Abstract
The ubiquitous apicomplexan parasite Toxoplasma gondii stimulates its host’s immune response to achieve quiescent chronic infection. Central to this goal are host dendritic cells. The parasite exploits dendritic cells to disseminate through the body, produce pro-inflammatory cytokines, present its antigens to the immune system and yet at the same time subvert their signaling pathways in order to evade detection. This carefully struck balance by Toxoplasma makes it the most successful parasite on this planet. Recent progress has highlighted specific parasite and host molecules that mediate some of these processes particularly in dendritic cells and in other cells of the innate immune system. Critically, there are several important factors that need to be taken into consideration when concluding how the dendritic cells and the immune system deal with a Toxoplasma infection, including the route of administration, parasite strain and host genotype.
Keywords: Toxoplasma gondii, CD8+ T cells, IFNγ, IL-12, Toll like receptors, blood-brain barrier, dendritic cells
Introducing Toxoplasma gondii
Imagine you are Toxoplasma gondii, arguably the most successful parasite on this planet.1-3 Your ultimate goal is to sexually replicate in a feline, whether it be an Asian leopard, an African lion, a South American puma or maybe the common European pet cat.4 The way you achieve this is to efficiently infect, yet not kill, an intermediate host and persist to chronicity. The immune system of your intermediate host presents challenges, but also opportunities. At the forefront of what you encounter are dendritic cells (DCs): secretors of defense molecules, mediators of crosstalk to T cells, but also potential shuttle rides to various locations within your host. The consequences of these interactions most likely affect human infections, for example in terms of the prevalence of particular parasite strains, their clinical impact and the way in which the parasite has evolved to manipulate an intermediate host.1,5-7
All warm-blooded mammals including humans and birds are potential intermediate hosts for Toxoplasma and the parasite exists in two inter-convertible stages: the lytic, invasive and active tachyzoites and the slow-growing, encysted bradyzoites. In the definitive host, the feline, the parasite presents as oocysts, which are shed for a limited period in the feces and are highly infective and long-lived.4 Natural infection usually proceeds by direct contact with oocysts or by ingesting undercooked meat containing bradyzoite cysts. Bradyzoite cysts convert to tachyzoites in the small intestine of the intermediate host and can infect almost all nucleated cells. Here they replicate within a parasitophorous vacuole (PV), egress by lysing the cell and infect neighboring cells. Tachyzoites elicit a potent immune response that eliminates most parasites. However, some tachyzoites can evade this response, convert back to bradyzoites and persist mostly in non-replicative cells such as those in the brain or heart of their intermediate host. Toxoplasma-infected intermediate hosts will present with a chronic infection of bradyzoite cysts for the rest of their lives. Tachyzoites that grow in the absence of a functioning immune system cause tissue destruction, which can be fatal. Alternatively, an overstimulation of the immune system can lead to hyperinflammation with equally fatal consequences to the host. Thus, Toxoplasma needs to carefully strike a balance between inducing and evading the immune response to reach its ultimate goal of quiescent chronic infection in the brain. Clinically, immunocompromised individuals are most at risk of developing encephalitic, ocular or pneumatic toxoplasmosis by reactivation of bradyzoite cysts to tachyzoites in neural or muscle cells.3,8 Moreover, vertical transmission of an acute infection from a mother to her unborn child can lead to spontaneous abortion, stillbirth or severe birth defects in the form of ocular or neurological deficits.9 To date no human vaccine is available, the chronic phase of infection is refractory to all anti-toxoplasmotic drugs and diagnosis of a recent infection remains challenging.10
Furthermore, it is important to note that Toxoplasma exists as strains of varying genotypes, resulting virulence and potential disease outcome. Isolates from humans and livestock in Europe and North America mostly fall within three clonal lineages, type I, II and III. Of these, type I is highly virulent in mice (lethal to mice at just one parasite), while type II and III Toxoplasma are much less virulent (lethal to 50% of a mouse colony at 103–105 parasites). Recent progress in sample collection from wildlife and more advanced genotyping methods have securely placed atypical strains on the Toxoplasma population map (reviewed in ref. 11). Currently, it is unclear how and why this population structure has evolved, what the natural hosts for different strains are and how this has impacted parasite selection by hosts’ immune responses. Moreover, distinct Toxoplasma protein products, such as Rop16, Rop18, Rop5, Gra2 and Gra15 have been identified as some of the causes of these differences in virulence at least in mice.12-17 In the future it will become increasingly important to assess studies of the immune response to Toxoplasma by carefully noting the strain of Toxoplasma utilized, its dose and potential attenuation state and the route of administration to which strain of mice.
Immune Control of Toxoplasma gondii
Toxoplasma is promiscuous and can infect virtually any nucleated host cell.18 Asymptomatic infection is achieved by the rapid induction of a strong cell-mediated immune response, which elicits production of high levels of gamma interferon (IFNγ) by natural killer (NK) cells, CD4+ and CD8+ T cells during the acute and chronic phase of infection. Interleukin 12 (IL-12) is the major cytokine inducing IFNγ production by lymphocytes and is derived mainly from dendritic cells, macrophages, neutrophils and monocytes.19 These two cytokines drive the strong Th1-biased phenotype of CD4+ and CD8+ T cells. Early in the acute phase of infection, NK cell-derived IFNγ is triggered by IL-12 production leading to protection against the infection.20,21 Essential in both the acute and chronic phase of infection is the IFNγ-producing capability of CD8+ T cells, ultimately aiding in the establishment of chronic infection.22,23 Eventually, the anti-inflammatory cytokines IL-10, TGFβ and IL-27 are responsible for dampening the inflammatory response and minimizing damage caused by inflammation.24-28 Toxoplasma seemingly has the ability to determine its own destiny by maximizing its persistence and minimizing host immunopathology, and all of this in the presence of one of the most powerful pro-inflammatory responses known. It is becoming increasingly clear that different types of Toxoplasma elicit different innate immune responses and in mice, virulent Toxoplasma fails to establish a life-long chronic infection, killing the host prematurely due to hyperinflammation or heavy parasite burden depending on mouse genotype.29-32
IFNγ activates different intracellular anti-parasitic defense mechanisms within infected cells. In both mice and humans the production of reactive nitrogen intermediates by NK and T cells, macrophages, antigen presenting cells (APC) and neutrophils leads to metabolic poisoning of the parasite.33-36 IFNγ activates indolamine 2,3-deoxygenase that in turn induces tryptophan degradation and thus inhibits parasite growth.37-39 The p47 GTPases, a class of large GTPases present in the mouse genome are transcriptionally upregulated in response to IFNγ in cells such as macrophages, astrocytes and fibroblasts, and confer resistance to Toxoplasma by mediating vacuolar degradation.40,41 In humans and in mice, IFNγ can also upregulate guanylate binding proteins, that are implicated in Toxoplasma vacuolar recognition42,43 and mediation of bacterial defense mechanisms, such as autophagy, control of reactive oxygen bursts and control of ubiquitinated cargo, reminiscent of potentially important anti-Toxoplasma measures.44
In this review, we focus on how DCs are manipulated by the apicomplexan parasite Toxoplasma gondii in its natural host to achieve a state of chronic infection. For a brief visual summary please refer to Figure 1.
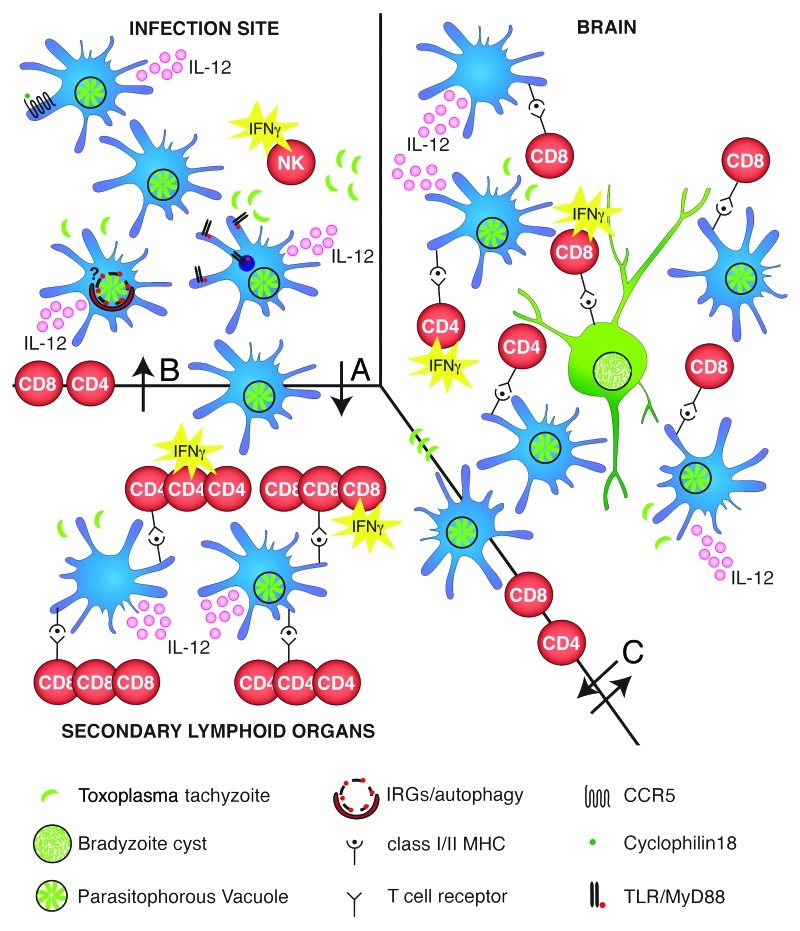
Figure 1. Complexity of dendritic cells interactions with Toxoplasma gondii on its way to achieve persistence in the host. Infection site: Toxoplasma enters an intermediate host’s body either via the natural route of infection in the gut or as in numerous studies covered here, after being intraperitoneally injected as tachyzoites. Regardless, dendritic cells (DCs) present the first line of defense. Toxoplasma infected and bystander DCs secrete the cytokine IL-12, which in turn stimulates the production of IFNγ by natural killer (NK) cells. Molecular recognition of Toxoplasma products by DCs proceeds via CCR5 sensing Toxoplasma cyclophilin18, TLR-mediated sensing of Toxoplasma profilin or other yet unknown parasite products. It remains to be investigated if IFNγ-upregulated GTPases and autophagy contribute to parasite elimination in DCs as already shown in macrophages. Arrow (A): Infected and activated bystander DCs travel from the infection site to the secondary lymphoid organ. Secondary lymphoid organ: Infected and activated bystander DCs produce IL-12 and activate CD4+ and CD8+ T cells to proliferate and produce IFNγ to activate effector molecules and mechanisms. Arrow (B): Proliferated and activated CD4+ and CD8+ T cells travel back to the infection site. Arrow (C): Infected DCs/monocytes are used as a Trojan horse by Toxoplasma to cross the blood brain barrier. Next activated CD4+ and CD8+ T cells cross the blood-brain barrier. Brain: Toxoplasma-infected and bystander DCs, astrocytes and microglia can present antigens to CD4+ and CD8+ T cells which secrete IFNγ. IFNγ secretion by CD8+ T cells is the dominant and necessary immune response for the parasite to be maintained in the bradyzoite stage and to avoid recrudescence to tachyzoites.
Molecular Recognition of Toxoplasma gondii by Dendritic Cells
Toxoplasma orchestrates a carefully balanced string of events between various cell types including neutrophils, DCs and macrophages upon first encountering the host’s innate immune defense. A complex network of molecular signaling pathways leads to the activation and regulation of cytokines and ultimately to the production of effector molecules. Here, we focus on the parasite molecules that stimulate or manipulate host responses in DCs. A more global view of the parasites interaction with other cells of the innate immune system has been expertly reviewed previously.19,45-47
IL-12 production by DCs is often used as a measure of Toxoplasma recognition by these immune cells. It had been found that the IL-12 response of splenic DCs to soluble parasite extract (STAg) exceeded that of lipopolysaccharide (LPS) and CpG oligonucleotides.48 In a seminal study, it was recognized that the Toll-like receptor (TLR) adaptor protein MyD88 is a molecule of major importance in host defense to Toxoplasma, with STAg being capable of mediating the induction of IL-12 production by DCs either in vivo or ex vivo (see Fig. 1, Infection Site).49 In the search for which TLR would be the major player in DC activation, TLR11 was identified to signal upon binding a Toxoplasma profilin-like molecule.50 The resulting IL-12 production was selective to the CD8α+ subset of DCs.50,51 In a more recent study, TLR11 was localized intracellularly in association with the nucleic acid-sensing TLR trafficking protein UNC93B1.52 Mice carrying a single point mutation in UNC93B1, retaining the protein in the endoplasmic reticulum thus preventing intracellular TLR trafficking, are highly susceptible to Toxoplasma and produce less IL-12 upon intraperitoneal (i.p.) Toxoplasma bradyzoite infection.52,53 As direct infection of DCs by Toxoplasma was not required, but in fact very low levels of Toxoplasma profilin were sufficient to induce cytokine production in a transwell assay, it can be speculated that the intracellular location of TLR11 is a very sensitive way to sense Toxoplasma products after phagocytosis.52 However, TLR11−/− mice survive acute Toxoplasma infection in contrast to the severe lethality seen for MyD88−/− animals, but display increased cyst burden in the chronic phase.50
Albeit not demonstrated specifically in DCs, other TLRs, such as TLR2, can also be activated in response to Toxoplasma.54 TLR2 and TLR4 both signal after binding Toxoplasma glycosylphosphatidylinositol (GPI) anchors,55 however single absence of either TLR2 or TLR4 in DCs did not reduce the production of IL-12 in response to STAg.49
The route of infection plays an important role in TLR recognition of Toxoplasma. It has long been established that C57BL/6 mice infected per oral (p.o.) with Toxoplasma develop severe pathology in the small intestine due to pro-inflammatory cytokines.56 DC maturation and migration to the draining lymph node (LN), as well as resulting CD8+ and CD4+ T cell activation are impaired in TLR9−/− mice infected orally with Toxoplasma.57,58 Parasite-induced damage of the intestinal mucosa is decreased in TLR4−/− mice59 and in mice treated with broad-spectrum antibiotics60 in association with decreased pro-inflammatory cytokines. In contrast, TLR2−/−, TLR4−/− and TLR9−/− mice infected systemically i.p. with Toxoplasma demonstrate limited susceptibility and no appreciable defect in IL-12 production in response to the infection as opposed to the same animals receiving the parasite orally.58 Germ-free mice fail to produce IL-12 upon p.o. Toxoplasma infection, an ability that can be rescued by co-administering LPS.58 The resulting model proposes that parasitic infection causes damage to the intestinal epithelium resulting in the translocation of microflora and subsequent MyD88-dependent signaling and IL-12 production.
DC mobilization and IL-12 production by DCs are moreover mediated by a MyD88-independent mechanism via the chemokine receptor CCR5 (see Fig. 1, Infection Site). Following Toxoplasma infection, increased parasite cyst numbers correlate with lower levels of serum IL-12 and IFNγ in CCR5−/− animals.61 Secreted parasitic cyclophilin (C18) was shown to trigger IL-12 production by DCs albeit to a lesser extent than STAg itself.62 It is important to note that both of these studies were performed by i.p. injection of STAg. When tachyzoites of type I vs. type II were injected i.p., different panels of chemokines were produced by macrophages at the site of infection possibly leading to the recruitment and retention of different cell populations.63,64 Natural Toxoplasma infection usually occurs by ingesting infectious oocysts or bradyzoite cysts. Few studies have addressed the role of DCs and how they sense parasite products after oral infection. Gr-1+ inflammatory monocytes were found to be required to mediate mucosal resistance of Toxoplasma after oral infection of B57BL/6 mice, a property not dependent on CD11c+ DCs, but on the presence of the chemokine receptor CCR2.65
It is possible that DCs can directly act as effector cells to eliminate Toxoplasma as suggested by their ability to display oxygen-dependent microbicidal activity after IFNγ activation.66 Moreover, plasmacytoid DCs (pDCs) have been shown to be efficient at autophagy,67 a process known to eliminate Toxoplasma in primed macrophages68-71 and to involve the family of p47 GTPases (see Fig. 1, Infection Site).69 The various subsets of DCs possibly recognize either direct infection with Toxoplasma or sense parasite products differently, and are thus important mediators of parasitic elimination and facilitators for the development of an efficient adaptive immune response. We will discuss which DC subsets are responding with IL-12 production after sensing the parasite in the next section.
Toxoplasma gondii Stimulates IL-12 Production by Dendritic Cells
Toxoplasma is a powerful inducer of DC-derived IL-12. IL-12 critically drives Th1 cell development.72 In the first report linking this cytokine to mouse DCs, splenic CD8α+ DCs stimulated in vivo intravenously with STAg produced IL-12 without priming by IFNγ.73 Subsequently, CD11c+ DCs, both of the CD8α+ or CD8α− type, and pDCs have all been shown to play an important role in host resistance to Toxoplasma through their capacity to produce IL-12. Additionally, besides DCs, inflammatory monocytes, neutrophils and macrophages are all implicated in IL-12 production during early phases of infection.20,74-76 Which of these cellular sources confer protection against the infection in vivo is currently being investigated and debated.
IL-12 is a heterodimer consisting of a p40 subunit that is also shared with the cytokine IL-23. The p40 subunit is covalently linked to a light chain p35 subunit to make biologically active IL-12, also known as IL-12p70. IL-12p40 deficient mice are more susceptible to Toxoplasma infection than IL-12p35 deficient mice, but both are more sensitive than wild-type mice.77 Mice deficient in IL-23p19, the subunit specific to IL-23, develop normal T cell responses upon Toxoplasma infection and can control parasite replication.77 Several cell types produce high levels of the heterodimer IL-12p70 and its production depends upon parasite genotype. In macrophages, it has been shown that acute type II infections induce both IL-12p40 and IL-12p70 production while type I infections primarily induce high levels of IL-12p40.76,78 An attenuated type I parasite in contrast to replicating type I Toxoplasma led to the production of IL-12p70 systemically and in peritoneal cells.79
Conventional CD11c+ DCs have been shown to play key roles in host resistance to Toxoplasma bradyzoite cysts administered i.p.80,81 In the first study, a lineage ablation approach was used by transgenic expression of simian diphtheria toxin receptor under control of the CD11c promoter. Diphtheria toxin administration to these mice causes transient deletion of CD11c-expressing cells and renders these animals more susceptible to i.p. Toxoplasma bradyzoite infection. In the second study, MyD88 was exclusively deleted by Cre recombinase in CD11c-expressing cells. This decreased early IL-12 production, again after i.p. infection with Toxoplasma bradyzoite cysts, and delayed the IFNγ response by NK cells, rendering the mice more susceptible to infection. While both elegant studies, they do not formally exclude the possibility that IL-12 is produced by CD11c-expressing macrophages. Besides conventional DCs, pDCs have been shown to expand after p.o. or i.p. infection with type II parasites.82 In vitro infected pDCs were shown to produce IL-12p40, a phenomenon dependent on TLR11.82 Upon deletion of the transcription factor interferon regulatory factor 8 (Irf8), mice infected i.p. with type II bradyzoite cysts failed to transcribe IL-12p40, a property ascribed to either macrophages and/or dendritic cells and rendering the mice susceptible to the infection.83 Batf3−/− mice are specifically defective in generating CD8α+ DCs and exhibited decreased IL-12 and IFNγ production and succumbed during the peak of acute infection to Toxoplasma type II tachyzoite administered i.p.84 Splenic CD8α+ DCs expanded from 2.5% to 17% of the total DC compartment after infection in wild-type mice, and interestingly the resulting CD8+ T cell response to two endogenous Toxoplasma antigens (Gra4 and Gra6) investigated was defective. Another report finds circulating Ly6C+ monocytes to be recruited to the site of Toxoplasma infection and to differentiate into macrophages and IL-12 producing CD11b+ CD8α− DCs. NK cell-derived IFNγ was deemed to be crucial for monocyte differentiation at the site of infection.85 This study was performed using i.p. infection with Toxoplasma type II bradyzoite cysts. Both studies reciprocally infect with bradyzoite vs. tachyzoites i.p. and confirm that this changes the major IL-12 producing DC subset originally described. Thus it seems imperative to correlate the original question asked with the type and route of Toxoplasma infection chosen.
It has been shown that injection of STAg renders DCs unresponsive to further IL-12 production triggered by subsequent Toxoplasma infection.86,87 This phenomenon of DC paralysis is induced by lipoxin A4, an arachidonate inhibitor of inflammation.86-88 Lipoxin A4 activates the receptors AhR and LXAR in DCs and thus triggers the expression of SOCS-2, a suppressor of cytokine signaling.89 Consequently, SOCS-2 partially dampens the pro-inflammatory IL-12 mediated response to Toxoplasma, in part by downregulating CCR5.89
Even though systemic administration of STAg alone induces rapid splenic DC-derived IL-12,73,86 maximal levels of bioactive IL-12p70 are produced only after receiving a second signal via CD40 ligation on DCs.90 Infection with type II Toxoplasma can also induce splenic CD8α+ and CD8α− DCs to become activated and produce IL-12 dependent upon CD40 cross-linking.91 CD40L knockout mice produce lower levels of IL-12, however, this is enough to induce IFNγ production to ensure the survival of the mice through acute infection.92 In human DCs, CD40-CD40L interaction is required for IL-12 production in response to Toxoplasma infection.93,94 Interestingly, this may explain why patients defective in CD40L expression are more susceptible to intracellular infection linked to T cell mediated immunity.95
Once Toxoplasma reaches the brain it encysts as bradyzoites. IL-12 production by CD11c+ DCs isolated from the brain has been found to persist for one year post-infection.96 Continued production of IL-12 in the chronic phase of infection prevents parasite recrudescence.97
What are the long-term consequences of an intact IL-12 response mostly mediated by DCs for the outcome of a Toxoplasma infection? In bacterial listeriosis, IL-12 via IL-12p35 is thought to promote the generation of effector memory CD8+ T cells, but dampen the differentiation of long-term central memory CD8+ T cells.98 For a replication attenuated type I Toxoplasma strain, similar results were found as in IL-12p35 deficient animals CD62LlowKLRG1+ CD8+ effector T cells did not develop.99 Also, IL-12 appeared dispensable or maybe even slightly negative for central memory CD8+ T cell differentiation.100 Nevertheless, IL-12p40 is required for protective immunity elicited by vaccination with the replication deficient strain and re-challenge with type I replicative RH type I Toxoplasma.101 Thus, replication deficient type I Toxoplasma probably induces enough IL-12p70 to mediate long-lasting CD8+ T cell-mediated immunity. Mice infected i.p. with type I vs. type II Toxoplasma tachyzoites both expressing and secreting ovalbumin develop fewer DCs at the site of infection and fewer antigen-specific CD8+ T cells. In this study, IL-12p70 administration during type I infection moderately rescued this deficiency.64 It remains to be investigated how different strains of Toxoplasma induce varying levels of IL-12 and what the consequences for long-term protective immunity are.
Toxoplasma gondii Modulates Dendritic Cell Interactions with T Cells
Dendritic cells are known as professional APCs that are specialized in loading peptides derived from exogenous and endogenous sources onto both MHC class I and II molecules for presentation to CD8+ and CD4+ T cells respectively.102 Toxoplasma is controlled in the acute and chronic phase of infection by CD8+ T cells22,23,103,104 which means that its antigens are effectively presented in the context of MHC class I (see Fig. 1, Secondary Lymphoid Organs). Potential problems arise when thinking about Toxoplasma antigen presentation from infected DCs. First, the PV has long been believed to be a nondegradative and nonfusogenic compartment.105 Thus, potential antigens contained in this compartment need to escape and with a pore limit of 1300 daltons, this seems an inexplicable task.106 Second, infection of DCs and macrophages by Toxoplasma interferes with several signaling pathways that are crucial to develop protective immunity.107 Toxoplasma can replicate in nonhematopoietic cells as well as professional APCs. It is not clear which cell type in general primes T cells in a Toxoplasma infection in vivo.
Most studies to date have been undertaken with model antigens such as ovalbumin expressed and secreted into the PV by type I or type II parasites.64,108-113 Recently, four endogenous Toxoplasma MHC class I epitopes were identified, restricted to two separate class I MHC alleles.100,114,115 The H-2Ld MHC locus expressed by BALB/c mice has been ascribed to mediate resistance to toxoplasmic encephalitis in the chronic phase of infection in H-2d mice,116-118 thus BALB/c mice were used for the two former studies. Epitopes from the two Toxoplasma’s dense granule proteins Gra4 and Gra6 were identified, as well as one from the inactive rhoptry kinase Rop7. In order to be able to use basic immunological tools confined to C57BL/6 mice, the last study identified another epitope from an unidentified Toxoplasma protein called T57 on this background. Moreover, using somatic cell nuclear transfer, antigen-specific transnuclear CD8+ T cell mice for all of these epitopes were generated and are easily maintained (ref. 119 and unpublished results).
Bone marrow-derived DCs infected in vitro with Toxoplasma tachyzoites expressing and secreting ovalbumin have been shown to induce CD8+ T cell proliferation dependent on the transporter associated with antigen processing (TAP).112 Additionally, the generation of the endogenous epitope GRA6 in DCs is dependent on the ER-associated aminopeptidase.114 Cross-presentation of dead parasite material out of uninfected DCs or general splenocytes was ruled out as a presentation pathway in a number of studies employing ovalbumin-secreting tachyzoites.108-110 However, two reports show cross-presentation by bystander DCs both in the LN early in infection as well as during toxoplasmic encephalitis.120,121 When investigating which antigens targeted to intra-parasitic and intra-vacuolar locations would be efficiently presented by bone-marrow DCs or macrophages ex vivo to OT I T cells, it was determined that only antigen secreted into the vacuole would be appropriate.113 Employing this strain of transgenic Ova-secreting parasites, the ER was speculated to fuse with the vacuole108 to enhance antigen presentation, a process dependent on Sec22b.122
The role of DCs in presenting Toxoplasma antigens to CD4+ T cells is less clear. It has been proposed that Toxoplasma profilin is a major immunodominant antigen that can simultaneously activate DCs and be processed to be presented to CD4+ T cells dependent upon TLR11.51 Active invasion by Toxoplasma tachyzoites blocks LPS-induced bone marrow-derived DC maturation in vitro and their subsequent capacity to activate CD4+ T cells.123 In contrast, pDCs expand during acute i.p. tachyzoite infection with Toxoplasma, upregulate MHC class II and co-stimulatory molecules and prime CD4+ T cells.82 IL-12 production and pDC maturation was dependent on TLR11 which suggests that this DC subset is important to control the infection in vivo.82 Recently, a Toxoplasma 15-mer epitope presented on I-Ab MHC molecules in C57/BL6 mice has been identified and immunization with this peptide was shown to confer significant protection against parasite challenge.124 A peptide-specific T cell response was observed by these authors even with heat-killed parasites, while another study found enhanced presentation of the secreted version of the model antigen ovalbumin.111 Further studies with this newly identified immunogenic CD4 Toxoplasma epitope will facilitate the understanding of the CD4 antigen processing pathway and the exact role CD4+ T cells play in controlling the infection.
After natural oral infection with Toxoplasma, lamina propria DCs are hampered in their ability to induce regulatory CD4+ T cells in vitro. Consequently, IL-2 production in the gastrointestinal tract and in the periphery is reduced leading to immunopathogenesis via heightened IFNγ-producing effector T cells.125 Also, gut DCs exposed to Toxoplasma antigen in vitro induce fewer regulatory T cells.125
It will be important to revisit some of the specifics of antigen presentation to CD8+ T cells using the knowledge of the true endogenous epitopes and their associated tools, as there may be crucial differences depending on parasite strain and epitope under study, antigen expression level, mode of infection and time-point post-infection. Moreover, antigen presentation to CD4+ T cells remains virtually uncharacterized, yet activated CD4+ T cells are found equally numerous as CD8+ T cells in a chronically Toxoplasma-infected mouse brain. Knowledge of how DCs manipulate the generation of this effector T cell population and control the levels of regulatory T cells may have profound influence on the generation of vaccine-mediated immunity.
Dendritic Cells are Hijacked by Toxoplasma gondii
Commonly, Toxoplasma infects its intermediate host via the oral route or in the case of a congenital infection it passes through the placenta. Oocysts or bradyzoites can be ingested by an intermediate host in contaminated water, soil or meat and will end up in the gut. The dissemination out of the gastrointestinal tract before activation of an immune response is crucial for the establishment of a chronic infection and Toxoplasma must cross the intestinal epithelium to achieve this. Bradyzoites and sporozoites released from oocysts infect cells of the small intestine where they convert to fast-replicating and highly invasive tachyzoites.18,126 This is a rapid process. Already one hour after oral infection with bradyzoites, parasites can be found in the lamina propia (LP).126,127 Within two hours, parasites are transported to the LNs and they are able to reach the brain within six days of initial contact with the host.126 To travel quickly Toxoplasma uses highways within the host’s body, namely the bloodstream and the lymphatic system. As extracellular parasites are more vulnerable to elimination from the blood than intracellular ones,128 Toxoplasma hijacks host cells and uses them as means of transportation (see Fig. 1A and C).
Toxoplasma can infect any nucleated cell, but it has a preference for cells of the immune system, mainly DCs.129-131 DCs are present in many tissues, scanning the body for invading pathogens. As described above, upon detection of an intruder, they raise an alarm by producing cytokines that attract and activate other cells of the immune system, and migrate to LNs to activate pathogen-specific T cells.132 It may seem paradoxical that Toxoplasma chooses to target the cell type that predominantly fights infections. However, the parasite does not want to kill its intermediate host. Hence, triggering the immune system in order to be kept under control while hitching a ride may thus be of interest to the parasite. Because of their motile properties, DCs are likely candidates to act as Trojan horses to disseminate Toxoplasma to other tissues. DCs infected with Toxoplasma exhibit a hypermotility phenotype.131,133-136 Type II tachyzoites are superior to type I at inducing migration of human DCs in vitro,131 and murine DCs in vivo.134 Lambert et al.133 showed that only live Toxoplasma can induce a migratory phenotype in DCs, suggesting that it is not simply the effect of recognition of the pathogen and maturation of the DCs, but active manipulation of the DCs by Toxoplasma. In contrast to mouse DCs, human DCs migrate in response to soluble antigens produced by both type I and type II strains of Toxoplasma without maturation.137 Toxoplasma is not the only pathogen that manipulates migratory function of DCs as Neospora caninum-infected DCs exhibit the same phenotype.135 Importantly, type II Toxoplasma use DCs more effectively as a shuttle, while type I parasites are predominantly using the extracellular route.134 This serotype difference in the infection/migration route may dictate by the greater ability of type I tachyzoites to cross the epithelial barriers as an extracellular parasite than type II tachyzoites.127 Toxoplasma hidden inside DCs can travel to the secondary lymphoid tissue and to other organs of the body away from the inflammatory site and into the circulation (see Fig. 1A).
Toxoplasma infects different subtypes of DCs including pDCs.131,134,138 Bierly et al.138 showed that in an i.p. infection with the type I Toxoplasma tachyzoites, CD11c+GR1+ DCs expressing pDC markers B220 and PDCA-1 were preferentially infected and responsible for shuttling Toxoplasma from the peritoneum to the spleen. Additionally, using CCR2−/− mice they demonstrated that this receptor unlike CCR5 was important for migration.138 Nevertheless, first contact of Toxoplasma with DCs in the course of a natural oral infection will occur in the small intestine, where Toxoplasma invades epithelial cells.127 Resident intestinal DCs are likely to be among the first leukocytes to be infected by Toxoplasma. Many different subtypes of conventional and pDCs residing in the intestinal mucosa have been described (reviewed in refs. 139 and 140). However, the question of which DC subsets are important for Toxoplasma dissemination in early mucosal infection has not been fully addressed. In vitro CD11c+MHCII+ DCs isolated from LP of the small intestine and Payer’s patches (PP) can be effectively infected by type I and type II Toxoplasma tachyzoites.134 When Courret et al.130 orally infected mice with type II Toxoplasma bradyzoite cysts, mimicking a natural infection, two days post-infection the majority of CD11c+CD11b+/− cells in the LP were parasitized. This suggests that CD11c+CD11b+/− cells present in the LP are likely to be used by the parasite to travel from the intestine to the mesenteric lymph nodes (MLN) and PP.
It is not clear whether Toxoplasma transported in DCs to the LNs needs to change its vehicle to disseminate further into the bloodstream and to other organs. It is generally accepted that DCs do not leave the secondary lymphoid organs once they have entered. However, there is indirect evidence for DCs leaving LNs to act as Trojan horses.141 It is conceivable that the hyper-migratory phenotype of DCs infected with Toxoplasma would allow them to exit the LNs and via the thoracic duct enter the bloodstream. This hypothesis still awaits verification. Another, more probable scenario is that the DCs carrying Toxoplasma to the LNs are being lysed, and released tachyzoites can in turn infect neighboring cells that enter the blood stream. As reported by Courret et al.130 parasitized leukocytes found in the blood are of a different phenotype (CD11c−CD11b+) than those in LNs (CD11c+CD11b+/−).
Toxoplasma gondii Employs Dendritic Cells to Enter Immune-Privileged Organs
The chronic phase of Toxoplasma infection is characterized by cysts of the bradyzoite stage localized in different body tissues of the intermediate host. However, preferential target organs for Toxoplasma are immune privileged sites like the brain or the eye.3,8 In these organs, as well as in the developing fetus (targeted by the parasite during acute infection of a pregnant female) immune responses are limited or prevented. This enables Toxoplasma to hide from surveillance by the cells of the immune system as well as from circulating antibodies. To reach these organs Toxoplasma has to pass barriers protecting them from exaggerated immune responses. In the case of the brain this involves crossing the blood-brain barrier (BBB) while to infect the fetus Toxoplasma must cross the placenta.
Entering the placenta
In the case of acute infection during pregnancy, Toxoplasma is able to pass the placental barrier and infect the fetus.142,143 The mechanism by which this happens is poorly understood. One possible route is directly via the maternal blood to the cells forming the fetal part of the placenta. Another option is that infected maternal leukocytes bring Toxoplasma to the decidua—the maternal part of the placenta that participates in the exchange of oxygen, nutrients and waste with the developing fetus as well as protecting the fetus from the maternal immune system.144 The infected maternal leukocytes will be killed by residing NK cells or lysed by the multiplying parasites. Released extracellular tachyzoites may cross to the fetus by infecting cells of the fetal part of the placenta. A number of in vitro studies have shown that placental cells can be infected by Toxoplasma; however, no strain differences were noted in infection capability.145
An alternative mechanism for Toxoplasma to traverse the placenta is to again use host cells as Trojan horses. Maternal leukocytes rarely travel to the fetus. However, it has been suggested that maternal APC, possibly decidual DCs cross the placenta to reside in fetal LNs where they induce the development of regulatory T cells.146 This type of DC could give Toxoplasma the opportunity to shuttle across the placenta to infect the fetus.
In an in vitro system, Toxoplasma type II exhibited a higher dependency on DC-mediated transmigration for efficient translocation across polarized cellular monolayers in contrast to type I parasite, which transmigrated as extracellular tachyzoites.135 These findings are consistent with the notion that Toxoplasma type I parasites preferentially disseminate extracellularly,127 whereas type II parasites preferentially exploit the shuttling-function of DCs.134 As Toxoplasma type II causes more vertical infections in comparison to type I,5 it is likely that the Trojan horse mechanism is more effective in crossing the placenta and infecting the fetus than extracellular transmigration.
Crossing the blood-brain barrier
Toxoplasma can invade endothelial cells, but its ability to cross the BBB as extracellular parasites in vivo needs clarification. Only few Toxoplasma tachyzoites injected i.v. in mice were observed in the brain in contrast to those injected i.v. as intracellular parasites.128 Thus, Toxoplasma most likely uses leukocytes as Trojan horses to enter the brain. Access of immune cells to the brain is limited but it does occur, not only during neuroinflammation, but also as an immune surveillance mechanism (reviewed in ref. 147). It is therefore reasonable to hypothesize that DCs transporting Toxoplasma from the infection site to the LNs could play the role of Trojan horses sneaking it into the brain (see Fig. 1C). However, a study by Courret et al.130 suggests CD11c−CD11b+ cells, most likely monocytes, play this role. They showed that both CD11c+ and CD11b+ cells circulating in blood are able to cross the BBB and can be detected in the brain of infected mice seven days after p.o. infection with type II Toxoplasma cysts. Nevertheless, at day seven post-infection (the earliest time point for parasite detection in the brain) the majority of brain mononuclear cells containing parasites were of CD11c−CD11b− or CD11b+ phenotype and only at day 15 post-infection more CD11c+ cells were found to be parasitized. That would suggest that CD11c−CD11b+ cells are carrying Toxoplasma across the BBB. CD11c−CD11b+ cells are in general considered to be monocytes or macrophages, however DCs of that phenotype have also been reported.148 In contrast, Lachenmaier et al.136 using i.v. injection of type I Toxoplasma-infected cells showed that there is no difference between the ability of CD11b+ and CD11c+ cells in crossing the BBB, suggesting that both macrophages and DCs are used by Toxoplasma as Trojan horses. Discrepancies between these two studies can probably be explained by the different strains of Toxoplasma used (type I vs. type II) and different routes of infection (i.v. vs. p.o.) where the model used by Courret et al. most closely reflects the natural course of infection.
To fully characterize which leukocytes are important for dissemination of Toxoplasma to the brain during natural oral infection additional studies should be performed taking into account different parasite strains, stage of infection and infection route.
DCs in the Infected Brain Facilitate Persistence of Toxoplasma gondii
Upon entry to the brain tachyzoites infect astrocytes, neurons and microglial cells (see Fig. 1, Brain). The rapidly replicating tachyzoites transform into the very slowly replicating bradyzoites, which form cysts that can persist throughout the lifetime of the host. Infiltration of the parasite is followed by expansion and recruitment of mononuclear cell populations in the brain. There are multiple reports indicating substantial increase in the number of DCs in the brain upon infection with Toxoplasma.96,149-151 Different sources of these DCs have been reported. Fischer at al.96 showed expansion of a population of brain DCs that originates from bone marrow precursors and expands in the brain upon i.p. infection with cysts of Toxoplasma type II. This occurs relatively late, four weeks after infection and is dependent on GM-CSF production.96 However, occurrence of this DC expansion is not only specific for an infection with Toxoplasma as a similar population arises in the brain upon induction of experimental autoimmune encephalomyelitis (EAE).152 Additionally, John et al.151 reported that DCs can be recruited to the Toxoplasma infected brain from the circulation and that this recruitment is dependent on Gαi-coupled receptor signaling and engagement of LFA-1.
What is the role of DCs in the brain in a Toxoplasma infection? DCs isolated from Toxoplasma-infected brains were shown to be the main producers of IL-12 and in vivo IL-12 production was associated with dividing parasites (see Fig. 1, Brain).96 IL-12 is important for maintaining IFNγ production by T cells.72 Regulated IL-12 production by DCs in the Toxoplasma-infected brain is a well-balanced mechanism essential to eliminate rapidly dividing tachyzoites that may be released from sporadically bursting cysts, but not responding to the latent bradyzoite form of the parasite thus preventing encephalitis.
With the development of new imaging techniques including two-photon microscopy, it is now possible to visualize real-time DC-T cells interactions in the brain.149,152 It has been shown that DCs in Toxoplasma-infected brain interact with T cells and that many of the DCs are localized proximal to infected cells or free tachyzoites.149,151 Schaeffer et al.149 demonstrated that DCs and CD11c−CD11b+ cells in Toxoplasma-infected brain form aggregates around isolated, mainly extracellular parasites, but not intact cysts suggesting that DCs can sense free parasites released from bursting cysts and shape a barrier around them to prevent the infection spreading.
Moreover, DCs isolated from the Toxoplasma-infected brains were shown to have a mature phenotype and to be able to trigger antigen-specific T cell responses.96,151 Aggregating DCs observed by Schaeffer et al.149 were surrounded by antigen-specific T cells suggesting their role in antigen presentation and T cell activation. These DCs were not parasitized by Toxoplasma, thus cross-presentation of the Toxoplasma-derived antigens to the CD8+ T cells is conceivable.149
Taken together, brain-infiltrating DCs may be crucial for local restimulation of Toxoplasma antigen specific effector T cells during Toxoplasma infection and may contribute to the chronicity of the host response.
Concluding Remarks and Outlook
Toxoplasma has learned to exploit and subvert DCs of its intermediate host’s immune system to achieve persistent chronic infection. It is becoming clear that Toxoplasma can stir cytokine production by DCs, use DCs to mediate interactions with T cells and employ DCs to circumnavigate the host. Identification of further molecular players of Toxoplasma that can differentially modulate DC function will be key to understanding the link between innate immune recognition and protective adaptive Th1-mediated immunity. Equally important is to identify host effector molecules and mechanisms that elicit defined immune responses to the parasite in DCs and other effector cells. Host-pathogen interactions are like two sides of the same coin and cannot be investigated without taking both into account. Careful dissection of parasite and host genotype, route of infection and stage of the parasite are essential to properly address and answer questions of how Toxoplasma became the most successful human parasite. The completion of several Toxoplasma strain genomes of different virulence combined with host genomes will facilitate identification of new host-pathogen interaction mechanisms.153,154 Furthermore, Toxoplasma might transcriptionally modify DCs to serve its desired purpose, which can now easily be studied using tools for epigenetic gene regulation. As it is becoming clear that there are major differences in how different strains of Toxoplasma exert their different virulence phenotypes, it will be imperative to distinguish between their ability to subvert the immune response in general and DCs in particular. This knowledge will be important in designing effective counter-measures, particularly vaccines.
Acknowledgments
E.-M.F. thanks the Wellcome Trust for supporting her to set up a lab with the Career Development Fellowship and the Medical Research Council for additional funding. A.S. is funded by the Medical Research Council. We would like to thank Stephanie Coomes and Barbara Clough for reviewing the manuscript and Hayley Wood for drawing the figure.
Glossary
Abbreviations:
- APC
antigen presenting cell
- BBB
blood-brain barrier
- DCs
dendritic cells
- i.p.
intraperitoneal
- IFNγ
interferon gamma
- LN
lymph node
- LP
lamina propria
- MLN
mesenteric LN
- NK cell
natural killer cell
- p.o.
per oral
- pDC
plasmacytoid DC
- PP
Peyer’s patches
- PV
parasitophorous vacuole
- STAg
soluble parasite extract
- TLR
Toll like receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/22833
References
- 1.Grigg ME, Sundar N. Sexual recombination punctuated by outbreaks and clonal expansions predicts Toxoplasma gondii population genetics. Int J Parasitol. 2009;39:925–33. doi: 10.1016/j.ijpara.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8:634–40. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 3.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 4.Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010;26:190–6. doi: 10.1016/j.pt.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Ajzenberg D, Cogné N, Paris L, Bessières MH, Thulliez P, Filisetti D, et al. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J Infect Dis. 2002;186:684–9. doi: 10.1086/342663. [DOI] [PubMed] [Google Scholar]
- 6.Boothroyd JC, Grigg ME. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr Opin Microbiol. 2002;5:438–42. doi: 10.1016/S1369-5274(02)00349-1. [DOI] [PubMed] [Google Scholar]
- 7.Baragaña B, Blackburn AG, Breccia P, Davis AP, de Mendoza J, Padrón-Carrillo JM, et al. Enantioselective transport by a steroidal guanidinium receptor. Chemistry. 2002;8:2931–6. doi: 10.1002/1521-3765(20020703)8:13<2931::AID-CHEM2931>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 8.Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–22. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 9.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39:1385–94. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Robert-Gangneux F, Dardé M-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264–96. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wendte JM, Gibson AK, Grigg ME. Population genetics of Toxoplasma gondii: new perspectives from parasite genotypes in wildlife. Vet Parasitol. 2011;182:96–111. doi: 10.1016/j.vetpar.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercier C, Howe DK, Mordue D, Lingnau M, Sibley LD. Targeted disruption of the GRA2 locus in Toxoplasma gondii decreases acute virulence in mice. Infect Immun. 1998;66:4176–82. doi: 10.1128/iai.66.9.4176-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KDC, et al. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med. 2011;208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinfeldt T, Könen-Waisman S, Tong L, Pawlowski N, Lamkemeyer T, Sibley LD, et al. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol. 2010;8:e1000576. doi: 10.1371/journal.pbio.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–80. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 16.Saeij JPJ, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, et al. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–3. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niedelman W, Gold DA, Rosowski EE, Sprokholt JK, Lim D, Farid Arenas A, et al. The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog. 2012;8:e1002784. doi: 10.1371/journal.ppat.1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carruthers V, Boothroyd JC. Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2007;10:83–9. doi: 10.1016/j.mib.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Miller CM, Boulter NR, Ikin RJ, Smith NC. The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol. 2009;39:23–39. doi: 10.1016/j.ijpara.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, et al. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–43. [PubMed] [Google Scholar]
- 21.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993;90:6115–9. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Grunvald E, Hieny S, et al. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–54. [PubMed] [Google Scholar]
- 23.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–8. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki Y, Sher A, Yap G, Park D, Neyer LE, Liesenfeld O, et al. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol. 2000;164:5375–82. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 25.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kühn R, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 26.Mennechet FJD, Kasper LH, Rachinel N, Minns LA, Luangsay S, Vandewalle A, et al. Intestinal intraepithelial lymphocytes prevent pathogen-driven inflammation and regulate the Smad/T-bet pathway of lamina propria CD4+ T cells. Eur J Immunol. 2004;34:1059–67. doi: 10.1002/eji.200324416. [DOI] [PubMed] [Google Scholar]
- 27.Villarino AV, Stumhofer JS, Saris CJM, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–47. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 28.Buzoni-Gatel D, Debbabi H, Mennechet FJD, Martin V, Lepage AC, Schwartzman JD, et al. Murine ileitis after intracellular parasite infection is controlled by TGF-beta-producing intraepithelial lymphocytes. Gastroenterology. 2001;120:914–24. doi: 10.1053/gast.2001.22432a. [DOI] [PubMed] [Google Scholar]
- 29.Saeij JPJ, Boyle JP, Boothroyd JC. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 2005;21:476–81. doi: 10.1016/j.pt.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol. 2001;167:4574–84. doi: 10.4049/jimmunol.167.8.4574. [DOI] [PubMed] [Google Scholar]
- 31.Gavrilescu LC, Denkers EY. IFN-gamma overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J Immunol. 2001;167:902–9. doi: 10.4049/jimmunol.167.2.902. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen TD, Bigaignon G, Markine-Goriaynoff D, Heremans H, Nguyen TN, Warnier G, et al. Virulent Toxoplasma gondii strain RH promotes T-cell-independent overproduction of proinflammatory cytokines IL12 and gamma-interferon. J Med Microbiol. 2003;52:869–76. doi: 10.1099/jmm.0.04860-0. [DOI] [PubMed] [Google Scholar]
- 33.Khan IA, Schwartzman JD, Matsuura T, Kasper LH. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc Natl Acad Sci U S A. 1997;94:13955–60. doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–73. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts F, Roberts CW, Ferguson DJ, McLeod R. Inhibition of nitric oxide production exacerbates chronic ocular toxoplasmosis. Parasite Immunol. 2000;22:1–5. doi: 10.1046/j.1365-3024.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 36.Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1:1397–406. doi: 10.1016/S1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 37.Fujigaki S, Saito K, Takemura M, Maekawa N, Yamada Y, Wada H, et al. L-tryptophan-L-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infect Immun. 2002;70:779–86. doi: 10.1128/IAI.70.2.779-786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacKenzie CR, Langen R, Takikawa O, Däubener W. Inhibition of indoleamine 2,3-dioxygenase in human macrophages inhibits interferon-gamma-induced bacteriostasis but does not abrogate toxoplasmastasis. Eur J Immunol. 1999;29:3254–61. doi: 10.1002/(SICI)1521-4141(199910)29:10<3254::AID-IMMU3254>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Silva NM, Rodrigues CV, Santoro MM, Reis LFL, Alvarez-Leite JI, Gazzinelli RT. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: induction by endogenous gamma interferon and requirement of interferon regulatory factor 1. Infect Immun. 2002;70:859–68. doi: 10.1128/IAI.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collazo CM, Yap GS, Sempowski GD, Lusby KC, Tessarollo L, Woude GF, et al. Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. J Exp Med. 2001;194:181–8. doi: 10.1084/jem.194.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liesenfeld O, Parvanova I, Zerrahn J, Han S-J, Heinrich F, Muñoz M, et al. The IFN-γ-inducible GTPase, Irga6, protects mice against Toxoplasma gondii but not against Plasmodium berghei and some other intracellular pathogens. PLoS One. 2011;6:e20568. doi: 10.1371/journal.pone.0020568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Degrandi D, Konermann C, Beuter-Gunia C, Kresse A, Würthner J, Kurig S, et al. Extensive characterization of IFN-induced GTPases mGBP1 to mGBP10 involved in host defense. J Immunol. 2007;179:7729–40. doi: 10.4049/jimmunol.179.11.7729. [DOI] [PubMed] [Google Scholar]
- 43.Virreira Winter S, Niedelman W, Jensen KD, Rosowski EE, Julien L, Spooner E, et al. Determinants of GBP recruitment to Toxoplasma gondii vacuoles and the parasitic factors that control it. PLoS One. 2011;6:e24434. doi: 10.1371/journal.pone.0024434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim B-H, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332:717–21. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- 45.Denkers EY, Butcher BA, Del Rio L, Bennouna S. Neutrophils, dendritic cells and Toxoplasma. Int J Parasitol. 2004;34:411–21. doi: 10.1016/j.ijpara.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Pifer R, Yarovinsky F. Innate responses to Toxoplasma gondii in mice and humans. Trends Parasitol. 2011;27:388–93. doi: 10.1016/j.pt.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pollard AM, Knoll LJ, Mordue DG. The role of specific Toxoplasma gondii molecules in manipulation of innate immunity. Trends Parasitol. 2009;25:491–4. doi: 10.1016/j.pt.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aliberti J, Jankovic D, Sher A. Turning it on and off: regulation of dendritic cell function in Toxoplasma gondii infection. Immunol Rev. 2004;201:26–34. doi: 10.1111/j.0105-2896.2004.00179.x. [DOI] [PubMed] [Google Scholar]
- 49.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, et al. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 50.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–9. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 51.Yarovinsky F, Kanzler H, Hieny S, Coffman RL, Sher A. Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4+ T cell response. Immunity. 2006;25:655–64. doi: 10.1016/j.immuni.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 52.Pifer R, Benson A, Sturge CR, Yarovinsky F. UNC93B1 is essential for TLR11 activation and IL-12-dependent host resistance to Toxoplasma gondii. J Biol Chem. 2011;286:3307–14. doi: 10.1074/jbc.M110.171025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melo MB, Kasperkovitz P, Cerny A, Könen-Waisman S, Kurt-Jones EA, Lien E, et al. UNC93B1 mediates host resistance to infection with Toxoplasma gondii. PLoS Pathog. 2010;6:e1001071. doi: 10.1371/journal.ppat.1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mun H-S, Aosai F, Norose K, Chen M, Piao L-X, Takeuchi O, et al. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int Immunol. 2003;15:1081–7. doi: 10.1093/intimm/dxg108. [DOI] [PubMed] [Google Scholar]
- 55.Debierre-Grockiego F, Campos MA, Azzouz N, Schmidt J, Bieker U, Resende MG, et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179:1129–37. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 56.Liesenfeld O, Kosek J, Remington JS, Suzuki Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minns LA, Menard LC, Foureau DM, Darche S, Ronet C, Mielcarz DW, et al. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J Immunol. 2006;176:7589–97. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- 58.Benson A, Pifer R, Behrendt CL, Hooper LV, Yarovinsky F. Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell Host Microbe. 2009;6:187–96. doi: 10.1016/j.chom.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heimesaat MM, Fischer A, Jahn H-K, Niebergall J, Freudenberg M, Blaut M, et al. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007;56:941–8. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177:8785–95. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 61.Aliberti J, Reis e Sousa C, Schito M, Hieny S, Wells T, Huffnagle GB, et al. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat Immunol. 2000;1:83–7. doi: 10.1038/76957. [DOI] [PubMed] [Google Scholar]
- 62.Aliberti J, Valenzuela JG, Carruthers VB, Hieny S, Andersen J, Charest H, et al. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat Immunol. 2003;4:485–90. doi: 10.1038/ni915. [DOI] [PubMed] [Google Scholar]
- 63.Lee CW, Sukhumavasi W, Denkers EY. Phosphoinositide-3-kinase-dependent, MyD88-independent induction of CC-type chemokines characterizes the macrophage response to Toxoplasma gondii strains with high virulence. Infect Immun. 2007;75:5788–97. doi: 10.1128/IAI.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tait ED, Jordan KA, Dupont CD, Harris TH, Gregg B, Wilson EH, et al. Virulence of Toxoplasma gondii is associated with distinct dendritic cell responses and reduced numbers of activated CD8+ T cells. J Immunol. 2010;185:1502–12. doi: 10.4049/jimmunol.0903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, et al. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–17. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aline F, Bout D, Dimier-Poisson I. Dendritic cells as effector cells: gamma interferon activation of murine dendritic cells triggers oxygen-dependent inhibition of Toxoplasma gondii replication. Infect Immun. 2002;70:2368–74. doi: 10.1128/IAI.70.5.2368-2374.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 68.Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest. 2006;116:2366–77. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJP, et al. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–71. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Subauste CS, Andrade RM, Wessendarp M. CD40-TRAF6 and autophagy-dependent anti-microbial activity in macrophages. Autophagy. 2007;3:245–8. doi: 10.4161/auto.3717. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Y, Wilson D, Matthews S, Yap GS. Rapid elimination of Toxoplasma gondii by gamma interferon-primed mouse macrophages is independent of CD40 signaling. Infect Immun. 2007;75:4799–803. doi: 10.1128/IAI.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 73.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, et al. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–29. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bliss SK, Zhang Y, Denkers EY. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-gamma-independent IL-12. J Immunol. 1999;163:2081–8. [PubMed] [Google Scholar]
- 75.Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J Immunol. 2000;165:4515–21. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 76.Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J Immunol. 2004;172:3686–94. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- 77.Lieberman LA, Cardillo F, Owyang AM, Rennick DM, Cua DJ, Kastelein RA, et al. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol. 2004;173:1887–93. doi: 10.4049/jimmunol.173.3.1887. [DOI] [PubMed] [Google Scholar]
- 78.Kim L, Butcher BA, Lee CW, Uematsu S, Akira S, Denkers EY. Toxoplasma gondii genotype determines MyD88-dependent signaling in infected macrophages. J Immunol. 2006;177:2584–91. doi: 10.4049/jimmunol.177.4.2584. [DOI] [PubMed] [Google Scholar]
- 79.Gigley JP, Fox BA, Bzik DJ. Cell-mediated immunity to Toxoplasma gondii develops primarily by local Th1 host immune responses in the absence of parasite replication. J Immunol. 2009;182:1069–78. doi: 10.4049/jimmunol.182.2.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu C-H, Fan YT, Dias A, Esper L, Corn RA, Bafica A, et al. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J Immunol. 2006;177:31–5. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- 81.Hou B, Benson A, Kuzmich L, DeFranco AL, Yarovinsky F. Critical coordination of innate immune defense against Toxoplasma gondii by dendritic cells responding via their Toll-like receptors. Proc Natl Acad Sci U S A. 2011;108:278–83. doi: 10.1073/pnas.1011549108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pepper M, Dzierszinski F, Wilson E, Tait E, Fang Q, Yarovinsky F, et al. Plasmacytoid dendritic cells are activated by Toxoplasma gondii to present antigen and produce cytokines. J Immunol. 2008;180:6229–36. doi: 10.4049/jimmunol.180.9.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scharton-Kersten T, Contursi C, Masumi A, Sher A, Ozato K. Interferon consensus sequence binding protein-deficient mice display impaired resistance to intracellular infection due to a primary defect in interleukin 12 p40 induction. J Exp Med. 1997;186:1523–34. doi: 10.1084/jem.186.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, et al. CD8α(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35:249–59. doi: 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, et al. NK cell-derived interferon-γ orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. 2012;36:1047–59. doi: 10.1016/j.immuni.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reis e Sousa C, Yap G, Schulz O, Rogers N, Schito M, Aliberti J, et al. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity. 1999;11:637–47. doi: 10.1016/S1074-7613(00)80138-7. [DOI] [PubMed] [Google Scholar]
- 87.Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat Immunol. 2002;3:76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- 88.Aliberti J, Serhan C, Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J Exp Med. 2002;196:1253–62. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, et al. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med. 2006;12:330–4. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- 90.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–62. doi: 10.1016/S1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 91.Straw AD, MacDonald AS, Denkers EY, Pearce EJ. CD154 plays a central role in regulating dendritic cell activation during infections that induce Th1 or Th2 responses. J Immunol. 2003;170:727–34. doi: 10.4049/jimmunol.170.2.727. [DOI] [PubMed] [Google Scholar]
- 92.Reichmann G, Walker W, Villegas EN, Craig L, Cai G, Alexander J, et al. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect Immun. 2000;68:1312–8. doi: 10.1128/IAI.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Subauste CS, Wessendarp M. Human dendritic cells discriminate between viable and killed Toxoplasma gondii tachyzoites: dendritic cell activation after infection with viable parasites results in CD28 and CD40 ligand signaling that controls IL-12-dependent and -independent T cell production of IFN-gamma. J Immunol. 2000;165:1498–505. doi: 10.4049/jimmunol.165.3.1498. [DOI] [PubMed] [Google Scholar]
- 94.Séguin R, Kasper LH. Sensitized lymphocytes and CD40 ligation augment interleukin-12 production by human dendritic cells in response to Toxoplasma gondii. J Infect Dis. 1999;179:467–74. doi: 10.1086/314601. [DOI] [PubMed] [Google Scholar]
- 95.Subauste CS, Wessendarp M, Sorensen RU, Leiva LE. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type 1 immune response that can be restored by soluble CD40 ligand trimer. J Immunol. 1999;162:6690–700. [PubMed] [Google Scholar]
- 96.Fischer HG, Bonifas U, Reichmann G. Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol. 2000;164:4826–34. doi: 10.4049/jimmunol.164.9.4826. [DOI] [PubMed] [Google Scholar]
- 97.Yap G, Pesin M, Sher A. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol. 2000;165:628–31. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- 98.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–81. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 99.Wilson DC, Matthews S, Yap GS. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii Infection. J Immunol. 2008;180:5935–45. doi: 10.4049/jimmunol.180.9.5935. [DOI] [PubMed] [Google Scholar]
- 100.Wilson DC, Grotenbreg GM, Liu K, Zhao Y, Frickel EM, Gubbels MJ, et al. Differential regulation of effector- and central-memory responses to Toxoplasma gondii Infection by IL-12 revealed by tracking of Tgd057-specific CD8+ T cells. PLoS Pathog. 2010;6:e1000815. doi: 10.1371/journal.ppat.1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sukhumavasi W, Egan CE, Warren AL, Taylor GA, Fox BA, Bzik DJ, et al. TLR adaptor MyD88 is essential for pathogen control during oral toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. J Immunol. 2008;181:3464–73. doi: 10.4049/jimmunol.181.5.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/S0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 103.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–80. [PubMed] [Google Scholar]
- 104.Parker SJ, Roberts CW, Alexander J. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin Exp Immunol. 1991;84:207–12. doi: 10.1111/j.1365-2249.1991.tb08150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sibley LD. Toxoplasma gondii: perfecting an intracellular life style. Traffic. 2003;4:581–6. doi: 10.1034/j.1600-0854.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 106.Schwab JC, Beckers CJ, Joiner KA. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad Sci U S A. 1994;91:509–13. doi: 10.1073/pnas.91.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saeij JPJ, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–7. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goldszmid RS, Coppens I, Lev A, Caspar P, Mellman I, Sher A. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med. 2009;206:399–410. doi: 10.1084/jem.20082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gubbels MJ, Striepen B, Shastri N, Turkoz M, Robey EA. Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infect Immun. 2005;73:703–11. doi: 10.1128/IAI.73.2.703-711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dzierszinski F, Pepper M, Stumhofer JS, LaRosa DF, Wilson EH, Turka LA, et al. Presentation of Toxoplasma gondii antigens via the endogenous major histocompatibility complex class I pathway in nonprofessional and professional antigen-presenting cells. Infect Immun. 2007;75:5200–9. doi: 10.1128/IAI.00954-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pepper M, Dzierszinski F, Crawford A, Hunter CA, Roos D. Development of a system to study CD4+-T-cell responses to transgenic ovalbumin-expressing Toxoplasma gondii during toxoplasmosis. Infect Immun. 2004;72:7240–6. doi: 10.1128/IAI.72.12.7240-7246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bertholet S, Goldszmid R, Morrot A, Debrabant A, Afrin F, Collazo-Custodio C, et al. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J Immunol. 2006;177:3525–33. doi: 10.4049/jimmunol.177.6.3525. [DOI] [PubMed] [Google Scholar]
- 113.Gregg B, Dzierszinski F, Tait E, Jordan KA, Hunter CA, Roos DS. Subcellular antigen location influences T-cell activation during acute infection with Toxoplasma gondii. PLoS One. 2011;6:e22936. doi: 10.1371/journal.pone.0022936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blanchard N, Gonzalez F, Schaeffer M, Joncker NT, Cheng T, Shastri AJ, et al. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat Immunol. 2008;9:937–44. doi: 10.1038/ni.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frickel EM, Sahoo N, Hopp J, Gubbels MJ, Craver MPJ, Knoll LJ, et al. Parasite stage-specific recognition of endogenous Toxoplasma gondii-derived CD8+ T cell epitopes. J Infect Dis. 2008;198:1625–33. doi: 10.1086/593019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McLeod R, Skamene E, Brown CR, Eisenhauer PB, Mack DG. Genetic regulation of early survival and cyst number after peroral Toxoplasma gondii infection of A x B/B x A recombinant inbred and B10 congenic mice. J Immunol. 1989;143:3031–4. [PubMed] [Google Scholar]
- 117.Brown CR, Hunter CA, Estes RG, Beckmann E, Forman J, David C, et al. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85:419–28. [PMC free article] [PubMed] [Google Scholar]
- 118.Johnson JJ, Roberts CW, Pope C, Roberts F, Kirisits MJ, Estes R, et al. In vitro correlates of Ld-restricted resistance to toxoplasmic encephalitis and their critical dependence on parasite strain. J Immunol. 2002;169:966–73. doi: 10.4049/jimmunol.169.2.966. [DOI] [PubMed] [Google Scholar]
- 119.Kirak O, Frickel EM, Grotenbreg GM, Suh H, Jaenisch R, Ploegh HL. Transnuclear mice with predefined T cell receptor specificities against Toxoplasma gondii obtained via SCNT. Science. 2010;328:243–8. doi: 10.1126/science.1178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.John B, Harris TH, Tait ED, Wilson EH, Gregg B, Ng LG, et al. Dynamic Imaging of CD8(+) T cells and dendritic cells during infection with Toxoplasma gondii. PLoS Pathog. 2009;5:e1000505. doi: 10.1371/journal.ppat.1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.John B, Ricart B, Tait Wojno ED, Harris TH, Randall LM, Christian DA, et al. Analysis of behavior and trafficking of dendritic cells within the brain during toxoplasmic encephalitis. PLoS Pathog. 2011;7:e1002246. doi: 10.1371/journal.ppat.1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cebrian I, Visentin G, Blanchard N, Jouve M, Bobard A, Moita C, et al. Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell. 2011;147:1355–68. doi: 10.1016/j.cell.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 123.McKee AS, Dzierszinski F, Boes M, Roos DS, Pearce EJ. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J Immunol. 2004;173:2632–40. doi: 10.4049/jimmunol.173.4.2632. [DOI] [PubMed] [Google Scholar]
- 124.Grover HS, Blanchard N, Gonzalez F, Chan S, Robey EA, Shastri N. The Toxoplasma gondii peptide AS15 elicits CD4 T cells that can control parasite burden. Infect Immun. 2012;80:3279–88. doi: 10.1128/IAI.00425-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–86. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dubey JP. Bradyzoite-induced murine toxoplasmosis: stage conversion, pathogenesis, and tissue cyst formation in mice fed bradyzoites of different strains of Toxoplasma gondii. J Eukaryot Microbiol. 1997;44:592–602. doi: 10.1111/j.1550-7408.1997.tb05965.x. [DOI] [PubMed] [Google Scholar]
- 127.Barragan A, Sibley LD. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J Exp Med. 2002;195:1625–33. doi: 10.1084/jem.20020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Unno A, Suzuki K, Xuan X, Nishikawa Y, Kitoh K, Takashima Y. Dissemination of extracellular and intracellular Toxoplasma gondii tachyzoites in the blood flow. Parasitol Int. 2008;57:515–8. doi: 10.1016/j.parint.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 129.Channon JY, Seguin RM, Kasper LH. Differential infectivity and division of Toxoplasma gondii in human peripheral blood leukocytes. Infect Immun. 2000;68:4822–6. doi: 10.1128/IAI.68.8.4822-4826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gâtel D, Tardieux I. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107:309–16. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Diana J, Persat F, Staquet MJ, Assossou O, Ferrandiz J, Gariazzo MJ, et al. Migration and maturation of human dendritic cells infected with Toxoplasma gondii depend on parasite strain type. FEMS Immunol Med Microbiol. 2004;42:321–31. doi: 10.1016/j.femsim.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 132.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 133.Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Microbiol. 2006;8:1611–23. doi: 10.1111/j.1462-5822.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- 134.Lambert H, Vutova PP, Adams WC, Loré K, Barragan A. The Toxoplasma gondii-shuttling function of dendritic cells is linked to the parasite genotype. Infect Immun. 2009;77:1679–88. doi: 10.1128/IAI.01289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Collantes-Fernandez E, Arrighi RB, Alvarez-García G, Weidner JM, Regidor-Cerrillo J, Boothroyd JC, et al. Infected dendritic cells facilitate systemic dissemination and transplacental passage of the obligate intracellular parasite Neospora caninum in mice. PLoS One. 2012;7:e32123. doi: 10.1371/journal.pone.0032123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lachenmaier SM, Deli MA, Meissner M, Liesenfeld O. Intracellular transport of Toxoplasma gondii through the blood-brain barrier. J Neuroimmunol. 2011;232:119–30. doi: 10.1016/j.jneuroim.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Diana J, Vincent C, Peyron F, Picot S, Schmitt D, Persat F. Toxoplasma gondii regulates recruitment and migration of human dendritic cells via different soluble secreted factors. Clin Exp Immunol. 2005;141:475–84. doi: 10.1111/j.1365-2249.2005.02856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bierly AL, Shufesky WJ, Sukhumavasi W, Morelli AE, Denkers EY. Dendritic cells expressing plasmacytoid marker PDCA-1 are Trojan horses during Toxoplasma gondii infection. J Immunol. 2008;181:8485–91. doi: 10.4049/jimmunol.181.12.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–46. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rescigno M. Before they were gut dendritic cells. Immunity. 2009;31:454–6. doi: 10.1016/j.immuni.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 141.Randolph GJ, Ochando J, Partida-Sánchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 142.Ferro EA, Bevilacqua E, Favoreto-Junior S, Silva DA, Mortara RA, Mineo JR. Calomys callosus (Rodentia : Cricetidae) trophoblast cells as host cells to Toxoplasma gondii in early pregnancy. Parasitol Res. 1999;85:647–54. doi: 10.1007/s004360050609. [DOI] [PubMed] [Google Scholar]
- 143.Abbasi M, Kowalewska-Grochowska K, Bahar MA, Kilani RT, Winkler-Lowen B, Guilbert LJ. Infection of placental trophoblasts by Toxoplasma gondii. J Infect Dis. 2003;188:608–16. doi: 10.1086/377132. [DOI] [PubMed] [Google Scholar]
- 144.Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, et al. Molecular cues to implantation. Endocr Rev. 2004;25:341–73. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 145.Robbins JR, Zeldovich VB, Poukchanski A, Boothroyd JC, Bakardjiev AI. Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect Immun. 2012;80:418–28. doi: 10.1128/IAI.05899-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120:1368–79. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Singh-Jasuja H, Thiolat A, Ribon M, Boissier MC, Bessis N, Rammensee HG, et al. The mouse dendritic cell marker CD11c is down-regulated upon cell activation through Toll-like receptor triggering. Immunobiology. 2013;218:28–39. doi: 10.1016/j.imbio.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 149.Schaeffer M, Han S-J, Chtanova T, van Dooren GG, Herzmark P, Chen Y, et al. Dynamic imaging of T cell-parasite interactions in the brains of mice chronically infected with Toxoplasma gondii. J Immunol. 2009;182:6379–93. doi: 10.4049/jimmunol.0804307. [DOI] [PubMed] [Google Scholar]
- 150.Wilson EH, Harris TH, Mrass P, John B, Tait ED, Wu GF, et al. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30:300–11. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–26. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- 152.Cahalan MD, Parker I, Wei SH, Miller MJ. Real-time imaging of lymphocytes in vivo. Curr Opin Immunol. 2003;15:372–7. doi: 10.1016/S0952-7915(03)00079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim K, Weiss LM. Toxoplasma: the next 100 years. Microbes Infect. 2008;10:978–84. doi: 10.1016/j.micinf.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, Gajria B, et al. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37(Database issue):D539–43. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]