Abstract
Judicial structural modifications of 5:7-fused ring-expanded nucleosides (RENs), based on molecular modeling studies with one of its known targets, human RNA helicase (hDDX3), led to the lead, novel, 5:7-5-fused tricyclic heterocycle (1). The latter exhibited promising broad-spectrum in vitro anticancer activity against a number of cancer cell lines screened. This paper describes our systematic, albeit limited, structure-activity relationship (SAR) studies on this lead compound, which produced a number of analogs with broad-spectrum in vitro anticancer activities against lung, breast, prostate, and ovarian cancer cell lines, in particular compounds 15i, 15j, 15m and 15n which showed IC50 values in submicromolar to micromolar range, and are worthy of further explorations. The SAR data also enabled us to propose a tentative SAR model for future SAR efforts for ultimate realization of optimally active and minimally toxic anticancer compounds based on the diimidazo[4,5-d:4′,5′-f][1,3]diazepine structural skeleton of the lead compound 1.
Keywords: Organic synthesis and medicinal chemistry; Diimidazo[4, 5-d:4′, 5′-f][1, 3]diazepines; Anti-cancer activity; Lung, breast, prostate and ovarian cancers; In vitro screening; Structure-activity relationship (SAR) studies; DDX3 as potential target
1. Introduction
The conserved RNA helicase ‘human DDX3’ (hDDX3) is a ubiquitously expressed 73 kD protein belonging to DEAD box family of ATP-dependent RNA helicases. The ‘DEAD box’ name comes from conserved amino acid sequence D-E-A-D (Asp-Glu-Ala-Asp) found in all members of the family. Different stages of RNA life cycle from their transcription, splicing, quality control, and transport to decay, are influenced by DDX3 in all species.1–3 hDDX3 has important roles in tumor proliferation and viral infections in human.4, 5 hDDX3 is of major medical importance due to its involvement in numerous cancers as well as viral infections, including but not limited to hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infections.6, 7 DDX3 has been validated as a target in anticancer and antiviral therapy.8 hDDX3 is aggressively expressed in certain forms of cancer. Benzopyran and benzopyran diepoxide (BPDE), constituents of cigarette smoke, are two among the many activators of DDX3. This activation in turn promotes growth, proliferation and neoplastic transformation of breast epithelial cells.9
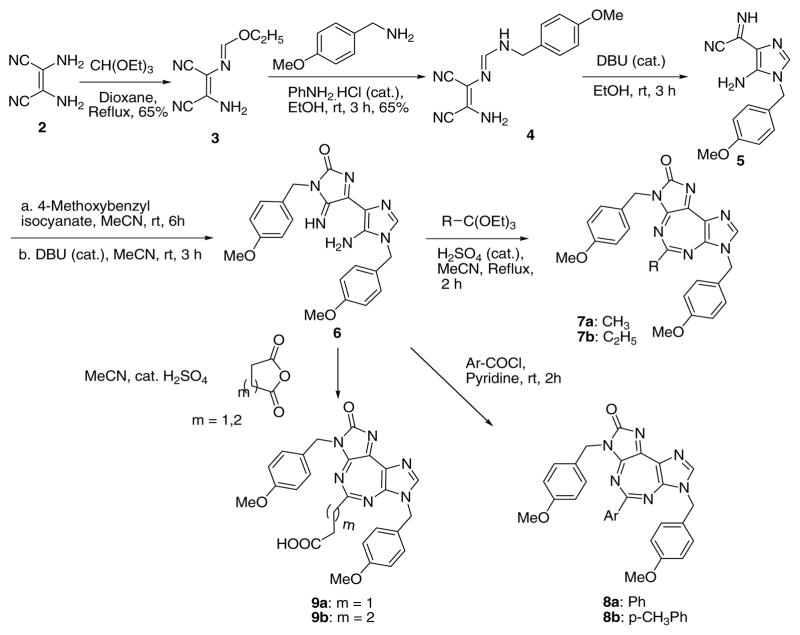
Our laboratory has been engaged in the design and synthesis of ring expanded nucleosides (RENs) possessing broad spectrum antiviral/anticancer activities, which were confirmed to be inhibitors of viral NTPases/helicases.10–12 Recently, two leading RENs were also experimentally demonstrated to be potent inhibitors of hDDX3 in vitro.13 RENs possess the characteristic 5:7-fused imidazodiazepine heterocyclic ring system, and mimic natural 5:6-fused purine nucleosides.
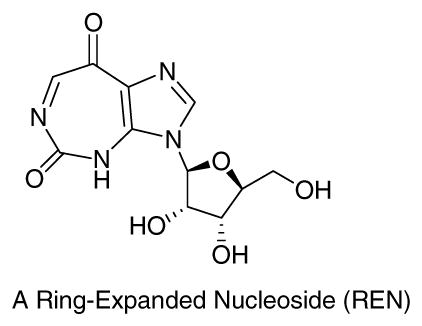
Inspired by positive biological results of many compounds bearing the 5:7-fused REN moiety,14 we proceeded to make further structural modifications of RENs through extensive molecular modeling studies, employing the coordinates from the reported crystal structure of hDDX3.15 These efforts ultimately led to the design and synthesis of a 5:7:5-fused diimidazo[4,5-d:4′,5′-f][1,3]diazepine-2-one, a novel tricyclic compound (1).16 The latter was indeed found to be a potent inhibitor of hDDX3 both in vitro and in vivo with an impressive selective cytotoxicity against cancer versus host.17, 18 Our preliminary biological studies with a limited number of compounds bearing the general structure 1 showed highly promising anticancer activity against a number of cancer cell lines.18, 19 Good anti-cancer activity coupled with an acceptable toxicity profile both in vitro and in vivo encouraged us to launch systematic structure-activity relationship studies (SARs) in order to improve upon efficacy and toxicity profiles of this series of compounds. To that end, we present here our SAR results that lead us to propose a tentative SAR model which we believe would assist in future SAR efforts for ultimate realization of one or more optimally potent and minimally toxic anti-cancer compounds based on the skeletal structure of 1.
2. Chemistry
2.1. DDX3 Crystal structure and Proposed SAR Model
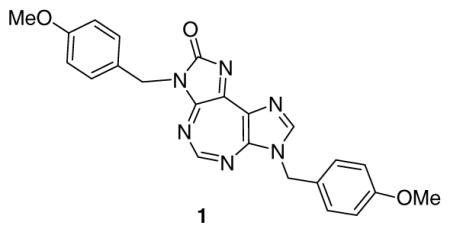
The reported crystal structure of a complex of hDDX3 with adenosine monophosphate [Protein Data Bank (PDB) code: 2I4I)] shows that it has two domains, N-terminal domain and C-terminal domain, which form a cleft where ATP binds (Figure 1).15 The nucleobase stays toward the surface of protein whereas the phosphate group buries deep inside the binding pocket.
Figure 1.
Visualization (Pymol®) of interactions between DDX3 and AMP from reported crystal structure in PDB.
We used the visualization software Pymol® to analyze interactions within 5Å. Purine nucleobase stacks over Tyr200 and mainly interacts with Q motif and the phosphate group interacts mainly with P-loop. Gln207 of Q-motif interacts with N6 and N-7 of purine. Arg202 interacts with N-1 of purine through a molecule of water.
A few important protein-ligand (AMP) interactions are shown in a cartoon representation in Figure 2A. It is known that the presence of a water molecule makes a large volume available around the purine binding region of active site, and results in relaxed substrate specificity.20 Favorable binding is achieved on displacement of water molecule from the active site as a result of favorable entropy. Introduction of structural features that mimic active site water molecule, such as urea, has been successfully used to design an anti-HIV protease inhibitor.21, 22
Figure 2.
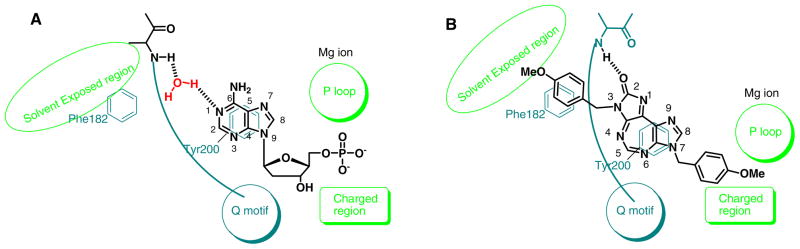
(A) Model for interactions between AMP and DDX3 (B) Proposed model for interactions between 1 and DDX3.
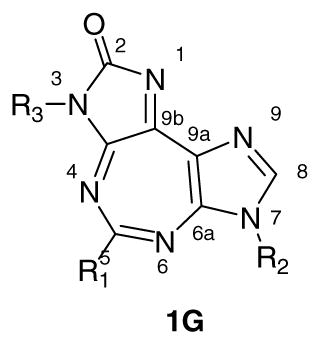
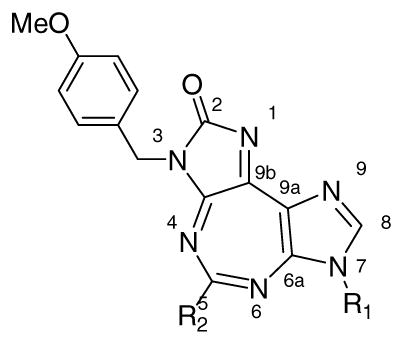
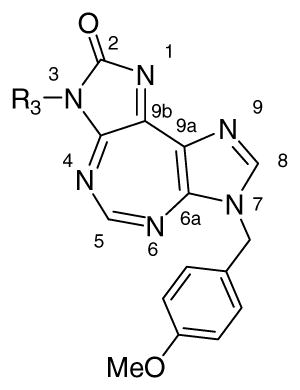
Taking into consideration these features as well as the molecular structure of 1, along with visualization of DDX3-AMP crystal structure through Pymol®, we constructed a working model for SAR studies. The cartoon representation of the proposed protein-ligand (1) interactions in this model is shown in Figure 2B. The central diazepine ring of 1 is expected to interact through π-π stacking interactions with Q motif residue Tyr200. The benzyl group attached to the ureidate portion of 1 is likely to embed in the hydrophobic region formed by backbone of residues 203–205 and Phe182, and is suitably placed to form π-π stacking interaction with Phe182. Likewise, the p-methoxybenzyl group present on the imidazole ring is expected to interact with the P loop. This is supported by our earlier observation that the p-methoxybenzyl group can replace ribose sugar on a nucleobase without significant loss of activity of the parent compound.23, 24 Three positions, N-3, N-5, and N-7, were selected for variations as shown in the general structure 1G based on the proposed interactions described above.
2.2. Organic Synthesis of Analogues of 1
5-Substituted analogues of 1 were synthesized as outlined in Scheme 1. Diaminomalenonitrile (2) was converted into formimidate 3 by reaction with ethyl orthoformate.16, 25 The reaction of 3 with p-methoxybenzylamine produced formamidine 4, which upon base catalyzed cyclization, furnished imidazole 5.16, 26 Treatment of 5 with 4-methoxybenzyl isocyanate produced the intermediate ureidate (not shown) that underwent base-catalyzed ring closure to furnish the important precursor diimidazole compound 6.16, 26 Ring-closure of the latter with appropriate reagents produced the desired tricyclic 5:7:5-fused heterocycles (7–9). For example, the reaction of 6 with triethyl orthoacetate and triethyl orthopropionate produced compounds with methyl (7a) and ethyl (7b) substitution at 5-position, respectively. Likewise, precursor 6 was treated with appropriate benzoyl chloride in the presence of a base to provide phenyl (8a) and toluyl (8b) analogues. Reactions with succinic and glutaric anhydrides gave compounds with alkyl chains bearing water-solublizing carboxylic acid group, 9a and 9b, respectively.
Scheme 1.
Synthesis of 5-substituted analogues of 1.
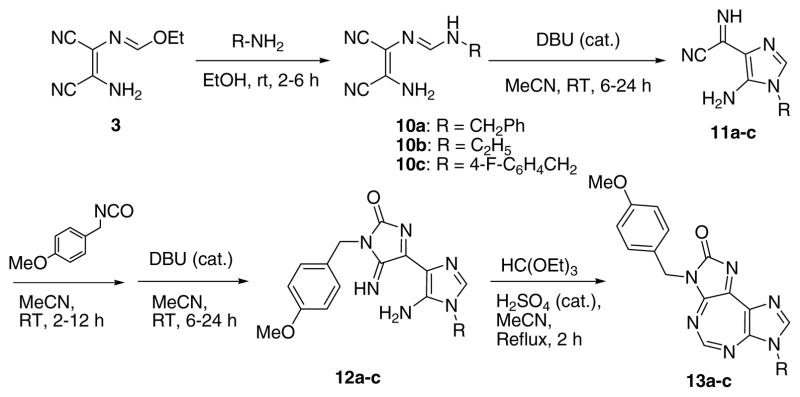
Compounds with substitution at 7-position were synthesized as shown in Scheme 2. Formimidate 3 was treated with appropriate amine to produce formamidines (10a–10c) which, under base catalyzed conditions, provided imidazole compounds 11a–11c. Treatment with p-methoxybenzylisocyanate provided the corresponding ureidate intermediates (not shown), which underwent DBU catalyzed cyclization to provide imidazolones 12a–c. The latter, upon treatment with ethyl orthoformate, furnished the target tricyclic compounds 13a–c.
Scheme 2.
Synthesis of 7-substituted analogues of 1
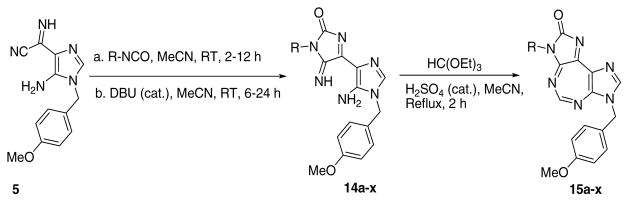
Compounds with substitution at 3-position were synthesized as shown in Scheme 3. Imidazole 5 was treated with appropriate isocyanates to give the respective ureidate intermediates (not shown). The latter were treated with DBU to obtain imidazolones 14a–x, followed by reaction with ethyl orthoformate to obtain the target 15a–x. It is to be noted that some necessary isocyanates were synthesized in the laboratory from the corresponding acid or amine. The preferred method for synthesis was from the corresponding acid by reaction with diphenylphosphoryl azide. For others, corresponding amine was treated with triphosgene in the presence of a base to produce isocyanate.
Scheme 3.
Synthesis of 3-substituted analogues of 1.
3. Biological Activity
The above compounds were tested against the cancer cell lines of lungs, prostate, breast and ovary. Activity against various cancer cell lines is collected in Tables 1 and 2.
Table 1.
Effect of substituent at R1 and R2 having 4-Methoxybenzyl at R3
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | R1 | R2 | IC50 (μM)a | ||||||
|
| |||||||||
| A549 Lung | H460 Lung | MCF-7 Breast | MDA-MB-231 Breast | OVCAR-3 Ovarian | PC-3 Prostate | ||||
| 1 | 4-MeO-C6H4CH2 | H | 2.56 | 2.87 | 7.59 | 4.05 | 14.50 | 4.90 | |
| 7a | 4-MeO- C6H4CH2 | CH3 | NE | NE | NE | NE | NE | NE | |
| 7b | 4-MeO- C6H4CH2 | CH2CH3 | NE | NE | NE | NE | NE | NE | |
| 8a | 4-MeO- C6H4CH2 | Ph | NE | NE | NE | NE | NE | NE | |
| 8b | 4-MeO- C6H4CH2 | 4-CH3Ph | NE | NE | NE | NE | NE | NE | |
| 9a | 4-MeO- C6H4CH2 | (CH2)2COOH | NE | NE | NE | NE | NE | NE | |
| 9b | 4-MeO- C6H4CH2 | (CH2)3COOH | NE | NE | NE | NE | NE | NE | |
| 13a | PhCH2 | H | NE | NE | NE | NE | NE | NE | |
| 13b | CH2CH3 | H | NE | NE | NE | NE | NE | NE | |
| 13c | 4-F- C6H4CH2 | H | 4.40 | 5.06 | 5.87 | 5.33 | NE | 7.67 | |
NE - Not effective. Maximum dose did not inhibit the growth/proliferation by ≤ 75%
Table 2.
Effect of substituent at R3
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | R3 | IC50 (μM)a | |||||
|
| |||||||
| A549 Lung | H460 Lung | MCF-7 Breast | MDA-MB-231 Breast | OVCAR-3 Ovarian | PC-3 Prostate | ||
| 15a | Ph | NE | NE | NE | NE | NE | NE |
| 15b | 4-MeO-Ph | NE | NE | NE | NE | NE | NE |
| 15c | 4-MeO- C6H4CH2CH2 | NE | NE | NE | NE | NE | NE |
| 15d | CH2CH3 | NE | NE | NE | NE | NE | NE |
| 15e | CH2COOEt | 33.50 | 34.50 | NE | 37.00 | NE | NE |
| 15f | CH2CH2Cl | 8.63 | 7.90 | NE | NE | NE | 9.33 |
| 15g | PhCH2 | 1.7 | 1.8 | 7.0 | 2.3 | 5.0 | 4.05 |
| 15h | 4-F- C6H4CH2 | 1.65 | 1.5 | 4.05 | 2.5 | 9.0 | 2.1 |
| 15i | 4-Cl- C6H4CH2 | 0.78 | 0.85 | 2.38 | 0.97 | 0.88 | 0.92 |
| 15j | 4-Br- C6H4CH2 | 0.63 | 0.57 | 1.02 | 1.16 | 1.62 | 0.98 |
| 15k | 3-MeO- C6H4CH2 | 1.50 | 1.55 | 6.00 | 2.60 | 2.50 | 5.15 |
| 15l | 2-MeO- C6H4CH2 | NE | NE | NE | NE | NE | NE |
| 15m | 3-F- C6H4CH2 | 1.20 | 1.24 | 1.64 | 2.70 | 3.18 | 1.83 |
| 15n | 3,4-Di-F- C6H3CH2 | 0.99 | 0.74 | 1.75 | 1.70 | 2.27 | 1.59 |
| 15o | 3-Cl- C6H4CH2 | 0.89 | 1.00 | 2.49 | 1.15 | 1.07 | 0.97 |
| 15p | 3-Br- C6H4CH2 | 1.37 | 1.17 | 1.85 | 1.53 | 1.54 | 2.57 |
| 15q | 3-F3C- C6H4CH2 | 3.19 | 3.10 | 4.31 | 4.61 | 3.68 | 8.30 |
| 15r | 4-OH- C6H4CH2 | 4.21 | 4.68 | 6.58 | 3.15 | 4.36 | 5.12 |
| 15s | 4-(CH3OCH2CH2)O-C6H4CH2 | 4.89 | 5.14 | 14.77 | 5.61 | 5.45 | 6.70 |
| 15t | 4-(HOCH2CH2)O- C6H4CH2 | 12.34 | 10.94 | 27.16 | 10.58 | 13.15 | 19.69 |
| 15u | 3-Pyridylmethyl | 8.05 | 8.30 | 19.83 | 8.68 | 9.89 | 26.87 |
| 15v | 4-(CH3)2N- C6H4CH2 | 8.81 | 8.44 | 15.10 | 8.82 | 9.43 | 202.18 |
| 15w | CH2CH2Piperidinyl | NE | NE | NE | NE | NE | NE |
| 15x | CH2CH2Morpholinyl | NE | NE | NE | NE | NE | NE |
NE - Not effective. Maximum dose did not inhibit the growth/proliferation by ≤ 75%
As revealed by the collected data, even the small alkyl groups, such as methyl (7a) and ethyl (7b) at 5-position, are detrimental to activity. Similarly, phenyl (8a), p-toluyl (8b) and carboxylic acid groups (9a and 9b) at 5-position lead to inactive compounds. Our proposed model shows this part of scaffold (5-position) in the vicinity of the backbone of Q motif. Hydrogen atom seems to be the right size to occupy the available volume. Substitution larger than hydrogen disrupts the binding of molecule. These observations suggest that the volume in active site is completely filled with the bulky 5:7:5-fused ring. It is possible that the water molecule of the active site, through which ATP interacts with backbone, is displaced. Such displacement of water molecule from active site is reported to provide favorable entropy.27
p-Methoxybenzyl group at 7-position was found to be optimum for activity. Fluoro in place of methoxy (13c) is tolerated, whereas, hydrogen (13a) is not. Changing this benzyl group with ethyl group (13b) led to loss of activity. The phosphate group of ATP interacts with P loop, which is highly charged, with hydrogen bond donating residues. Hydrogen bond acceptor such as methoxy or fluoro at this position is necessary for molecule to interact successfully with this region. The benzyl group is the right scaffold to orient and hold the hydrogen bond acceptor in place for successful interaction.
As evident from results in Table 1, H at 5-position and p-methoxy group at 7-position were optimum for activity. Keeping these groups, we planned further changes at the 3-position. Greater degree of change was tolerated at the 3-position and significant modulation of activity was observed.
Phenyl ring at 3-position is proposed to be involved in π-π stacking interactions with Phe182. One carbon linker connects this phenyl ring with the rest of molecule. Increasing (15c) or decreasing (15a, 15b) the length of linker between phenyl and imidazolone ring resulted in inactive compounds. π-π Stacking between phenyl ring of molecule and Phe182 appeared to be critical for activity and one methylene spacer puts phenyl ring of the molecule at the right distance for this interaction. Compounds (15e, 15f) bearing other polar groups in place of phenyl on methylene linker showed weak activity.
At this 3-benzyl group, unlike one at 7-position, hydrogen in place of methoxy group (15g) showed higher potency. Replacement of the methoxy group by halogens also improved potency (15h–j). The anti-cancer activity is likely related to size and/or polarizability of halogens. p-Bromo analogue is the most active compound of this series with activity in the range of 0.5 to 1 μM against all cell lines. Changing the position of methoxy group from p-position to m-position (15k) conferred higher potency whereas taking it to o-position (15l) led to loss of activity. An ortho substitution might interfere with the orientation of the benzyl group. Similar gain in activity was seen when fluoro group was shifted from p-position to m-position (15h vs 15m). 3,4-Difluoro compound (15n) was more potent than both monosubstituted analogues. Moving bulkier halogens to m-position (15i vs 15o and 15j vs 15p) did not offer any advantage over corresponding p-analogue.
We also incorporated groups that increase water solubility in the core. Phenolic analogue (15r) as well as p-(2-methoxy)ethoxy analogue (15s) showed activity comparable to the parent compound. p-(2-Hydroxy)ethoxy analogue (15t) was 2–3 fold less active than p-(2-methoxy)ethoxy analogue (15s). Replacing phenyl ring with pyridine (15u) led to 2–3-fold loss of activity. Similar activity was seen for p-dimethylamino analogue (15v). Piperidinylethyl (15w) and morpholinylethyl (15x) analogues were completely inactive.
4. Conclusions
Structural scrutiny of the lead compound 1, in conjunction with the reported crystal structure of DDX3-AMP complex, guided the initial, albeit limited, SAR studies and led way to develop a tentative model for better understanding of the activity of the lead compound as well as for future undertaking of extensive SAR studies. The azepine ring was not amenable for changes. Limited changes were carried out on the imidazole and the imidazolone rings of 1. In particular, the imidazolone ring provided a useful handle for improvement of activity. In summary, our initial structure activity relationship studies on the title diimidazo[4,5-d:4′,5′-f][1,3]diazepine series of compounds based on the hypothesized model led to potent anticancer compounds worthy of further explorations. Compounds 15i, 15j, 15m and 15n showed excellent broad-spectrum anti-cancer activity in the submicromolar to micromolar range.
5. Experimental
5.1 General
The 1H and 13C NMR spectra were recorded on a JEOL-400 NMR spectrometer, operating at 400MHz for 1H and 100 MHz for 13C NMR, respectively. Thin layer chromatography was performed on Merck Kieselgel 60 F254 (0.2 mm thickness). Flash chromatography was performed using 32–63 mesh silica gel. Mass spectra were recorded on a Brucker Daltonics Esquire-3000 LC-Quadrupole ion trap spectrometer. High resolution mass spectra were recorded a Bruker Daltonics (Billerica, MA) Apex IV FTICR. Melting points were recorded on Hoover capillary melting point apparatus and are uncorrected. Anhydrous solvents were purchased and used without further drying.
5.2. Synthetic Organic Chemistry
5.2.1. General procedure for synthesis of isocyanates from acid
To a suspension of acid (1.5 g, 6.25 mmol, 1.0 equi) in toluene, under inert atmosphere, was added triethylamine (0.76 g, 7.5 mmol, 1.2 equi) followed by slow addition of diphenylphsophoryl azide (1.90 g, 6.87 mmol, 1.1 equi ). Reaction mixture was stirred at room temperature for 15 min., followed by heating to 80°C for 1 h during which evolution of nitrogen was observed. Solvent was removed at reduced pressure. Unless otherwise mentioned, isocyanate was extracted by the following method. Residue was stirred in hexane (10 mL/mmol) and supernatant was collected. Hexane extraction was repeated twice. Combined hexane layer was evaporated to get crude isocyanate, which was used for next step without further purification.
5.2.2. 3,7-Bis(4-methoxybenzyl)-5-methyl-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (7a)
To a solution of 6 (0.1 g, 0.239 mmol, 1.0 equi.) in acetonitrile (3 mL) was added triethyl orthoacetate (0.31 g, 1.90 mmol, 8.0 equi.) and a drop of H2SO4 and heated under reflux for 6 h under inert atmosphere. Solution was cooled to room temperature. The precipitate was washed with acetonitrile and dried under vacuum to get product (Yield = 0.08 g, 75%); m.p.: 228–229°C; 1H-NMR (DMSO-d6): δ = 2.81 (s, 3H, -CH3), 3.7 (s, 6H, -OCH3), 5.02 (s, 2H, -CH2), 5.45 (s, 2H, -CH2), 6.85–6.91 (m, 4H, Ar-H), 7.31 (d, J= 8.7 Hz, 2H, Ar-H), 7.38 (d, J= 8.7 Hz, 2H, Ar-H), 8.83 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 30.35, 42.71, 46.33, 55.04, 55.09, 113.87, 114.06, 127.84, 128.09, 128.31, 129.43, 129.54, 147.76, 149.39, 155.22, 158.30, 158.69, 158.99, 160.42, 165.81; MS (m/z): 443.2 (M+1); Anal. Calcd for C24H22N6O3. 0.25 H2O: C, 64.49; H, 5.07; N, 18.80; found C, 64.58; H, 4.89; N, 18.81.
5.2.3. 3,7-Bis(4-methoxybenzyl)-5-ethyl-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (7b)
The procedure is similar to that of 7a above, but using triethyl orthopropionate (16 equi.), Yield = 0.12 g, 55%; m.p.: 208–210°C; 1H-NMR (DMSO-d6): δ = 1.35 (t, J = 7.32 Hz, 3H, -CH3), 3.08 (q, J = 7.32 Hz, 2H, -CH2), 3.70 (s, 3H, -OCH3), 3.73 (s, 3H, -OCH3), 5.03 (s, 2H, -CH2), 5.47 (s, 2H, -CH2), 6.85–6.91 (m, 4H, Ar-H), 7.31 (d, J = 8.7 Hz, 2H, Ar-H), 7.38 (d, J = 8.7 Hz, 2H, Ar-H), 8.86 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 12.57, 35.89, 42.8, 46.47, 55.11, 55.15, 113.93, 114.12, 127.79, 128.23, 128.42, 129.49, 129.54, 147.89, 149.5, 155.24, 158.46, 158.75, 159.05, 164.11, 165.95; MS (m/z): 457.2 (M+1); Anal. Calcd for C25H24N6O3: C; 65.78; H; 5.30; N; 18.41; found: C; 65.75; H; 5.33; N; 18.38.
5.2.4. 3,7-Bis(4-methoxybenzyl)-5-phenyl-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (8a)
To a solution of 6 (50 mg, 0.12 mmol, 1.0 equi.) in pyridine (5 mL) was added benzoyl chloride (0.020 mg, 0.144 mmol, 1.2 equi.) and stirred at room temperature for 6 h under inert atmosphere. The reaction mixture was poured on crushed ice and the precipitate formed was filtered. The residue was washed with water and dried to get crude product which as purified by recrystallization from acetone (Yield = 52 mg, 87.6%); m.p.: >260°C. 1H-NMR (DMSO-d6): δ = 3.68 (s, 6 H, 2 X OCH3), 5.19 (s, 2 H, CH2), 5.62 (s, 2 H, CH2), 6.86 (d, J = 8.7 Hz, 2 H, Ar-H), 6.89 (d, J = 8.7 Hz, 2 H, Ar-H), 7.36 (d, J = 8.7 Hz, 2 H, Ar-H), 7.40 (d, J = 8.7 Hz, 2 H, Ar-H), 7.58 (m, 3H), 8.58 (m, 2H), 8.93 (s, 1 H, Imid-H). 13C-NMR (DMSO-d6): δ = 42.89, 46.51, 54.95, 54.99, 113.84, 114.02, 128.23, 128.43, 128.77, 129.05, 129.12, 129.47, 131.62, 138.64, 148.56, 149.44, 154.36, 155.43, 158.59, 158.70, 158.88, 165.90. HRMS (m/z): Calcd for C29H25N6O3 (MH+) 505.1943; found, 505.1982; Anal. Calcd for C29H24N6O3: C, 69.04; H, 4.79; N, 16.66; found: C, 68.77; H, 4.90; N, 16.65.
5.2.5. 3,7-Bis(4-methoxybenzyl)-5-(4-methylphenyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (8b)
Procedure analogous to that of 8a above; m.p.: >260°C. 1H-NMR (DMSO-d6): δ = 2.40 (s, 3 H, CH3), 3.68 (s, 6 H, 2 X OCH3), 5.17 (s, 2 H, CH2), 5.60 (s, 2 H, CH2), 6.87 (d, J = 8.7 Hz, 2 H, Ar-H), 6.89 (d, J = 8.7 Hz, 2 H, Ar-H), 7.37 (m, 6 H, Ar-H), 8.47 (d, J = 8.24 Hz, 2 H, Ar-H), 8.91 (s, 1 H, Imid-H). 13C-NMR (DMSO-d6): δ = 21.07, 42.96, 46.57, 55.04, 55.07, 113.93, 114.10, 128.37, 128.54, 129.15, 129.22, 129.51, 129.61, 136.07, 141.94, 146.62, 148.55, 149.64, 154.62, 155.43, 158.68, 158.88, 158.96, 166.00; MS (ESI, m/z) 519.4 (M +1). Anal. Calcd. for C30H26N6O3. 0.5H2O: C, 68.30; H, 5.16; N, 15.93; found: C, 68.19; H, 5.56; N, 14.26.
5.2.6. 3,7-Bis(4-methoxybenzyl)-5-(2-carboxyethyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (9a)
To a solution of 6 (0.1 g, 0.24 mmol, 1.0 equi.) in DMF (2 mL) was added succinic anhydride (0.12 g, 1.2 mmol, 5 equi.) and a drop of H2SO4 and heated at 60°C for 12 h under inert atmosphere. Reaction mixture was cooled and poured over crushed ice. The precipitate was filtered and washed with water and dried under vacuum to get crude product (0.12 g). The compound was purified by recrystallization in acetone (Yield = 20 mg, 16.7%); m.p.: 229–231°C; 1H-NMR (DMSO-d6): δ = 2.78 (t, J = 6.6 Hz, 2H), 3.30 (m, 2H), 3.66 (s, 3 H, OCH3), 3.68 (s, 3 H, OCH3), 5.00 (s, 2 H, CH2), 5.43 (s, 2 H, CH2), 6.82 (d, J = 8.7 Hz, 2H, Ar-H), 6.86 (d, J = 8.7 Hz, 2H, Ar-H), 7.29 (d, J = 8.7 Hz, 2H, Ar-H), 7.36 (d, J = 8.7 Hz, 2H, Ar-H), 8.81 (s, 1H, Imid-H), 12.23 (s, 1H, -COOH); 13C-NMR (DMSO-d6): δ = 31.40, 37.04, 42.76, 46.23, 55.05, 55.09, 113.88, 114.10, 127.67, 128.14, 128.36, 129.51, 129.56, 147.83, 149.14, 155.27, 158.33, 158.71, 159.02, 161.80, 165.84, 174.08; MS (m/z) 501.4 (M+1); Anal. Calcd. for C26H24N6O5.0.5H2O:C, 61.29; H, 4.95; N, 16.49; found C, 61.65; H, 4.69; N, 16.53.
5.2.7. 3,7-Bis(4-methoxybenzyl)-5-(2-carboxypropyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (9b)
Procedure similar to that of 9a (Yield = 30 mg, 25%); m.p.: 148–150°C; 1H-NMR (DMSO-d6): δ = 2.09 (m, 2H, CH2), 2.33 (t, 2H, CH2), 3.07 (t, 2H, CH2), 3.70 (s, 6H), 5.02 (s, 2H, CH2), 5.45 (s, 2H, CH2), 6.85 (d, J = 8.7 Hz, 2 H, Ar-H), 6.88 (d, J = 8.7 Hz, 2 H, Ar-H), 7.32 (d, J = 8.7 Hz, 2 H, Ar-H), 7.38 (d, J = 8.7 Hz, 2 H, Ar-H), 8.86 (s, 1H, Imid-H), 12.08 (s, 1H, -COOH); 13C-NMR (DMSO-d6): δ = 23.72, 33.33, 42.15, 43.31, 46.98, 55.58, 55.63, 114.40, 114.59, 128.38, 128.68, 128.85, 130.06, 130.13, 148.34, 149.91, 155.90, 159.02, 159.24, 159.54, 163.17, 166.41, 174.91; MS (ESI) (m/z) 515.3 (M+1); Anal. Calcd. for C27H26N6O5: C, 63.03; H, 5.09; N, 16.33; found C, 62.66; H, 5.13; N, 16.05.
5.2.8. 2-(5-Amino-1-benzyl-1H-imidazol-4-yl)-2-iminoaceto-nitrile (11a)
To a solution of formimidate 3 (1.0 g., 6.1 mmol) in ethanol (5 mL) was added benzylamine (0.65 g., 6.1 mmol) and aniline hydrochloride (5 mg), under inert atmosphere, at room temperature and stirred for 4 h (starting material completely consumed on TLC). The solvent was evaporated under reduced pressure. Resulting residue was suspended in ethanol (5 mL) under inert atmosphere and 2 drops DBU was added at room temperature followed by stirring for 5 h (starting material completely consumed on TLC). Reaction mixture was diluted with diethyl ether (20 mL). Precipitate formed was filtered, washed with diethyl ether (2 X 10 mL) and dried to obtain the product (Yield = 0.67 g, 50.0%); 1H-NMR (DMSO-d6): δ = 5.12 (s, 2H, -CH2), 6.78 (brs, 2H, -NH2), 7.22–7.36 (m, 6H, Ar-H), 10.90 (s, 1H, -NH); HRMS (m/z): Calcd.for C12 H12N5 (MH+): 226.1087; found, 226.1087.
5.2.9. 2-(5-Amino-1-ethyl-1H-imidazol-4-yl)-2-iminoaceto-nitrile (11b)
To a acetonitrile (7 mL) solution of 3 (2.2 g, 13.41 mmol, 1 equi.) was added ethylamine hydrochloride (1.20 g, 14.75 mmol, 1.1 equi.) and triethylamine (1.63 g, 16.09, 1.2 equi.), at room temperature under inert atmosphere, and stirred for 12 h. Reaction mixture was filtered and washed with diethyl ether. The filtrate was concentrated and purified by column chromatography (gradient elution using ethyl acetate in hexane) (Yield = 1.0 g, 45.6%); 1H-NMR (DMSO-d6): δ = 1.27 (t, J = 7.3 Hz, 3H, -CH3), 3.86 (q, J = 7.3 Hz, 2H, -CH2), 6.70 (brs, 2H, -NH2), 7.25 (s, 1H, Imid-H), 10.83 (s, 1H, -NH).
5.2.10. 2-(1-(4-Fluorobenzyl)-5-amino-1H-imidazol-4-yl)-2-iminoacetonitrile (11c)
To a solution of formimidate 3 (0.5 g., 3.05 mmol) in acetonitrile (5 mL) was added 4-fluorobenzylamine (0.38 g., 3.05 mmol) and aniline hydrochloride (2 mg), under inert atmosphere, at 0°C. The reaction mixture was allowed to come to room temperature followed by stirring at for 12 h. The solvent was evaporated under reduced pressure. Resulting residue was suspended in ethyl acetate (5 mL) under inert atmosphere and 3 drops DBU was added at room temperature followed by stirring for 24 h (starting material completely consumed on TLC). Reaction mixture was diluted with diethyl ether (20 mL). Supernatant was decanted, and residue was washed with diethyl ether (2 X 10 mL). The combined organic layer was evaporated to dryness. Residue was triturated with diethyl ether (2X 10 mL) and dried to get product (Yield = 0.540 g., 73%); 1H-NMR (DMSO-d6): δ = 5.12 (s, 2H, -CH2), 6.78 (brs, 2H, -NH2), 7.17–7.23 (m, 2H, Ar-H), 7.28–7.32 (m, 2H, Ar-H), 7.35 (s, 1H, Imid-H), 10.91 (brs, 1H, -NH).
5.2.11. 1-(4-Methoxybenzyl)-4-(5-amino-1-benzyl-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (12a)
To a suspension of 11a (0.6 g., 2.67 mmol, 1.0 equi.) in 5 mL acetonitrile was added 4-methoxybenzyl isocyanate (1.30 g., 8.0 mmol, 3.0 equi.) and stirred at room temperature for 12 h. The precipitate formed was filtered, washed with diethyl ether (2X10 mL) and dried. This precipitate was suspended in 5 mL acetonitrile. DBU (2 drops) was added to it and stirred for 3 h. The resulting precipitate was filtered, washed with diethyl ether and dried to give 12a (Yield = 0.45 g., 43.5%); m.p.: 214–216°C; 1H-NMR (DMSO-d6): δ = 3.70 (s, 3H, -OCH3), 4.62 (s, 2H, -CH2), 5.20 (s, 2H, -CH2), 6.85 (d, J=8.7 Hz, 2H, Ar-H), 7.20–7.26 (m, 4H, Ar-H), 7.30–7.33 (m, 1H, Ar-H), 7.37–7.40 (m, 2H, Ar-H), 7.75 (s, 1H, Imid-H), 7.92 (brs, 2H, -NH2), 9.78 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 41.12, 45.83, 55.03, 113.73, 127.15, 127.85, 128.79, 128.83, 129.64, 135.83, 139.07, 151.89, 156.99, 158.37, 159.57, 166.74; HRMS (m/z): Calcd. For C21H21N6O2 (MH+): 389.1720; found 389.1720; Anal. Calcd. for C21H20N6O2.0.2H2O: C, 64.34; H, 5.25; N, 21.44; found: C, 64.41; H, 5.22; N, 21.22.
5.2.12. 1-(4-Methoxybenzyl)-4-(5-amino-1-ethyl-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (12b)
Procedure analogous to that of 12a (Yield = 0.24 g., 30.4 %); m.p.:200–202°C; 1H-NMR (DMSO-d6): δ = 1.27 (t, J = 7.1 Hz, 3H, -CH3), 3.71 (s, 3H, -OCH3), 3.90 (q, J = 7.1 Hz, 2H, -CH2), 4.66 (s, 2H, -CH2), 6.86 (d, J = 8.7 Hz, 2H, -CH2), 7.21 (d, J = 8.7 Hz, 2H, -CH2), 7.68 (s, 1H, Imid-H), 7.81 (brs, 2H, -NH2), 9.78 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 14.33, 39.29, 39.92, 55.04, 113.74, 113.87, 128.84, 129.64, 138.61, 151.66, 157.01, 158.37, 159.59, 166.79; MS (m/z): 327.1 (M+1); Anal. Calcd. for C16H18N6O2: C, 58.88; H, 5.56; N, 25.75; found: C, 58.58; H, 5.60, N, 25.64.
5.2.13. 4-(1-(4-Fluorobenzyl)-5-amino-1H-imidazol-4-yl)-1-(4-methoxybenzyl)-5-imino-1H-imidazol-2(5H)-one (12c)
Procedure similar to that of 12a (Yield = 0.30 g., 40.8 %); m.p.: 222–224°C; 1H-NMR (DMSO-d6): δ = 3.72 (s, 3H, -OCH3), 4.62 (s, 2H, -CH2), 5.19 (s, 2H, -CH2), 6.85 (d, J = 8.7 Hz, 2H, Ar-H), 7.20–7.24 (m, 4H, Ar-H), 7.33 (dd, J = 5.5 Hz, 8.7 Hz, 2H, Ar-H), 7.76 (s, 1H, Imid-H), 7.90 (brs, 2H, -NH2), 9.79 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 41.65, 45.71, 55.55, 114.20, 116.04, 116.26, 129.36, 130.01, 130.09, 130.12, 132.49, 139.33, 152.13, 157.74, 158.90, 160.03, 161.04, 163.48, 167.25; Anal. Calcd. for C21H19FN6O2.0.2H2O: C,61.52; H,4.77; N,20.50; found: C,61.62; H,4.65; N,20.41. HRMS (m/z): Calcd. for C21H20FN6O2(MH+): 407.1626; found 407.1626.
5.2.14. 7-Benzyl-3-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (13a)
To a solution of 12a (0.2 g, 0.51 mmol, 1.0 equi.) in acetonitrile (5 mL) was added triethyl orthoformate (0.42 g, 4.12 mmol, 8.0 equi.) and a drop of H2SO4 and heated under reflux for 2 h under inert atmosphere. The reaction mixture was cooled to room temperature. Precipitate formed was filtered, washed with diethyl ether (2X10 mL), and dried to get product (Yield = 0.16 g, 79%); m.p.: 168–170°C; 1H-NMR (DMSO-d6): δ = 3.70 (s, 3H, -OCH3), 5.08 (s, 2H, -CH2), 5.61 (s, 2H, -CH2), 6.86 (d, J = 8.7 Hz, 2H, Ar-H), 7.28–7.35 (m, 7H, Ar-H), 8.73 (s, 1H, azepine H), 8.95 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 42.86, 46.90, 55.07, 113.89, 127.48, 127.95, 128.75, 129.12, 136.32, 148.53, 149.88, 149.93, 155.88, 158.69, 159.77, 165.79; HRMS (m/z): Calcd. for C22H19N6O2: 399.1564; found 399.1564; Anal. Calcd for C22H18N6O2.0.5H2O: C, 64.85; H, 4.70; N, 20.63; found C, 64.65; H, 4.50; N, 20.44.
5.2.15. 7-Ethyl-3-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (13b)
Procedure similar to that of 13a except for washing of the precipitate with ethyl acetate (2X5 mL), instead of diethyl ether (Yield = 0.13 g, 63%); m.p.:176–179°C; 1H-NMR (DMSO-d6): δ = 1.44 (t, J = 7.1 Hz, 3H, -CH3), 3.7 (s, 3H, -OCH3), 4.37 (q, J = 7.1 Hz, 2H, -CH2), 5.09 (s, 2H, -CH2), 6.86 (d, J = 8.7 Hz, 2H, Ar-H), 7.31 (d, J = 8.7 Hz, 2H, Ar-H), 8.75 (s, 1H, diazepine-H), 8.87 (s, 1H, Imid-H); 13 C-NMR (DMSO-d6): δ = 15.26, 42.91, 55.12, 113.95, 128.03, 128.91, 129.17, 148.44, 149.62, 149.95, 155.66, 158.74, 159.61, 165.85; MS (m/z): 337.1 (M+1); Anal. Calcd for C17H16N6O2: C, 60.71; H, 4.79; N, 24.99; found C, 60.57; H, 4.75; N, 24.88.
5.2.16. 7-(4-Fluorobenzyl)-3-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (13c)
Procedure similar to that of 13a (Yield = 0.12 g, 43.4 %); m.p.: 196–198°C. 1H-NMR (DMSO-d6): δ = 3.70 (s, 3H, -OCH3), 5.08 (s, 2H, -CH2), 5.58 (s, 2H, -CH2), 6.86 (d, J = 8.7 Hz, 2H, Ar-H), 7.18 (m, 2H, Ar-H), 7.29 (d, J = 8.7 Hz, 2H, Ar-H), 7.42 (dd, J = 5.5 Hz, 8.7 Hz, 2H, Ar-H), 8.73 (s, 1H, diazepine- H), 8.94 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 42.87, 46.23, 55.07, 113.88, 115.45, 115.67, 127.93, 128.70, 129.12, 129.85, 129.92, 132.52, 132.55, 148.42, 149.89, 155.88, 158.70, 159.77, 160.55, 162.97, 165.78; Anal. Calcd for C22H17FN6O2: C, 63.46; H,4.11; N,20.18; found C,63.19; H,4.08; N,20.12; HRMS (m/z): Calcd. For C23H18FN6O2 (MH+) 417.1469; found 417.1469.
5.2.17. 4-(1-(4-Methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1-phenyl-1H-imidazol-2(5H)-one (14a)
To a suspension of 5 (0.25 g., 0.98 mmol) in 5 mL acetonitrile was added phenyl isocyanate (0.35 g, 2.94 mmol, 3 equi.) and stirred at room temperature, under inert atmosphere, till TLC showed complete consumption of starting material (3 h). The precipitate formed was filtered, washed with diethyl ether (3 X 10 mL) and dried. This precipitate was suspended in 4 mL acetonitrile under inert atmosphere. DBU (2 drops) was added to it and stirred for 2 h. The resulting precipitate was filtered, washed with diethyl ether and dried to give 14a (Yield = 0.17 g. 46.4 %); m.p.: 192–193°C; 1H-NMR (DMSO-d6): δ = 3.73 (s, 3H, -OCH3), 5.14 (s, 2H, -CH2), 6.94 (d, J = 8.7 Hz, 2H, Ar-H), 7.27 (d, J = 8.7 Hz, 2H, Ar-H), 7.32 (m, 1H), 7.45 (d, J = 4.1 Hz, 4H, Ar-H), 7.78 (s, 1H, Imid-H), 8.00 (brs, 2H, -NH2), 10.06 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 41.47, 55.14, 113.83, 114.20, 126.61, 127.59, 128.49, 128.93, 133.92, 139.17, 151.92, 156.76, 159.00, 159.60, 165.49; Anal. Calcd for C20H18N6O2: C,64.16; H,4.85; N,22.45; found C,64.17; H,4.79; N,22.32. HRMS (m/z) Calcd for C20H19N6O2 (MH+) 375.1564; found 375.1564.
5.2.18. 4-(1-(4-Methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1-(4-methoxyphenyl)-1H-imidazol-2(5H)-one (14b)
The necessary isocyanate was synthesised by the general procedure described above. The procedure for 14 b is similar to that of 14a. (Yield = 0.2 g., 42%). The initial reaction of isocyanate was carried out for 24 h; m.p.: 176–178°C. 1H-NMR (DMSO-d6): δ = 3.73 (s, 6H, -OCH3), 5.11 (s, 2H, -CH2), 6.94 (d, J = 8.7 Hz, 2H, Ar-H), 7.00 (d, J = 8.7 Hz, 2H, Ar-H), 7.27 (d, J = 8.7 Hz, 2H, Ar-H), 7.31 (d, J = 8.7 Hz, 2H, Ar-H), 7.75 (s, 1H, Imid-H), 8.1 (brs, 2H, -NH2), 9.90 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 45.35, 55.13, 55.31, 113.77, 113.96, 114.17, 126.63, 127.73, 128.03, 128.92, 139.28, 152.18, 156.35, 157.76, 158.97, 159.98, 165.77. MS (m/z): 405.2 (M+1); Anal. Calcd for C21H20N6O3: C, 62.37; H, 4.98; N, 20.58; found C, 62.65; H, 5.00; N, 20.98.
5.2.19. 4-(1-(4-Methoxybenzyl)-5-amino-1H-imidazol-4-yl)-1-(4-methoxyphenethyl)-5-imino-1H-imidazol-2(5H)-one (14c)
The necessary isocyanate was synthesised by the general procedure. The procedure for 14c is similar to that of 14a. (Yield = 0.14 g., 55%). The initial reaction of isocyanate was carried out for 24 h; m.p.: 232–233°C; 1H-NMR (DMSO-d6): δ = 2.83 (t, J = 7.3 Hz, 2H, -CH2), 3.68–3.73 (m, 8H, -OCH3, -CH2), 5.10 (s, 2H, -CH2), 6.82 (d, J = 8.7 Hz, 2H, Ar-H), 6.95 (d, J=8.7 Hz, 2H, Ar-H), 7.1 (d, J=8.7 Hz, 2H, Ar-H), 7.27 (d, J = 8.7 Hz, 2H, Ar-H), 7.72 (s, 1H, Imid-H), 7.86 (brs, 2H, -NH2), 9.74 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 32.24, 45.40, 54.92, 55.14, 113.61, 113.76, 114.18, 127.66, 129.00, 129.60, 130.44, 138.72, 151.49, 156.99, 157.71, 158.99, 159.59, 166.64. MS (m/z): 433.2 (M+1); Anal. Calcd for C23H24N6O3: C, 63.88; H, 5.59; N, 19.43; found C, 63.62; H, 5.54; N, 19.31.
5.2.20. 4-(1-(4-Methoxybenzyl)-5-amino-1H-imidazol-4-yl)-1-ethyl-5-imino-1H-imidazol-2(5H)-one (14d)
Procedure similar to that of 14a. (Yield = 0.25 g., 40%). Reaction of isocyanate was carried out for 12 h; m.p.: 193–195°C; 1H-NMR (DMSO-d6): δ = 1.1 (t, J = 7.3 Hz, 3H, -CH3), 3.54 (q, J = 7.3 Hz, 2H, -CH2), 3.73 (s, 3H, -OCH3), 5.11 (s, 2H, -CH2), 6.94 (d, J = 8.7 Hz, 2H, Ar-H), 7.25 (d, J = 8.7 Hz, 2H, Ar-H), 7.71 (s, 1H, Imid-H), 7.83 (brs, 2H, -NH2), 9.73 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 13.15, 33.18, 45.41, 55.13, 113.56, 114.17, 127.63, 128.93, 138.59, 151.37, 157.31, 158.98, 159.44, 166.64. MS (m/z): 327.1 (M+1); Anal. Calcd for C16H18N6O2 0.25 H2O: C, 58.08; H, 5.64; N, 25.40; found C, 58.33; H, 5.51; N, 25.44.
5.2.21. Ethyl 2-(4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-2-oxo-2H-imidazol-1(5H)-yl)acetate (14e)
Procedure similar to that of 14a. (Yield = 0.1 g, 33.3%). Reaction of isocyanate was carried out for 12 h; m.p.: 200–202°C; 1H-NMR (DMSO-d6): δ = 1.19 (t, J = 7.1 Hz, 3H, CH3), 3.73 (s, 3H, OCH3), 4.12 (q, J = 7.1 Hz, 2H, CH2), 4.28 (s, 2H, CH2), 5.12 (s, 2H, CH2), 6.94 (d, J = 8.7 Hz, 2H, Ar-H), 7.26 (d, J = 8.7 Hz, 2H, Ar-H), 7.75 (s, 1H, Imid-H), 7.96 (brs, 2H, -NH2), 9.72 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ =14.56, 45.98, 55.68, 61.53, 114.43, 114.73, 128.11, 129.49, 139.77, 152.40, 157.5, 159.59, 166.82, 168.52; MS (m/z): 385.2 (M+1); Anal. Calcd for C18H20N6O4: C, 56.24; H, 5.24; N, 21.86; found C, 56.02; H, 5.14; N, 21.77.
5.2.22. 4-(1-(4-Methoxybenzyl)-5-amino-1H-imidazol-4-yl)-1-(2-chloroethyl)-5-imino-1H-imidazol-2(5H)-one (14f)
Procedure similar to that of 14a. (Yield = 0.1 g., 68%). Initial reaction of isocyanate was carried out for 3 h; m.p.:174–175°C; 1H-NMR (DMSO-d6): δ = 3.73 (s, 3H, -OCH3), 3.85 (s, 4H, -CH2), 5.12 (s, 2H, -CH2), 6.93 (d, J = 8.7 Hz, 2H, Ar-H), 7.23–7.27 (m, 2H, Ar-H), 7.75 (s, 1H, Imid-H), 7.91 (brs, 2H, -NH2), 9.78 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 40.96, 42.08, 43.39, 45.35, 45.43, 55.12, 55.17, 113.77, 114.19, 127.60, 128.93, 139.02, 151.68, 156.90, 158.99, 159.41, 166.47; MS (m/z): 361.1 (M+1); Anal. Calcd for C16H17ClN6O2.0.5 H2O: C, 51.97; H, 4.91; N, 22.73; found C, 51.62; H, 4.68; N, 22.38.
5.2.23. 4-(1-(4-Methoxybenzyl)-5-amino-1H-imidazol-4-yl)-1-benzyl-5-imino-1H-imidazol-2(5H)-one (14g)
Procedure similar to that of 14a. (Yield = 0.2 g, 63%). Initial reaction of isocyanate was carried out for 12 h; m.p.: 230–231°C; 1H-NMR (DMSO-d6): δ = 3.73 (s, 3H, OCH3), 4.71 (s, 2H, CH2), 5.13 (s, 2H, CH2), 6.94 (d, J = 8.7 Hz, 2H, Ar-H), 7.28 (m, 7 H, Ar-H), 7.74 (s, 1H, Imid-H), 7.92 (brs, 2H, -NH2), 9.80 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 41.53, 45.33, 55.03, 113.69, 114.07, 126.95, 127.14, 127.51, 128.27, 128.84, 137.48, 138.83, 151.54, 157.04, 158.88, 159.44, 166.66; MS (ESI) m/z = 389.3 (M+1); Anal. Calcd for C21H20N6O2: C, 64.94; H, 5.19; N, 21.64; found C, 64.76; H, 5.12; N, 21.61.
5.2.24. 1-(4-Fluorobenzyl)-4-(1-(4-methoxybenzyl)-5-amino-1 H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14h)
Procedure similar to that of 14a. (Yield = 0.32 g, 40.2%). Initial reaction of isocyanate was carried out for 12 h; m.p.: 233–235°C; 1H-NMR (DMSO-d6): δ = 3.69 (s, 3H, OCH3), 4.68 (s, 2H, CH2), 5.11 (s, 2H, CH2), 6.93 (d, J = 8.7 Hz, 2H, Ar-H), 7.12 (m, 2 H, Ar-H), 7.25 (d, J = 8.7 Hz, 2H, Ar-H), 7.28 (m, 2H, Ar-H), 7.73 (s, 1H, Imid-H), 7.89 (brs, 2H, NH2), 9.78 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 40.95, 45.41, 55.12, 113.86, 114.16, 115.02, 115.23, 127.62, 128.93, 129.38, 129.45, 133.78, 139.02, 151.74, 157.02, 158.98, 159.48, 166.67; MS (m/z): 407.3 (M+1); Anal. Calcd for C21H19FN6O2: C, 62.06; H, 4.71; N, 20.68; found C, 61.92; H, 4.76; N, 20.62.
5.2.25. 1-(4-Chlorobenzyl)-4-(1-(4-methoxybenzyl)-5-amino-1 H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14i)
The necessary isocyanate was synthesised by the general procedure described above. The procedure for 14i is similar to that of 14a. (Yield = 0.45 g., 56.6 %). Initial reaction of isocyanate was carried out for 12 h; m.p.: 248–249°C; 1H-NMR (DMSO-d6): δ = 3.72 (s, 3H, OCH3), 4.68 (s, 2H, -CH2), 5.11 (s, 2H, -CH2), 6.93 (d, J = 8.7 Hz, 2H, -CH2), 7.25 (d, J = 8.7 Hz, 2H, -CH2), 7.28 (d, J = 8.7 Hz, 2H, -CH2), 7.36 (d, J = 8.7 Hz, 2H, -CH2), 7.73 (s, 1H, Imid-H), 7.92 (br s, 2H, -NH2), 9.77 (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 40.13, 45.43, 55.14, 113.85, 114.19, 127.58, 128.35, 128.94, 129.19, 131.67, 136.57, 139.03, 151.72, 157.07, 158.99, 159.44, 166.64; HRMS (m/z): Calcd. for C21H20ClN6O2 (MH+) 423.1330; found: 423.1333; Anal. Calcd for C21H19ClN6O2: C, 59.65; H, 4.53; N, 19.87; found C, 59.87; H, 4.46; N, 19.82.
5.2.26. 1-(4-Bromobenzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14j)
The necessary isocyanate was synthesised by the general procedure. Procedure for 14j is similar to that of 14a. (Yield = 0.21 g., 77.7%). Initial reaction of isocyanate was carried out for 3 h; m.p.: 236–238°C; 1H-NMR (DMSO-d6): δ = 3.73 (s, 3H, -OCH3), 4.67 (s, 2H, -CH2), 5.12 (s, 2H, -CH2), 6.94 (d, J = 8.7 Hz, 2H, Ar-H), 7.22 (d, J = 8.7 Hz, 2H, Ar-H), 7.25 (d, J = 8.7 Hz, 2H, Ar-H), 7.48 (d, J = 8.7 Hz, 2H, Ar-H), 7.74 (s, 1H, Imid-H), 7.95 (brs, 2H, -NH2), 9.77 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 41.06, 45.41, 55.12, 55.15, 113.86, 114.17, 120.15, 127.59, 128.93, 129.53, 131.27, 137.00, 139.04, 151.73, 157.03, 158.98, 159.43, 166.62; MS (m/z): 467.2, 469.2 (M, M+2); Anal. Calcd for C21H19BrN6O2: C, 53.97; H, 4.10; N, 17.98; found C, 53.83; H, 4.00; N, 17.74.
5.2.27. 1-(3-Methoxybenzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14k)
The necessary isocyanate was synthesised by the general procedure. Procedure for 14k is similar to that of 14a. (Yield = 0.56 g, 68%). Initial reaction of isocyanate was carried out for 12 h; m.p.: 213–215°C; 1H-NMR (DMSO-d6): δ = 3.73 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 4.68 (s, 2H, CH2), 5.08 (s, 2H, CH2), 6.81–6.82 (m, 3H, Ar-H), 6.94 (d, J = 8.7 Hz, 2H, Ar-H), 7.21 (t, J = 7.8 Hz, 1 H, Ar-H), 7.27 (d, J = 8.7 Hz, 2H, Ar-H), 7.74 (s, 1H, Imid-H), 7.91 (s, 2H, -NH2), 9.80 (s, 1H, Ar-H); 13C-NMR (DMSO-d6): δ = 41.59, 45.43, 54.96, 55.14, 112.31, 113.06, 113.80, 114.18, 119.29, 127.61, 128.95, 129.49, 138.95, 139.16, 151.66, 157.13, 158.99, 159.26, 159.55, 166.75; MS (ESI) m/z = 419.3 (M+1); Anal. Calcd for C22H22N6O3.0.4H2O: C, 62.08; H, 5.40; N, 19.74; found C, 62.22; H, 5.11; N, 19.41.
5.2.28. 1-(2-Methoxybenzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14l)
The necessary isocyanate was synthesised by the general procedure described above. Procedure for 14l is similar to that of 14a. (Yield = 0.43 g, 52%). Initial reaction of isocyanate was carried out for 12 h; m.p.: 198–199°C; 1H-NMR (DMSO-d6): δ = 3.73 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.67 (s, 2H, CH2), 5.13 (s, 2H, CH2), 6.81–6.84 (m, 2H, Ar-H), 6.83 (d, J = 8.2 Hz, 2H, Ar-H), 6.99 (d, J = 8.2 Hz, 1H, Ar-H), 7.22 (m, 1H), 7.28 (d, J = 8.2 Hz, 2H, Ar-H), 7.75 (s, 1H, Imid-H), 7.93 (brs, 2H, -NH2), 9.76 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 36.87, 45.45, 55.15, 55.38, 110.49, 113.80, 114.18, 120.15, 124.77, 125.88, 127.64, 127.95, 128.98, 138.88, 151.63, 156.28, 157.26, 159.00, 159.67, 166.83; MS (ESI) (m/z) 419.3 (M+1). Anal. Calcd for C22H22N6O3: C, 63.15; H, 5.30; N, 20.08; found C, 62.89; H, 5.30; N, 19.87.
5.2.29. 1-(3-Fluorobenzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14m)
The necessary isocyanate was synthesised by the general procedure. Procedure for 14m is similar to that of 14a. (Yield = 0.45 g., 56.5%). Initial reaction of isocyanate was carried out for 12 h; m.p.:208–211°C; 1H-NMR (DMSO-d6): δ = 3.71 (s, 3H, -OCH3), 4.71 (s, 2H, -CH2), 5.1 (s, 2H, -CH2), 6.94 (d, J = 8.7 Hz, 2H, Ar-H), 7.05–7.1(m, 3H, Ar-H), 7.2 (d, J = 8.7 Hz, 2H, Ar-H), 7.32–7.36 (m, 1H, Ar-H), 7.74 (s, 1H, Imid-H), 8.01 (brs, 2H, -NH2), 9.77 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 41.17, 45.39, 55.14, 114.03, 114.09, 114.17, 123.17, 127.67, 128.94, 130.37, 130.46, 139.20, 140.55, 151.99, 156.78, 158.98, 159.54, 166.68; MS (m/z): 407.2 (M+1); HRMS (m/z) Calcd for C21H20FN6O2 (MH+) 407.1626 ; found 407.1609
5.2.30. 1-(3,4-Difluorobenzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14n)
The necessary isocyanate was synthesised by the general procedure described above. Procedure for 14n is similar to that of 14a. (Yield = 0.27 g., 65%). Initial reaction of isocyanate was carried out for 12 h; m.p.:253–256°C; 1H-NMR (DMSO-d6): δ = 3.73 (s, 3H, -OCH3), 4.69 (s, 2H, -CH2), 5.12 (s, 2H, -CH2), 6.93 (d, J = 8.7 Hz, 2H, Ar-H), 7.10–7.12 (m, 1H, Ar-H), 7.25–7.37 (m, 4H, Ar-H), 7.75 (s, 1H, Imid-H), 7.93 (brs, 2H, -NH2), 9.78 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 40.72, 45.42, 55.11, 55.16, 113.90, 114.17, 116.34, 116.48, 117.35, 117.52, 124.05, 127.59, 128.93, 135.32, 139.05, 147.27, 147.39, 147.90, 148.02, 149.70, 149.83, 150.34, 150.47, 151.74, 157.07, 158.98, 159.42, 166.58; MS (m/z): 425.2 (M+1); Anal. Calcd for C21H18F2N6O2: C, 59.43; H, 4.27; N, 19.80; found C, 59.20; H, 4.25; N, 19.70.
5.2.31. 1-(3-Chlorobenzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14o)
The necessary isocyanate was synthesised by the general procedure described above. Procedure for 14o is similar to that of 14a. (Yield = 0.12 g, 50.8%). Initial reaction of isocyanate was carried out for 12 h; m.p.: 185–186°C; 1H-NMR (DMSO-d6): δ = 3.73 (s, 3H, -OCH3), 4.71 (s, 2H, -CH2), 5.12 (s, 2H, -CH2), 6.94 (d, J= 8.2 Hz, 2H, Ar-H), 7.12–7.36 (m, 6H, Ar-H), 7.75 (s, 1H, Imid-H), 7.95 (brs, 2H, -NH2), 9.79 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 41.12, 45.43, 55.15, 113.91, 114.18, 125.93, 127.08, 127.40, 127.59, 128.93, 130.36, 132.99, 139.08, 140.10, 151.77, 158.99, 159.45, 166.64; MS (ESI) (m/z) 423.1 (M+1); HRMS (m/z) Calcd for C21H20ClN6O2 (MH+): 423.1330; found : 423.1314.
5.2.32. 1-(3-Bromobenzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14p)
The necessary isocyanate was synthesised by general procedure. To a suspension of 2 (0.15 g, 0.580 mmol, 1.0 equi.) in 5 mL acetonitrile was added isocyanate (3 equi.) and stirred at room temperature for 12 h. Precipitate formed was filtered off. The solution was purified by column chromatography (gradient elution using methanol in dichloromethane) to obtain the solid. The solid obtained was suspended in 5 mL acetonitrile. 2 drops DBU was added to it and stirred for 1 h. The resulting precipitate was filtered, washed with diethyl ether and dried to give 14p (Yield = 0.13 g, 47.4%); m.p.: 224–225°C; 1H NMR (DMSO-d6): δ = 3.73 (s, 3H, -OCH3), 4.68 (s, 2H, -CH2), 5.07 (s, 2H, -CH2), 6.92 (d, J = 8.7 Hz, 2H, Ar-H), 7.23–7.27 (m, 4H, Ar-H), 7.44–7.45 (m, 2H, Ar-H), 7.71 (s, 1H, Imid-H), 7.98 (br s, 2H, -NH2), 9.66 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 41.01, 45.17, 55.13, 113.97, 114.16, 121.58, 126.32, 127.62, 128.92, 129.95, 130.64, 139.16, 140.37, 151.90, 158.97, 159.46, 166.62; MS (ESI) (m/z) 467.1 (M+1); Anal. Calcd for C21H19BrN6O2: C, 53.97; H, 4.10; N, 17.98; found C, 53.98; H, 4.06; N, 17.69.
5.2.33. 1-(3-(Trifluoromethyl)benzyl)-4-(1-(4-methoxybenzyl) -5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14q)
The necessary isocyanate was synthesised by the general procedure described above. Procedure is similar to that of 14p (Yield = 0.16 g, 45.7%); The product from isocyanate reaction was purified by column chromatography (gradient elution using methanol in dichloromethane); m.p.: 218–220°C. 1H-NMR (DMSO-d6): δ = 3.73 (s, 3H, -OCH3), 4.8 (s, 2H, -CH2), 5.08 (s, 2H, -CH2), 6.93 (d, J = 8.7 Hz, 2H, Ar-H), 7.26 (d, J = 8.7 Hz, 2H, Ar-H), 7.53–7.56 (m, 2H, Ar-H), 7.59–7.63 (m, 2H, Ar-H), 7.75 (s, 1H, Imid-H), 7.94 (brs, 2H, -NH2), 9.78 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 41.24, 45.41, 55.13, 108.28, 113.95, 114.17, 122.78, 123.80, 123.84, 125.49, 127.59, 128.93, 129.23, 129.58, 131.38, 139.04, 139.15, 151.86, 156.93, 158.97, 159.48, 166.64; MS (ESI): 457.2 (M+1); Anal. Calcd for C22H19F3N6O2: C, 57.89; H, 4.20; N, 18.41; found C, 58.18; H, 4.14; N, 18.34.
5.2.34. 1-(4-((2-Methoxyethoxy)methoxy)benzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14r)
The synthesis of the title compound comprises the following four steps:
(a) Ethyl 4-Hydroxyphenylacetate
To ethanolic solution (50 mL) of 4-hydroxy-phenylacetic acid (10 g) was added a 0.2 mL of conc. H2SO4 and refluxed for 12 h. Solvent was removed at reduced pressure, residue was cooled and carefully neutralized with saturated aquesous NaHCO3. Resulting solution was diluted with ethyl acetate and washed with water, brine, dried over MgSO4 and evaporated to dryness to get 11.2 g of the product, which was directly used in the next step.
(b) Ethyl 2-(4-((2-Methoxyethoxy)methoxy)phenyl) acetate
To a solution of Ethyl 4-hydroxyphenylacetate (1.5 g, 8.33 mmol) and DIPEA (3.2 g, 25.0 mmol) in DCM was added MEMCl (1.56 g, 12.50 mmol) under intert atmosphere at 0–5°C. The reaction mixture was allowed to come to room temperature and stirred overnight. Reaction mixture was diluted with ethyl acetate and washed wih water, brine, dried over MgSO4 and evaportated to get product. The crude was purified by column chromatography (Yield = 2.0 g, 89.7 %), and directly employed in the next step.
(c) 2-(4-((2-Methoxyethoxy)methoxy)phenyl)acetic acid
To a solution of Ethyl 2-(4-((2-methoxyethoxy)methoxy)phenyl) acetate (1.9g, 7.09 mmol) in 30 mL THF: water (1:1) was added LiOH.H2O (2.38g, 56.71 mmol) and stirred at room temperature for 4 h. Reaction mixture was diluted with water, pH adjusted to 4–5 by citric acid, and extracted with ethyl acetate. The organic layer was washed wih water, brine, dried over MgSO4 and evaportated to get product (Yield = 1.5g, 88.2%); 1H-NMR (CDCl3): δ = 3.36 (s, 3H), 3.52–3.54 (m, 2H), 3.57 (s, 2H), 3.79–3.81 (m, 2H), 5.24 (s, 2H), 7.00 (d, J = 8.7 Hz, 2H), 7.17 (d, J = 8.7 Hz, 2H).
(d) 1-(4-((2-Methoxyethoxy)methoxy) benzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14r)
The necessary isocyanate was synthesised by the general procedure. Procedure for 14r is similar to that of 14p (Yield = 0.45 g, 70.4%). Product from isocyanate reaction was purified by column chromatography (gradient elution using methanol in dichloromethane); m.p.: 200–202°C; 1H-NMR (DMSO-d6): δ = 3.20 (s, 3H), 3.43 (t, J = 4.8 Hz, 2H), 3.68 (t, J = 4.8 Hz, 2H), 3.73 (s, 3H), 4.63 (s, 2H), 5.11 (s, 2H), 5.20 (s, 2H), 6.92- 6.95 (m, 4H), 7.20 (d, J = 8.7 Hz, 2H), 7.25 (d, J = 8.7 Hz, 2H), 7.73 (s, 1H), 7.89 (brs, 2H), 9.77 (s, 1H); 13C-NMR (DMSO-d6): δ = 41.10, 45.41, 55.14, 58.01, 67.27, 70.96, 92.88, 113.74, 114.18, 116.05, 127.61, 128.71, 128.93, 130.80, 138.89, 151.60, 155.95, 157.13, 158.98, 159.51, 166.72; Anal. Calcd for C25H28N6O5: C, 60.96; H, 5.73; N, 17.06; found C, 60.76; H, 5.78; N, 17.01; HRMS (m/z) Calcd for C25H29N6O5 (MH+) 493.2194; found 493.2194.
5.2.35. 1-(4-(2-Methoxyethoxy)benzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14s)
The synthesis of the title compound comprises the following three steps:
(a) Ethyl 2-(4-(2-Methoxyethoxy)phenyl)acetate
To a solution of Ethyl 4-hydroxyphenylacetate (1.0 g, 5.55 mmol) in DMF under inert atmosphere was added potassium carbonate (2.30g, 16.65 mmol) and 2-Methoxyethylbromide (0.92g, 6.66 mmol) and stirred overnight at 60°C. Reaction mixture was diluted with ethyl acetate and washed with water, brine, dried over MgSO4 and evaportated to get product (Yield = 1.2 g., 91.0 %), which was used for the next step without further purification.
(b) 2-(4-(2-Methoxyethoxy)phenyl)acetic Acid
To a solution of ester (1.2g, 5.04 mmol) obtained in previous step, in 20 mL THF: water (1:1) was added LiOH.H2O (0.32g, 7.56 mmol) and stirred at room temperature for 4 h. Reaction mixture was diluted with water and washed with ethyl acetate. The pH of aqueous layer was adjusted to 4–5 by citric acid, and extracted with ethyl acetate. The organic layer was washed wih water, brine, dried over MgSO4 and evaportated to obtain the product (Yield = 0.95 g., 90%); 1H-NMR (CDCl3,): δ = 3 43 (s, 3H), 3.55 (s, 2H), 3.72 (t, J = 4.8 Hz, 2H), 4.08 (t, J = 4.8 Hz, 2H), 6.86 (d, J = 8.7 Hz, 2H), 7.16 (d, J=8.7 Hz, 2H).
(c) 1-(4-(2-Methoxyethoxy)benzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14s)
The necessary isocyanate was synthesised by the general procedure. Procedure for 14s is similar to that of 14a. (Yield = 0.2 g., 44.0%); m.p.: 205–207°C. 1H-NMR (DMSO-d6,): δ = 3.27 (s, 3H), 3.62 (t, J = 4.6Hz, 2H), 3.72 (s, 3H), 4.03 (t, J = 4.6 Hz, 2H), 4.62 (s, 2H), 5.11 (s, 2H), 6.86 (d, J = 8.7 Hz, 2H), 6.93 (d, J = 8.7 Hz, 2H), 7.19 (d, J = 8.7 Hz, 2H), 7.25 (d, J = 8.7 Hz, 2H), 7.73 (s, 1H), 7.90 (brs, 2H), 9.77 (s, 1H); 13C-NMR (DMSO-d6): δ = 41.09, 45.38, 55.14, 58.13, 66.82, 70.34, 113.80, 114.16, 114.26, 127.66, 128.81, 128.93, 129.71, 138.96, 151.71, 156.96, 157.57, 158.97, 159.56, 166.75; Anal. Calcd for C24H26N6O4: C, 62.33; H, 5.67; N, 18.17; found C, 61.97; H, 5.64; N, 18.01; HRMS (m/z) Calcd for C24H27N6O4 (MH+) 463.2088; found 463.2086.
5.2.36. 1-(4-(2-((2-Methoxyethoxy)methoxy)ethoxy)benzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14t)
The synthesis of the title compound comprises the following four steps:
(a) 2-((2-Methoxyethoxy)methoxy)ethyl 4-Methylbenzene Sulfonate
To a solution of tosylate (1.0 g., 4.63 mmol) in DCM, under inert atmosphere, at room temperature was added diisopropyl ethylamine (1.49 g., 11.57 mmol) followed by dropwise addition of MEM-Cl (0.69 g., 5.55 mmol) and stirred for 2 h. Reaction mixture was diluted with DCM and washed with water. The organic layer was washed with brine, dried over MgSO4 and evaporated to get product. The product was used for the next reaction without further purification.
(b) 2-(4-(2-((2-Methoxyethoxy)methoxy)ethoxy)phenyl)-acetic Acid Ethyl ester
To a suspension of 4-hydroxyphenyl-acetic acid (0.36 g, 2.0 mmol) in acetontirle (5 mL), under inert atmosphere, was added potassium carbonate (0.83g, 6.0 mmol) followed by tosylate (0.67g, 2.2 mmol), and refluxed for 6 h. Reaction mixture was cooled and filtered. The filtrate was washed with water, brine, dried over MgSO4, and evaporated to give desired product. (Yield: 0.6 g, 96%); 1H-NMR (CDCl3): δ = 1.23 (t, 3H), 3.38 (s, 3H), 3.53–3.55 (m, 4H), 3.72 (t, J = 4.6Hz, 2H), 3.90 (t, J = 4.6Hz, 2H), 4.10 (m, 4H), 4.80 (s, 2H), 6.86 (d, J = 8.3 Hz, 2H), 7.18 (d, J = 8.3 Hz, 2H).
(c) 2-(4-(2-((2-Methoxyethoxy)methoxy)ethoxy)phenyl)-acetic Acid
To a solution of ester (0.6g, 1.92 mmol) obtained in previous step, in 20 mL THF: water (1:1) was added LiOH.H2O (0.64g, 15.38 mmol) and stirred at room temperature for 6 h. Reaction mixture was diluted with water and washed with ethyl acetate. The pH of aqueous layer was adjusted to 4–5 by citric acid, and extracted with ethyl acetate. The organic layer was washed wih water, brine, dried over MgSO4 and evaportated to get product (Yield = 0.42 g., 78.0%); 1H-NMR (CDCl3): δ = 3.37 (s, 3H), 3.53–3.55 (m, 4H), 3.72 (m, 2H), 3.88 (m, 2H), 4.10 (m, 2H), 4.80 (s, 2H), 6.85 (d, J = 8.7 Hz, 2H), 7.17 (d, J = 8.7 Hz, 2H).
(d) 1-(4-(2-((2-Methoxyethoxy)methoxy)ethoxy)benzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14t)
The necessary isocyanate was synthesised by the general procedure. Procedure for 14t is similar to that of 14p (Yield = 0.225g, 59%). Product from isocyanate reaction was purified by column chromatography (gradient elution using methanol in dichloromethane). The final product 14t was also purified by column chromatography (gradient elution using methanol in dichloromethane); m.p.: 181–183°C; 1H-NMR (DMSO-d6): δ = 3.00 (s, 3H), 3.43 (m, 2H), 3.57 (m, 2H), 3.72 (s, 3H), 3.76 ( m, 2H), 4.06 (m, 2H), 4.62 (s, 2H), 4.66 (s, 2H), 5.10 (s, 2H), 6.86 (d, J = 8.2 Hz, 2H), 6.93 (d, J = 8.2 Hz, 2H), 7.20 (d, J = 8.2 Hz, 2H), 7.25 (d, J = 8.2 Hz, 2H), 7.72 (s, 1H), 7.91 (brs, 2H), 9.76 (s, 1H); 13C-NMR (DMSO-d6): δ = 41.09, 45.39, 55.13, 57.98, 65.55, 66.23, 66.98, 71.13, 94.70, 113.77, 114.16, 114.29, 127.63, 128.81, 128.92, 129.73, 138.93, 151.68, 157.54, 158.97, 159.54, 166.73; Anal. Calcd for C27H32N6O6: C, 60.44; H, 6.01; N, 15.66; found C, 60.27; H, 5.95; N, 15.54; HRMS (m/z) Calcd for C27H33N6O6 (MH+): 537.2456; found 537.2450.
5.2.37. 4-(1-(4-Methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1-((pyridin-3-yl)methyl)-1H-imidazol-2(5H)-one (14u)
The necessary isocyanate was synthesised by the general procedure described above. Procedure is similar to that of 14a. (Yield = 0.1 g, 44.5%). Initial reaction of isocyanate was carried out for 12 h; m.p.: 238–241°C; 1H-NMR (DMSO-d6): δ = 3.73 (s, 3H, -OCH3), 4.74 (s, 2H, -CH2), 5.08 (s, 2H, -CH2), 6.92–6.95 (m, 2H, Ar-H), 7.24–7.35 (m, 3H, Ar-H), 7.65–7.70 (m, 1H, Ar-H), 7.74 (s, 1H, Imid-H), 7.93 (br s, 2H, -NH2), 8.45–8.52 (m, 2H, Ar-H), 9.78 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 45.5, 55.13, 113.92, 114.17, 123.56, 127.59, 128.92, 133.11, 135.19, 139.10, 148.42, 148.82, 151.80, 158.97, 159.43, 166.59; MS (ESI): 390.2 (M+1); Anal. Calcd for C20H19N7O2: C, 61.69; H, 4.92; N, 25.18; found C, 61.41; H, 4.89; N, 25.02.
5.2.38. 1-(4-(Dimethylamino)benzyl)-4-(1-(4-methoxybenzyl)-5-amino-1H-imidazol-4-yl)-5-imino-1H-imidazol-2(5H)-one (14v)
The necessary isocyanate was synthesised by the general procedure. Procedure is similar to that of 14a. (Yield = 0.11 g, 43.4%). Initial reaction of isocyanate was carried out for 12 h; m.p.: 216–218°C; 1H-NMR (DMSO-d6): δ = 2.86 (s, 6H, -CH3), 3.72 (s, 3H, -OCH3), 4.56 (s, 2H, -CH2), 5.11 (s, 2H, -CH2), 6.64 (d, J = 8.7 Hz, 2H, Ar-H), 6.93 (d, J= 8.7 Hz, 2H, Ar-H), 7.12 (d, J= 8.7 Hz, 2H, Ar-H), 7.25 (d, J = 8.7 Hz, 2H, Ar-H), 7.72 (s, 1H, Imid-H), 7.87 (brs, 2H, -NH2, 9.77 (s, 1H, -NH); 13C-NMR (DMSO-d6): δ = 41.23, 45.38, 55.12, 112.24, 113.66, 114.16, 125.15, 127.62, 128.52, 128.91, 138.77, 149.67, 151.53, 158.96, 159.57, 166.77. MS (ESI) (m/z) 432.2 (M+1); Anal. Calcd for C23H25N7O2: C, 64.02; H, 5.84; N, 22.72; found C, 63.83; H, 5.82; N, 22.47.
5.2.39. 7-(4-Methoxybenzyl)-3-phenyl-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15a)
To a suspension of 14a (0.12 g., 0.33 mmol) in 3 mL acetonitrile was added triethyl orthoformate (0.39 g, 2.67 mmol, 8.0 equi.) and a drop of H2SO4 and heated under reflux for 1 h under inert atmosphere. The reaction mixture was cooled to room temperature. Precipitate formed was filtered, washed with diethyl ether (2 X 10 mL), and dried to get the product (Yield = 0.65 g, 51.3 %); m.p.: 249–251°C; 1H-NMR (DMSO-d6): δ = 3.71 (s, 3H, -OCH3), 5.53 (s, 2H, -CH2), 6.91 (d, J = 8.7 Hz, 2H, Ar-H), 7.34 (d, J = 8.7 Hz, 2H, Ar-H), 7.48–7.52 (m, 3H, Ar-H), 7.56–7.60 (m, 2H, Ar-H), 8.62 (s, 1H, diazepine- H), 8.97 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 46.55, 55.12, 114.12, 127.67, 128.22, 128.61, 128.80, 128.93, 129.27, 132.87, 148.40, 149.70, 150.05, 156.08, 159.03, 160.17, 164.99; HRMS (m/z): Calcd for C21H16N6O2 (MH+) 385.1407; found 385.1407; Anal. Calcd for C21H16N6O2.0.5H2O: C, 64.11; H, 4.36; N, 21.36; found C, 63.84; H, 4.04; N, 21.29.
5.2.40. 7-(4-Methoxybenzyl)-3-(4-methoxyphenyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15b)
Procedure similar to that of 15a (Yield = 0.1g, yield: 81.3%); m.p.: 255–257°C; 1H-NMR (DMSO-d6): δ = 3.73 (s, 3H, -OCH3), 3.83 (s, 3H, -OCH3), 5.53 (s, 2H, -CH2), 6.91 (d, J = 6.8 Hz, 2H, Ar-H), 7.11 (d, J = 6.8 Hz, 2H, Ar-H), 7.34 (d, J = 8.7 Hz, 2H, Ar-H), 7.41 (d, J = 8.7 Hz, 2H, Ar-H), 8.61 (s, 1H, diazepine- H), 8.95 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 46.55, 55.13, 55.48, 114.13, 114.19, 125.39, 128.26, 128.71, 128.85, 129.27, 148.35, 149.71, 150.05, 156.03, 159.03, 159.23, 160.31, 165.25; MS (m/z) 415.2 (M+1); Anal. Calcd for C22H18N6O3: C, 63.76; H, 4.38; N, 20.28; found C, 63.52; H, 4.31; N, 20.16.
5.2.41. 7-(4-Methoxybenzyl)-3-(4-methoxyphenethyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15c)
Procedure similar to that of 15a (Yield = 0.04g, yield: 32.7%).; m.p.: 156–157°C; 1H-NMR (DMSO-d6): δ = 2.9 (t, J = 7.32 Hz, 2H, -CH2), 3.62 (s, 3H, -OCH3), 3.66 (s, 3H, -OCH3), 4.07 (t, J = 7.32 Hz, 2H, -CH2), 5.46 (s, 2H, -CH2), 6.75 (d, J = 8.7 Hz, 2H, Ar-H), 6.87 (d, J = 8.7 Hz, 2H, Ar-H), 7.05 (d, J = 8.7 Hz, 2H, Ar-H), 7.30 (d, J = 8.7 Hz, 2H, Ar-H), 8.67 (s, 1H, diazepine- H), 8.87 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 32.38, 41.65, 46.48, 55.09, 113.80, 114.12, 129.29, 129.72, 129.78, 148.35, 149.70, 155.57, 157.88, 159.02, 159.72, 165.66; MS (m/z) 443.2 (M+1); Anal. Calcd for C24H22N6O3: C, 65.15; H, 5.01; N, 18.99; found C, 64.88; H, 5.01; N, 18.70.
5.2.42. 3-Ethyl-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15d)
Procedure similar to that of 15a; the precipitated product was washed with ethyl acetate instead of diethyl ether (Yield = 0.13 g, 50%); m.p.: 186–188°C; 1H-NMR (DMSO-d6): δ = 1.24 (t, J = 7.1 Hz, 3H, -CH3), 3.71 (s, 3H, -OCH3), 3.98 (q, J = 7.1 Hz, 2H, -CH2), 5.52 (s, 2H, -CH2), 6.91 (d, J = 8.7 Hz, 2H, Ar-H), 7.33 (d, J = 8.7 Hz, 2H, Ar-H), 8.74 (s, 1H, diazepine- H), 8.91 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 13.11, 38.89, 46.46, 55.12, 114.10, 128.27, 128.43, 129.25, 148.25, 149.68, 149.74, 155.80, 159.01, 159.67, 165.74. MS (m/z) 337.2 (M+1); Anal. Calcd for C17H16N6O2. 0.25 H2O: C, 59.90; H, 4.88; N, 24.66; found C, 60.17; H, 4.64; N, 24.27.
5.2.43. 3-Ethoxycarbonylmethyl-7-(4-Methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15e)
Procedure similar to that of 15a; the precipitated product was washed with ethyl acetate instead of diethyl ether (Yield = 0.04 g, 39.2%); m.p.: 179–180°C; 1H-NMR (DMSO-d6): δ = 1.20 (t, J = 7.1 Hz, 3H, CH3), 3.71 (s, 3H, OCH3), 4.16 (q, J= 7.1 Hz, 2H, CH2), 4.78 (s, 2H, CH2), 5.53 (s, 2H, CH2), 6.89 (d, J= 8.7 Hz, 2H, Ar-H), 7.34 (d, J = 8.7 Hz, 2H, Ar-H), 8.75 (s, 1H, diazepine- H), 8.98 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 13.97, 40.14, 46.59, 55.11, 61.57, 114.12, 128.11, 128.87, 129.27, 148.91, 149.72, 149.95, 155.69, 159.02, 159.41, 165.12, 166.97; MS (m/z) 395.3 (M+1); Anal. Calcd for C19H18N6O4 : C, 57.86; H, 4.60; N, 21.31; found C, 57.78; H, 4.58; N, 21.20.
5.2.44. 3-(2-Chloroethyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15f)
Procedure similar to that of 15a (Yield = 0.04g, yield: 39.2%); m.p.:183–184°C; 1H-NMR (DMSO-d6): δ = 3.71 (s, 3H, -OCH3), 3.96 (t, J = 6.4 Hz, 2H, -CH2), 4.3 (t, J = 6.4 Hz, 2H, -CH2), 5.52 (s, 2H, -CH2), 6.9 (d, J = 8.7 Hz, 2H, Ar-H), 7.34 (d, J = 8.7 Hz, 2H, Ar-H), 8.76 (s, 1H, diazepine- H), 8.95 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 41.34, 42.25, 47.11, 55.67, 114.66, 128.73, 129.19, 129.82, 149.14, 150.27, 150.32, 156.22, 159.58, 160.41, 166.07; MS (m/z) 371.1 (M+1); Anal. Calcd for C17H15ClN6O2: C, 55.07; H, 4.08; N, 22.66; found C, 54.86; H, 3.99; N, 22.43.
5.2.45. 3-Benzyl-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15g)
Procedure similar to that of 15a (Yield = 0.12 g, 67%); m.p.: 181–183°C; 1H-NMR (DMSO-d6): δ = 3.70 (s, 3H, OCH3), 5.15 (s, 2H, CH2), 5.52 (s, 2H, CH2), 6.88 (d, J = 8.7 Hz, 2H, Ar-H), 7.32 (m, 7 H, Ar-H), 8.90 (s, 1H,, diazepine-H), 8.94 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 43.32, 46.47, 55.11, 114.09, 127.43, 128.23, 128.51, 128.65, 129.26, 135.97, 148.41, 149.81, 155.91, 159.01, 159.87, 165.79; MS (ESI) (m/z) 399.3 (M+1); Anal. Calcd. for C22H18N6O2: C, 66.32; H, 4.55; N, 21.09; found C, 66.29; H, 4.45; N, 21.08.
5.2.46. 3-(4-Fluorobenzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15h)
Procedure similar to that of 15a (Yield = 0.15 g, 73.5%); m.p.: 178–180°C; 1H-NMR (DMSO-d6): δ = 3.70 (s, 3H, OCH3), 5.13 (s, 2H, CH2), 5.52 (s, 2H, CH2), 6.89 (d, J = 8.24 Hz, 2H, Ar-H), 7.12 (t, J = 8.9 Hz, 2 H, Ar-H), 7.32–7.41 (m, 4H, Ar-H), 8.73 (s, 1H, diazepine-H), 8.99 (s, 1H, Imiid-H); 13C-NMR (DMSO-d6): δ = 42.64, 46.49, 55.12, 114.10, 115.18, 115.39, 128.24, 128.78, 129.27, 129.67, 129.75, 132.17, 148.41, 149.80, 156.0, 159.02, 159.84, 165.74; MS (m/z) 417.3 (M+1); Anal. Calcd for C22H17FN6O2: C, 63.46; H, 4.11; N, 20.18; found C, 63.23; H, 3.90; N, 19.89.
5.2.47. 3-(4-Chlorobenzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15i)
Procedure similar to that of 15a (Yield = 150 mg, yield: 69.4%); m.p.: 188–189°C; 1H-NMR (DMSO-d6): δ = 3.71 (s, 3H, OCH3), 5.14 (s, 2H, -CH2), 5.52 (s, 2H, -CH2), 6.89 (d, J = 8.7 Hz, 2H, -CH2), 7.25 (d, J = 8.7 Hz, 2H, -CH2), 7.33–7.36 (m, 6H, Ar-H), 8.71 (s, 1H, diazepine-H), 8.94 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 42.68, 46.50, 55.12, 114.66, 128.78, 129.01, 129.34, 129.83, 129.98, 132.70, 135.52, 148.98, 150.35, 156.56, 159.58, 160.41, 166.27; HRMS (m/z): calcd for C22H18ClN6O2 (MH+) 433.1174; found 433.1178; Anal. Calcd for C22H17ClN6O2: C, 61.04; H, 3.96; N, 19.41; found C, 61.31; H, 3.85; N, 19.48.
5.2.48. 3-(4-Bromobenzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15j)
Procedure similar to that of 15a (Yield = 0.09g, yield: 49.2%); m.p.: 147–148°C; 1H-NMR (DMSO-d6): δ = 3.71 (s, 3H, -OCH3), 5.12 (s, 2H, -CH2), 5.52 (s, 2H, -CH2), 6.89 (d, J = 8.7 Hz, 2H, Ar-H), 7.30 (d, J = 8.7 Hz, 2H, Ar-H), 7.32 (d, J = 8.7 Hz, 2H, Ar-H), 7.5 (d, J = 8.7 Hz, 2H, Ar-H), 8.73 (s, 1H, diazepine- H), 8.94 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 42.73, 46.49, 55.14, 114.10, 120.65, 128.23, 128.79, 129.27, 129.76, 131.38, 135.39, 148.43, 149.79, 156.02, 159.02, 159.87, 165.71; MS (m/z) 477.1 and 479.1 (M+, M+2). Anal.: Calcd for C22H17BrN6O2: C, 55.36; H, 3.59; N, 17.61; found C, 55.04; H, 3.54; N, 17.29.
5.2.49. 3-(3-Methoxybenzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15k)
Procedure similar to that of 15a (Yield = 0.15 g, 71%); m.p.: 202–203°C; 1H-NMR (DMSO-d6): δ = 3.70 (s, 6H, 2XOCH3), 5.12 (s, 2H, CH2), 5.51 (s, 2H, CH2), 6.81–6.90 (m, 5H, Ar-H), 7.20 (t, J = 7.8 Hz, 1 H, Ar-H), 7.34 (d, J = 8.72 Hz, 2H, Ar-H), 8.74 (s, 1H, diazepine-H), 8.94 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 43.27, 46.48, 55.02, 55.12, 112.74, 113.26, 114.10, 119.43, 128.24, 128.77, 129.28, 129.64, 137.49, 148.40, 149.81, 155.94, 159.00, 159.35, 159.87, 165.79. MS (ESI): (m/z) 429.3 (M+1); Anal. Calcd for C23H20N6O3: C, 64.48; H, 4.71; N, 19.62; found C, 64.73; H, 4.59; N, 19.75.
5.2.50. 3-(2-Methoxybenzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15l)
Procedure similar to that of 15a (Yield = 0.08 g, 78%); m.p.: 225–227°C; 1H-NMR (DMSO-d6): δ = 3.71 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 5.09 (s, 2H, CH2), 5.52 (s, 2H, CH2), 6.76–6.84 (m, 2H, Ar-H), 6.90 (d, J = 8.72 Hz, 2H, Ar-H), 7.03 (d, J = 8.24 Hz, 1H, Ar-H), 7.24 (m, 1H), 7.35 (d, J = 8.72 Hz, 2H, Ar-H), 8.67 (s, 1H, diazepine-H), 8.95 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 46.49, 55.11, 55.50, 110.61, 114.10, 120.21, 123.21, 126.48, 128.24, 128.45, 128.73, 129.30, 148.37, 149.77, 156.02, 156.29, 159.02, 160.12, 165.77; MS (ESI) (m/z) 429.3 (M+1); Anal. Calcd. for C23H20N6O3.H2O: C, 61.87; H, 4.97; N, 18.82; found C, 61.48; H, 4.54; N, 18.68.
5.2.51. 3-(3-Fluorobenzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15m)
Procedure similar to that of 15a; the residue was washed with acetonitrile and diethyl ether followed by drying. (Yield = 0.15 g, 36.6%); m.p.: 187–188°C; 1H-NMR (DMSO-d6): δ = 3.71 (s, 3H, -OCH3), 5.17 (s, 2H, -CH2), 5.53 (s, 2H, -CH2), 6.88–6.92 (m, 2H, Ar-H), 7.06–7.12 (m, 1H, Ar-H), 7.16–7.18 (m, 2H, Ar-H), 7.33–7.38 (m, 3H, Ar-H), 8.73 (s, 1H, diazepine-H), 8.99 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 42.79, 46.50, 55.13, 114.11, 114.29, 114.39, 123.37, 128.25, 128.84, 129.29, 130.46, 130.55, 138.75, 138.83, 148.37, 149.82, 156.13, 159.04, 159.98, 165.74; MS (m/z): 417.1 (M+1); Anal. Calcd for C22H17FN6O2: C, 63.46; H, 4.11; N, 20.18; found C, 63.25; H, 4.03; N, 20.08.
5.2.52. 3-(3,4-DifluoroBenzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15n)
Procedure similar to that of 15a; the residue was washed with acetonitrile and diethyl ether followed by drying. (Yield = 0.12 g, 48.9%); m.p.:188–189°C; 1H-NMR (DMSO-d6): δ = 3.71 (s, 3H, -OCH3), 5.14 (s, 2H, -CH2), 5.53 (s, 2H, -CH2), 6.9 (d, J=8.7 Hz, 2H, Ar-H), 7.21 (m, 1H, Ar-H), 7.34 - 7.44 (m, 4H, Ar-H), 8.73 (s, 1H, diazepine- H), 8.95 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 42.29, 46.49, 55.11, 114.09, 116.51, 116.69, 117.40, 117.57, 124.29, 128.23, 128.81, 129.28, 133.64, 148.33, 149.72, 149.78, 156.17, 159.03, 159.96, 165.66; MS (m/z): 435.1 (M+1); Anal. Calcd for C22H16F2N6O2: C, 60.83; H, 3.71; N, 19.35; found C, 60.75; H, 3.63; N, 19.21.
5.2.53. 3-(3-Chlorobenzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15o)
Procedure similar to that of 15a; the residue was washed with acetonitrile and diethyl ether followed by drying. (Yield = 0.039 g, 38.2%); m.p.: 183–185°C; 1H-NMR (DMSO-d6): δ = 3.71 (s, 3H, -OCH3), 5.16 (s, 2H, -CH2), 5.53 (s, 2H, -CH2), 6.9 (d, J= 8.7 Hz, 2H, Ar-H), 7.23–7.45 (m, 6H, Ar-H), 8.74 (s, 1H, diazepine-H), 8.95 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 42.73, 46.48, 55.12, 114.11, 126.09, 127.17, 127.48, 128.25, 128.83, 129.28, 130.38, 133.18, 138.45, 148.34, 149.81, 156.19, 159.03, 160.00, 165.76; MS (ESI): 433.1 (M+1); Anal. Calcd for C22H17ClN6O2: C, 61.04; H, 3.96; N, 19.41; found C, 61.09; H, 3.93; N, 19.37.
5.2.54. 3-(3-Bromobenzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15p)
Procedure similar to that of 15a; the residue was washed with acetonitrile and diethyl ether followed by drying. (Yield = 0.05 g, 49%); m.p.: 172–173°C; 1H-NMR (DMSO-d6): δ = 3.71 (s, 3H, -OCH3), 5.16 (s, 2H, -CH2), 5.53 (s, 2H, -CH2), 6.9 (d, J= 8.7 Hz, 2H, Ar-H), 7.25–7.36 (m, 4H, Ar-H), 7.46–7.48 (m, 1H, Ar-H), 7.56 (s, 1H, Ar-H), 8.74 (s, 1H, diazepine-H), 8.94 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 42.68, 46.48, 55.12, 114.10, 121.78, 126.49, 128.23, 128.82, 129.28, 130.04, 130.39, 130.66, 138.68, 148.34, 149.79, 156.16, 159.02, 159.97, 165.75; MS (ESI) (m/z) 477.1 (M+1); Anal. Calcd for C22H17BrN6O2: C, 55.36; H, 3.59; N, 17.61; found C, 55.39; H, 3.53; N, 17.50.
5.2.55. 3-(3-(Trifluoromethyl)benzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15q)
Procedure similar to that of 15a; the residue was washed with acetonitrile and diethyl ether followed by drying. (Yield = 0.039 g, 19%); m.p.: 176–177°C; 1H-NMR (DMSO-d6): δ = 3.74 (s, 3H, -OCH3), 5.25 (s, 2H, -CH2), 5.53 (s, 2H, -CH2), 6.9 (d, J= 8.7 Hz, 2H, Ar-H), 7.35 (d, J= 8.7 Hz, 2H, Ar-H), 7.52–7.65 (m, 3H, Ar-H), 7.75 (s, 1H, Ar-H), 8.74 (s, 1H, diazepine-H), 8.95 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 42.87, 46.49, 55.12, 114.11, 122.74, 124.25, 125.46, 128.23, 128.83, 129.04, 129.29, 129.61, 131.54, 137.42, 148.36, 149.79, 156.21, 159.03, 160.05, 165.79; MS (ESI) (m/z) 467.1 (M+1); Anal. Calcd for C23H17F3N6O2: C, 59.23; H, 3.67; N, 18.02; found C, 59.17; H, 3.54; N, 17.78.
5.2.56. 3-(4-Hydroxybenzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15r)
The synthesis of the title compound involved the following two steps:
(a) 7-(4-Methoxybenzyl)-3-(4-((2-methoxyethoxy) methoxy)benzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (14r-ii)
To a suspension of 14r (0.425 g, 0.86 mmol) in acetonitrile (5 mL) was added triethyl orthoformate (1.02 g, 6.91 mmol) and a drop of conc. H2SO4 and refluxed for 1 h. Solvent was evaporated and the residue was recrystalized from ethyl acetate (Yield = 0.375 g, 87%); m.p.: 126–128°C; 1H-NMR (DMSO-d6): δ = 3.19 (s, 3H), 3.42 (m, 2H), 3.66 (m, 2H), 3.71 (s, 3H), 5.09 (s, 2H), 5.20 (s, 2H), 5.52 (s, 2H), 6.90 (d, J=8.7 Hz, 2H), 6.95 (d, J=8.7 Hz, 2H), 7.28 (d, J=8.7 Hz, 2H), 7.33 (d, J=8.7 Hz, 2H), 8.75 (s, 1H), 8.93 (s, 1H); 13C-NMR (DMSO-d6): δ = 42.83, 46.46, 55.10, 57.99, 67.27, 70.93, 93.40, 114.64, 116.71, 128.77, 129.53, 129.68, 129.78, 148.93, 150.35, 156.39, 156.81, 159.55, 160.29, 166.32; HRMS (m/z) Calculated for C26H27N6O5 (MH+) 503.2037; found 503.2040; Anal. Calcd for C26H26N6O5: C, 62.14; H, 5.21; N, 16.72; found C, 61.84; H, 5.23; N, 16.69.
(b) 3-(4-Hydroxybenzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15r)
To a solution of 14r-ii (0.3g) in DCM (5 mL) was added TFA (0.6 mL) and stirred for 4 h. The reaction mixture was diluted with DCM, washed with water, brine and dried over MgSO4 and evaporated to get product (Yield = 0.21g, 85%); m.p.: 216–218°C; 1H-NMR (DMSO-d6): δ = 3.71 (s, 3H), 5.03 (s, 2H), 5.51 (s, 2H), 6.67 (d, J = 8.7 Hz, 2H), 6.89 (d, J = 8.7 Hz, 2H), 7.17 (d, J = 8.7 Hz, 2H), 7.33 (d, J = 8.7 Hz, 2H), 8.75 (s, 1H), 8.92 (s, 1H), 9.38 (s, 1H); 13C-NMR (DMSO-d6): δ = 42.98, 46.47, 55.12, 114.11, 115.19, 126.21, 128.25, 128.66, 129.16, 129.24, 148.39, 149.82, 155.77, 156.86, 159.00, 159.70, 165.80; HRMS (m/z) Calculated for C22H19N6O3 (MH+) 415.1513; found 415.1517; Anal. Calcd for C22H18N6O3.H2O: C, 61.10; H, 4.66; N, 19.43; found C, 61.39; H, 4.33; N, 19.26;
5.2.57. 3-(4-(2-Methoxyethoxy)benzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15s)
Procedure similar to that of 15a; the residue was washed with acetonitrile and diethyl ether followed by drying. (Yield = 0.090 g, 48.9%); m.p.:160–161°C. 1H-NMR (DMSO-d6): δ = 3.27 (s, 3H), 3.61 (t, J = 4.6 Hz, 2H), 3.71 (s, 3H), 4.03 (t, J = 4.6 Hz, 2H), 5.08 (s, 2H), 5.52 (s, 2H), 6.86 (d, J = 8.7 Hz, 2H), 6.89 (d, J = 8.7 Hz, 2H), 7.27 (d, J = 8.7 Hz, 2H), 7.33 (d, J = 8.7 Hz, 2H), 8.75 (s, 1H), 8.93 (s, 1H); 13C-NMR (DMSO-d6): δ = 42.85, 46.47, 55.11, 58.12, 66.85, 70.32, 114.09, 114.39, 128.02, 128.22, 128.76, 129.11, 129.25, 148.39, 149.80, 155.79, 157.91, 158.99, 159.70, 165.77; HRMS (m/z) Calcd for C25H25N6O4 (MH+) 473.1931; found 473.1936; Anal. Calcd for C25H24N6O4: C, 63.55; H, 5.12; N, 17.796; found C, 63.42; H, 5.01; N, 17.68.
5.2.58. 3-(4-(2-Hydroxyethoxy)benzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15t)
The synthesis of the title compound involved the following two steps:
(a) 7-(4-Methoxybenzyl)-3-(4-(2-((2-Methoxyethoxy) methoxy)ethoxy)benzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (14t-ii)
To a solution of 14t (0.2g, 0.37 mmol) in acetonitrile (4 mL) was added triethyl orthoformate (0.44g, 3.0 mmol) and a drop of conc. H2SO4 and refluxed for 1 h. All volatiles were removed under reduced pressure. Residue was recrystalised from ethyl acetate. (Yield = 0.18 g, 88.9%); m.p.: 121–123°C; 1H-NMR (DMSO-d6): δ = 3.21 (s, 3H), 3.42 (m, 2H), 3.56 (m, 2H), 3.71 (s, 3H), 3.76 (m, 2H), 4.06 (m, 2H), 4.65 (s, 2H), 5.08 (s, 2H), 5.52 (s, 2H), 6.86 (d, J = 8.2 Hz, 2H), 6.90 (d, J = 8.2 Hz, 2H), 7.28 (d, J = 8.2 Hz, 2H), 7.33 (d, J = 8.2 Hz, 2H), 8.75 (s, 1H), 8.92 (s, 1H); 13C-NMR (DMSO-d6): δ = 42.83, 46.46, 55.10, 57.96, 65.51, 66.22, 66.99, 71.11, 94.68, 114.09, 114.42, 128.04, 128.22, 129.08, 129.24, 148.39, 149.80, 155.83, 157.87, 158.99, 159.73, 165.77; HRMS (m/z) Calcd for C28H31N6O6 (MH+) 547.2299; found 547.2301; Anal. Calcd for C28H30N6O6.0.25H2O: C, 61.03; H, 5.58; N, 15.25; found C, 60.97; H, 5.34; N, 15.11.
(b) 3-(4-(2-Hydroxyethoxy)benzyl)-7-(4-methoxybenzyl) -3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15t)
To a solution of 14t-ii (0.15g, 0.27 mmol) in DCM (5 mL) was added TFA (1.1 mL) and stirred for 8 h. The reaction mixture was diluted with DCM, washed with water, brine and dried over MgSO4 and evaporated to get product (Yield = 0.10 g, 79.5%); m.p.:213–215°C; 1H-NMR (DMSO-d6): δ = 3.66 (m, 2H), 3.71 (s, 3H), 3.92 (t, J = 5.0 Hz, 2H), 4.81 (t, J = 5.7 Hz, 1H), 5.08 (s, 2H), 5.52 (s, 2H), 6.86 (d, J = 8.7 Hz, 2H), 6.89 (d, J = 8.7 Hz, 2H), 7.27 (d, J = 8.7 Hz, 2H), 7.33 (d, J = 8.7 Hz, 2H), 8.73 (s, 1H), 8.92 (s, 1H); 13C-NMR (DMSO-d6): δ = 42.86, 46.47, 55.11, 59.50, 69.47, 114.10, 114.43, 127.89, 128.24, 128.71, 129.08, 129.24, 148.40, 149.81, 155.83, 158.13, 159.00, 159.74, 165.79; HRMS (m/z) Calcd for C24H23N6O4 (MH+) 459.1775; found 459.1773; Anal. Calcd for C24H22N6O4: C, 62.87; H, 4.84; N, 18.33; found C, 62.59; H, 4.91; N, 18.25.
5.2.58. 7-(4-Methoxybenzyl)-3-((pyridin-3-yl)methyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15u)
Procedure similar to that of 15a; the residue was washed with acetonitrile and diethyl ether followed by drying. (Yield = 0.045 g, 54.8%); m.p.: 162–163°C; 1H-NMR (DMSO-d6): δ = 3.71 (s, 3H, -OCH3), 5.19 (s, 2H, -CH2), 5.53 (s, 2H, -CH2), 6.9 (d, J= 8.7 Hz, 2H, Ar-H), 7.31–7.35 (m, 3H, Ar-H), 7.73–7.75 (m, 1H, Ar-H), 8.48 (d, 1H, Ar-H), 8.62 (s, 1H, Ar-H), 8.74 (s, 1H, diazepine-H), 8.94 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 41.06, 46.48, 55.11, 114.09, 123.58, 128.23, 128.81, 129.24, 131.66, 135.41, 148.37, 148.74, 148.95, 149.77, 156.09, 159.01, 159.91, 165.68. MS (ESI) (m/z) 400.2 (M+1); Anal. Calcd for C21H17N7O2: C, 63.15; H, 4.29; N, 24.55; found C, 62.86; H, 4.26; N, 24.53.
5.2.59. 3-(4-(Dimethylamino)benzyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15v)
Procedure similar to that of15a; the residue was washed with acetonitrile and diethyl ether followed by drying. (Yield = 0.065 g, 79.4%); m.p.: 150–152°C; 1H-NMR (DMSO-d6): δ = 2.86 (s, 6H, -CH3), 3.7 (s, 3H, -OCH3), 5.02 (s, 2H, -CH2), 5.51 (s, 2H, -CH2), 6.62 (d, J = 8.7 Hz, 2H, Ar-H), 6.89 (d, J= 8.7 Hz, 2H, Ar-H), 7.19 (d, J = 8.7 Hz, 2H, Ar-H), 7.32 (d, J = 8.7 Hz, 2H, Ar-H), 8.75 (s, 1H, diazepine-H), 8.91 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 43.05, 46.44, 55.09, 112.22, 114.08, 123.30, 128.22, 128.61, 128.82, 129.21, 148.38, 149.78, 149.91, 155.68, 158.99, 159.59, 165.82. MS (ESI) (m/z) 442.2 (M+1); Anal. Calcd for C24H23N7O2: C, 65.29; H, 5.25; N, 22.21; found C, 64.99; H, 5.22; N, 22.13.
5.2.60. 3-((2-Piperidin-1-yl)ethyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15w)
To a suspension of 15f (0.1g, 0.27 mmol, 1.0 equi.) in 4 ml DMF was added piperidine (0.034 g, 0.40 mmol, 1.5 equi.), potassium iodide (0.004g, 0.027 mmol, 0.1 equi.), potassium carbonate (0.055g, 0.40 mmol, 1.5 equi.). The mixture was stirred for 18 h at 60°C. It was allowed to cool to room temperature and water and ethyl acetate was added. The organic layer was washed with water. After the solvent was concentrated under reduced pressure, to the residue was added EtOAc/Et2O. The resulting precipitate was filtered, washed with diethyl ether and dried to give product (Yield = 0.03 g, 26.5%); m.p.: 221–223°C; 1H-NMR (DMSO-d6): δ = 1.12–1.24 (m, 8H, CH2), 2.11–2.16 (m, 2H, CH2), 3.53–3.58 (m, 1H, -CH2), 3.67 (s, 3H, -OCH3), 3.81–3.84 (m, 2H, -CH2), 4.42 (t, J = 8.7 Hz, 1H, -CH2), 5.2 (q, J = 15.12 Hz, 2H, -CH2), 6.81 (d, J = 8.7 Hz, 2H, Ar-H), 7.12 (d, J = 8.7 Hz, 2H, Ar-H), 7.56 (s, 1H, diazepine- H), 7.99 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 24.10, 25.15, 43.76, 44.33, 45.43, 52.29, 55.07, 97.07, 113.73, 121.65, 128.35, 129.54, 138.78, 141.10, 147.05, 158.62, 171.39, 172.58; MS (m/z) 420.2 (M+1); Anal. Calcd for C22H25N7O2. 0.5 H2O: C, 61.67; H, 6.12; N, 22.88; found C, 61.69; H, 5.84; N, 22.75.
5.2.61. 3-((2-Morpholin-4-yl)ethyl)-7-(4-methoxybenzyl)-3,7-dihydro-2H-diimidazo[4,5-d:4′,5′-f][1,3]diazepin-2-one (15x)
Procedure similar to that of 15w (Yield = 0.027 g. 23.8%); m.p.: 214–216°C; 1H-NMR (DMSO-d6): δ = 2.17–2.19 (m, 2H, CH2), 2.32–2.50 (m, 2H, CH2), 3.2–3.41 (m, 4H, CH2), 3.58–3.62 (m, 1H, -CH2), 3.71 (s, 3H, -OCH3), 3.84–3.89 (m, 2H, -CH2), 4.44 (t, J = 8.7 Hz, 1H, -CH2), 5.24 (q, J =15.12 Hz, 2H, -CH2), 6.86 (d, J = 8.7 Hz, 2H, Ar-H), 7.16 (d, J = 8.7 Hz, 2H, Ar-H), 7.60 (s, 1H, diazepine- H), 8.03 (s, 1H, Imid-H); 13C-NMR (DMSO-d6): δ = 43.73, 43.98, 45.51, 52.39, 55.14, 65.75, 96.74, 113.86, 128.43, 129.52, 139.10, 141.31, 158.67, 172.44. MS (m/z) 422.2 (M+1); Anal. Calcd for C21H23N7O3.0.25 H2O : C, 59.21; H, 5.56; N, 23.02; found C, 59.14; H, 5.35; N, 22.76.
5.3. Biological Procedures
In Vitro Screening: New anticancer compounds were screened using the following cell lines: lung cancer: A549 and H460; breast cancer: MCF-7 and MDA-MB-231; ovarian cancer: OVCAR-3 and prostate cancer: PC-3. Cells (0.5–1.7 × 103 cells/50 μL/well) were seeded in RPMI + 10% FBS in 96-well plate the day before adding the drug dilutions. DMSO was used to dissolve all compounds (stock concentrations ranged from 100 mM to 200 mM). Each compound was tested at nine different concentrations ranging from 100 μM to 5 nM. Every drug dilution for each drug was tested in 4-replicates within each experiment, and each experiment was repeated 1x or 2x. Cells treated with DMSO (equivalent volume) were used as a “vehicle control”. After addition of the drug, cells were cultured for 72h at 37°C, 5% CO2. The experiment was terminated by adding WST-1 Cell Proliferation Reagent (Roche, Mannheim, Germany) to each well and additional incubation for 4h at 37°C, 5% CO2. The colorimetric readouts of cellular metabolic activity was performed by measuring absorbance at 450–690 nm using a Synergy HT Multi-Detection Microplate Reader and Gen5 software (Bio-Tek, Winoski, VT). Data analysis and IC50 calculation was done using GraphPad Prism software, v.5 (La Jolla, CA).
Acknowledgments
This paper is dedicated to RSH’s doctoral mentor Professor Dr. Stewart W. Schneller of Auburn University, Alabama on the occasion of his 70th birthday. The research was supported by a grant (#1R01 GM087738-01A1) from the National Institute of General Medical Sciences of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eckhard J. Trends Biochem Sci. 2011;36:19. doi: 10.1016/j.tibs.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuller-Pace FV. Nucleic Acids Res. 2006;34:4206. doi: 10.1093/nar/gkl460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarn W-Y, Chang T-H. RNA Biol. 2009;6:17. doi: 10.4161/rna.6.1.7440. [DOI] [PubMed] [Google Scholar]
- 4.Garbelli A, Beermann S, Di Cicco G, Dietrich U, Maga G. PLoS One. 2011;6:e19810. doi: 10.1371/journal.pone.0019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi Y-J, Lee S-G. J Cell Biochem. 2012;113:985. doi: 10.1002/jcb.23428. [DOI] [PubMed] [Google Scholar]
- 6.Lee C-S, Dias AP, Jedrychowski M, Patel AH, Hsu JL, Reed R. Nucleic Acids Res. 2008;36:4708. doi: 10.1093/nar/gkn454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosmane RS. Curr Top Med Chem. 2002;2:1093. doi: 10.2174/1568026023393147. [DOI] [PubMed] [Google Scholar]
- 8.Maga G, Falchi F, Radi M, Botta L, Casaluce G, Bernardini M, Irannejad H, Manetti F, Garbelli A, Samuele A, Zanoli S, Esté JA, Gonzalez E, Zucca E, Paolucci S, Baldanti F, De Rijck J, Debyser Z, Botta M. Chem Med Chem. 2011;6:1371. doi: 10.1002/cmdc.201100166. [DOI] [PubMed] [Google Scholar]
- 9.Botlagunta M, Vesuna F, Mironchik Y, Raman A, Lisok A, Winnard P, Jr, Mukadam S, Van Diest P, Chen JH, Farabaugh P, Patel AH, Raman V. Oncogene. 2008;27:3912. doi: 10.1038/onc.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sood RK, Bhadti VS, Fattom AI, Naso RB, Korba BE, Kern ER, Chen H-M, Hosmane RS. Antivir Res. 2002;53:159. doi: 10.1016/s0166-3542(01)00204-2. [DOI] [PubMed] [Google Scholar]
- 11.Zhang N, Chen H-M, Koch V, Schmitz H, Minczuk M, Stepien P, Fattom AI, Naso RB, Kalicharran K, Borowski P, Hosmane RS. J Med Chem. 2003;46:4776. doi: 10.1021/jm030277k. [DOI] [PubMed] [Google Scholar]
- 12.Zhang N, Chen H-M, Koch V, Schmitz H, Liao C-L, Bretner M, Bhadti VS, Fattom AI, Naso RB, Hosmane RS, Borowski P. J Med Chem. 2003;46:4149. doi: 10.1021/jm030842j. [DOI] [PubMed] [Google Scholar]
- 13.Yedavalli VSRK, Zhang N, Cai H, Zhang P, Starost MF, Hosmane RS, Jeang K-T. J Med Chem. 2008;51:5043. doi: 10.1021/jm800332m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosmane RS. Prog Heterocycl Chem. 2009;21:35. [Google Scholar]
- 15.Hogbom M, Collins R, van den Berg S, Jenvert R-M, Karlberg T, Kotenyova T, Flores A, Hedestam GBK, Schiavone LH. J Mol Biol. 2007;372:150. doi: 10.1016/j.jmb.2007.06.050. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Ujjinamatada RK, Hosmane RS. Org Lett. 2008;10:4681. doi: 10.1021/ol8020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.A separate manuscript based on biological results of inhibition of hDDX3 by 1, along with supportive molecular modeling data, is currently being prepared for submission by Dr. V. Raman, Johns Hopkins University School of Medicine, Baltimore, MD
- 18.Hosmane RS, Raman V, Kumar R. 2009-US5273 2010039187. Application: WO Patent. 2010:175.
- 19.Kondaskar AN, Hosmane RS. Abstr. Papers, 239th ACS Nat. Mtg; San Francisco, CA, United States. March 21–25, 2010; MEDI. [Google Scholar]
- 20.Franca R, Belfiore A, Spadari S, Maga G. Proteins. 2007;67:1128. doi: 10.1002/prot.21433. [DOI] [PubMed] [Google Scholar]
- 21.Lam PYS, Ru Y, Jadhav PK, Aldrich PE, DeLucca GV, Eyermann CJ, Chang C-H, Emmett G, Holler ER, Daneker WF, Li L, Confalone PN, McHugh RJ, Han Q, Li R, Markwalder JA, Seitz SP, Sharpe TR, Bacheler LT, Rayner MM, Klabe RM, Shum L, Winslow DL, Kornhauser DM, Jackson DA, Erickson-Viitanen S, Hodge CN. J Med Chem. 1996;39:3514. doi: 10.1021/jm9602571. [DOI] [PubMed] [Google Scholar]
- 22.Lam P, Jadhav P, Eyermann C, Hodge C, Ru Y, Bacheler L, Meek J, Otto M, Rayner M, Wong Y, et al. Science. 1994;263:380. doi: 10.1126/science.8278812. [DOI] [PubMed] [Google Scholar]
- 23.Zhang P, Zhang N, Korba BE, Hosmane RS. Bioorg Med Chem Lett. 2005;15:5397. doi: 10.1016/j.bmcl.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P, Zhang N, Korba BE, Hosmane RS. Bioorg Med Chem Lett. 2007;17:2225. doi: 10.1016/j.bmcl.2007.01.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Z, Hosmane RS. Synth Commun. 2001;31:549. [Google Scholar]
- 26.Kondaskar A, Kondaskar S, Kumar R, Fishbein JC, Muvarak N, Lapidus RG, Sadowska M, Edelman MJ, Bol GM, Vesuna F, Raman V, Hosmane RS. ACS Med Chem Lett. 2011;2:252. doi: 10.1021/ml100281b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michel J, Tirado-Rives J, Jorgensen WL. J Am Chem Soc. 2009;131:15403. doi: 10.1021/ja906058w. [DOI] [PMC free article] [PubMed] [Google Scholar]