Abstract
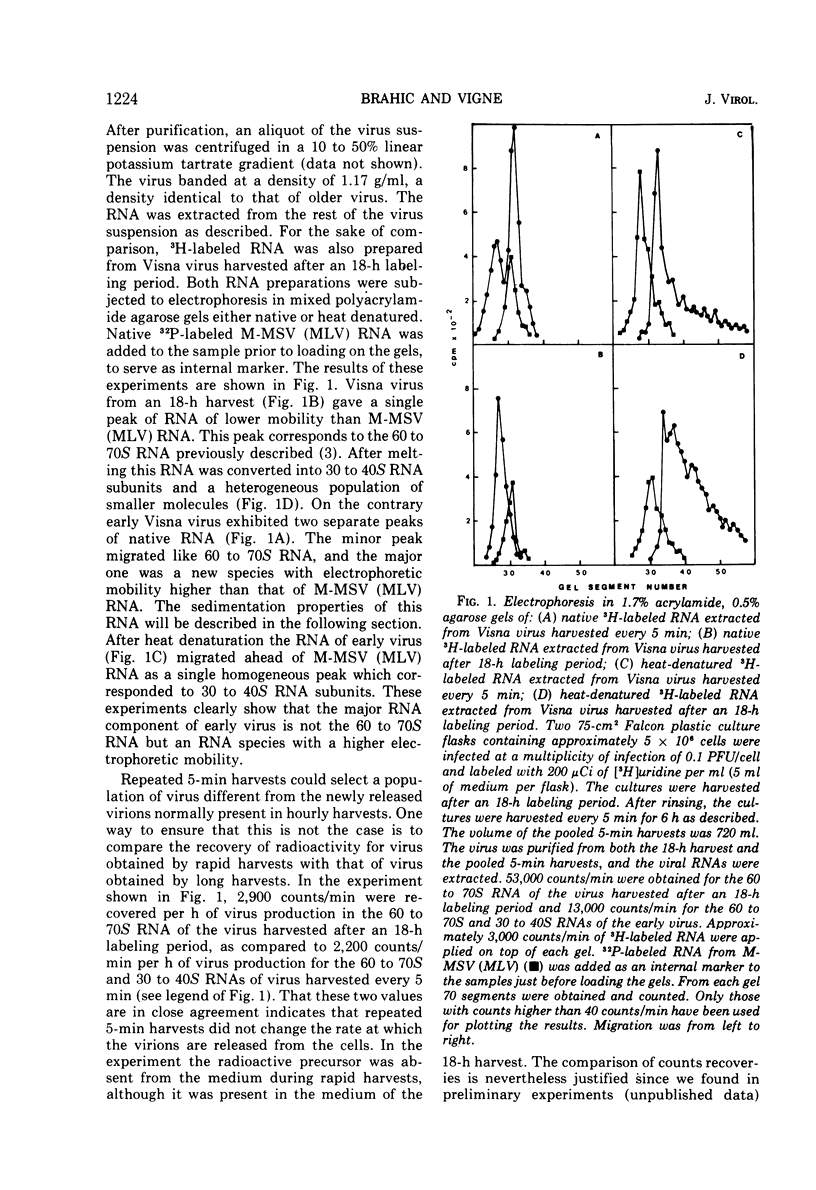
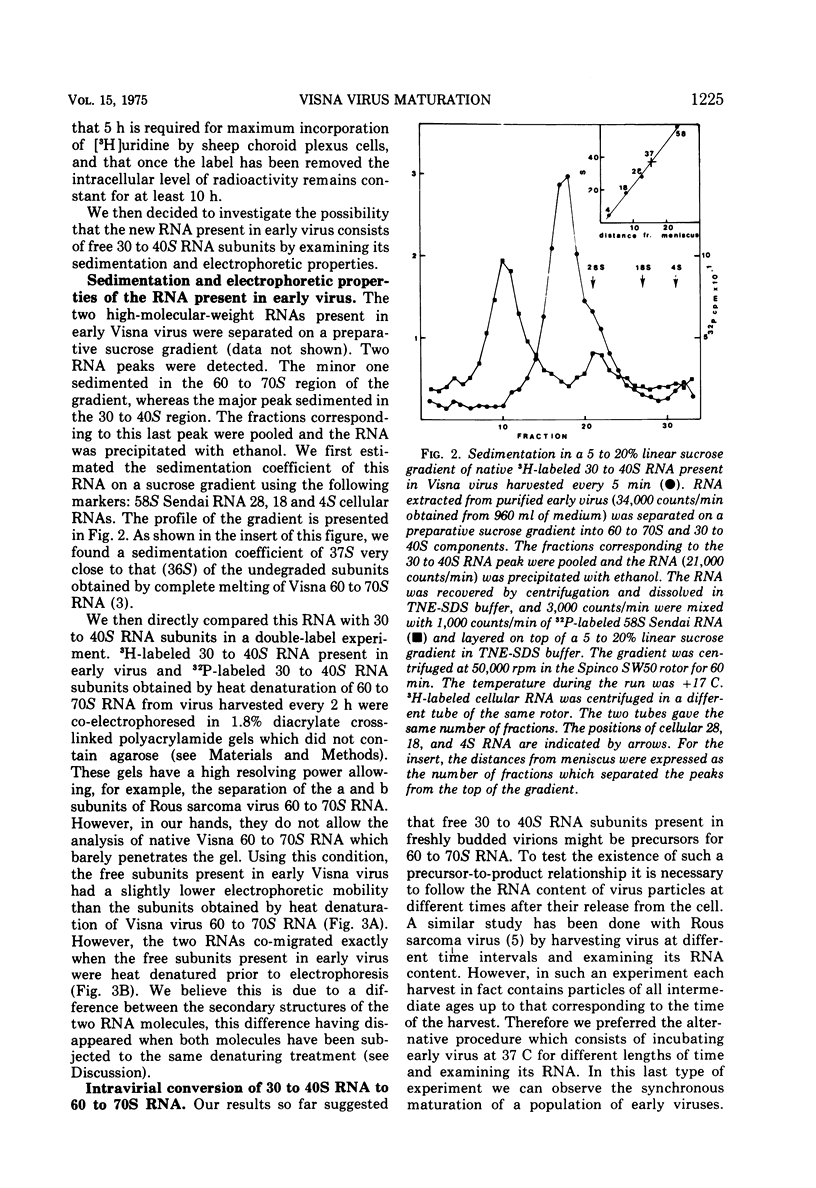
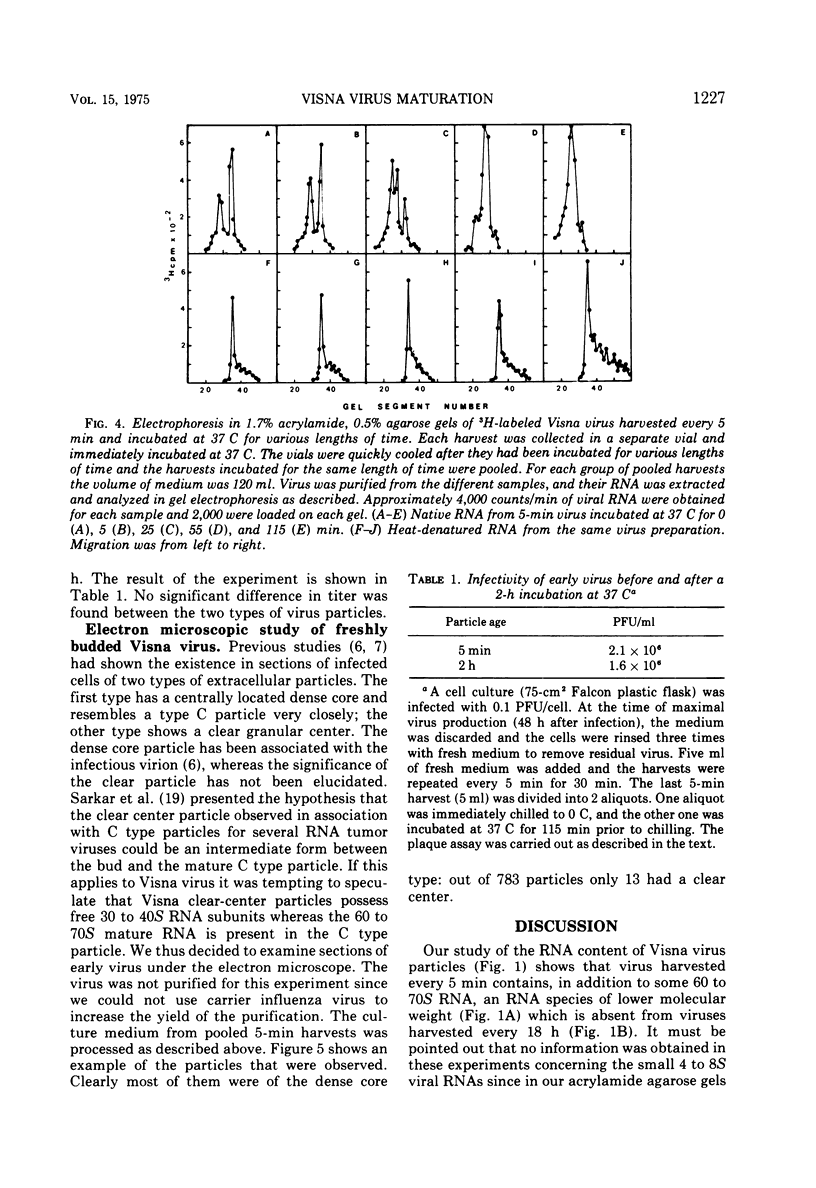
The major RNA component of Visna virus harvested at short intervals of time (5 min) is not the 60 to 70S RNA but a molecule of higher electrophoretic mobility. This RNA has been isolated and characterized. Its sedimentation coefficient is identical to that of 30 to 40S RNA subunits obtained by heat denaturation of the 60 to 70S RNA. In 1.8% acrylamide gels without agarose the electrophoretic mobility of 30 to 40S RNA subunits present in rapidly harvested virus is slightly lower than that of the subunits obtained by denaturation of the 60 to 70S RNA; after heat denaturation the mobilities are identical. These free RNA subunits present in early virus particles assemble into a 60 to 70S RNA complex as shown by following the RNA content of early virus incubated at 37 C for various lengths of time. The rate of this maturation process is slow. There is no difference between the infectivity of immature and mature virus particles. Both particles have a dense core when examined in sections of virus pellets.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascione R., Arlinghaus R. B. Characterization and cell-free activity of polyribosomes isolated from baby hamster kidney cells. Biochim Biophys Acta. 1970 Apr 15;204(2):478–488. doi: 10.1016/0005-2787(70)90168-1. [DOI] [PubMed] [Google Scholar]
- Brahic M., Tamalet J., Filippi P., Delbecchi L. The high molecular weight RNA of Visna virus. Biochimie. 1973;55(8):885–891. doi: 10.1016/s0300-9084(73)80165-8. [DOI] [PubMed] [Google Scholar]
- Canaani E., Helm K. V., Duesberg P. Evidence for 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1973 Feb;70(2):401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
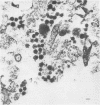
- Chippaux-Hyppolite C., Taranger C., Tamalet J., Pautrat G., Brahic M. Aspects ultrastructuraux du virus visna en cultures cellulaires. Ann Inst Pasteur (Paris) 1972 Sep;123(3):409–420. [PubMed] [Google Scholar]
- Coward J. E., Harter D. H., Morgan C. Electron microscopic observations of Visna virus-infected cell cultures. Virology. 1970 Apr;40(4):1030–1038. doi: 10.1016/0042-6822(70)90149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. The RNA of influenza virus. Proc Natl Acad Sci U S A. 1968 Mar;59(3):930–937. doi: 10.1073/pnas.59.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Takemoto K., Robert M., Gallo R. C. Polyadenylic acid in Visna virus RNA. Science. 1973 Mar 30;179(4080):1328–1330. doi: 10.1126/science.179.4080.1328. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Garapin A. C., Faras A. J., Taylor J. M., Bishop J. M. A comparison of the high molecular weight RNAs of visna virus and Rous sarcoma virus. Virology. 1974 Jan;57(1):259–270. doi: 10.1016/0042-6822(74)90126-3. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Varmus H. E. Demonstration of a DNA provirus in the lytic growth of visna virus. Nat New Biol. 1973 Oct 24;245(147):237–239. doi: 10.1038/newbio245237a0. [DOI] [PubMed] [Google Scholar]
- Harter D. H., Axel R., Burny A., Gulati S., Schlom J., Spiegelman S. The relationship of visna, maedi and RNA tumor viruses as studied by molecular hybridization. Virology. 1973 Mar;52(1):287–291. doi: 10.1016/0042-6822(73)90418-2. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Bruschi A. Molecular weight determination of Sendai RNA by dimethyl sulfoxide gradient sedimentation. J Virol. 1973 May;11(5):615–620. doi: 10.1128/jvi.11.5.615-620.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Characterization of ribonucleic acid from visna virus. J Virol. 1971 May;7(5):582–587. doi: 10.1128/jvi.7.5.582-587.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Properties of maedi nucleic acid and the presence of ribonucleic acid- and deoxyribonucleic acid-dependent deoxyribonucleic acid polymerase in the virions. J Virol. 1972 Aug;10(2):228–233. doi: 10.1128/jvi.10.2.228-233.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Ribonucleic acid-dependent deoxyribonucleic acid polymerase in visna virus. J Virol. 1970 Nov;6(5):702–704. doi: 10.1128/jvi.6.5.702-704.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautrat G., Tamalet J., Chippaux-Hyppolite C., Brahic M. Etude de la structure du virus Visna en microscopie électronique. C R Acad Sci Hebd Seances Acad Sci D. 1971 Aug 9;273(6):653–655. [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P., GRIMSSON H. Visna, a demyelinating transmissible disease of sheep. J Neuropathol Exp Neurol. 1957 Jul;16(3):389–403. doi: 10.1097/00005072-195707000-00010. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Nowinski R. C., Moore D. H. Helical nucleocapsid structure of the oncogenic ribonucleic acid viruses (oncornaviruses). J Virol. 1971 Oct;8(4):564–572. doi: 10.1128/jvi.8.4.564-572.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlom J., Harter D. H., Burny A., Spiegelman S. DNA polymerase activities in varions of visna virus, a causative agent of a "slow" neurological disease. Proc Natl Acad Sci U S A. 1971 Jan;68(1):182–186. doi: 10.1073/pnas.68.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M. L., Wirthlin L. S., Scott J. F., Zamecnik P. C. The 3'-terminal nucleosides of the high molecular weight RNA of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1972 May;69(5):1176–1180. doi: 10.1073/pnas.69.5.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone L. B., Scolnick E., Takemoto K. K., Aaronson S. A. Visna virus: a slow virus with an RNA dependent DNA polymerase. Nature. 1971 Jan 22;229(5282):257–258. doi: 10.1038/229257a0. [DOI] [PubMed] [Google Scholar]
- Stone L. B., Takemoto K. K., Martin M. A. Physical and biochemical properties of progressive pneumonia virus. J Virol. 1971 Oct;8(4):573–578. doi: 10.1128/jvi.8.4.573-578.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORMAR H., CRUICKSHANK J. G. THE STRUCTURE OF VISNA VIRUS STUDIED BY THE NEGATIVE STAINING TECHNIQUE. Virology. 1965 Jan;25:145–148. doi: 10.1016/0042-6822(65)90262-x. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Stone L. B. Transformation of murine cells by two "slow viruses," visna virus and progressive pneumonia virus. J Virol. 1971 Jun;7(6):770–775. doi: 10.1128/jvi.7.6.770-775.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiollais P., Galibert F., Lepetit A., Auger M. A. L'électrophorèse des acides ribonucléiques en gel de polyacrylamide. Biochimie. 1972;54(3):339–354. doi: 10.1016/s0300-9084(72)80213-x. [DOI] [PubMed] [Google Scholar]
- Trowbridge R. S. Evaluation of a plaque assay for the maedi-progressive pneumonia-visna viruses. Appl Microbiol. 1974 Sep;28(3):366–373. doi: 10.1128/am.28.3.366-373.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]