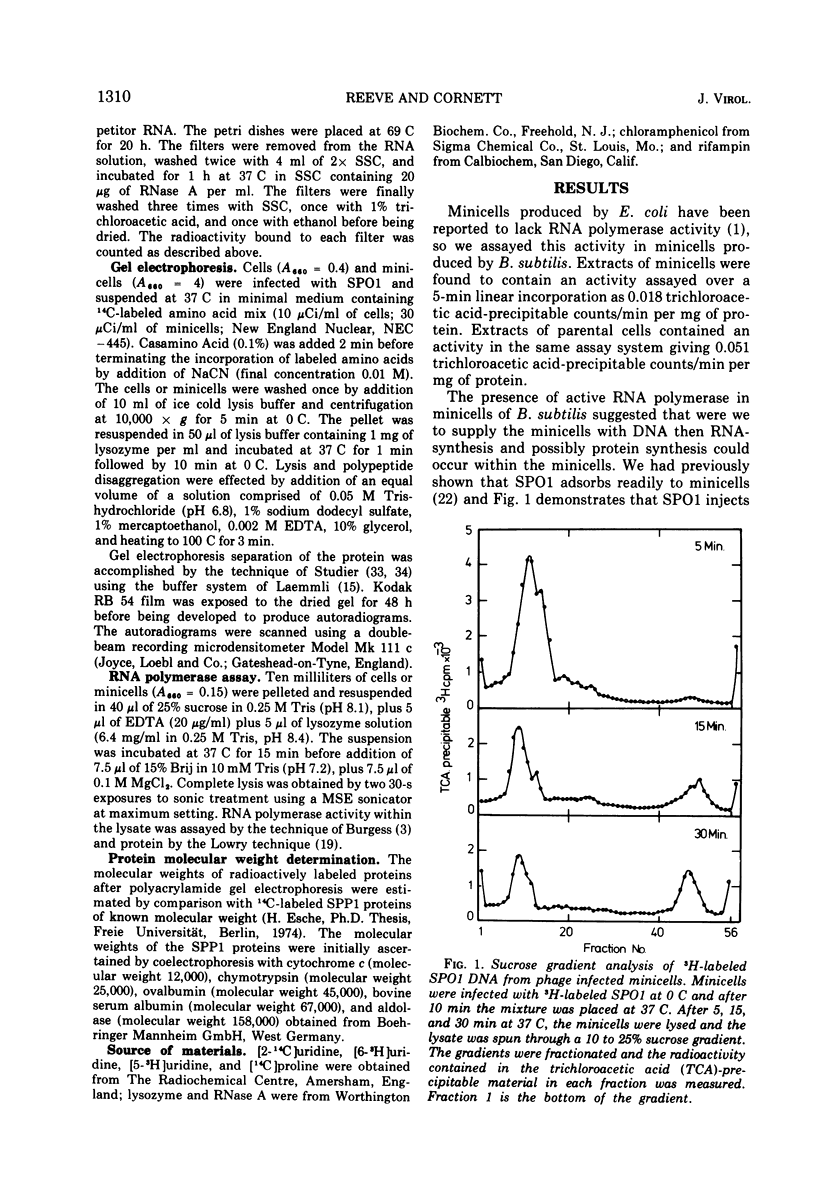
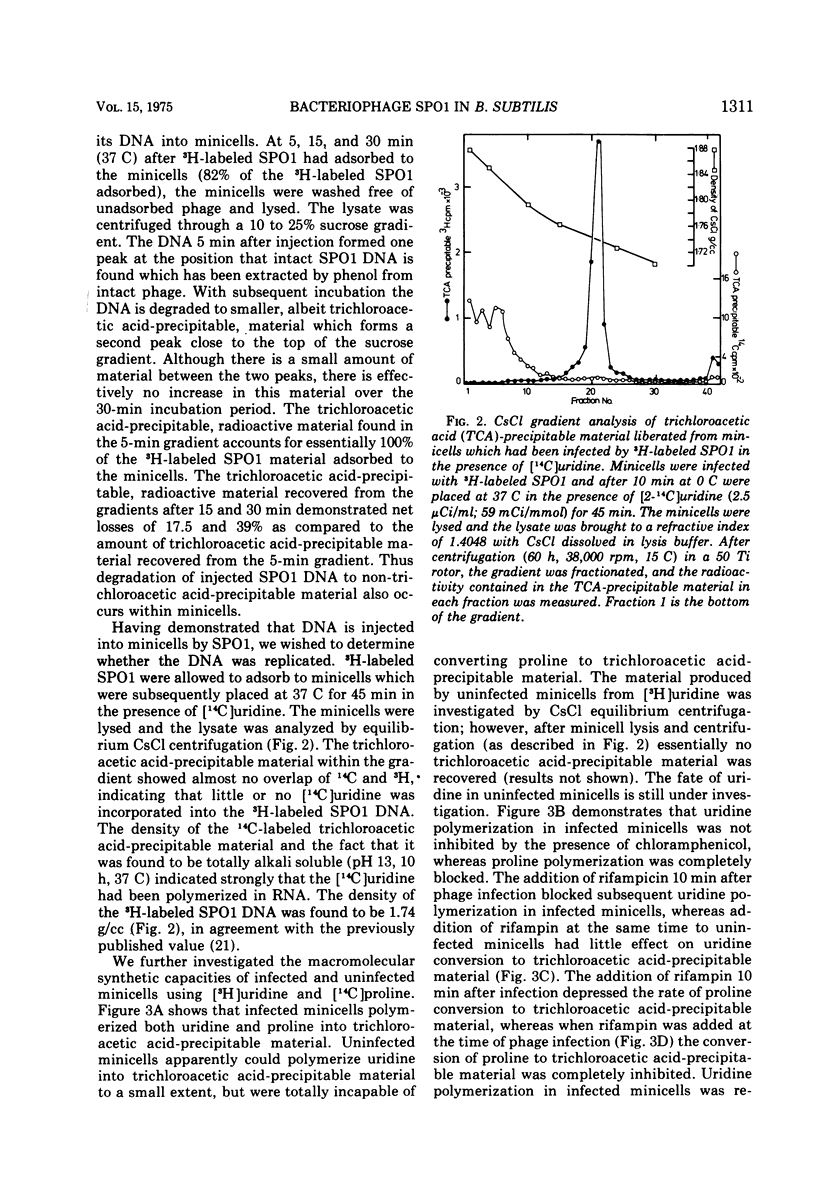
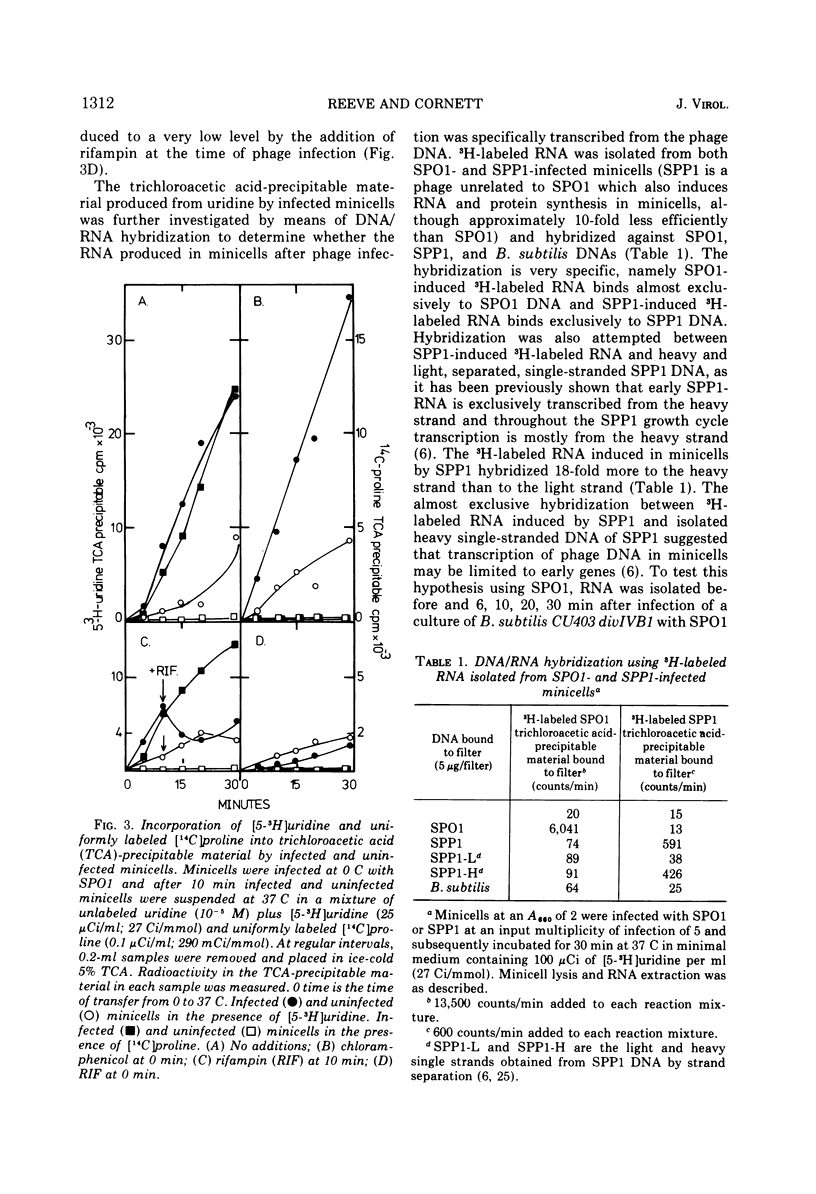
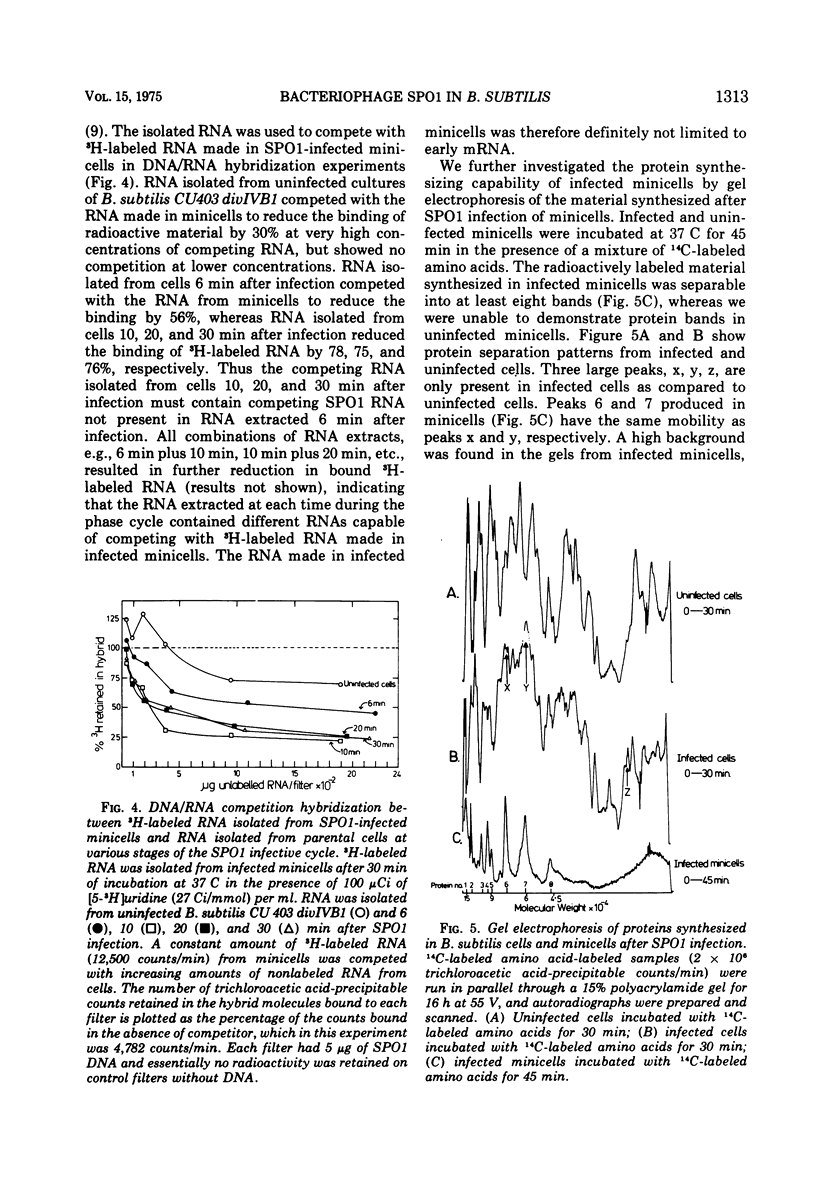
Abstract
SPO1 bacteriophage injects its DNA into minicells produced by Bacillus subtilis CU403 divIVB1. The injected DNA is partially degraded to small trichloracetic acid-precipitable material and trichloroacetic acid-soluble material. The injected DNA is not replicated; however, it serves as a template for RNA and protein synthesis. The RNA produced specifically hybridizes to SPO1 DNA, and the amount of RNA hybridized can be reduced by competition with RNA isolated at all stages of the phage cycle from infected nucleate cells of the B. subtilis CU403 divIVB1. An unrelated phage, SPP1, also induces phage-specific RNA in infected minicells. Translation occurs in SPO1-infected minicells resulting in at least eight proteins which have been separated by gel electrophoresis, and two of these proteins have mobilities similar to proteins found only in infected B. subtilis CU403 divIVB1 nucleate cells. A large proportion of the polypeptide material synthesized in infected minicells is very small and heterogeneous in size.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal N., Kleinschmidt A. K., Spatz H. C., Trautner T. A. Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet. 1967;100(1):39–55. doi: 10.1007/BF00425774. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Cohen A., Fisher W. D., Curtiss R., 3rd, Adler H. I. The properties of DNA transferred to minicells during conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:635–641. doi: 10.1101/sqb.1968.033.01.071. [DOI] [PubMed] [Google Scholar]
- Esche H., Spatz H. C. Asymmetric transcription of SPP1 in vivo. Mol Gen Genet. 1973 Jul 31;124(1):57–63. doi: 10.1007/BF00267164. [DOI] [PubMed] [Google Scholar]
- Fox T. D., Pero J. New phage-SPO1-induced polypeptides associated with Bacillus subtilis RNA polymerase. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2761–2765. doi: 10.1073/pnas.71.7.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Derepression of anthranilate synthase in purified minicells of Escherichia coli containing the Col-trp plasmid. J Bacteriol. 1973 Aug;115(2):615–622. doi: 10.1128/jb.115.2.615-622.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriophage SPO1 development: six classes of SPO1 RNA. J Mol Biol. 1971 Apr 28;57(2):279–297. doi: 10.1016/0022-2836(71)90346-9. [DOI] [PubMed] [Google Scholar]
- Hori H., Takata R., Muto A., Osawa S. Ribosomal RNA synthesis in the F'14 episome-containing minicells of Escherichia coli. Mol Gen Genet. 1974;128(4):341–347. doi: 10.1007/BF00268521. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Segregation into and replication of plasmid deoxyribonucleic acid in chromosomeless segregants of Escherichia coli. J Bacteriol. 1970 Jun;102(3):642–647. doi: 10.1128/jb.102.3.642-647.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass L. R., Yarmolinsky M. B. Segregation of functional sex factor into minicells. Proc Natl Acad Sci U S A. 1970 Jul;66(3):815–822. doi: 10.1073/pnas.66.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool A. J., Pranger M., Nijkamp H. J. Proteins synthesized by a non-induced bacteriocinogenic factor in minicells of Escherichia coli. Mol Gen Genet. 1972;115(4):314–323. doi: 10.1007/BF00333170. [DOI] [PubMed] [Google Scholar]
- Kool A. J., van Zeben M. S., Nijkamp H. J. Identification of messenger ribonucleic acids and proteins synthesized by the bacteriocinogenic factor Clo DF13 in purified minicells of Escherichia coli. J Bacteriol. 1974 Apr;118(1):213–224. doi: 10.1128/jb.118.1.213-224.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levner M. H., Cozzarelli N. R. Replication of viral DNA in SPO1-infected Bacillus subtilis. I. Replicative intermediates. Virology. 1972 May;48(2):402–416. doi: 10.1016/0042-6822(72)90051-7. [DOI] [PubMed] [Google Scholar]
- Levy S. B., Norman P. Segregation of transferable R factors into Escherichia coli minicells. Nature. 1970 Aug 8;227(5258):606–607. doi: 10.1038/227606a0. [DOI] [PubMed] [Google Scholar]
- Levy S. B. Physical and functional characteristics of R-factor deoxyribonucleic acid segregated into Escherichia coli minicells. J Bacteriol. 1971 Oct;108(1):300–308. doi: 10.1128/jb.108.1.300-308.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H., Reeve J. N., Cole R. M. Physiological studies of Bacillus subtilis minicells. J Bacteriol. 1974 Mar;117(3):1312–1319. doi: 10.1128/jb.117.3.1312-1319.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H., Coyne S. I., Hallock L. L., Cole R. M. Minicells of Bacillus subtilis. J Bacteriol. 1973 May;114(2):860–873. doi: 10.1128/jb.114.2.860-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H. Minicells of Bacillus subtilis. A unique system for transport studies. Biochim Biophys Acta. 1974 Jun 13;352(2):298–306. doi: 10.1016/0005-2736(74)90221-1. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H. Minicells of Bacillus subtilis: a new bacteriophage-blocking agent. J Virol. 1973 Oct;12(4):944–945. doi: 10.1128/jvi.12.4.944-945.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva S., Polsinelli M., Falaschi A. A new phage of Bacillus subtilis with infectious DNA having separable strands. J Mol Biol. 1968 Jul 28;35(2):347–356. doi: 10.1016/s0022-2836(68)80029-4. [DOI] [PubMed] [Google Scholar]
- Rogerson A. C., Stone J. E. Beta-beta' subunits of ribonucleic acid polymerase in episome-free minicells of Escherichia coli. J Bacteriol. 1974 Jul;119(1):332–333. doi: 10.1128/jb.119.1.332-333.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe D. H., Tucker R. G. The biosynthesis of 5-hydroxymethyldeoxyuridylic acid in bacteriophage-infected Bacillus subtilis. Virology. 1966 May;29(1):157–166. doi: 10.1016/0042-6822(66)90205-4. [DOI] [PubMed] [Google Scholar]
- Spatz H. C., Trautner T. A. One way to do experiments on gene conversion? Transfection with heteroduplex SPP1 DNA. Mol Gen Genet. 1970;109(1):84–106. doi: 10.1007/BF00334048. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Maizel J. V., Jr T7-directed protein synthesis. Virology. 1969 Nov;39(3):575–586. doi: 10.1016/0042-6822(69)90105-6. [DOI] [PubMed] [Google Scholar]
- Veltkamp E., Barendsen W., Nijkamp H. J. Influence of protein and ribonucleic acid synthesis on the replication of the bacteriocinogenic factor Clo DF13 in Escherichia coli cells and minicells. J Bacteriol. 1974 Apr;118(1):165–174. doi: 10.1128/jb.118.1.165-174.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vörös J., Goodman R. N. Filamentous forms of Erwinia amylovora. Phytopathology. 1965 Aug;55(8):876–879. [PubMed] [Google Scholar]
- Yehle C. O., Ganesan A. T. Deoxyribonucleic acid synthesis in bacteriophage SP01-infected Bacillus subtilis. II. Purification and catalytic properties of a deoxyribonucleic acid polymerase induced after infection. J Biol Chem. 1973 Nov 10;248(21):7456–7463. [PubMed] [Google Scholar]
- Yehle C. O., Ganesan A. T. Deoxyribonucleic acid synthesis in bacteriophage SPO1-infected Bacillus subtilis. I. Bacteriophage deoxyribonucleic acid synthesis and fate of host deoxyribonucleic acid in normal and polymerase-deficient strains. J Virol. 1972 Feb;9(2):263–272. doi: 10.1128/jvi.9.2.263-272.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Embden J., Cohen S. N. Molecular and genetic studies of an R factor system consisting of independent transfer and drug resistance plasmids. J Bacteriol. 1973 Nov;116(2):699–709. doi: 10.1128/jb.116.2.699-709.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


