SUMMARY
Resveratrol induces mitochondrial biogenesis and protects against metabolic decline but whether SIRT1 mediates these benefits is the subject of debate. To circumvent the developmental defects of germ-line SIRT1 knockouts, we have developed the first inducible system that permits whole-body deletion of SIRT1 in adult mice. Mice treated with a moderate dose of resveratrol showed increased mitochondrial biogenesis and function, AMPK activation and increased NAD+ levels in skeletal muscle, whereas SIRT1 knockouts displayed none of these benefits. A mouse overexpressing SIRT1 mimicked these effects. A high dose of resveratrol activated AMPK in a SIRT1-independent manner, demonstrating that resveratrol dosage is a critical factor. Importantly, at both doses of resveratrol no improvements in mitochondrial function were observed in animals lacking SIRT1. Together these data indicate that SIRT1 plays an essential role in the ability of moderate doses of resveratrol to stimulate AMPK and improve mitochondrial function both in vitro and in vivo.
INTRODUCTION
The mammalian sirtuins (SIRT1-7) are a conserved family of NAD+-dependent deacetylases and ADP-ribosyltransferases involved in numerous fundamental cellular processes including gene silencing, DNA repair, and metabolic regulation (Baur et al., 2010; Donmez and Guarente, 2010; Haigis and Sinclair, 2010). Deletion of SIRT1 in outbred strains of mice abrogates the effect caloric restriction (CR) on physical activity (Chen et al., 2005) and lifespan extension (Boily et al., 2008), whereas overexpression of SIRT1 mimics many of the salutary effects of CR, including a reduced incidence of cardiovascular and metabolic diseases (Banks et al., 2008; Bordone et al., 2007; Pfluger et al., 2008), cancer (Herranz et al., 2010; Oberdoerffer et al., 2008), and neurodegeneration (Donmez et al., 2010; Qin et al., 2006). Recent human genetic studies also support a role for SIRT1 in maintaining human health status with age (Dong et al., 2011; Rutanen et al., 2010).
The polyphenol resveratrol (2,3,4′-trihydroxystilbene) first attracted scientific attention when it was linked to the cardiovascular benefits of red wine and was subsequently found to possess potent anti-tumor activity (Jang et al., 1997). In 2003, a screen for small molecule activators of SIRT1 identified 21 different SIRT1-activating molecules, the most potent of which was resveratrol (Howitz et al., 2003). In the majority of studies to date, resveratrol has been found to increase lifespan in Saccharomyces cerevisiae, Caenorhabditis elegans, and Drosophila melanogaster in a sirtuin-dependent manner, although the lifespan extension in yeast and flies, and the Sir2-dependence in worms have been challenged (Agarwal and Baur, 2011). In addition, resveratrol extends life and delays the onset of age-related phenotypes in a short-lived species of fish (Valenzano et al., 2006).
In obese rodents, treatment with resveratrol produces a variety of health benefits including improved metabolic and vascular function, decreased hepatic steatosis, reduced inflammation, greater endurance, and a gene expression pattern resembling calorie restriction (Barger et al., 2008a; Barger et al., 2008b; Baur et al., 2006; Lagouge et al., 2006; Pearson et al., 2008; Ramadori et al., 2009). Recent clinical studies show that resveratrol also confers metabolic benefits in humans (Brasnyo et al., 2011; Crandall JP, 2012). Understanding how resveratrol exerts its effects is important, not only for the potential insights into the biological causes of age-related diseases, but also to allow the development of more potent and specific molecules.
One of the most robust and reproducible effects of resveratrol treatment is an increase in mitochondrial mass (Baur et al., 2006; Lagouge et al., 2006). SIRT1 promotes mitochondrial biogenesis through deacetylation and activation of PGC-1α (Gerhart-Hines et al., 2007; Rodgers et al., 2005), a master regulator of mitochondrial biogenesis that co-activates the nuclear respiratory factors (NRF-1 and NRF-2), which induce the transcription of genes involved in mitochondrial biogenesis (Scarpulla, 2011). PGC-1α is also activated by another important metabolic sensor, the AMP-activated protein kinase (AMPK) (Jager et al., 2007). Though the effects of resveratrol and SIRT1 on PGC-1α are well established, there is considerable debate about the mechanism by which this regulation is achieved. One school of thought is that the direct activation of SIRT1 by resveratrol is an in vitro artifact (Beher et al., 2009; Borra et al., 2005; Kaeberlein et al., 2005; Pacholec et al., 2010) and that resveratrol works primarily by activating AMPK (Canto et al., 2009), potentially by inhibition of phospodiesterases (PDE), ATPase, or complex III (Gledhill et al., 2007; Hawley et al., 2010; Park et al., 2012; Zini et al., 1999). It has been proposed that AMPK then activates SIRT1 indirectly by elevating intracellular levels of its co-substrate, NAD+ (Canto et al., 2009; Fulco et al., 2008). Alternatively, resveratrol may first activate SIRT1 in vivo, leading to AMPK activation via deacetylation and activation of the AMPK kinase LKB1 (Hou et al., 2008; Ivanov et al., 2008; Lan et al., 2008).
Unfortunately, studies to date have been unable to determine which model is most relevant under physiological conditions. We and others have shown that resveratrol activates AMPK in cell culture and in vivo (Baur et al., 2006; Dasgupta and Milbrandt, 2007) and a study of AMPK knockout mice established that AMPK is required for many of the beneficial effects of resveratrol on metabolic function (Um et al., 2010). On the other hand, recent enzymological studies have presented evidence for direct SIRT1 activation by small molecules (Dai et al., 2010) and there is a growing literature of cell culture studies in which the effects of resveratrol are lost after knocking down or inhibiting SIRT1 (Breen et al., 2008; Csiszar et al., 2009; Fischer-Posovszky et al., 2010; Gracia-Sancho et al., 2010; He et al., 2010; Ivanov et al., 2008; Kao et al., 2010; Kim et al., 2011; Li et al., 2010; Lin et al., 2010; Ohguchi et al., 2010; Park et al., 2010; Shindler et al., 2010; Sulaiman et al., 2010; Tanno et al., 2010; Ungvari et al., 2009; Vetterli et al., 2011; Xia et al., 2011; Yang et al., 2010; Yoshizaki et al., 2010). Moreover, resveratrol’s central effects on liver gluconeogenesis (Ramadori et al., 2009) are abrogated when SIRT1 activity is impaired in the hypothalamus (Knight et al., 2011), and treatment of mice with a SIRT1 activator that is structurally unrelated to resveratrol, SRT1720, increases mitochondrial capacity in skeletal muscle (Feige et al., 2008) and liver in a SIRT1-dependent manner (Minor et al., 2011), while improving the health and survival of mice on a high fat diet, similar to what has been observed with resveratrol (Minor et al., 2011).
This study was aimed at testing whether the ability of resveratrol to activate AMPK and increase mitochondrial function requires SIRT1 in vivo, and whether SIRT1 overexpression is sufficient to mimic these effects. The main impediment to studying SIRT1 in vivo has been the poor survival, impaired growth, and developmental defects of germline SIRT1 knockout mice (Cheng et al., 2003; McBurney et al., 2003; Sequeira et al., 2008). While studies performed in outbred mice suggest that the effects of resveratrol treatment on cancer may be impaired in SIRT1 knockout mice (Boily et al., 2009; Boily et al., 2008), the knockout mice used in these studies were small, sterile and suffered from craniofacial abnormalities and eyelid inflammatory conditions, making interpretation of the data extremely difficult. To circumvent these issues, we have developed an adult-inducible SIRT1 knockout mouse. This mouse shows efficient deletion of SIRT1 across a variety of tissues and appears grossly normal and healthy beyond 1 year of age. Using these animals, as well as cell culture models, we observe that the stimulation of AMPK activity and increase in NAD+ levels, mitochondrial biogenesis, and mitochondrial function in skeletal muscle by a moderate dose of resveratrol is entirely dependent upon SIRT1. Further, overexpression of SIRT1 mimics the effects of resveratrol on both mitochondria and AMPK activation. Interestingly, a ~10-fold higher dose of resveratrol activates AMPK in a SIRT1-independent manner, though improvements in mitochondrial function are SIRT1-dependent. Taken together, these data highlight the differences resveratrol treatment can have at different doses and demonstrate an important role for SIRT1 in activating AMPK and mediating the benefits of resveratrol in vivo.
RESULTS
Resveratrol Improves Mitochondrial Function and Increases Mitochondrial Biogenesis in a SIRT1-Dependent Manner
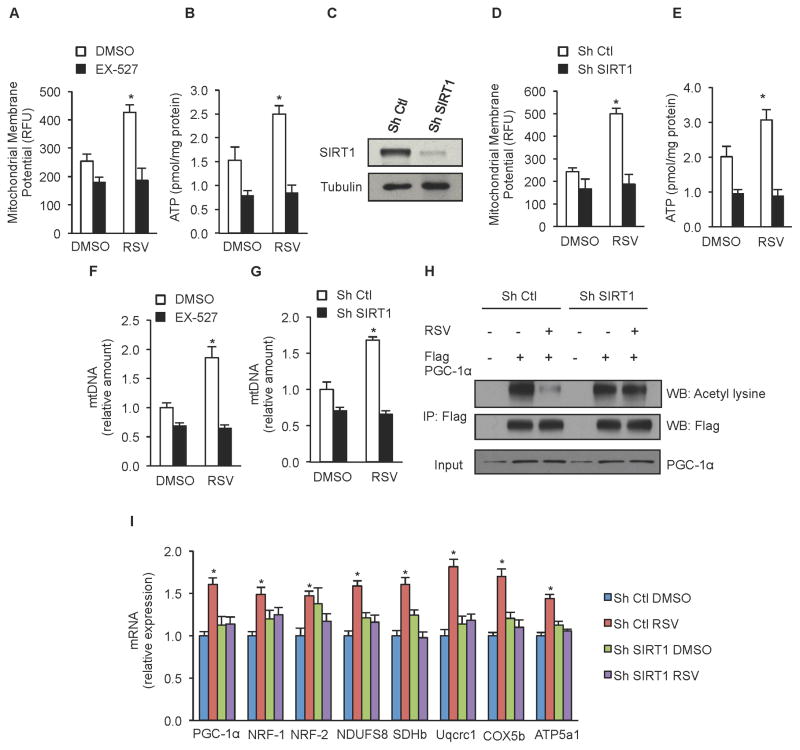
While both overexpression of SIRT1 and treatment with resveratrol have been shown to increase mitochondrial content via activation of PGC-1α, it remains to be established whether SIRT1 is required for resveratrol to improve mitochondrial function in skeletal muscle. Our initial investigation was performed using C2C12 myoblasts, a murine skeletal muscle cell line. Following treatment with 25 μM resveratrol, C2C12 cells showed a significant increase in mitochondrial membrane potential (Figure 1A) and cellular ATP content (Figure 1B). Treatment with the SIRT1 inhibitor EX-527 or shRNA knockdown of SIRT1 consistently reduced mitochondrial membrane potential and ATP content and completely abolished the ability of resveratrol to improve these parameters (Figure 1A–E).
Figure 1. Improved mitochondrial function and increased mitochondrial biogenesis in response to resveratrol treatment requires SIRT1.
(A) Mitochondrial membrane potential and (B) ATP content in C2C12 cells treated with 25 μM resveratrol and 10 μM EX-527 for 24h.
(C) Representative immunoblot for SIRT1 and tubulin in C2C12 cells infected with SIRT1 or non-targeting shRNA.
(D) Mitochondrial membrane potential and (E) ATP content in C2C12 cells infected with SIRT1 or non-targeting shRNA and treated with 25 μM resveratrol for 24h.
(F) Mitochondrial DNA content analyzed by means of quantitative PCR in C2C12 cells treated with 10 μM EX-527 or (G) infected with SIRT1 or non-targeting shRNA and treated with 25 μM resveratrol. Relative expression values were normalized to untreated cells.
(H) C2C12 cells infected with SIRT1 or non-targeting shRNA, and expressing Flag-HA-PGC-1α were treated with resveratrol 25 μM for 24h and PGC-1α acetylation was tested in Flag immunoprecipitates. Total PGC-1α was evaluated on total extracts as input.
(I) PGC-1α, NRF-1, NDUFS8, SDHb, Uqcrc1, COX5b, ATP5a1 mRNA analyzed by means of quantitative RT-PCR in C2C12 cells infected with SIRT1 or non-targeting shRNA after 24h treatment with 25 μM resveratrol. Relative expression values were normalized to untreated cells.
Values are expressed as mean ± SEM. (*p < 0.05 versus DMSO).
Further analysis revealed that resveratrol treatment increased mtDNA copy number in control cells (Figure 1F and 1G), suggesting that increased mitochondrial biogenesis may underlie the ability of resveratrol to improve mitochondrial function. Similar to the results with mitochondrial membrane potential and ATP, knockdown of SIRT1 or EX-527 treatment completely blocked the ability of resveratrol to increase mtDNA copy number. Immunoprecipitation of the SIRT1 target PGC-1α showed a substantial decrease in PGC-1α acetylation in resveratrol-treated cells, consistent with previous reports (Baur et al., 2006; Lagouge et al., 2006). Importantly, resveratrol treatment had no effect on PGC-1α acetylation in cells lacking SIRT1 (Figure 1H). Consistent with these findings, resveratrol treatment increased mRNA expression of a number of genes downstream of PGC-1α including transcription factors responsible for stimulating mitochondrial biogenesis (NRF-1, NRF-2) and components of the mitochondrial electron transport chain (NDUFS8, SDHb, Uqcrc1, COX5b, ATP5a1). All of the increases in gene expression were absent in cells treated with EX-527 or in which SIRT1 expression was knocked down (Figure 1I, data not shown).
Generation of Adult Inducible SIRT1 Knockout Mice
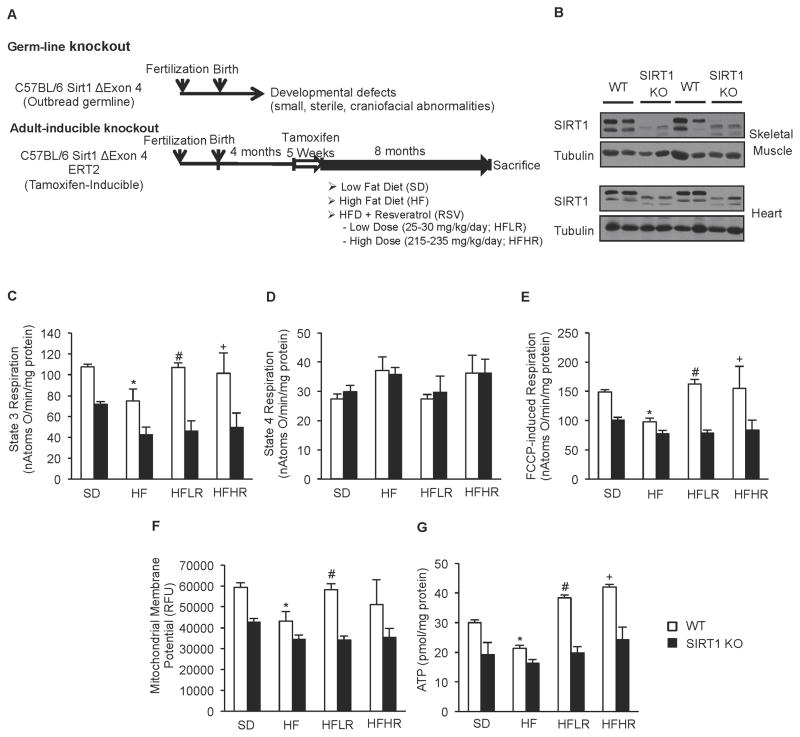
To test whether these findings were relevant in vivo, we generated an adult-inducible whole body SIRT1 knockout mouse designed to circumvent the developmental issues commonly observed in germline SIRT1 knockouts. We backcrossed tamoxifen inducible cre-ERT2 mice (Ruzankina et al., 2007) to C57BL/6J and combined this with floxed SIRT1Δex4 mice (Cheng et al., 2003). Tamoxifen treatment did not result in detectable liver damage, as reflected by serum aminotransferase levels 7 or 30 days after the final dose (Supplementary Figure S1E–H). Following tamoxifen treatment, S1Δex4ERT2 and control mice were placed on one of four different diets: a standard diet (SD), a high fat diet (HF; 60% FDC), a high fat diet supplemented with 400 mg resveratrol/kg of food (HFLR) or a high fat diet supplemented with a high dose of 4 g resveratrol/kg of food (HFHR). The former is a relatively low dose used in our laboratories’ previous studies, while the latter dose is similar to the concentrations used by other groups (Lagouge et al., 2006) (Figure 2A). Feeding of these diets resulted in an approximate daily dose of 25–30 mg/kg/day and 215–230 mg/kg of body weight/day, respectively.
Figure 2. Generation of adult inducible SIRT1 KO mice revealed that ability of resveratrol to improve mitochondrial function requires SIRT1 in vivo.
(A) Schematic representation of induction of SIRT1 KO and treatment with the different diets.
(B) Representative immunoblot for SIRT1 and tubulin in skeletal muscle and heart of WT and SIRT1 KO mice.
(C) State 3 respiration of isolated mitochondria from skeletal muscle of WT and SIRT1 KO mice on experimental diets (n=8) (*p<0.05 versus WT SD, #p<0.05 versus WT HFD).
(D) State 4 respiration of isolated mitochondria from skeletal muscle of WT and SIRT1 KO mice on experimental diets (n=8)
(E) FCCP-induced respiration of isolated mitochondria from skeletal muscle of WT and SIRT1 KO mice on experimental diets (n=8) (*p<0.05 versus WT SD, #p<0.05 versus WT HFD).
(F) Mitochondrial membrane potential of isolated mitochondria from skeletal muscle of WT and SIRT1 KO mice on experimental diets (n=8) (*p<0.05 versus WT SD, #p<0.05 versus WT HFD).
(G) Cellular ATP content from gastrocnemius of WT and SIRT1 KO mice on experimental diets (n=8) (*p<0.05 versus WT SD, #p<0.05 versus WT HFD, +p<0.05 versus WT HFD).
Deletion of the catalytic region of SIRT1 in S1ΔE4-ERT2 (SIRT1 KO) mice was evaluated by Western blot analysis. Full-length SIRT1 protein was nearly undetectable in skeletal and cardiac muscle of SIRT1 KO mice (Figure 2B) and substantially reduced (>85%) in all other tissues examined (Supplementary Figure S1D). In contrast to the much smaller size and developmental abnormalities reported for the germline knockouts (Boily et al., 2009; Boily et al., 2008), induction of SIRT1 knockout in adult mice did not result in any overt phenotype (Supplemental Figure S1A and S1B). Similarly, whole body measurements of various metabolic parameters did not reveal any obvious differences between SIRT1 KO and WT mice, although weight gain was slightly lower in KO mice fed the standard diet (Table S1).
Resveratrol Improves Mitochondrial Function in Skeletal Muscle of WT Mice, but has no Effect on Adult Inducible SIRT1 KO Mice
The function of mitochondria isolated from skeletal muscle was significantly impaired by feeding of a high fat diet, while treatment with both high and low doses of resveratrol prevented these deleterious effects. At both low and high doses, resveratrol produced substantial increases in ADP-induced respiration (State 3), maximal respiration (FCCP-induced), mitochondrial membrane potential, and cellular ATP levels (Figure 2C–G). Strikingly, none of the significant increases in mitochondrial function seen in the WT mice treated with resveratrol were observed in SIRT1 KO mice (Figure 2C–G). While the beneficial effects of resveratrol were clearly evident in animals treated with both doses of resveratrol, the variability between animals receiving the higher dose was considerably greater. For this reason, the majority of our subsequent analyses focused on the cohorts receiving the lower dose of resveratrol (i.e. 25–30 mg/kg/day).
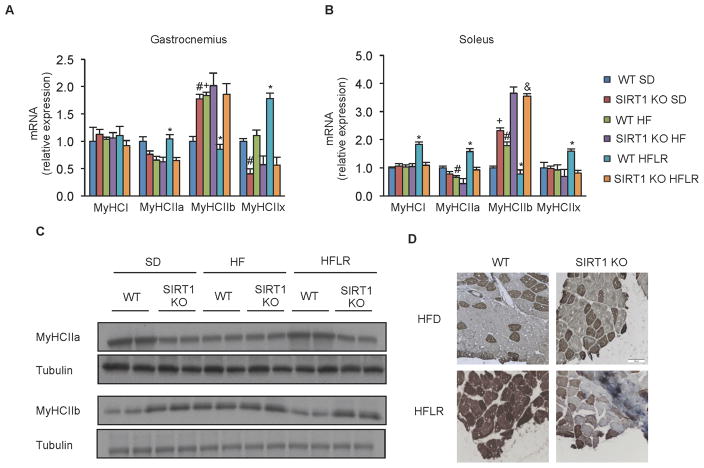
Treatment with Resveratrol Induces a SIRT1-Dependent Shift Toward more Oxidative Muscle Fibers
In addition to impairment in mitochondrial function, feeding of a high fat diet is known to cause an increased abundance of glycolytic muscle fibers. Thus, we tested whether treatment with resveratrol counteracted these changes and, if so, whether SIRT1 was required. Gene expression analysis of myosin heavy-chain genes from gastrocnemius muscle indicated that while the number of Type I muscle fibers was not altered by resveratrol, the abundance of highly glycolytic fast twitch (Type IIb) muscle fibers was lower, while more oxidative fast twitch (Type IIa and IIx) fibers were more abundant in resveratrol-treated mice (Figure 3A), consistent with the findings of Lagouge et al (2006). Together, these changes indicate an overall shift toward more oxidative fiber types in response to resveratrol treatment.
Figure 3. Resveratrol induces a shift toward more oxidative fibers in a SIRT1 dependent manner.
(A–B) MyHCI, MyHCIIa, MyHCIIb and MyHCIIx mRNA analyzed by quantitative RT-PCR in gastrocnemius (A) and soleus (B) of WT and SIRT1 KO mice on experimental diets. Relative expression values were normalized to WT SD mice (n=4) *p<0.05 versus WT HFD, #p<0.05 versus WT SD, +p<0.05 versus WT SD).
(C) Representative immunoblot for MyHCIIa, MyHCIIb and tubulin in gastrocnemius of WT and SIRT1 KO mice on experimental diets.
(D) Representative MyHCIIa immunostaining in gastrocnemius of WT and SIRT1 KO mice on experimental diets.
Values are expressed as mean ± SEM.
Interestingly, as with the measures of mitochondrial function, these changes in muscle type were entirely dependent upon SIRT1 (Figure 3A). The shift towards more oxidative fibers and the SIRT1-dependence of these effects was not exclusive to gastrocnemius, as similar gene expression changes were observed in the soleus muscle as well (Figure 3B). This induction of oxidative type II fibers in response to resveratrol treatment in WT but not SIRT1 KO mice was confirmed by Western blot and histological analysis (Figure 3C and 3D). These data demonstrate that the ability of resveratrol to induce a shift toward more oxidative muscle fibers, improve mitochondrial function, and increase cellular ATP requires SIRT1.
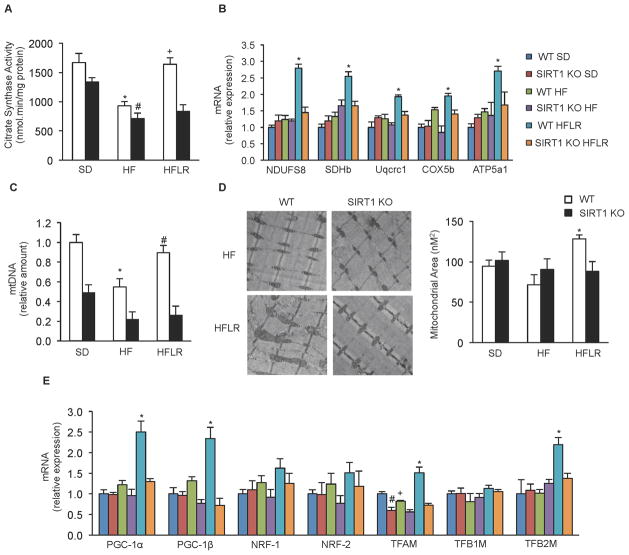
Stimulation of Mitochondrial Biogenesis in Skeletal Muscle Requires Functional SIRT1
We next sought to understand more precisely the mechanisms by which resveratrol increases mitochondrial function and ATP production, and to investigate their relationship to SIRT1. Based on previous work, we expected that resveratrol treatment would increase mitochondrial biogenesis and we hypothesized that these effects would be dependent upon SIRT1. We first assessed citrate synthase activity, a commonly used marker of mitochondrial content. Consistent with the changes observed in fiber type and mitochondrial function, the high fat diet decreased citrate synthase activity in the gastrocnemius of WT mice and resveratrol treatment completely prevented this decrease. Similarly, mRNA levels of components of the mitochondrial ETC were increased by resveratrol treatment. Interestingly, none of these effects were observed in SIRT1 KO mice (Figure 4A and 4B).
Figure 4. Resveratrol improves mitochondrial biogenesis in skeletal muscle of WT but not SIRT1 KO mice.
(A) Citrate synthase activity measured in gastrocnemius from WT and SIRT1 KO mice on experimental diets (n=8) (*p<0.05 versus WT SD, #p<0.05 versus SIRT1 KO SD, +p<0.05 versus WT HFD).
(B) NDUFS8, SDHb, Uqcrc1, COX5b and ATP5a1 mRNA analyzed by quantitative RT-PCR in gastrocnemius of WT and SIRT1 KO mice on experimental diets. Relative expression values were normalized to WT SD mice. (n=4 experiments *p<0.05 versus WT HFD).
(C) Mitochondrial DNA content analyzed by quantitative PCR in gastrocnemius of WT and SIRT1 KO mice on experimental diets. Relative expression values were normalized to WT SD mice. (n=8 experiments *p<0.05 versus WT SD, #p<0.05 versus WT HFD).
(D) Electronic microscopy analysis of gastrocnemius from WT and SIRT1 KO mice on experimental diets and the respective mitochondrial area quantification (n=4) (*p<0.05 versus WT HFD).
(E) PGC-1α, PGC-1β, NRF-1, NRF-2, TFAM, TFB1M and TFB2M mRNA analyzed by means of quantitative RT-PCR in gastrocnemius of WT and SIRT1 KO mice on experimental diets. Relative expression values were normalized to WT SD mice. (n=4) (*p<0.05 versus WT HFD, #p < 0.05 versus WT SD, +p<0.05 versus WT SD).
Values are expressed as mean ± SEM.
Consistent with the results in C2C12 cells, the decrease in mtDNA copy number in HFD mice was prevented by resveratrol treatment in the gastrocnemius of WT mice, while the SIRT1 KO mice showed no response to resveratrol treatment (Figure 4C). Measurement and quantification of mitochondrial mass by electron microscopy showed that resveratrol treatment induced an increase in mitochondrial area in WT but not in SIRT1 KO mice (Figure 4D, Supplemental Figure S2). Additionally, transcript levels of PGC-1α, PGC-1β, and the mitochondrial transcription factors TFAM and TFB2M, were increased in resveratrol-treated mice in a SIRT1-dependent manner (Figure 4E). Importantly, these effects were not exclusive to gastrocnemius, as similar changes in citrate synthase activity, mtDNA copy number, and the transcription of both ETC components and mitochondrial biogenesis factors were also seen in the soleus and cardiac tissue of WT mice in response to resveratrol treatment but were absent in SIRT1 KO mice (Supplemental Figure S3). Overall, our findings from adult-inducible SIRT1 KO mice demonstrate that resveratrol increases mitochondrial biogenesis, induces a shift toward more oxidative muscle fibers, and improves mitochondrial function in mice on a high fat diet, and that all of these beneficial effects require SIRT1.
Overexpression of SIRT1 Mimics the Effects of Resveratrol Treatment in Skeletal Muscle
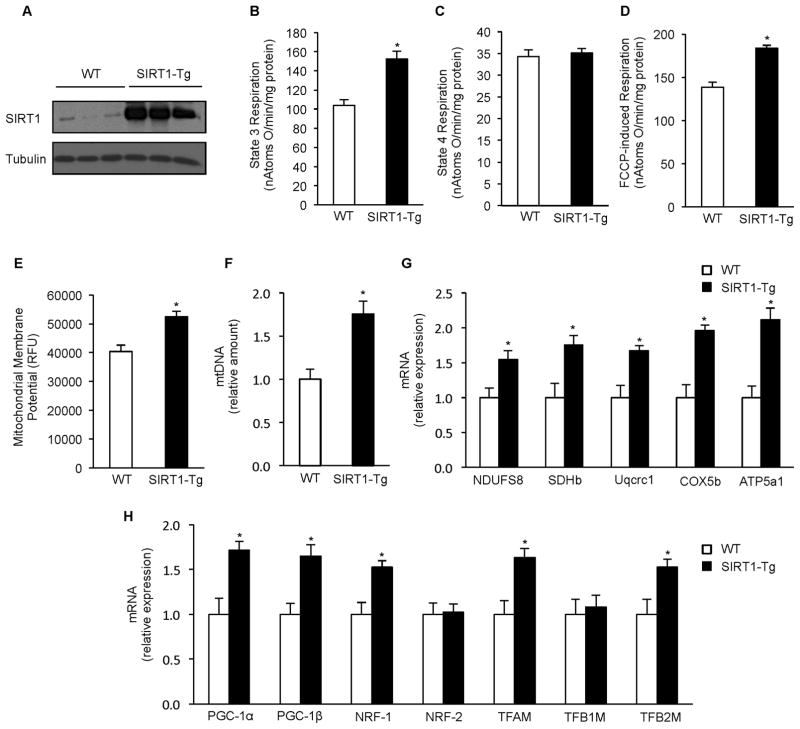
These data are consistent with the hypothesis that resveratrol acts via SIRT1 to increase mitochondrial function in vivo. This hypothesis also predicts that SIRT1 overexpression should be sufficient to mimic the effects of resveratrol. To test this, we generated a SIRT1 transgenic mouse (SIRT1-Tg) that constitutively expresses high levels of SIRT1 in skeletal muscle and other tissues (Figure 5A, Supplemental Figure S4A). This mouse differs from previous whole-body SIRT1 transgenics that either overexpress SIRT1 at lower levels (1.5 – 2 fold) (Banks et al., 2008; Pfluger et al., 2008) or predominately in brain and adipose tissue (Bordone et al., 2007). Interestingly, despite the high levels of SIRT1 expression in multiple tissues (>5X), there was no detectable difference in overall appearance, body weight, or home cage behavior.
Figure 5. Mice overexpressing SIRT1 mimic the effects of resveratrol on mitochondrial function and biogenesis.
(A) Representative immunoblot for SIRT1 and tubulin in gastrocnemius of 6 months old WT and SIRT1 Tg mice.
(B) State 3 respiration of isolated mitochondria from skeletal muscle of WT and SIRT1 Tg mice (n=6).
(C) State 4 respiration of isolated mitochondria from skeletal muscle of WT and SIRT1 Tg mice (n=6)
(D) FCCP-induced respiration of isolated mitochondria from skeletal muscle of WT and SIRT1 Tg mice (n=6).
(E) Mitochondrial membrane potential of isolated mitochondria from skeletal muscle of WT and SIRT1 Tg mice (n=6).
(F) Mitochondrial DNA content analyzed by means of quantitative PCR in skeletal muscle of WT and SIRT1 Tg mice. Relative expression values were normalized to WT mice. (n=6).
(G) NDUFS8, SDHb, Uqcrc1, COX5b and ATP5a1 mRNA analyzed by quantitative RT-PCR in gastrocnemius of WT and SIRT1 Tg mice. Relative expression values were normalized to WT mice. (n=6).
(H) PGC-1α, PGC-1β, NRF-1, NRF-2, TFAM, TFB1M and TFB2M mRNA analyzed by quantitative RT-PCR in gastrocnemius of WT and SIRT1 Tg mice. Relative expression values were normalized to WT mice. (n=6).
Values are expressed as mean ± SEM (*p < 0.05 versus WT).
In a striking recapitulation of the effects of resveratrol treatment, mitochondria isolated from the skeletal muscle of the SIRT1-Tg mice had significantly greater mitochondrial membrane potential, State 3 respiration, and maximal respiration (Figure 5B–E). Additionally, mtDNA copy number (Figure 5F) as well as mRNA levels of ETC components and regulators of mitochondrial biogenesis that were induced by resveratrol treatment were similarly upregulated in mice overexpressing SIRT1, while those unaffected by resveratrol were also unchanged in SIRT1-Tg mice (Figure 5G and 5H). Together these data show for the first time that overexpression of SIRT1 in skeletal muscle is sufficient to induce mitochondrial biogenesis and improve mitochondrial function. When coupled with the SIRT1 KO data establishing that SIRT1 is required for the beneficial effects of resveratrol, these results provide strong evidence that the ability of resveratrol to improve mitochondrial function in skeletal muscle is due to SIRT1-mediated stimulation of mitochondrial biogenesis.
Resveratrol Alters Metabolism in SIRT1 KO Livers but not Primary KO Hepatocytes
As resveratrol has previously been found to improve metabolic function in mice on a high fat diet (Baur et al., 2006; Lagouge et al., 2006) and mitochondrial dysfunction is known to play an important role in the development of glucose imbalance in diabetes (Lowell and Shulman, 2005), we sought to determine whether the ability of resveratrol to improve glucose homeostasis was lost in our inducible SIRT1 knockout mice. As expected, blood glucose levels were elevated in high fat diet fed mice under both fed and fasted conditions. Resveratrol treatment partially prevented the increase in both fed and fasted blood glucose levels induced by high fat diet in both WT and SIRT1 KO mice, suggesting that these effects are at least partially independent of SIRT1 (Supplementary Figure S5A and B). Similarly, resveratrol treated WT and SIRT1 KO mice performed better in a glucose tolerance test (Supplementary Figure S5D and E). As the liver is known to play a key role in regulating glucose homeostasis and resveratrol was previously shown to improve liver function (Baur et al., 2006; Baur and Sinclair, 2006), we evaluated liver metabolism in response to resveratrol treatment. Liver weight and triglycerides were reduced in both WT and SIRT1 KO mice (Supplementary Figure S5C and F). Analysis of gene expression in the livers of these mice revealed an overall shift toward utilization of fatty acids (Supplementary Figure S5G) and increased mitochondrial biogenesis (Supplementary Figure S6D), which in many cases occurred in both WT and SIRT1 KO mice. Resveratrol-treated mice also showed a significant increase in COX activity and cellular ATP levels in both WT and SIRT1 KO mice, while citrate synthase activity was unaltered (Supplementary Figure S6A–C).
In contrast to the knockout mouse, treatment of primary hepatocytes isolated from liver-specific SIRT1 KO (Alb-Cre; SIRT1ΔE4) mice or in HepG2 cells demonstrated that the ability of resveratrol (25 μM) to induce changes in gene expression, AMPK activation and mitochondrial function of hepatocytes is SIRT1-dependent (Supplementary Figure S6H–L and Supplementary Figure S7J). These data indicate that the benefits of resveratrol on liver physiology in the SIRT1 KO mice may be due to signaling from other tissues or the incomplete deletion of SIRT1 in the livers of these mice. As further evidence of the important role signaling between tissues can play we have observed that overexpression of SIRT1 exclusively in SF1 neurons is sufficient to induce expression of genes involved in mitochondrial biogenesis in skeletal muscle (Supplementary Figure S6M)
SIRT1 is Required for Activation of AMPK by Resveratrol
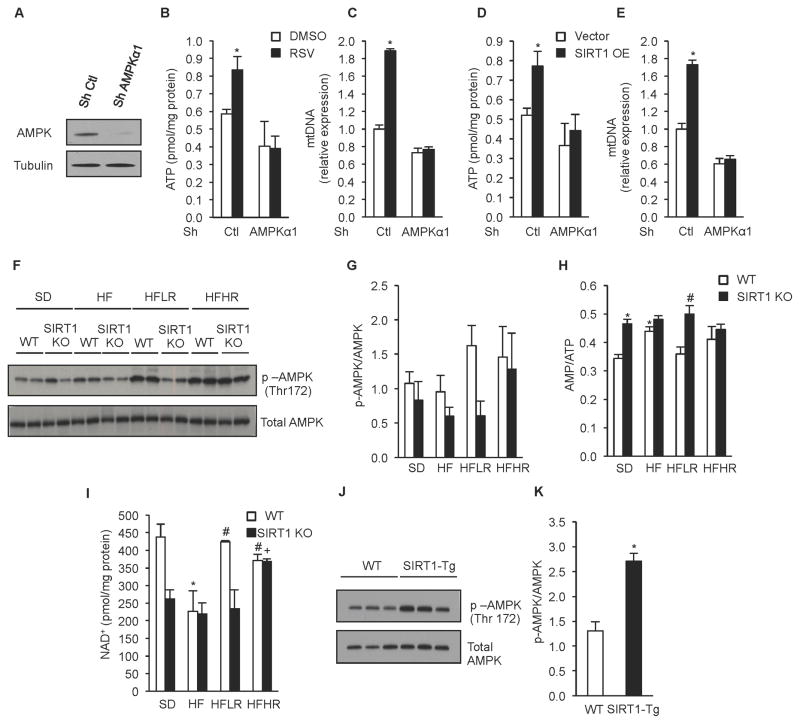
Given the complex interplay between SIRT1 and AMPK, it has been difficult to untangle their roles in mediating the effects of resveratrol but the inducible SIRT1 KO mouse presented us with an opportunity to test their epistasis. Our previous work showed that resveratrol increases levels of activated (phosphorylated) AMPK in vivo (Baur et al., 2006), indicating that AMPK may play a role in mediating resveratrol’s benefits. This was supported by a subsequent study demonstrating that AMPK is required for many of the metabolic effects of resveratrol in vivo (Um et al., 2010). Consistent with this, the ability of resveratrol and SIRT1 overexpression to boost cellular ATP and mtDNA copy number was prevented by knockdown of the AMPKα1 subunit or treatment with Compound C, an AMPK inhibitor (Figure 6A–E and Supplementary Figure S7A–E).
Figure 6. Resveratrol activates AMPK through SIRT1 dependent and independent mechanisms depending on dose.
(A) Representative immunoblot for AMPKα and tubulin in C2C12 cells infected with AMPKα or non-targeting shRNA.
(B) ATP content in C2C12 cells infected with AMPKα or non-targeting shRNA and treated with 25 μM resveratrol for 24h (n=5) (*p<0.05 versus DMSO).
(C) Mitochondrial DNA content analyzed by quantitative PCR in C2C12 cells infected with AMPKα or non-targeting shRNA and treated with 25 μM resveratrol for 24h. Relative expression values were normalized to WT. (n=5) (*p<0.05 versus DMSO).
(D) ATP content in C2C12 cells infected with AMPKα or non-targeting shRNA and treated with adenovirus overexpressing SIRT1 or empty vector (n=5) (*p<0.05 versus empty DMSO).
(E) Mitochondrial DNA content analyzed by quantitative PCR in C2C12 cells infected with AMPKα or non-targeting shRNA and with adenovirus overexpressing SIRT1 or empty vector. Relative expression values were normalized to control. (n=5) experiments (*p<0.05 versus empty DMSO).
(F) Representative immunoblot for p-AMPK (Thr172) and total AMPK in gastrocnemius of WT and SIRT1 KO mice on experimental diets.
(G) Quantification of AMPK activity evaluated by the ratio of p-AMPK and AMPK in gastrocnemius of WT and SIRT1 KO mice on experimental diets (n=8).
(H) NAD+ content in gastrocnemius of WT and SIRT1 KO mice on experimental diets (n=4) (*p<0.05 versus WT SD, #p<0.05 versus WT HF, +p<0.05 versus SIRT1 KO HF). (J) Representative immunoblot for p-AMPK (Thr172), and total AMPK in gastrocnemius of WT and SIRT1 Tg mice.
(K) Quantification of AMPK activity evaluated by the ratio of quantification of p-AMPK and AMPK in gastrocnemius of WT and SIRT1 Tg mice (n=6) (*p<0.05 versus WT).
Values are expressed as mean ± SEM.
Treatment with a moderate dose of resveratrol increased the levels of phosphorylated AMPK and NAD+ in gastrocnemius of WT mice (Figure 6F–I). Strikingly, none of these changes were observed in SIRT1 KO mice. Consistent with these findings, the regulation of AMPK-regulated genes involved in fatty acid metabolism (LCAD, MCAD, CPT1b, FAS and Scd1) was also dependent upon SIRT1. Interestingly, at the higher dose of resveratrol, many of these effects were SIRT1-independent, demonstrating that the dose is critical to the outcome (Figure 6F and 6G, Supplementary Figure S7F and S7G). SIRT1 overexpressing mice had significantly increased levels of AMPK phosphorylation (Figure 6J and 6K). Together these data demonstrate that SIRT1 is necessary for moderate doses of resveratrol to activate AMPK and increase NAD+ and that SIRT1 acts upstream of AMPK. Interestingly, despite the different requirements for resveratrol to activate AMPK in the SIRT1 KO mice, neither dose of resveratrol improved mitochondrial function in the absence of SIRT1 (Figure 2).
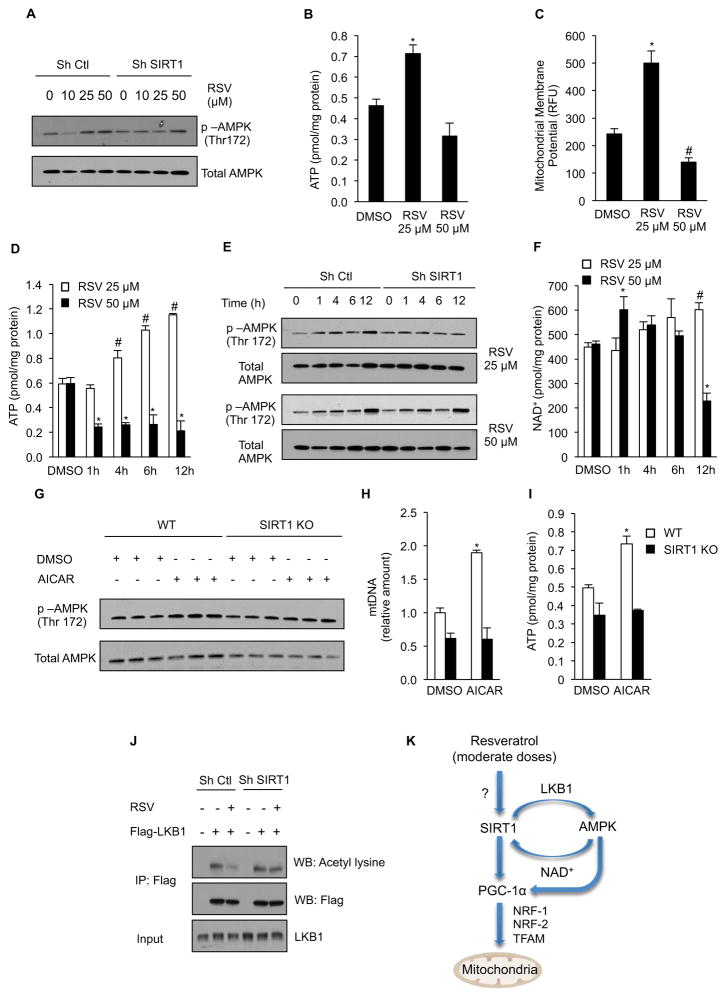
Resveratrol has been implicated in the direct modulation of numerous targets (Baur and Sinclair, 2006), but it has been difficult to discern which of these targets are physiologically relevant. Part of the difficulty has arisen from the fact that doses of resveratrol given to animals are wildly variable and concentrations used on cells vary greatly as well (Kim et al., 2007; Park et al., 2012). To provide some clarity, we performed a series of dose- and time-course experiments with resveratrol. Treatment of C2C12 cells with a moderate dose of resveratrol (25 μM) activated AMPK in a SIRT1-dependent manner, while at higher dose of resveratrol (50 μM) AMPK was activated in a SIRT1-independent manner (Figure 7A). Similarly, treatment with the lower dose of resveratrol for 24h mimicked the effects on muscle in vivo by increasing ATP and mitochondrial membrane potential. In contrast, the 50 μM dose reduced mitochondrial membrane potential and cellular ATP levels, indicative of mitochondrial dysfunction, an effect of resveratrol that was not observed in vivo (Figure 7B and 7C).
Figure 7. Resveratrol activates AMPK in a SIRT1 dependent manner through deacetylation of LKB1.
(A) Representative immunoblot for p-AMPK (Thr172) and total AMPK in C2C12 cells infected with SIRT1 or non-targeting shRNA and treated with 10, 25 or 50 μM resveratrol for 24h.
(B) ATP content in C2C12 cells treated with 25 or 50 μM resveratrol for 24h (n=4) (*p<0.05 versus DMSO).
(C) Mitochondrial membrane potential in C2C12 cells treated with 25 or 50 μM resveratrol for 24h (n=4) (*p<0.05 versus DMSO).
(D) ATP content in C2C12 cells treated with 25 or 50 μM resveratrol for 1, 4, 6 and 12h (n=4) (*p<0.05 versus DMSO).
(E) Representative immunoblot for for p-AMPK (Thr172), and total AMPK in C2C12 cells treated with 25 or 50 μM resveratrol for 1, 4, 6 and 12h.
(F) NAD+ content in C2C12 cells treated with 25 or 50 μM resveratrol for 1, 4, 6 and 12h (n=4) (*p<0.05 versus 50 μM DMSO, #p<0.05 versus 25 μM DMSO).
(G) Representative immunoblot for for p-AMPK (Thr172), and total AMPK in primary myoblasts isolated from wild type and SIRT1 knockout mice and treated with 500 μM AICAR for 24h.
(H) Mitochondrial DNA content analyzed by quantitative PCR in primary myoblasts isolated from wild type and SIRT1 knockout mice and treated with 500 μM AICAR for 24h. Relative expression values were normalized to control. (n=3) experiments (*p<0.05 versus empty DMSO).
(I) ATP content in primary myoblasts isolated from wild type and SIRT1 knockout mice and treated with 500 μM AICAR for 24h (n=3) (*p<0.05 versus DMSO).
(J) C2C12 cells infected with SIRT1 or non-targeting shRNA, and expressing Flag-LKB1 were treated with resveratrol 25 μM for 24h and LKB1 acetylation was tested in Flag immunoprecipitates. Total LKB1 was evaluated in total extracts as input.
(K) Moderate doses of resveratrol activate AMPK and stimulate mitochondrial biogenesis in a SIRT1-dependent manner that results in improvement of mitochondrial function.
Values are expressed as mean ± SEM.
Treatment with 25 μM resveratrol elevated ATP levels at 4, 6 and 12 hours, consistent with what we observed in vivo (Figure 7D). In contrast, the 50 μM dose significantly decreased ATP levels early as 1 hour after treatment. At the 25 μM dose, activation of AMPK occurred in a SIRT1-dependent manner, while the 50 μM dose activated AMPK independently of SIRT1 (Figure 7E). Importantly, the increase in ATP was evident before any changes in cellular NAD+ levels were detected (Figure 7F), indicating that improvements in mitochondrial function and elevation of cellular ATP levels are both dependent upon SIRT1 and occur prior to increases in cellular NAD+.
To further elucidate the epistasis of SIRT1 and AMPK, we treated primary hepatocytes and myoblasts isolated from SIRT1 KO mice with AICAR, an AMP mimetic that directly activates AMPK and promotes its phosphorylation by LKB1, Phosphorylation of AMPK by AICAR was blunted in both the primary hepatocytes and primary myoblasts lacking SIRT1 (Figure 7G and Supplementary Figure S7J). Moreover, the ability of AICAR to increase mitochondrial DNA copy number and ATP in the liver and muscle KO cells was completely blocked (Figure 7H–I and Supplementary Figure S7H–I). In further support of SIRT1 acting upstream of AMPK, treatment of C2C12 cells with 25 μM resveratrol resulted in a SIRT1-dependent decrease in LKB1 acetylation (Figure 7J). These findings are consistent with previous work done in C2C12 cells (Canto et al., 2009) and support previous findings that resveratrol-stimulated, SIRT1-mediated deacetylation of LKB1 plays a direct role in the activation of AMPK (Ivanov et al., 2008; Lan et al., 2008). Taken together, these findings show that treatment of mice on a high fat diet with moderate doses of resveratrol results in increased phosphorylation of AMPK, induction of mitochondrial biogenesis, increased ATP and NAD+ levels, and a shift toward more oxidative muscle fibers, all of which are SIRT1-dependent effects.
DISCUSSION
Genetic disorders with impaired mitochondrial function are characterized by a rapid onset of symptoms commonly seen in the elderly, such as type II diabetes, muscle loss, and neurodegeneration (Finsterer, 2004; Sahin et al., 2011; Wallace, 2010). Moreover, there is increasing evidence that declining mitochondrial function in normal individuals may underlie a number of common age-related diseases (de Moura et al., 2010; Figueiredo et al., 2009; Wallace, 2005) and that treatments that stimulate mitochondrial function can delay the progression of some of these diseases (Baur et al., 2006; Fillmore et al., 2010; Horvath et al., 2011; Hwang et al., 2009; Lagouge et al., 2006; Wenz et al., 2009). Skeletal muscle is one of the primary tissues responsible for insulin stimulated glucose uptake and reduced mitochondrial function has been shown to play an important role in the development of insulin resistance with obesity (Morino et al., 2006; Wallace, 2005). Therefore stimulation of pathways upstream of mitochondrial biogenesis may prove effective in delaying and treating a variety of rare and common diseases.
Treatment of rodents with resveratrol, SRT1720, or overexpression of SIRT1 has been shown to activate PGC-1α and prevent diseases commonly associated with mitochondrial dysfunction and aging (Banks et al., 2008; Baur et al., 2006; Bordone and Guarente, 2005; Herranz et al., 2010; Lagouge et al., 2006; Pearson et al., 2008). Interestingly, skeletal muscle-restricted overexpression of PGC-1α is sufficient to delay many age-related phenotypes and extend mouse lifespan (Wenz et al., 2009). In cell culture, many of the effects of resveratrol are no longer observed in cells with impaired SIRT1 activity (Breen et al., 2008; Csiszar et al., 2009; Fischer-Posovszky et al., 2010; Gracia-Sancho et al., 2010; He et al., 2010; Ivanov et al., 2008; Kao et al., 2010; Kim et al., 2011; Li et al., 2010; Lin et al., 2010; Ohguchi et al., 2010; Park et al., 2010; Shindler et al., 2010; Sulaiman et al., 2010; Tanno et al., 2010; Ungvari et al., 2009; Vetterli et al., 2011; Xia et al., 2011; Yang et al., 2010; Yoshizaki et al., 2010). However, the ability of resveratrol to elicit cellular changes in a SIRT1-independent manner (Bjorklund et al., 2011; Centeno-Baez et al., 2011; Mader et al., 2010; Zhang, 2006), and the dependency of resveratrol’s effects on AMPK in vivo (Um et al., 2010), have fueled an active debate about how resveratrol is able to mimic CR, protect against a high fat diet, and improve mitochondrial function in vivo.
The adult-inducible SIRT1 knockout mouse strain described here has allowed us to directly assess the effects of resveratrol treatment in otherwise healthy adult animals lacking functional SIRT1. Using this model, we clearly demonstrate that the ability of resveratrol to stimulate mitochondrial biogenesis, increase mitochondrial function, and raise both ATP and NAD+ levels in skeletal muscle is dependent on SIRT1 in vivo. While further work is needed to fully determine the importance of SIRT1 in the ability of resveratrol to prevent metabolic syndrome and other age-related diseases, this study provides the first in vivo evidence that beneficial effects of resveratrol on mitochondrial function require SIRT1.
Given our observation that resveratrol increases the transcript levels of PGC-1α, mitochondrial transcription factors, and components of the electron transport chain, the beneficial effects of resveratrol on skeletal muscle are likely due to PGC-1α-mediated increases in mitochondrial biogenesis and a shift toward more oxidative muscle fibers. Consistent with this, overexpression of PGC-1α was recently reported to result in a similar shift toward more oxidative fiber types (Rasbach et al., 2010). This model is also consistent with the data from our SIRT1-Tg mouse, which showed similar changes in mitochondrial biogenesis, mitochondrial function, and cellular energy status as mice treated with resveratrol. This mouse further corroborates the striking parallels between resveratrol treatment, CR, and increased SIRT1 activity (Baur et al., 2006; Cohen et al., 2004; Pearson et al., 2008; Smith et al., 2009).
SIRT1 and AMPK have been shown to play many similar roles, including their ability to respond to stress and nutrient status, induce mitochondrial biogenesis, regulate glucose homeostasis, and control the activity of important transcriptional regulators such as PGC-1α, FOXO’s and p300 (Fulco and Sartorelli, 2008). Similarly, the beneficial effects of both CR and resveratrol have been suggested to involve activation of SIRT1 and AMPK (Boily et al., 2009; Boily et al., 2008; Um et al., 2010). As such it has been difficult to untangle the epistasis of SIRT1 and AMPK. There is clearly a dynamic interaction between these two pathways. AMPK has been shown to activate SIRT1, likely through an indirect increase in cellular NAD+ levels (Canto et al., 2009), while SIRT1 deacetylates the AMPK kinase LKB1, leading to increased phosphorylation and activation of AMPK (Ivanov et al., 2008; Lan et al., 2008).
In light of the data generated using our adult-inducible SIRT1 KO mice, we can reassess the physiologic relevance of proposed models of resveratrol’s action. If resveratrol induces AMPK by acting as an ATPase or Complex III inhibitor (Gledhill et al., 2007; Hawley et al., 2010; Zini et al., 1999), then ATP levels should be lower in the resveratrol-treated mice, and AMPK activation should occur independently of SIRT1. We do not observe such effects using moderate doses of resveratrol in vitro or in vivo. In time-course cell culture experiments, ATP levels were not altered after 1 hour and steadily increased at the 4, 6, and 12 hour time points, while NAD+ levels were not significantly increased until 12 hours of treatment. Moreover, we found no evidence of a decrease in ATP or increase in AMP in animals treated with either a low or high dose of resveratrol. Thus, when moderate doses are used, it seems unlikely that resveratrol activates AMPK by altering AMP and/or ATP levels. In contrast, 50 μM resveratrol caused a dramatic decline in mitochondrial membrane potential and ATP levels. Thus, SIRT1-independent activation of AMPK by high concentrations of resveratrol may be secondary to inhibition of mitochondrial respiration and ATP synthesis.
In a recent study, a new model was presented in which resveratrol increases mitochondrial biogenesis by inhibiting PDE leading to increased levels of cAMP. This increases cellular calcium levels, thereby stimulating phosphorylation of AMPK by CamKKβ. As cellular NAD+ levels were found to be elevated under these conditions, this was proposed as a mechanism by which resveratrol activates SIRT1. This model is not consistent with the effects we have observed with moderate doses of resveratrol in vitro or in vivo, as increases in p-AMPK, NAD+, LKB1 acetylation, and mitochondrial function were entirely SIRT1-dependent. Inhibition of PDE may, however, provide an explanation for some of the effects seen in animals treated with a higher dose of resveratrol where phosphorylation of AMPK and increased levels of NAD+ are observed independently of SIRT1.
In experiments using pharmacological agents, it is well recognized that care should be taken to use the lowest effective dose to minimize the chances of off target effects. Our study exemplifies the risk of using high doses of resveratrol: we clearly show that the ability of resveratrol treatment to increase phosphorylation of AMPK both in vivo and in vitro was dependent upon SIRT1 but only at moderate doses. Moreover treatment of cells with low doses of resveratrol mimicked the in vivo situation but high doses of resveratrol (≥50 μM) resulted not only in SIRT1-independent activation of AMPK but toxic effects that included a dramatic reduction in mitochondrial membrane potential and cellular ATP levels.
In this study we chose to test the SIRT1-dependence of resveratrol in cardiac and skeletal muscle, two organs with high energetic demands requiring efficient mitochondrial function. Cell culture experiments performed with hepatocytes from liver-specific SIRT1 knockout mice demonstrated that SIRT1 is required for resveratrol and AICAR to induce phosphorylation of AMPK and increase mitochondrial biogenesis and function. In the mouse, however, resveratrol improved glucose homeostasis and liver function in both WT and SIRT1 KO mice. It remains unclear whether the SIRT1-independent effects we observed are due to an accumulation of resveratrol in specific organs, the inefficiency of SIRT1 knockdown in liver, or differences in the actions of resveratrol between cell types. The discrepancy may also be due to contributions from other tissues. For example, direct administration of resveratrol to the brain of rodents has been found to improve whole body glucose homeostasis (Ramadori et al., 2009), and these effects were lost when SIRT1 function in the hypothalamus was impaired (Knight et al., 2011). Additionally, overexpression of SIRT1 in SF1 neurons is sufficient to induce the expression of genes involved in mitochondrial biogenesis in skeletal muscle, further demonstrating the complex interplay between tissues.
Using adult-inducible SIRT1 knockout mice we have been able to test for the first time whether beneficial effects of resveratrol treatment on muscle mitochondrial function are dependent upon SIRT1 in vivo. Our data clearly show that treatment with moderate doses of resveratrol results in AMPK activation, induction of mitochondrial biogenesis, and improved mitochondrial function in a manner that is dependent upon SIRT1. It is worth noting that even when AMPK was induced in a SIRT1-independent manner in vivo, the ability of high doses of resveratrol to improve mitochondrial function still required SIRT1. We favor a model whereby moderate doses of resveratrol first activate SIRT1, which leads to deacetylation of LKB1 and activation of AMPK (Figure 7K). Clearly, SIRT1 and AMPK do not function independently or linearly. The subsequent increase in NAD+ levels likely contributes to a positive feedback cycle that may serve to sustain the effects of increased SIRT1 activity beyond the activating stimulus. This model supports the enticing possibility of designing and developing potent small molecules that provide the health benefits of resveratrol by activating SIRT1 and downstream pathways to treat metabolic and other age-related diseases.
EXPERIMENTAL PROCEDURES
Generation of a Whole Body Adult-Inducible SIRT1 Knockout Mouse
Mice harboring a Cre-ERT2 fusion protein consisting of Cre recombinase fused to a triple mutant form of the human estrogen receptor; which does not bind its natural ligand (17β-estradiol) at physiological concentrations but will bind the synthetic estrogen receptor ligands 4-hydroxytamoxifen (OHT) and tamoxifen citrate have been described previously (Ruzankina and Brown, 2007). Cre-ERT2 is restricted to the cytoplasm and can only enter nuclear compartment following treatment with tamoxifen. These mice were crossed to SIRT1Δex4 mice (Cheng et al., 2003) to generate SIRT1Δex4ERT2 mice in which the catylic region of SIRT1 can be deleted upon treatment with tamoxifen. Cre induction was carried out by 6 week feeding of a diet containing 360 mg/kg tamoxifen citrate to SIRT1Δex4ERT2 and control mice. Controls included animals with a WT SIRT1 allele and ERT2 and SIRT1Δex4 mice lacking ERT2. Following tamoxifen treatment efficiency of deletion in DNA from tail samples was determined by PCR (Supplemental Figure 2C) and animals were maintained on experimental diets for eight months. Diets included AIN-93G standard diet (SD), AIN-93G modified to provide 60% of calories from fat (HF), or HF diet with the addition of 0.04% resveratrol (HFLR) as previously described (Baur 2006) as well as a HF diet containing 0.4% resveratrol (HFHR).
Generation of a Whole Body SIRT1 Overexpressor Mouse
A Cre-inducible SIRT1 transgenic mice harboring a transcriptional STOP element flanked with loxP sites inserted between a CAGGS promoter and the murine SIRT1 cDNA was described previously (Firestein et al., 2008). To generate constitutive SIRT1 transgenic animals (SIRT1-Tg), SIRT1STOP mice were crossed with CMV-Cre transgenic mice strains obtained in the C57/BL6J background from Jackson Labs (Bar Harbor, ME). SIRT1tg;CMV-Cre double transgenics were then backcrossed to C57BL/6J to outcross the CMV-Cre allele.
C2C12 Cell Cultures Treatments, Adenoviral Infections and SIRT1 Gene Silencing
Methods for cell culture treatments, adenoviral infections and SIRT1 and AMPKα gene silencing in C2C12 cells can be found in the supplemental information.
Primary Myoblasts and Hepatocytes Isolation and Treatments
The isolation methods of primary myoblasts from the adult-inducible SIRT1 KO animals and primary hepatocytes from liver-specific SIRT1 KO animals can be found in the supplemental information as well as the cell culture procedures and treatments.
Mitochondrial Function and Citrate Synthase Activity
Skeletal muscle mitochondria were isolated as described previously (Frezza et al., 2007) with minor modifications. Oxygen consumption of isolated mitochondria was polarographically determined with a Clark oxygen electrode as described before (Rolo et al., 2000). Mitochondrial membrane potential and citrate synthase activity was also determined as described previously (Rolo et al., 2003; Srere, 1963). Additional details can be found in the supplemental information. ATP content was measured with a commercial kit according to the manufacturer’s instructions (Roche). AMP/ATP ratio was measured by HPLC and the detailed methods can also be found in the supplemental information.
Immunohistochemical Analysis and Electron Microscopy
Immunohistochemical and Electron microscopy methodologies can be found in the supplemental information.
Gene Expression and mtDNA Analysis
Gene expression and mtDNA methodologies can be found in the Supplemental information as well as the primer sequences.
Immunoprecipitation and Immunoblot
Immunoprecipitation and immunoblot methodologies can be found in the supplemental information.
NAD+ measurement
NAD+ from C2C12 cells and skeletal muscle was quantified with a commercially available kit (BioVision) according to the manufacturer’s instructions and as described before (Gomes et al., 2012).
Statistical Analysis
Data were analyzed by a two-tailed unpaired Student’s t-test. All data are reported as mean ± SEM. Statistical analysis was performed using Excel software.
Supplementary Material
HIGHLIGHTS.
Resveratrol’s ability to improve mitochondrial function requires SIRT1 in vivo
Moderate doses of resveratrol activate AMPK and raise NAD+ in SIRT1-dependent manner
Activation of AMPK in the absence of SIRT1 does not improve mitochondrial function
Overexpression of SIRT1 mimics resveratrol’s effects on AMPK and mitochondria
Acknowledgments
This project was supported by the National Center for Research Resources and the new funding component of the National Institutes of Health through Grant Number R01AG028730. DAS was supported by grants from the NIH/NIA. The Sinclair lab is grateful to the Glenn Foundation for Medical Research and the Ellison Medical Foundation for funding support. BN was supported by an NIH/NIA training grant. BPH was supported by an NSERC PGS-D. APG is recipient of an individual fellowship from the Portuguese Foundation for Science and Technology (SFRH/BD/44674/2008). JAB was supported by a Pathway to Independence Award from NIA/NIH and a New Scholar Award from the Ellison Medical Foundation. RC was supported by grants from the American Heart Association (Scientist Development Grant) and the National Institutes of Health (DK080836). We would like to thank Chen Yan, Maria Elena Correa, Andrew Le, Mitesh Sanghvi, and Anuradha Ramamoorthy for their assistance on this project, Pere Puigserver and Zachary Gerhart-Hines for providing PGC1α and SIRT1 adenoviral vectors, Mengwei Zang and Jason Dyck for providing the LKB1 adenoviral vector and Eric Brown for kindly providing ERT2 mice. DAS is a consultant to Cohbar and Ovascience, and consultant and inventor on patents licensed to Sirtris, a GlaxoSmithKline company developing drugs based on sirtuin modulation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal B, Baur JA. Resveratrol and life extension. Ann N Y Acad Sci. 2011;1215:138–143. doi: 10.1111/j.1749-6632.2010.05850.x. [DOI] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell metabolism. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Pugh TD, Prolla TA, Weindruch R. Short-term consumption of a resveratrol-containing nutraceutical mixture mimics gene expression of long-term caloric restriction in mouse heart. Exp Gerontol. 2008a;43:859–866. doi: 10.1016/j.exger.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008b;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Chen D, Chini EN, Chua K, Cohen HY, de Cabo R, Deng C, Dimmeler S, Gius D, Guarente LP, et al. Dietary restriction: standing up for sirtuins. Science. 2010;329:1012–1013. doi: 10.1126/science.329.5995.1012. author reply 1013–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Bjorklund M, Roos J, Gogvadze V, Shoshan M. Resveratrol induces SIRT1- and energy-stress-independent inhibition of tumor cell regrowth after low-dose platinum treatment. Cancer Chemother Pharmacol. 2011 doi: 10.1007/s00280-011-1640-x. [DOI] [PubMed] [Google Scholar]
- Boily G, He XH, Pearce B, Jardine K, McBurney MW. SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene. 2009;28:2882–2893. doi: 10.1038/onc.2009.147. [DOI] [PubMed] [Google Scholar]
- Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, Mikolas E, Szijarto IA, Merei A, Halmai R, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- Breen DM, Sanli T, Giacca A, Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem Biophys Res Commun. 2008;374:117–122. doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno-Baez C, Dallaire P, Marette A. Resveratrol inhibition of inducible nitric oxide synthase in skeletal muscle involves AMPK but not SIRT1. Am J Physiol Endocrinol Metab. 2011 doi: 10.1152/ajpendo.00530.2010. [DOI] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Crandall JPOV, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot Study of Resveratrol in Older Adults With Impaired Glucose Tolerance. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP, et al. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem. 2010;285:32695–32703. doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura MB, dos Santos LS, Van Houten B. Mitochondrial dysfunction in neurodegenerative diseases and cancer. Environ Mol Mutagen. 2010;51:391–405. doi: 10.1002/em.20575. [DOI] [PubMed] [Google Scholar]
- Dong Y, Guo T, Traurig M, Mason CC, Kobes S, Perez J, Knowler WC, Bogardus C, Hanson RL, Baier LJ. SIRT1 is associated with a decrease in acute insulin secretion and a sex specific increase in risk for type 2 diabetes in Pima Indians. Mol Genet Metab. 2011 doi: 10.1016/j.ymgme.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez G, Guarente L. Aging and disease: connections to sirtuins. Aging Cell. 2010;9:285–290. doi: 10.1111/j.1474-9726.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell metabolism. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Figueiredo PA, Powers SK, Ferreira RM, Appell HJ, Duarte JA. Aging impairs skeletal muscle mitochondrial bioenergetic function. J Gerontol A Biol Sci Med Sci. 2009;64:21–33. doi: 10.1093/gerona/gln048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore N, Jacobs DL, Mills DB, Winder WW, Hancock CR. Chronic AMP-activated protein kinase activation and a high-fat diet have an additive effect on mitochondria in rat skeletal muscle. J Appl Physiol. 2010;109:511–520. doi: 10.1152/japplphysiol.00126.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J. Mitochondriopathies. Eur J Neurol. 2004;11:163–186. doi: 10.1046/j.1351-5101.2003.00728.x. [DOI] [PubMed] [Google Scholar]
- Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Posovszky P, Kukulus V, Tews D, Unterkircher T, Debatin KM, Fulda S, Wabitsch M. Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. Am J Clin Nutr. 2010;92:5–15. doi: 10.3945/ajcn.2009.28435. [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Sartorelli V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle. 2008;7:3669–3679. doi: 10.4161/cc.7.23.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. The EMBO journal. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gledhill JR, Montgomery MG, Leslie AG, Walker JE. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci U S A. 2007;104:13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AP, Duarte FV, Nunes P, Hubbard BP, Teodoro JS, Varela AT, Jones JG, Sinclair DA, Palmeira CM, Rolo AP. Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing SIRT1-dependent mitochondrial biogenesis. Biochim Biophys Acta. 2012;1822:185–195. doi: 10.1016/j.bbadis.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Sancho J, Villarreal G, Jr, Zhang Y, Garcia-Cardena G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res. 2010;85:514–519. doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell metabolism. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Andersson G, Lindgren U, Li Y. Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochem Biophys Res Commun. 2010;401:356–362. doi: 10.1016/j.bbrc.2010.09.053. [DOI] [PubMed] [Google Scholar]
- Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Erion DM, Elsworth JD, Roth RH, Shulman GI, Andrews ZB. GPA protects the nigrostriatal dopamine system by enhancing mitochondrial function. Neurobiol Dis. 2011;43:152–162. doi: 10.1016/j.nbd.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Kim DW, Jo EJ, Kim YK, Jo YS, Park JH, Yoo SK, Park MK, Kwak TH, Kho YL, et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58:965–974. doi: 10.2337/db08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VN, Partridge MA, Johnson GE, Huang SX, Zhou H, Hei TK. Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Exp Cell Res. 2008;314:1163–1176. doi: 10.1016/j.yexcr.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Kao CL, Chen LK, Chang YL, Yung MC, Hsu CC, Chen YC, Lo WL, Chen SJ, Ku HH, Hwang SJ. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J Atheroscler Thromb. 2010;17:970–979. doi: 10.5551/jat.4333. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. Embo J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Jung YJ, Lee JE, Lee AS, Kang KP, Lee S, Park SK, Han MK, Lee SY, Ramkumar KM, et al. SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am J Physiol Renal Physiol. 2011;301:F427–435. doi: 10.1152/ajprenal.00258.2010. [DOI] [PubMed] [Google Scholar]
- Knight CM, Gutierrez-Juarez R, Lam TK, Arrieta-Cruz I, Huang L, Schwartz G, Barzilai N, Rossetti L. Mediobasal Hypothalamic Sirtuin 1 Is Essential for Resveratrol’s Effects on Insulin Action in Rats. Diabetes. 2011 doi: 10.2337/db10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol. 2010;177:1065–1071. doi: 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JN, Lin VC, Rau KM, Shieh PC, Kuo DH, Shieh JC, Chen WJ, Tsai SC, Way TD. Resveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activation. J Agric Food Chem. 2010;58:1584–1592. doi: 10.1021/jf9035782. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- Mader I, Wabitsch M, Debatin KM, Fischer-Posovszky P, Fulda S. Identification of a novel proapoptotic function of resveratrol in fat cells: SIRT1-independent sensitization to TRAIL-induced apoptosis. Faseb J. 2010;24:1997–2009. doi: 10.1096/fj.09-142943. [DOI] [PubMed] [Google Scholar]
- McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin Y-K, Canto C, Scheibye-Knudsen M, et al. SRT1720 improves survival and healthspan of obese mice. Scientific Reports. 2011;1 doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–918. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohguchi K, Itoh T, Akao Y, Inoue H, Nozawa Y, Ito M. SIRT1 modulates expression of matrix metalloproteinases in human dermal fibroblasts. Br J Dermatol. 2010;163:689–694. doi: 10.1111/j.1365-2133.2010.09825.x. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Kim TH, Bae JS, Kim MY, Kim KS, Ahn YH. Role of resveratrol in FOXO1-mediated gluconeogenic gene expression in the liver. Biochem Biophys Res Commun. 2010;403:329–334. doi: 10.1016/j.bbrc.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Gautron L, Fujikawa T, Vianna CR, Elmquist JK, Coppari R. Central administration of resveratrol improves diet-induced diabetes. Endocrinology. 2009;150:5326–5333. doi: 10.1210/en.2009-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasbach KA, Gupta RK, Ruas JL, Wu J, Naseri E, Estall JL, Spiegelman BM. PGC-1alpha regulates a HIF2alpha-dependent switch in skeletal muscle fiber types. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21866–21871. doi: 10.1073/pnas.1016089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rolo AP, Oliveira PJ, Moreno AJ, Palmeira CM. Bile acids affect liver mitochondrial bioenergetics: possible relevance for cholestasis therapy. Toxicol Sci. 2000;57:177–185. doi: 10.1093/toxsci/57.1.177. [DOI] [PubMed] [Google Scholar]
- Rolo AP, Palmeira CM, Wallace KB. Mitochondrially mediated synergistic cell killing by bile acids. Biochim Biophys Acta. 2003;1637:127–132. doi: 10.1016/s0925-4439(02)00224-7. [DOI] [PubMed] [Google Scholar]
- Rutanen J, Yaluri N, Modi S, Pihlajamaki J, Vanttinen M, Itkonen P, Kainulainen S, Yamamoto H, Lagouge M, Sinclair DA, et al. SIRT1 mRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes. 2010;59:829–835. doi: 10.2337/db09-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y, Brown EJ. Relationships between stem cell exhaustion, tumour suppression and ageing. Br J Cancer. 2007;97:1189–1193. doi: 10.1038/sj.bjc.6604029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira J, Boily G, Bazinet S, Saliba S, He X, Jardine K, Kennedy C, Staines W, Rousseaux C, Mueller R, et al. sirt1-null mice develop an autoimmune-like condition. Exp Cell Res. 2008;314:3069–3074. doi: 10.1016/j.yexcr.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neuroophthalmol. 2010;30:328–339. doi: 10.1097/WNO.0b013e3181f7f833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, et al. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere PA, Brazil H, Gonen L. The citrate condensing enzyme of pigeon breast muscle and moth flight muscle. Acta Chem Scand. 1963;17:S129–S134. [Google Scholar]
- Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2010;298:H833–843. doi: 10.1152/ajpheart.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Vetterli L, Brun T, Giovannoni L, Bosco D, Maechler P. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E beta-cells and human islets through a SIRT1-dependent mechanism. J Biol Chem. 2011;286:6049–6060. doi: 10.1074/jbc.M110.176842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial DNA mutations in disease and aging. Environ Mol Mutagen. 2010;51:440–450. doi: 10.1002/em.20586. [DOI] [PubMed] [Google Scholar]
- Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xia L, Ding F, Zhu JH, Fu GS. Resveratrol attenuates apoptosis of pulmonary microvascular endothelial cells induced by high shear stress and proinflammatory factors. Hum Cell. 2011;24:127–133. doi: 10.1007/s13577-011-0031-2. [DOI] [PubMed] [Google Scholar]
- Yang J, Wang N, Li J, Zhang J, Feng P. Effects of resveratrol on NO secretion stimulated by insulin and its dependence on SIRT1 in high glucose cultured endothelial cells. Endocrine. 2010;37:365–372. doi: 10.1007/s12020-010-9314-8. [DOI] [PubMed] [Google Scholar]
- Yoshizaki T, Schenk S, Imamura T, Babendure JL, Sonoda N, Bae EJ, Oh DY, Lu M, Milne JC, Westphal C, et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298:E419–428. doi: 10.1152/ajpendo.00417.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem J. 2006;397:519–527. doi: 10.1042/BJ20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini R, Morin C, Bertelli A, Bertelli AA, Tillement JP. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp Clin Res. 1999;25:87–97. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.