“Immunity’s chain of command begins with antigen-presenting cells.”—Website describing the work of Ralph M. Steinman, MD.1
The dendritic cell (DC) is the key antigen-presenting cell that has the unique capacity to stimulate naïve T cells.2-4 The central importance of DCs in orchestrating the adaptive immune response was not always fully appreciated. In the late 1960s and 1970s, the lymphocyte was king. Indeed, most immunology laboratories focused on the role of lymphocytes and their role in health and disease. Antigen-presenting cells including macrophages were simply tools to stimulate lymphocytes into action. Nonetheless, some laboratories remained focus on understanding the rules that govern macrophage activation of lymphocytes. Dr. Zanvil Cohn, at the Rockefeller Institute, studied mononuclear phagocytes using light, phase and electron microscopy.5 In 1970, Ralph Steinman, a physician scientist trained at Harvard Medical School and then at the Massachusetts General Hospital, joined Cohn to understand more about a rare cell type seen in mouse spleen cell cultures. These cells were irregular in shape with long dendritic projections. These motile cells resembled “swimmers treading water.”6 At that time, nothing was known about function. Later, publications from multiple laboratories indicated that these “dendritic” cells played an important role in T cell activation and immunologic rejection.7,8 Despite these seminal observations, it took until the mid-1980s where basic investigations into DCs demonstrated the central importance into immune responses to pathogens.9 Indeed, DCs have been now used in clinical trials to serve as powerful tools in the fight against cancer and stubborn chronic infections including HIV-1.10-13 Steinman’s relentless pursuit of the function of these cells, leading to pioneering work that now has translated into clinical trials, led to his being awarded the Nobel Prize in Medicine or Physiology in 2011.
Dendritic cells are professional antigen presenting cells. They are located throughout the body poised to capture invading pathogens. Engagement of Toll-like receptors and generation of phagosomes permit activation of DCs.4 Instead of rapid killing of the organism, the environment of the phagosome is tightly regulated such that peptide antigens can be generated for loading onto class I and class II MHC molecules. The capacity of DCs to generate antigen loaded MHC molecules and rapid deployment of these molecules to the cell surface is essential for its ability to prime naïve T cells. In addition to incredible remodeling taking place intracellularly, DCs also modulate its phagocytic machinery and begin to adopt shapes that permit rapid locomotion toward regional lymph nodes.
Looking for Mr. Right
How does an antigen-loaded DC find an antigen-specific T cell within the lymph node? Here, technical advancements of 2-photon microscopy provided breathtaking images that supported a model of prolonged interaction of DC-T cell conjugates.14,15 T cells and DC migrate along a fine meshwork of stroma that exists in lymph nodes. Antigen-specific CD4+ T cells cooperate with DCs to produce CCL3/4 chemokines to induce migration of CD8+ T cells. These T cells undergo three phases of interactions with antigen-loaded DC.15 Initially, T cells migrate along the network looking for specific DCs. To interrogate each other, DC and T cells form short-term interactions. Upon engagement of antigen-specific T cells with DCs expressing the appropriate class II MHC-peptide complex, long-term stable contacts are formed, typically lasting about 24 h. These interactions serve to activate T cells and they resume rapid migration and undergo cellular division.
The special focus in this edition of Virulence serves to provide insight into DC function into a myriad of infectious diseases including CMV,16 HIV,17 HCV,18 influenza A,19 Salmonella enterica,20 Mycobacterium tuberculosis,21 Toxoplasma gondii,22 helminths23 and pathogenic fungi24 as well as the role of microscopy in the study of DC function25 (Fig. 1). Currently, our detailed molecular understanding of the role of DCs in these infections varies tremendously. In some infection models such as CMV, our knowledge is advanced; with molecular details of immune subversion within the antigen presenting cell has been detailed. In other infection models including helminthes, much work is still needed to be done to appreciate fully how DCs serve to generate productive immunity in hosts. In each case (with the sole exception being HIV-1) infection, our incomplete knowledge has contributed to the lack of clinical trials to augment immune responses in patients with these types of infections. Indeed, we have much work to do.
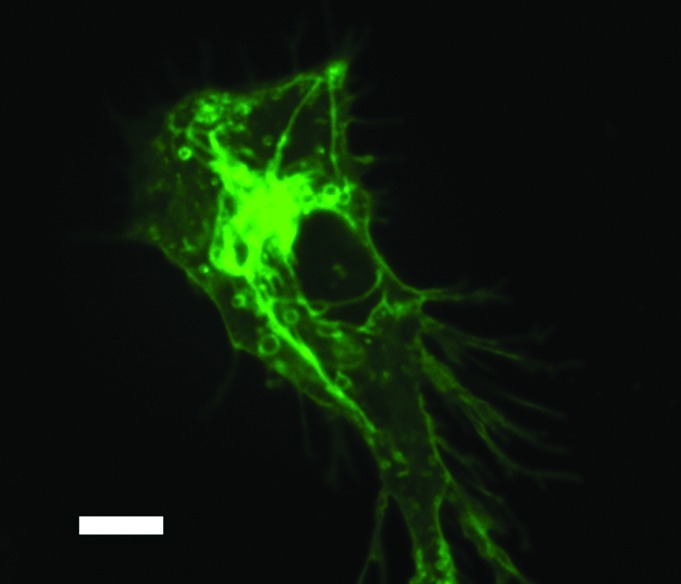
Figure 1. Image of an immature bone-marrow derived dendritic cell expressing class II MHC-GFP. Image is obtained using spinning disk confocal microscope. Size bar indicates 5 µm.
Barriers to Systematic Study of DCs
Many technical problems require solutions before great strides can be made in this field. Heretofore, we have treated DCs as a monolithic cell type with no subdivisions. In fact, this characterization of DCs is not correct. Considerable work has been done to define DC subsets that serve non-overlapping functions.26-29 Additionally, DCs in different anatomic sites appear to operate under different rules of activation and tolerance. A robust cell line that recapitulates all of the essential properties of primary DCs is lacking. Hence, the harvesting of primary cells is the main modality for cellular and molecular work, rendering more sophisticated biochemical and proteomic approaches less feasible. Additionally, many labs differentiate DCs ex vivo using synthetic cytokines. Indeed, multiple protocols exist for this cellular expansion. The method of generating these cells will likely have a significant impact on the interpretation of these experiments. To be sure, none of these shortcomings is insurmountable, yet these issues will need to be addressed by investigators in the field to continue important studies of DCs.
Concluding Remarks
Unlocking the secrets to DC recognition of pathogens, activation and communication with other cells of the immune system is a necessary step to realize the full potential of these cells. If we are to realize the full potential of these cells (as envisioned by Ralph Steinman), much work still needs to be done. However, the promise that DCs can be trained to serve as a powerful ally in the war that rages between host and pathogens necessitates us to carry on these important studies.
Acknowledgments
J.M.V. is supported by NIH grant R01 AI092084 and MGH Support Funds. The author thanks all of the current and former members of the laboratory.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/22975
References
- 1.http://www.rockefeller.edu/about/awards/nobel/rsteinman 2012. Accessed on November 15, 2012.
- 2.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–69. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 3.Coombes JL, Robey EA. Dynamic imaging of host-pathogen interactions in vivo. Nat Rev Immunol. 2010;10:353–64. doi: 10.1038/nri2746. [DOI] [PubMed] [Google Scholar]
- 4.Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat Rev Immunol. 2008;8:607–18. doi: 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowley DA, Fitch FW. The road to the discovery of dendritic cells, a tribute to Ralph Steinman. Cell Immunol. 2012;273:95–8. doi: 10.1016/j.cellimm.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 6.http://www.laskerfoundation.org/2007videoawards/steinman4.html 2012.November 15.
- 7.Inaba K, Young JW, Steinman RM. Direct activation of CD8+ cytotoxic T lymphocytes by dendritic cells. J Exp Med. 1987;166:182–94. doi: 10.1084/jem.166.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owens T, Czitrom AA, Gascoigne NR, Crispe IN, Ratcliffe MJ, Lai PK, et al. The presentation of cell surface alloantigens to T cells. Immunobiology. 1984;168:189–201. doi: 10.1016/S0171-2985(84)80110-2. [DOI] [PubMed] [Google Scholar]
- 9.Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–29. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tada F, Abe M, Hirooka M, Ikeda Y, Hiasa Y, Lee Y, et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int J Oncol. 2012;41:1601–9. doi: 10.3892/ijo.2012.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cintolo JA, Datta J, Mathew SJ, Czerniecki BJ. Dendritic cell-based vaccines: barriers and opportunities. Future Oncol. 2012;8:1273–99. doi: 10.2217/fon.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhargava A, Mishra D, Banerjee S, Mishra PK. Engineered dendritic cells for gastrointestinal tumor immunotherapy: opportunities in translational research. J Drug Target. 2012 doi: 10.3109/1061186X.2012.731069. In press. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi RT, O’Neill D, Bosch RJ, Chan ES, Bucy RP, Shopis J, et al. AIDS Clinical Trials Group A5130 team A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine. 2009;27:6088–94. doi: 10.1016/j.vaccine.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Germain RN, Robey EA, Cahalan MD. A decade of imaging cellular motility and interaction dynamics in the immune system. Science. 2012;336:1676–81. doi: 10.1126/science.1221063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kastenmüller W, Gerner MY, Germain RN. The in situ dynamics of dendritic cell interactions. Eur J Immunol. 2010;40:2103–6. doi: 10.1002/eji.201040482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gredmark-Russ S, Söderberg-Nauclér C. Dendritic cell biology in human cytomegalovirus infection and the clinical consequences for host immunity and pathology. Virulence. 2012;3:621–34. doi: 10.4161/viru.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wonderlich ER, Barratt-Boyes SM. A dendrite in every pie: Myeloid dendritic cells in HIV and SIV infection. Virulence. 2012;3:647–53. doi: 10.4161/viru.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Losikoff PT, Self AA, Gregory SH. Dendritic cells, regulatory T cells and the pathogenesis of chronic hepatitis C. Virulence. 2012;3:610–20. doi: 10.4161/viru.21823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waithman J, Mintern JD. Dendritic cells and influenza A virus infection. Virulence. 2012;3:603–9. doi: 10.4161/viru.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swart L, Hensel M. Interactions of Salmonella enterica with dendritic cells. Virulence. 2012;3:660–7. doi: 10.4161/viru.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihret A. The role of dendritic cells in Mycobacterium tuberculosis infection. Virulence. 2012;3:654–9. doi: 10.4161/viiru.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanecka A, Frickel E. Use and abuse of dendritic cells by Toxoplasma gondii. Virulence. 2012;3:678–89. doi: 10.4161/viru.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White R, Artavanis-Tsakonas K. How helminths use excretory secretory fractions to modulate dendritic cells. Virulence. 2012;3:668–77. doi: 10.4161/viru.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez-Ortiz ZG, Means TK. The role of dendritic cells in the innate recognition of pathogenic fungi (A. fumigatus, C. neoformans and C. albicans) Virulence. 2012;3:635–646. doi: 10.4161/viru.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vyas J. Insights into dendritic cell function using advanced imaging modalities. Virulence. 2012;3:690–4. doi: 10.4161/viru.22981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faivre V, Lukaszewicz AC, Alves A, Charron D, Payen D, Haziot A. Human Monocytes Differentiate into Dendritic Cells Subsets that Induce Anergic and Regulatory T Cells in Sepsis. PLoS One. 2012;7:e47209. doi: 10.1371/journal.pone.0047209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helft J, Manicassamy B, Guermonprez P, Hashimoto D, Silvin A, Agudo J, et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J Clin Invest. 2012;122:4037–47. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin ML, Zhan Y, Villadangos JA, Lew AM. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol Cell Biol. 2008;86:353–62. doi: 10.1038/icb.2008.3. [DOI] [PubMed] [Google Scholar]
- 29.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]


