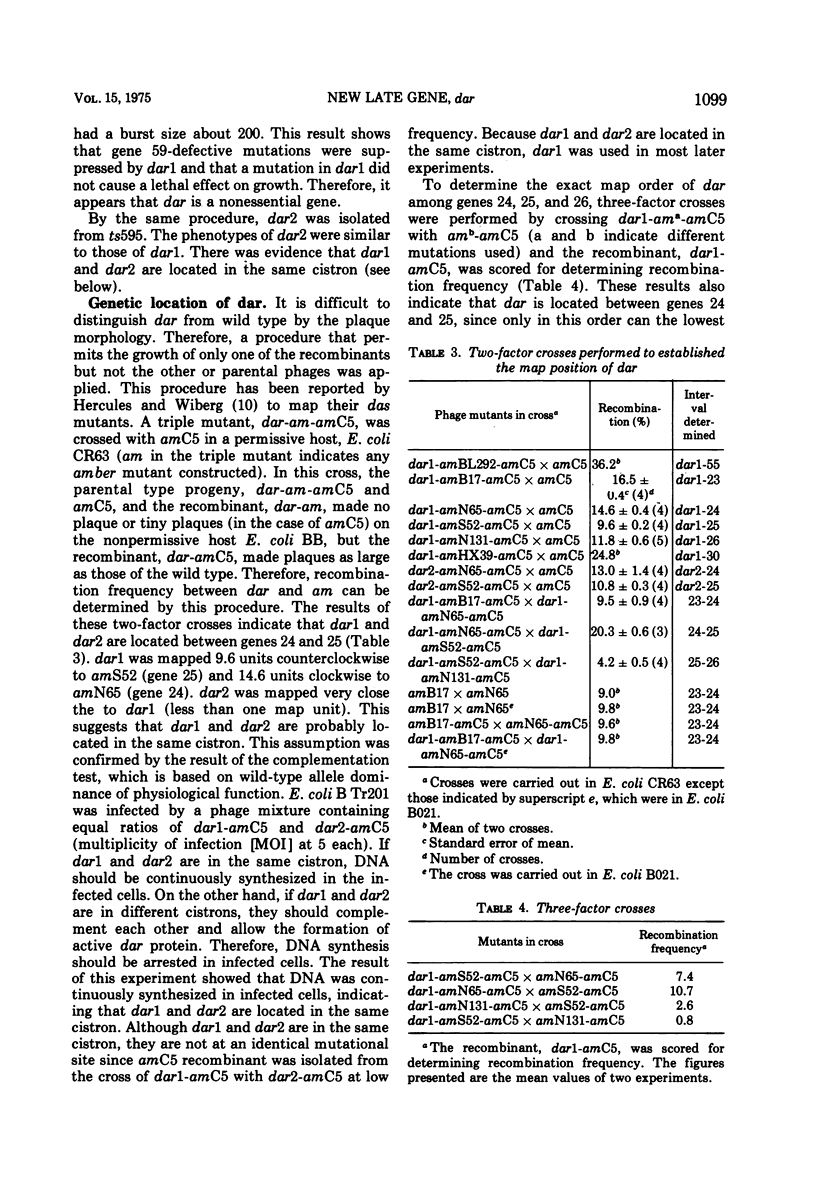
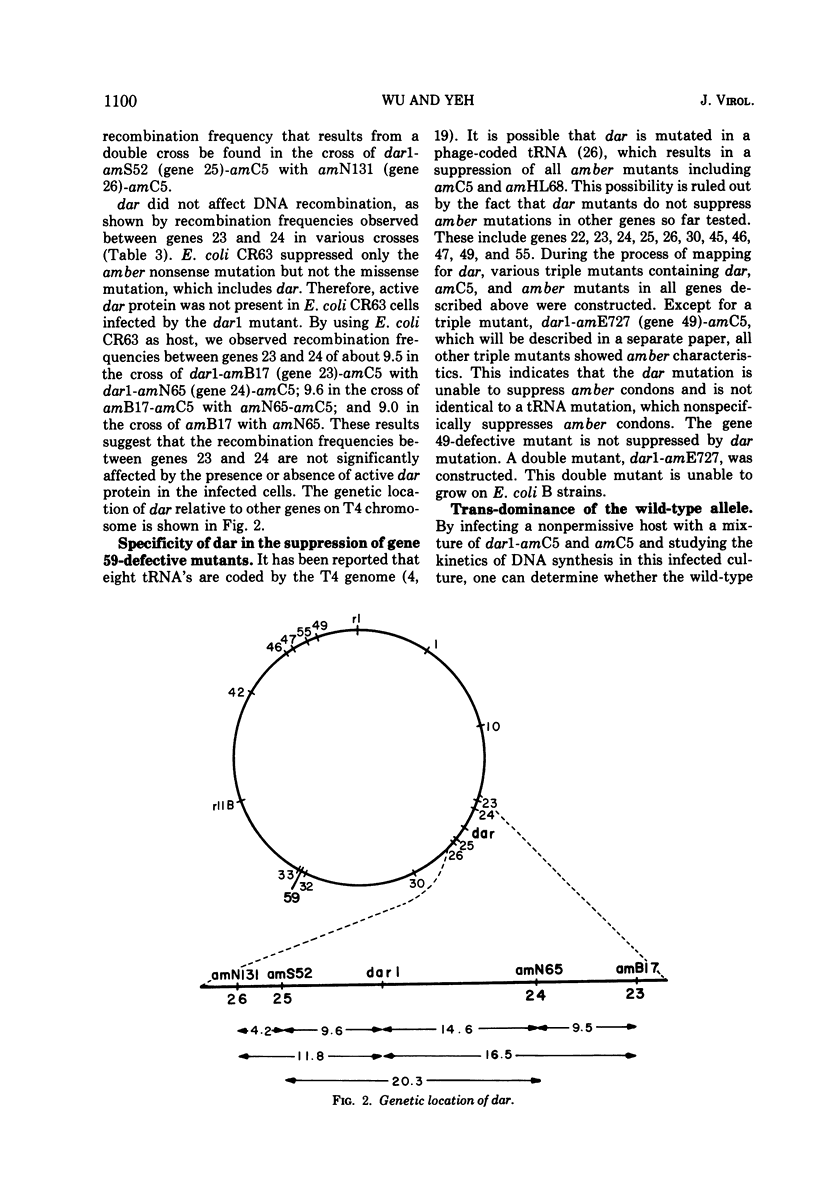
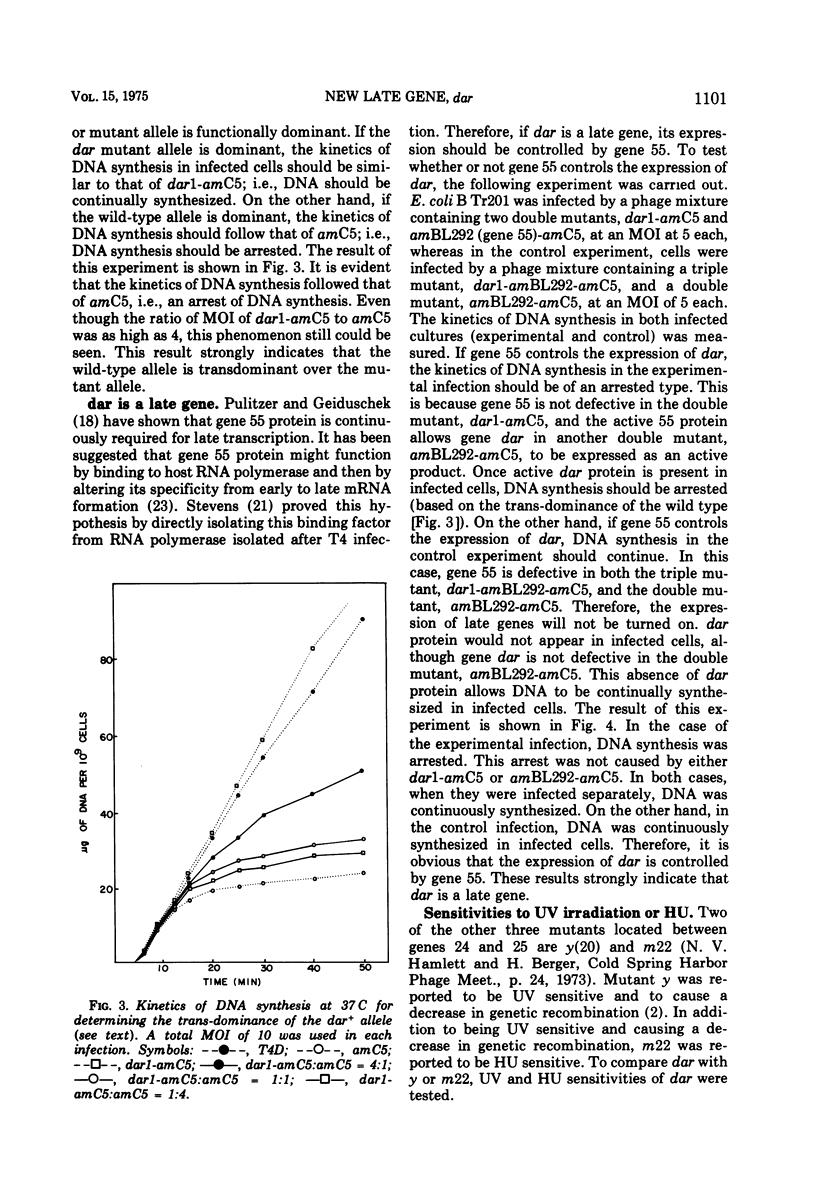
Suppressors of gene 59-defective mutants were isolated by screening spontaneous, temperature-sensitive (ts) revertants of the amber mutant, amC5, in gene 59. Six ts revertants were isolated. No gene 59-defective ts recombinant was obtained by crossing each ts revertant with the wild type, T4D. However, suppressors of gene 59-defective mutants were obtained from two of these ts revertants. These suppressor mutants are referred to as dar (DNA arrested restoration). dar mutants specifically restored the abnormalities, both in DNA synthesis and burst size, caused by gene 59-defective mutants to normal levels. It is unlikely that dar mutants are nonsense suppressors since theý failed to suppress amber mutations in 11 other genes investigated. The genetic expression of dar is controlled by gene 55; therefore, dar is a late gene. The genetic location of dar has been mapped between genes 24 and 25, a region contiguous to late genes. dar appears to be another nonessential gene of T4 since burst sizes of dar were almost identical to those of the wild type. Mutations in dar did not affect genetic recombination and repair of UV-damaged DNA, but caused a sensitivity to hydroxyurea in progeny formation. The effect of the dar mutation on host DNA degradation cannot account for its hydroxyurea sensitivity. dar mutant alleles were recessive to the wild-type allele as judged by restoration of arrested DNA synthesis. The possible mechanisms for the suppression of defects in gene 59 are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Meselson M. A T4-induced endonuclease which attacks T4 DNA. Proc Natl Acad Sci U S A. 1970 Jul;66(3):716–721. doi: 10.1073/pnas.66.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Symonds N. Radiation-sensitive mutants of T4D. I. T4y: a new radiation-sensitive mutant; effect of the mutation on radiation survival, growth and recombination. Mutat Res. 1969 Nov-Dec;8(3):431–439. doi: 10.1016/0027-5107(69)90060-8. [DOI] [PubMed] [Google Scholar]
- Chung S. T., Greenberg G. R. Temperature-sensitive revertants. Biochem Biophys Res Commun. 1971 Dec 3;45(5):1337–1343. doi: 10.1016/0006-291x(71)90166-5. [DOI] [PubMed] [Google Scholar]
- Daniel V., Sarid S., Littauer U. Z. Bacteriophage induced transfer RNA in Escherichia coli. New transfer RNA molecules are synthesized on the bacteriophage genome. Science. 1970 Mar 27;167(3926):1682–1688. doi: 10.1126/science.167.3926.1682. [DOI] [PubMed] [Google Scholar]
- EDGAR R. S., DENHARDT G. H., EPSTEIN R. H. A COMPARATIVE GENETIC STUDY OF CONDITIONAL LETHAL MUTATIONS OF BACTERIOPHAGE T4D. Genetics. 1964 Apr;49:635–648. doi: 10.1093/genetics/49.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R., Batcheler M. L., Clark C. K. The role of gene 49 in DNA replication and head morphogenesis in bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):439–463. doi: 10.1016/0022-2836(71)90147-1. [DOI] [PubMed] [Google Scholar]
- Gorini L., Beckwith J. R. Suppression. Annu Rev Microbiol. 1966;20:401–422. doi: 10.1146/annurev.mi.20.100166.002153. [DOI] [PubMed] [Google Scholar]
- Goscin L. A., Hall D. H. Hydroxyurea-sensitive mutants of T4. II. Degradation and utilization of bacterial DNA. Virology. 1973 Nov;56(1):207–217. doi: 10.1016/0042-6822(73)90300-0. [DOI] [PubMed] [Google Scholar]
- Hercules K., Munro J. L., Mendelsohn S., Wiberg J. S. Mutants in a nonessential gene of bacteriophage T4 which are defective in the degradation of Escherichia coli deoxyribonucleic acid. J Virol. 1971 Jan;7(1):95–105. doi: 10.1128/jvi.7.1.95-105.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules K., Wiberg J. S. Specific suppression of mutations in genes 46 and 47 by das, a new class of mutations in bacteriophage T4D. J Virol. 1971 Nov;8(5):603–612. doi: 10.1128/jvi.8.5.603-612.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R. Polypeptide bound to the host RNA polymerase is specified by T4 control gene 33. Nat New Biol. 1973 Aug 1;244(135):137–140. doi: 10.1038/newbio244137a0. [DOI] [PubMed] [Google Scholar]
- Kemper B., Hurwitz J. Studies on T4-induced nucleases. Isolation and characterization of a manganese-activated T4-induced endonuclease. J Biol Chem. 1973 Jan 10;248(1):91–99. [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. II. Studies on the maturation of gene 49-defective head intermediates. J Virol. 1972 Feb;9(2):377–389. doi: 10.1128/jvi.9.2.377-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Kozinski A. W., Litwin S. Molecular Recombination in T4 Bacteriophage Deoxyribonucleic Acid: III. Formation of Long Single Strands During Recombination. J Virol. 1970 Mar;5(3):368–380. doi: 10.1128/jvi.5.3.368-380.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulitzer J. F., Geiduschek E. P. Function of T4 gene 55. II. RNA synthesis by temperature-sensitive gene 55 mutants. J Mol Biol. 1970 Apr 28;49(2):489–507. doi: 10.1016/0022-2836(70)90259-7. [DOI] [PubMed] [Google Scholar]
- Scherberg N. H., Weiss S. B. Detection of bacteriophage T4- and T5-coded transfer RNAs. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1164–1171. doi: 10.1073/pnas.67.3.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Symonds N. The unexpected location of a gene conferring abnormal radiation sensitivity on phage T4. Nature. 1973 Feb 9;241(5389):395–396. doi: 10.1038/241395a0. [DOI] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESSMAN I. GENETIC ULTRAFINE STRUCTURE IN THE T4RII REGION. Genetics. 1965 Jan;51:63–75. doi: 10.1093/genetics/51.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. Bacteriophage sigma factor for RNA polymerase. Nature. 1969 Sep 13;223(5211):1107–1110. doi: 10.1038/2231107a0. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Effect of hydroxyurea on replication of bacteriophage T4 in Escherichia coli. J Virol. 1969 Mar;3(3):331–336. doi: 10.1128/jvi.3.3.331-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Snustad P., Jorgensen S. E., Koerner J. F. Isolation of bacteriophage T4 mutants defective in the ability to degrade host deoxyribonucleic acid. J Virol. 1970 Jun;5(6):700–708. doi: 10.1128/jvi.5.6.700-708.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. H., Kells S. Bacteriophage T4 transfer RNA. I. Isolation and characterization of two-phage-coded nonsense suppressors. J Mol Biol. 1972 Aug 14;69(1):39–56. doi: 10.1016/0022-2836(72)90022-8. [DOI] [PubMed] [Google Scholar]
- Wu J. R., Yeh Y. C. Requirement of a functional gene 32 product of bacteriophage T4 in UV, repair. J Virol. 1973 Oct;12(4):758–765. doi: 10.1128/jvi.12.4.758-765.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Ma F. J., Yeh Y. C. Suppression of DNA-arrested synthesis in mutants defective in gene 59 of bacteriophage T4. Virology. 1972 Jan;47(1):147–156. doi: 10.1016/0042-6822(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Wu R., Yeh Y. C. DNA arrested mutants of gene 59 of bacteriophage T4. II. Replicative intermediates. Virology. 1974 May;59(1):108–122. doi: 10.1016/0042-6822(74)90209-8. [DOI] [PubMed] [Google Scholar]
- Young C. W., Schochetman G., Karnofsky D. A. Hydroxyurea-induced inhibition of deoxyribonucleotide synthesis: studies in intact cells. Cancer Res. 1967 Mar;27(3):526–534. [PubMed] [Google Scholar]


