Abstract
Prostate cancer is an ideal target for chemoprevention. To date, chemoprevention clinical trials with 5α-reductase inhibitors (5-ARI) have yielded encouraging yet ultimately confounding results. Using a pre-clinical mouse model of high-grade prostatic intraepithelial neoplasia (HG-PIN) induced by PTEN loss, we observed unprecedented deteriorating effects of androgen deprivation, where surgical castration or MDV3100 treatment accelerated disease progression of the otherwise stable HG-PIN to invasive castration-resistant prostate cancer (CRPC). As an alternative, targeting the PI3K signaling pathway via either genetic ablation of PI3K components or pharmacological inhibition of PI3K pathway reversed the PTEN loss-induced HG-PIN phenotype. Finally, concurrent inhibition of PI3K and MAPK pathways was effective in blocking the growth of PTEN-null CRPC. Together, these data have revealed the potential adverse effects of anti-androgen chemoprevention in certain genetic contexts (such as PTEN loss) while demonstrating the promise of targeted therapy in the clinical management of this complex and prevalent disease.
Introduction
Prostate cancer is the most commonly diagnosed cancer in men and second only to lung cancer in the number of cancer deaths, with a total of 241,740 new cases and 28,170 deaths from prostate cancer projected to occur in 2012 (1). Despite early detection, there is currently no cure for the advanced stage of the disease. Prostate cancer is an age-associated disease, whose incidence dramatically increases in men older than 65 years. The fact that there will be a 76% increase in men older than 65 years by the year of 2050 (WHO report) has called for effective management of this deadly disease.
Prostate cancer appears to be an ideal target for chemoprevention because of its prevalence and established hormonally mediated pathogenesis. Androgen deprivation with 5α-reductase inhibitors (5-ARI), which function to decrease serum levels of dihydrotestosterone (DHT), reduced the overall risk of low-grade prostate cancer in two landmark randomized, placebo-controlled prostate cancer chemoprevention trials: the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial and the Prostate Cancer Prevention Trial (PCPT) with Finasteride (2, 3). However, the cumulative risk of high-grade prostate cancers at the end of both trials has generated widespread debates and concern, partly due to the intrinsic limitations of clinical trials (such as time frame, patient selection, flaws in methodology) and the genetic heterogeneity of prostate cancer(4).
Results
HG-PIN is considered a major precursor to prostate cancer. To re-evaluate the effects of androgen deprivation on prostate cancer prevention, here we conducted a preclinical trial utilizing a genetically engineered mouse model (GEMM) in which HG-PIN induced by PTEN loss recapitulates the features of its human counterpart (5). In mouse strain used in this study, a HG-PIN phenotype is induced by 8 weeks of age at nearly 100% penetrance in all three mouse prostate lobes, namely ventral prostate (VP), anterior prostate (AP) and dorsal lateral prostate (DLP) (Fig. 1a, left, and Supplementary Fig. 1). This HG-PIN phenotype features an intact smooth muscle layer and remains stable with no noticeable invasiveness up to 1 year of age (Fig. 1a, right, and data not shown). To study the biological effects of androgen deprivation in preclinical setting, we surgically castrated mice with HG-PIN at 8 weeks of age and monitored tumor growth over time. Consistent with previous reports (5–7), androgen deprivation induced extensive apoptosis (Fig. 1b, left), rapidly shrinking the HG-PIN in all lobes of the prostate glands (Fig. 1c). However a subpopulation of PTEN-deficient prostate tumor cells displayed castration-resistant growth (Fig. 1b, right) and repopulated the shrunken glands by 4–8 weeks post castration (Fig. 1c and data not shown), a phenotype mostly evident in the VP. Strikingly, in contrast to the sham operation group, we found an unprecedented deteriorating effect of androgen deprivation within 16–18 weeks post castration, in which surgical castration accelerated progression of the otherwise stable HG-PIN to invasive CRPC, characterized by broken layers of smooth muscle (Fig. 1d, and Supplementary Fig. 2 and 3). Paralleling androgen deprivation in men, the circulating and intra-prostatic testosterone levels in the CRPC mice dropped significantly to 5–15% of those seen in intact mice (Supplementary Fig. 2)
Fig. 1. Androgen deprivation potentiated the disease progression from HG-PIN to invasive CRPC.
(a) Genetic ablation of PTEN in prostatic epithelium caused HG-PIN. IF: pAKT/SMA. (b) Surgical castration induced extensive apoptosis in HG-PIN lesions (left, IF: TUNEL), whereas a subpopulation of tumor cells continued to proliferate (right, IHC: anti-BrdU). (c) PTEN-null prostate tumor mass initially shrank in response to surgical castration but gradually grew back. (d) Androgen deprivation accelerated progression of PTEN-null HG-PIN to invasive CRPC, arrows indicating invasive lesions. Shown are representative lesions observed in 30/32 (93.75%) mice. IHC: anti-SMA. (e) AR staining in CRPC vs. castration naïve HG-PIN. IHC: anti-AR. (f) Western blot of p53 and AR in age-matched wide-type prostate (WT), HG-PIN and CRPC. (g) Chemical castration accelerated progression of PTEN-null HG-PIN to invasive CRPC, arrows indicating invasive lesions. Shown are representative lesions observed in 8/10 (80%) mice. IHC: anti-SMA. Mice harboring HG-PIN at 8 weeks of age were surgically or chemically castrated for another 16–18 weeks, representative data are shown in Fig. 1d, Fig. 1e, Fig. 1f and Fig. 1g. (h) A comparison between the clinical and preclinical trials over the time. High-grade cancer is seen in human trials, whereas invasive CRPC is evident in the preclinical mouse studies.
Notably, these invasive CRPC lesions carry the common features of hormone-refractory recurrent prostate cancer in the clinic. We and others have reported that human CRPC retains widespread AR positivity within the tumor cell nuclei (8). Consistently, here we observed widespread nuclear AR staining in the epithelial lesions in both castration-naive HG-PIN and CRPC (Fig. 1e), accompanied by slightly increased AR expression as measured by western blot (Fig. 1f, and Supplementary Fig. 4). In line with its function as a safeguard in cancer(9), p53 protein loss (Fig. 1f, and Supplementary Fig. 5), inactivating somatic point mutations and gain of function mutation (Supplementary Fig. 6, and Supplementary Table. 1) occurred during the development of invasive CRPC, a process accompanied by down-regulation of the anti-metastatic SMAD4 (10) (Supplementary Fig. 5). Of note, androgen deprivation enriched tumor cells with positive p63 nuclear staining, especially in the invasive lesions (Supplementary Fig. 7, and 8), which is consistent with the recently suggested concepts of a basal cell of origin for human prostate cancer (11). In addition, chemical castration using MDV3100 (12), an androgen receptor antagonist that blocks the binding of DHT to androgen receptor, also accelerated the progression of HG-PIN to invasive CRPC in the VP at 16 weeks post castration, even though treatment did decrease the size of both the AP and DLP as compared to mock treated tumors (Fig. 1g, Supplementary Fig. 9, and data not shown). Together, our studies demonstrate that along with initial shrinkage of the HG-PIN tumor mass, androgen deprivation profoundly influenced the outcome of HG-PIN by accelerating disease progression (primarily in the VP), a result in line with the observed increased risk of progression to high-grade prostate cancer seen in clinical trials. A comparison between clinical vs. preclinical studies is summarized in Figure. 1h.
To explore the preventive potential of targeted therapy, we evaluated the effects of genetically ablating PI3K isoform(s) along with PTEN in the VP, the gland most responsive to androgen deprivation. Interestingly, we found lobe-specific and PI3K isoform-dependent effects on prostate tumorigenesis. Unlike the dominant role of p110β in the tumorigenesis of PTEN-deficient AP (13) (Fig. 2a–2e, and Supplementary Fig. 10), genetic ablation of either p110β or p110α was insufficient to block PTEN-controlled tumorigenesis in VP (Fig. 2f-2i). Instead, concurrent ablation of both p110β and p110α restored a normal glandular appearance to the PTEN-null VP, accompanied by a concomitant diminution of AKT phosphorylation (Fig. 2j, Supplementary Fig. 11 and data not shown). A similar result was also observed in DLP (data not shown). These data suggest that genetic ablation of particular PI3K isoforms is effective in preventing prostate tumor initiation and continued development. However, we can’t rule out the involvement of p110γ and p110δ, the other class I PI3K isoforms, in this process (14).
Fig. 2. Genetic ablation of PI3K components blocked the prostate tumorigenesis caused by PTEN loss.

(a–d) In anterior prostates, genetic ablation of p110β, not p110α impaired PTEN loss-induced tumorigenesis. (f–i) In VP, genetic ablation of p110α or p110β failed to affect PTEN loss-induced tumorigenesis. (j) Concurrent ablation of both p110α and p110β restored the normal ductual structure of PTEN-null prostate in VP. Note the HG-PIN lesions labeled in arrow, and regular ductal structure in arrowhead featuring single epithelial layer, small amounts of stroma, abundant secreted proteins. Prostates shown are at age of 12 weeks.
To evaluate the therapeutic effects of pharmacologically blocking PI3K kinase activity on established HG-PIN, we treated HG-PIN-bearing mice at age of 8 weeks with Placebo or BEZ235, an orally delivered pan-PI3K/mTOR dual inhibitor (Fig. 3a top panel). At the end of 4 weeks of daily oral gavage, BEZ235-treated PTEN-null prostate tumors were easily distinguished from placebo-treated counterparts because of their smaller size, dramatically reduced weight, and translucent gross appearance, resembling wild-type prostates (Fig. 3b, and data not shown). Histologically, in contrast to the multilayer HG-PIN and activated stroma in placebo group, the BEZ235 treatment resulted in an almost normal luminal architecture in PTEN-null VP compared to the multilayer HG-PIN and activated stroma in placebo group (Fig. 3a, bottom panel). Such therapeutic effects of PI3K inhibition were maintained upon prolonged treatment of up to 8 weeks (data not shown). Mechanistically, BEZ235 potently blocked tumor growth (Fig. 3c) and induced apoptosis (Fig. 3d). At signaling level, BEZ235 blocked the phosphorylation of AKT and RPS6, the molecular surrogates for signaling downstream of PI3K, in PTEN-null prostate tumors (Fig. 3e), though MAPK signaling was not affected (data not shown). Consistent with the effects of BEZ235, BKM120, a small molecule targeting pan-class I PI3Ks only, also reversed the HG-PIN phenotype (Fig. 3f). Thus, PI3K-targeted therapy could be useful in the prevention of HG-PIN with PTEN deficiency.
Fig. 3. Pharmacological inhibition of PI3K pathway reversed the PTEN-controlled HG-PIN phenotype.
(a) Scheme of BEZ235 drug treatment in PTEN-null HG-PIN (top panel), and histology of ventral prostates (lower panel). Green arrow indicates epithelium and blue arrow for stroma. In BEZ235 treatment group, note the single layer of luminal epithelial cells surrounded by a thin rim of fibromuscular stroma, loose connective tissue extending between individual ducts, and eosinophilic secretions in the gland lumens. (b) Weight of ventral prostates from Fig. 3a. (c) The effect of BEZ235 on cell proliferation in HG-PIN lesions. BrdU was administered at 1-hour post treatment and prostate tissues were harvested 5 hours later. (d) Quantification of apoptosis in HG-PIN lesions after 1 week of daily treatment via oral gavage. (e) Western blotting using HG-PIN prostate tissues harvested at 1-hour post treatment. (f) Histology of prostate tumors after BKM120-treatment, following the same scheme as in Fig. 3a. 12-week-old mice harboring HG-PIN were used above, unless otherwise indicated.
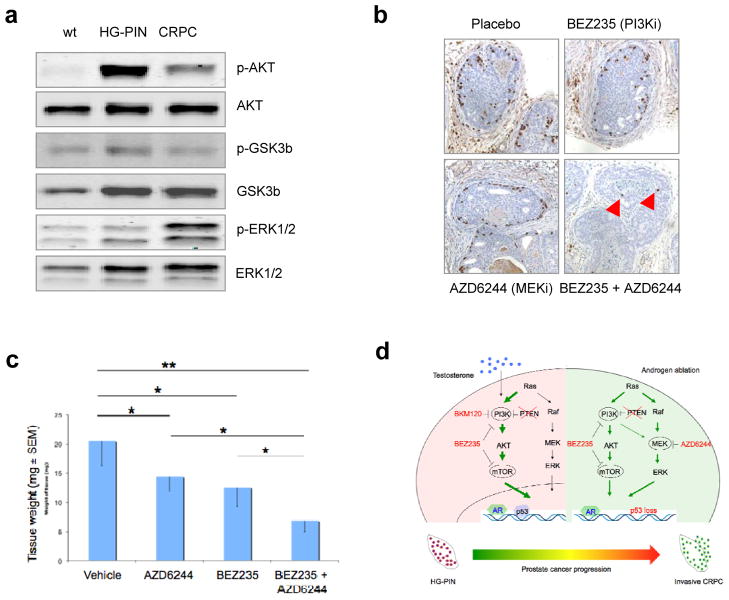
We were curious if BEZ235 could also reverse the more severe tumor phenotype of CRPC. To determine this, we surgically castrated HG-PIN-harboring mice at age of 8 weeks and allowed the CRPC tumor cells to grow for 16 weeks. We then treated animals with either BEZ235 or placebo. Surprisingly, we found that PTEN-null CRPC tumors were not as responsive to BEZ235 treatment as the PTEN-null castration-naïve primary tumors had been (Supplementary Fig. 12). This led us to wonder if CRPC had an altered signaling profile. In addition to the slightly increased AR expression (Fig. 1f and Supplementary Fig. 4), we consistently found a signaling shift in CRPC featuring modestly increased MAPK/ERK activation and a compromised activation of PI3K signaling (Fig 4a and Supplementary Fig. 13), a result in line with previous findings that activation of MAPK is associated with clinical prostate cancer progression(15) and combinatorial activities of AKT and B-RAF/ERK signaling conferred androgen resistance (16). Notably, this alteration of the PI3K-MAPK-AR signaling axis differs slightly from the recently reported PI3K-AR signaling crosstalk in PTEN-null prostate tumors (6, 7), a discrepancy that may arise from the change in predominant cell type in our model or from differences in the genetic background of GEMMs used in various studies (17). To ask whether inhibition of such altered signaling would affect the growth of CRPC, we first tested the status of BrdU-labeled cell proliferation after pharmacologically blocking PI3K signaling with BEZ235 and/or MAPK/ERK signaling with AZD6224, a MEK inhibitor that is currently tested in the clinic. In line with an earlier report (18), our results show that concurrent inhibition of PI3K and MAPK signaling pathways substantially suppressed cell division of CRPC, in addition to the partial effects by single agent activity (Fig. 4b and Supplementary Fig. 14), though no effects on apoptosis were seen (data not shown). To assess the effects of combination therapy on the development of CRPC, we surgically castrated 8-week-old mice harboring HG-PIN and let them recovered for 5 days prior to treatment with BEZ235 and/or AZD6224. At the end of 2 months of treatment, the combined use of BEZ235 and AZD6224 effectively suppressed the growth of CRPC as compared to placebo treatment (Fig. 4c). Thus, combination of targeted therapies aimed at the PI3K and/or RAF-MAPK pathways is effective in the management of PTEN-deficient CRPC.
Fig. 4. Combined inhibition of PI3K and MAPK signaling suppressed the growth of CRPC.
(a) Tissue western. Mice harboring HG-PIN at 8-weeks were surgically castrated and prostate tissues were harvested 16 weeks later. WT: wild-type prostate, HG-PIN: PTEN-null HG-PIN, and CRPC: PTEN-null CRPC. (b) Proliferation index of CRPC tumors post treatment. Mice harboring PTEN-null HG-PIN at 8 weeks of age underwent surgical castration for 16 weeks. BrdU was administered at 1-hour post drug treatment and prostate tissues were harvested 5 hours later. IHC: anti-BrdU. (c) The effects of drug treatment on the weight of CRPC prostate tissues. (d) Targeted therapy in the clinical management of prostate diseases with PTEN deficiency.
Discussion
It has long been thought that androgen deprivation would impair the progression of primary prostate diseases, a concept extensively tested in preventive clinical trials. Our data suggest that androgen deprivation could inadvertently accelerate the progression of the stable HG-PIN to invasiveness in certain genetic contexts (such as PTEN loss). These findings may have clinical relevance for several reasons. First, PTEN loss has been found in 9–45% of HG-PIN (19–21), the occurrence of which is associated with disease progression in clinical studies. For instance, one third of men diagnosed with HG-PIN eventually developed prostate cancer in a larger cohort study (245 patients) (22). Second, the levels of circulating androgen and the effects of anti-androgen treatments on testosterone levels are comparable in mice and humans (Supplementary Fig. 2). Finally it is intriguing that, in mice, treatment of HG-PIN with compounds targeting the PI3K pathway effectively reduces tumor size without causing invasiveness during the period studied (Fig. 3). However the clinical translation of this result would require finding effective doses of a PI3K targeting drug that could be tolerated over long periods of time.
There are caveats to the interpretation of the current results. Obviously mice are not men and it remains to be seen whether similar processes might be occurring in humans. Notably both of the anti-androgen treatments used in the current work affect the levels or action of both testosterone and DHT, while the anti-androgen drugs (5ARI) used in the clinical trials only altered DHT. It would also be interesting to measure androgen levels in patients on dutasteride or finasteride for cancer prevention and compare these to the PTEN-null animal model used here. Moreover, it remains unclear how the dynamics of hormone levels (i.e. hormone flare) might affect the clinical responsiveness to an LHRH agonist, despite the ultimate androgen deprivation by 5-ARI and LHRH agonists in prostate cancer patients. Of note, the model used here behaves differently from an earlier report where the F2 generation of offspring from a cross of 129/Balb/c and C57BL/6 mice displayed an invasive phenotype as early as 9 wks and died by 29 wks (5). In the current study, PTENflox/flox;PbCre4+ compound mice in a C57BL/6 background displayed HG-PIN phenotype but do not progress beyond HG-PIN even in mice up to 60 weeks old. This discrepancy in tumor phenotype between the current and previous studies might be attributed to these differences in genetic background (17). Finally it remains to be seen if changes in the relative strength of signaling in the ERK and PI3K pathways observed in CRPC in the PTEN null mice are also seen in the clinic.
Prostate cancer therapy is poised to enter a new era of personalized medicine. The recent advent of diagnostics technology has enabled clinical detection of PTEN status for patients with HG-PIN. Our findings raise a note of caution about the potential risk of chemoprevention for these patients, while providing the proof-of-principle demonstration of targeted therapy in the clinical management of this complex and prevalent disease (Fig. 4d). Further identification of the genetic, epigenetic or clinical features that predict patients prone to developing high-grade prostate cancer in clinical trials would help stratify patients for future chemoprevention and provide a rational for targeted therapy.
METHODS
Mice
PTENflox/flox (PTENf/f) mice, p110αflox/flox (p110αf/f) mice, p110βflox/flox (p110βf/f) mice and PbCre4 transgenic mice (PbCre4)(13) were used in this study. All protocols for mouse experiments were approved by the Institutional Animal Care and Use Committee of the Dana-Farber Cancer Institute, Boston, Massachusetts.
Drug treatment
We used BEZ235 (dual PI3K-mTOR inhibitor, 45mg/kg/day), AZD6244 (MEK inhibitor, also named ARRY-142886, 25mg/kg/day), BKM120 (PI3K inhibitor, 50mg/kg/day), and MDV3100 (AR inhibitor, 30mg/kg/day) as previously described (12, 23, 24). The doses used here do not affect the body weight or the growth of normal prostates in wild-type mice. BEZ235 and BKM were obtained from Novartis Institutes for Biomedical Research while MDV3100 and ARRY were obtained from a commercial source. Unless otherwise indicated, the drugs were administered by oral gavage on a daily basis, 7 days a week.
Surgical castration
Surgical castration was performed as previously described (5).
BrdU labeling
BrdU labeling and staining was performed according to the manufacturer’s instructions (Invitrogen). Mice were treated with drug for 1 hour followed by a five-hour BrdU labeling.
Western blotting and quantification
Western blot assays were performed as described previously (13) with antibodies against AR PG-21 (06-680, Upstate), AR c-19 (sc-815, Santa Cruz) p53 (sc-126, Santa Cruz), Tubulin (T9026, Sigma), Vinculin (V9131, Sigma), and antibodies from Cell Signaling Technology: phospho-AKT (9271), AKT (9272), phospho-p44/42 MAP kinase (9101), p44/42 MAP kinase (9102), phospho-S6 ribosomal protein (2211), S6 ribosomal protein (2217). The quantification of western blots was performed with Odyssey software (LI-COR Biosciences, Lincoln, NE, USA). The values were referred to control samples after normalized to reference proteins, either Tubulin or Vinculin.
Testosterone measurement
The androgen levels both in circulation and intra-prostate were measured using a mouse testosterone ELISA kit (Calbiotech) based on manufacture’s instructions. Basically, mouse blood samples were extracted from retro-orbital sinus and serum was prepared using serum separator tubes. For intra-prostate measurement, mouse prostates were micro-dissected in cold PBS and lysed in NP-40 buffer. The androgen levels were calculated as total amount per milliliter or per gram.
Histology and immunohistochemistry
Prostate tissues were processed and stained as described previously (8). FFPE blocks were sectioned (5 μm) and stained with hematoxylin and eosin (H&E) at Center for Molecular Oncologic Pathology, DFCI. We performed immunohistochemistry staining using the antibodies against PTEN (138G6, Cell Signaling), pAKT (3787, Cell Signaling), p63 (sc-8431, Santa Cruz Biotechnology), CK8 (MMS-162P, Covance), AR (06-680, Upstate), and BrdU (B35128, Invitrogen). We performed apoptosis TUNEL assay using Roche In Situ Cell Death Detection Kit according to the manufacturer’s instructions.
Statistical analyses
All statistical analyses were performed with Student’s t-test and are represented as means ± s.d. unless otherwise stated. * indicates P < 0.05; ** indicates P < 0.01.
Supplementary Material
Significance.
Chemoprevention with anti-androgen therapies is attractive for prostate cancer given its prevalence and established hormonally mediated pathogenesis. However, since PTEN loss has been found in 9–45% of HG-PIN in the clinic, the current findings suggest that patients with PTEN-deficient prostate tumors might be better treated with PI3K-targeted therapies.
Acknowledgments
We thank Drs P. Kantoff and W. Sellers for insightful discussions. We thank H. Wu, and P. Roy-Burman for animals, and I. Aspalter and J. Kye for technical assistance. We thank C. Priolo, J. Wang, P. Bayliss, Z. Ding, S. Xie, Y. Geng and members of the Roberts/Zhao/Loda/Signoretti labs and the Novartis Oncology Decision Board for helpful discussions. We apologize to colleagues whose primary papers were not cited due to space constraints. This work was supported by grants from Dana-Farber/Harvard Cancer Center Specialized Program of Research Excellence (P.A.R.T Investigatorship Award to S.J.), the National Institutes of Health (CA030002 and CA089021 to T.M.R.; CA089021 to ML; CA134502 to JJZ; and CA148164-01 to T.M.R. and J.J.Z). In compliance with Harvard Medical School guidelines, we disclose the consulting relationship: Novartis Pharmaceuticals, Inc. (M.L., J.J.Z. and T.M.R.).
Footnotes
AUTHOR CONTRIBUTIONS
S.J. X. G. J.J.Z. and T.M.R. conceived and designed the study; S.J. and X.G. carried out the experiments; S-M. M. provided PI3K inhibitors and expert advise on the measurement of their effects and methods of administration. X.W. and S.H.L. provided technical assistance. M.L., E.S. and S.S. supervised mouse pathological analysis; S.J., X.G. and T.M.R. wrote the paper with input from all authors.
Disclosure of Potential Conflicts of Interest: S. Jia is an employee of Genentech Inc and shareholder of Roche Pharmaceuticals. Sauveur-Michel Maira is an employee of the Novartis Institutes for BioMedical Research, Inc.. S.H.L. is an employee and shareholder of Incyte Corporation. M. Loda, J. Zhao and T. Roberts are consultants for the Novartis Institutes for BioMedical Research, Inc.
No potential conflicts of interest were disclosed by the other authors.
References
- 1.Liu Y, Hegde P, Zhang F, Hampton G, Jia S. Prostate cancer - a biomarker perspective. Front Endocrinol (Lausanne) 2012;3:72. doi: 10.3389/fendo.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 3.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 4.Walsh PC. Chemoprevention of prostate cancer. N Engl J Med. 2010;362:1237–8. doi: 10.1056/NEJMe1001045. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–21. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 6.Mulholland DJ, Tran LM, Li Y, Cai H, Morim A, Wang S, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer cell. 2011;19:792–804. doi: 10.1016/j.ccr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal Feedback Regulation of PI3K and Androgen Receptor Signaling in PTEN-Deficient Prostate Cancer. Cancer Cell. 2011;19:575–86. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92:1918–25. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 9.Koivisto PA, Rantala I. Amplification of the androgen receptor gene is associated with P53 mutation in hormone-refractory recurrent prostate cancer. The Journal of pathology. 1999;187:237–41. doi: 10.1002/(SICI)1096-9896(199901)187:2<237::AID-PATH224>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang J, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–73. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–71. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–9. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia S, Roberts TM, Zhao JJ. Should individual PI3 kinase isoforms be targeted in cancer? Current opinion in cell biology. 2009;21:199–208. doi: 10.1016/j.ceb.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gioeli D, Mandell JW, Petroni GR, Frierson HF, Jr, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59:279–84. [PubMed] [Google Scholar]
- 16.Gao H, Ouyang X, Banach-Petrosky WA, Gerald WL, Shen MM, Abate-Shen C. Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc Natl Acad Sci U S A. 2006;103:14477–82. doi: 10.1073/pnas.0606836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman D, Lesche R, Kertesz N, Wang S, Li G, Gao J, et al. Genetic background controls tumor development in PTEN-deficient mice. Cancer research. 2006;66:6492–6. doi: 10.1158/0008-5472.CAN-05-4143. [DOI] [PubMed] [Google Scholar]
- 18.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. The Journal of clinical investigation. 2008;118:3051–64. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimoto M, Cutz JC, Nuin PA, Joshua AM, Bayani J, Evans AJ, et al. Interphase FISH analysis of PTEN in histologic sections shows genomic deletions in 68% of primary prostate cancer and 23% of high-grade prostatic intra-epithelial neoplasias. Cancer Genet Cytogenet. 2006;169:128–37. doi: 10.1016/j.cancergencyto.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Han B, Mehra R, Lonigro RJ, Wang L, Suleman K, Menon A, et al. Fluorescence in situ hybridization study shows association of PTEN deletion with ERG rearrangement during prostate cancer progression. Mod Pathol. 2009;22:1083–93. doi: 10.1038/modpathol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bettendorf O, Schmidt H, Staebler A, Grobholz R, Heinecke A, Boecker W, et al. Chromosomal imbalances, loss of heterozygosity, and immunohistochemical expression of TP53, RB1, and PTEN in intraductal cancer, intraepithelial neoplasia, and invasive adenocarcinoma of the prostate. Genes Chromosomes Cancer. 2008;47:565–72. doi: 10.1002/gcc.20560. [DOI] [PubMed] [Google Scholar]
- 22.Kronz JD, Allan CH, Shaikh AA, Epstein JI. Predicting cancer following a diagnosis of high-grade prostatic intraepithelial neoplasia on needle biopsy: data on men with more than one follow-up biopsy. Am J Surg Pathol. 2001;25:1079–85. doi: 10.1097/00000478-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buonamici S, Williams J, Morrissey M, Wang A, Guo R, Vattay A, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Science translational medicine. 2010;2:51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.