Abstract
Objective
To describe the physical and social environment of sleep self-management in postpartum socioeconomically disadvantaged women.
Design
Descriptive, exploratory design.
Setting
Participants were recruited in the hospital after giving birth. Data were collected in participant homes after discharge.
Participants
Postpartum women on Medicaid with normal healthy infants.
Methods
Participants completed a survey about features within their physical and social sleep environment at 2 weeks postpartum. Participants then completed three days and nights of sleep diaries at both 4 and 8 weeks postpartum to document perceived awakenings, select sleep hygiene practices, bed sharing, and reasons for sleep disruption.
Results
The sleep environments of participants were dynamic from night to night. Bed sharing was common with nearly half of participants sharing with a partner, approximately 25 percent with the infant, and 20 percent with older children. Fifty-two percent of participants slept with the television on part (31%) or all (69%) of the night. Eight-five percent of participants drank caffeine and 24 percent smoked.
Conclusions
These results inform theory-driven postpartum sleep interventions. Modifications to the physical and social sleep environment that attend specifically to how sleep hygiene and environmental factors are manifested in the postpartum period have the potential to improve sleep for socioeconomically disadvantaged women. Future research is needed to articulate which changes can be effectively self-managed by mothers through nursing interventions.
Keywords: sleep hygiene, sleep environment, postpartum, bed sharing, socioeconomic disadvantage
Poor sleep costs society billions of dollars through negative effects on physical, cognitive, and behavioral functioning (Rosekind et al., 2011; Wade, 2011; Walsh & Engelhardt, 1999). The postpartum period is a key point in a woman’s life course when poor sleep is not only common, but also a risk factor for depression independent of factors such as prenatal depression, stressful life events, and poor partner relationship (Dorheim, Bondevik, Eberhard-Gran, & Bjorvatn, 2009; Lee, Baker, Newton, & Ancoli-Israel, 2008). Poor sleep and postpartum depression may be even more amplified in women living in socioeconomic disadvantage (Goyal, Gay, & Lee, 2010; Moore, Adler, Williams, & Jackson, 2002). While the infant is often the main source of maternal sleep disruption, other factors in the physical and social sleep environment, such as poor sleep hygiene and bed sharing practices, can further disrupt sleep (Lee et al., 2008; Mezick et al., 2008; Yang, Lin, Hsu, & Cheng, 2010). The objective of this study was to describe the physical and social sleep environment in a sample of socioeconomically disadvantaged postpartum women for the purpose of informing elements of a self-management sleep intervention that provides postpartum mothers tools to control modifiable factors within their sleep contexts and, subsequently, allows nursing to study the effects of modifications on sleep outcomes.
Background
Sleep Hygiene and Sleep Environment
Interventions that intend to promote sleep and its benefits often include sleep hygiene as a supportive component of the larger intervention (Perlis, Jungquist, Smith, & Posner, 2005; Stremler et al., 2006). The construct of sleep hygiene presents a set of principles that pertain to the practices and behaviors that influence the quality and quantity of sleep (Hauri, 1998; Mastin, Bryson, & Corwyn, 2006). Examples of these principles include limiting caffeine, avoiding alcohol and nicotine, exercise, nocturnal light and noise exposure, emotional distress or worry, and comfort of sleeping surfaces (Yang et al., 2010). Evidence provides the guidance for recommendations for sleep hygiene principles such as in the examples of how sleep quality is affected by caffeine (Smith, 2002), alcohol (Geoghegan, O’Donovan, & Lawlor, 2012), nicotine (Lexcen & Hicks, 1993), noise (Kawada, 2011), and bed-sharing (Troxel, Robles, Hall, & Buysse, 2007). Although sleep hygiene is often taught in sleep promotion interventions, the principles of sleep hygiene are a-theoretical. Sleep hygiene is usually taught as a supportive component of a sleep intervention, but itself has no particular theory underlying the selection or delivery of any set of principles. The development of evidence-based science and interventional practice around sleep faces serious barriers due to inconsistencies in the delivery, client interpretation, and ultimately, demonstrated efficacy of these sleep hygiene principles (Yang et al., 2010).
The sleep environment is comprised of those factors that promote or hinder a person’s ability to fall asleep or stay asleep after sleep onset (National Sleep Foundation, 2011). This global, all-encompassing definition supports several sleep hygiene principles that target components of the sleep environment such as noise and light abatement, room temperature, and mattress comfort. People (adults and children) and any pets within that environment are physical components of the sleep environment not included in sleep hygiene principles (Perlis et al., 2005).
Although researchers in fields such as aviation have extensively studied and adopted practices that promote optional functioning through sleep and sleep hygiene, few have examined or described sleep hygiene or sleep environments in postpartum mothers (Drury, Ferguson, & Thomas, 2012; Lee & Gay, 2011). Studies after childbirth more often focus on problematic infant sleep (Hiscock et al., 2007), relationships between maternal-infant sleep, bed sharing and infant feeding (Quillin & Glenn, 2004), or infant safety in relation to cases of Sudden Infant Death Syndrome (SIDS) or Sudden Unexpected Infant Deaths (SUID) (Fu, Moon, & Hauck, 2010; Quillin & Glenn, 2004; Shapiro-Mendoza, Kimball, Tomashek, Anderson, & Blanding, 2009) For example, one study retrospectively investigated the context surrounding infants who died of accidental strangulation or suffocation such as room ventilation (fan use and open windows), sleep surface, coverings over and under the infant, room temperature, smoking, bed sharing, and pacifier use (Coleman-Phox, 2008). Attention to reducing the risk of infant death is well justified, (Fu et al., 2010); however, a gap in nursing’s understanding of which modifiable factors in the postpartum mother’s physical and social sleep environment exists and intervention development should be targeted by theory-driven sleep promotion interventions.
Sleep Environments and Socioeconomic Disadvantage
Although people spend up to one-third of their lives asleep, few detailed analyses of the physical and social environments in which people sleep are available (Kim, 2009), especially in impoverished populations, despite evidence that persons living in poverty have lower sleep quality and quantity and suffer greater prevalence of sleep disorders (Friedman et al., 2007; Mezick et al., 2008; Moore et al., 2011; Spilsbury et al., 2006). In a sample of 187 adults between ages 45–75, Mezick et al. (2008) found that the contextual variables of room temperature, outside noise, and health worries mediated the association between socioeconomic status and subjective sleep quality.
Exploring postpartum sleep for women in different socioeconomic conditions, Lee and Gay (2011) tested how a modified behavioral-educational sleep hygiene intervention affected maternal postpartum sleep. Specifically, they tested the feasibility and efficacy of infant proximity, dim light, and noise masking in socioeconomically advantaged (n = 118) and disadvantaged (n = 122) primiparous mothers. These researchers concluded that their study’s modifications provided more benefit to maternal sleep in the less advantaged group than the more advantaged group, perhaps because at baseline, the less advantaged group of women’s sleep hygiene and sleep environments were less conducive to sleep and thus, benefited more from the study’s intervention (Lee & Gay). This study’s findings suggest that further research is needed to explore modifications to the sleep context of socioeconomically disadvantaged postpartum mothers as a strategy to improve this population’s health outcomes.
Theoretical Framework
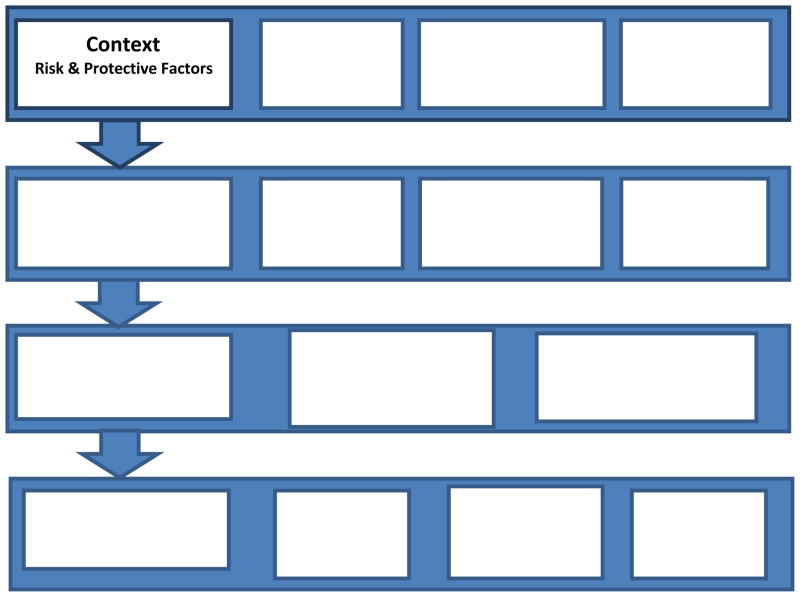
The Individuals and Families Self-Management Theory (IFSMT) underpinned this study (Ryan & Sawin, 2009). Although self-management theory has traditionally addressed chronic illness, the IFSMT expands self-management into the arena of health promotion. According to the IFSMT, self-management is: “…a process by which individuals and families use knowledge and beliefs, self-regulation skills and abilities, and social facilitation to achieve health-related outcomes” (UWM Self-Management Science Center Working Group, 2011, http://www4.uwm.edu/smsc/framework/). The self-management process occurs within a context of risk and protective factors that fall into three broad categories: 1) Condition specific, 2) Physical and Social Environment, and 3) Individual and Family (see Figure 1).
Figure 1.
The Individual and Family Self-management Theory. Reprinted from Ryan, P. & Sawin, K. J. (2009). The individual and family self-management theory: Background and perspectives on content, process, and outcomes. Nursing Outlook, 57(4), 217–225 with permission from Elsevier.
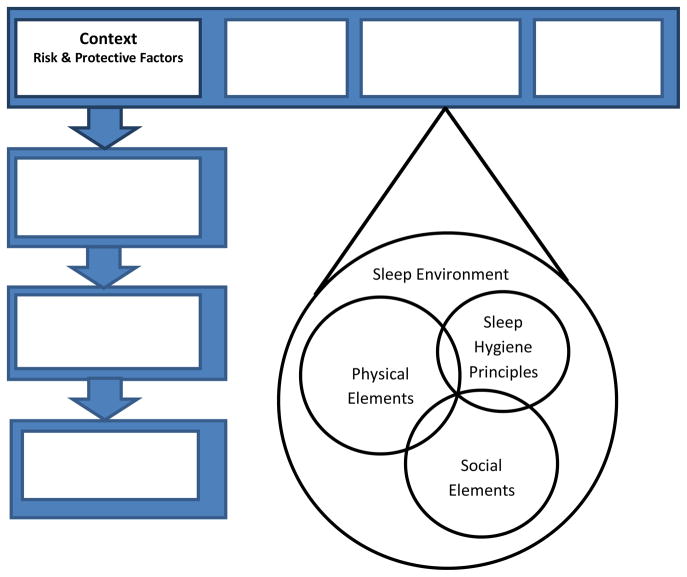
Focusing on the second of these three categories, this study sought to describe the physical and social environment in which the self-management of sleep takes places. The physical and social environment encompasses a broad definition of ‘sleep environment,’ which itself includes sleep hygiene principles, social elements (e.g., socioeconomic status), and the physical elements (see Figure 2). This study sought to describe: (a) Those principles of sleep hygiene relevant to postpartum mothers (e.g., caffeine, smoking, emotional distress); and (b) The physical elements of the sleep environment that may not be addressed by sleep hygiene (e.g., bed sharing, nighttime television use), but still may affect sleep quality and quantity and reflect the larger sleep environment of postpartum mothers in which sleep self-management occurs.
Figure 2.
Elements within the Physical and Social Environment of Sleep Self-Management. Modified from Ryan, P. & Sawin, K. J. (2009). The individual and family self-management theory: Background and perspectives on content, process, and outcomes. Nursing Outlook, 57(4), 217–225, with permission from Elsevier.
Description of postpartum women’s physical and social sleep environment of sleep self-management is essential for the integration of these factors into sleep interventions in a manner that accommodates the factors both common and unique to postpartum women and does so in a theory-driven manner. The self-management of sleep is an emerging frontier in nursing science. Efficacious interventions will be those that attend to the physical and social environmental factors that are relevant within specific populations such as postpartum women. Ultimately, the self-management of sleep has the potential to provide mothers not only with knowledge of the practices and behaviors that influence the quality and quantity of sleep, but will go beyond knowledge to facilitate self-efficacy, decision-making, enable mothers to monitor their response to changes in their physical and social sleep environments, and secure social support to promote sleep in the postpartum period.
Specific Aims
The aims of this study were to describe the physical and social environment of sleep self-management through exploration of:
Specific elements of sleep hygiene in postpartum mothers including use of caffeine, alcohol, and nicotine; and
Factors within the postpartum sleep environment including household size, participant sleep location, infant and bed sharing practices, nocturnal television use and reasons for nighttime awakenings.
Methods
Design, Setting, Sampling, and Procedure
As a part of a broader study on postpartum sleep this study used a repeated measures, descriptive, exploratory design to assess various aspects of the physical and social sleep environment at 2, 4, and 8 weeks postpartum. Approval was obtained from an Institutional Review Board before recruitment began. Recruitment of a convenience sample occurred within two urban, tertiary inpatient postpartum units in the Midwest United States. Nurses on those units gave potential participants a study brochure and form to sign granting permission to the researchers to enter the room. Potential participants who expressed an interest in the study (n = 277) were screened against eligibility criteria, solicited for consent, and enrolled (n = 183). Eligibility criteria were: 18 years or older, multiparous, insured by Medicaid, and had access to a telephone. Exclusion criteria were: history of sleep disorders, mental illness, current use of sleeping medications, alcohol or illegal drug use, current intimate partner violence, or hospitalization of the newborn in the intensive care unit. The most common reason for participant exclusion was a history of depression diagnosis (n = 20).
Data collection occurred in the participant’s home at 2, 4, and 8 weeks postpartum. Attrition was highest (n = 40) between enrollment and the first home study visit at 2 weeks postpartum during which time participants were lost to follow-up (phone disconnected or did not answer after repeated attempts to contact). Sample size at each time point was n = 143 (2 weeks), n = 121 (4 weeks), and n = 114 (8 weeks) postpartum.
Sample
Enrolled participants were on average 25 years old and ranged from 18 to 38 (see Table 1). On average participants had completed high school with a sample range of 6 to 19 years of education completed. The majority of the sample participants were a minority race or ethnicity. Most participants (78%) were partnered, whether married or unmarried. The participant’s newborn was child number two (42%), three (33%), four (13%), or five to nine (12%). Based upon reported income in the past month at enrollment, approximately half the sample qualified as being in ‘deep poverty’ as defined as an annual income below 50% the federal poverty level (i.e., < $11,025 for a family of four in 2009) (Haskins, 2001; U.S. Department of Health and Human Services, 2009).
Table 1.
Sample Characteristics
| Mean(SD) or % | |
|---|---|
| Age | 25(4.6) |
| Years of education | 11.9(1.7) |
| Race/Ethnicity | |
| African-American | 72% |
| Hispanic | 10% |
| White | 14% |
| Multiple race | 4% |
| Other race | 7% |
| Marital Status | |
| Married | 16% |
| Single & partnered | 62% |
| Single, no partner | 20% |
| Past month income | |
| <$1000 | 53% |
| $1,000–$1,999 | 35% |
| $2,000–2,999 | 10% |
| $3,000+ | 2% |
Measures
Physical and Social Sleep Environment Survey
At 2 weeks postpartum, basic questions about current smoking status (yes/no and typical daily number), nocturnal television and radio use, common noises in the sleep environment, participant and infant sleep location, and household size were assessed. These questions were generally designed to be open-ended, and the researcher read to participants and recorded their verbal responses. Survey questions were read to participants, because they were usually occupied holding their infant or other children, so having the researcher record the response was easier for participants and improved data legibility and accuracy. Open-ended questions were used when possible to elicit a more comprehensive and quality response than forced-choice response given the many diverse living situations represented in this population.
Nocturnal television and radio use was assessed by asking whether the device remained on all night or, if turned off, after what duration. Nighttime noise asked, the yes or no question, “Do you often wake up to sounds or noises at night?” after which the open-ended question, “What sounds wake you up?” followed. To assess household size, the open-ended question, “Who lives in your home most of the time” elicited the relationships of persons disclosed by the participant (e.g., myself, my 3 children and my mother), which were documented and later summed for analysis. Participant sleep location was assessed by, “Where do you sleep most nights?” followed by, “Who usually sleeps in bed with you?” to which participants responded with the relationships of persons who slept in bed with them (e.g., me, baby and 3 year old). With the exception of the ‘alone’ category, participants could choose multiple categories (e.g., partner, infant, and older child). Infant sleep location at 2 weeks was assessed as: in bed with the mother; in crib or bassinet in the same room as the mother; in crib or bassinette in a different room; or ‘other’ with space for a written response. Participants were encouraged to select all that applied, since infants can sleep in multiple locations within a single night (Joyner, Oden, Ajao, & Moon, 2010).
Sleep diary
Participants kept a sleep diary for 72 continuous hours at 4 weeks postpartum, and again at 8 weeks for 6 total days and nights of data. Each day, participants recorded the total number of cigarettes smoked, and the total number and type of caffeinated drink (e.g., soda, tea, coffee, energy drink). Alcohol use was assessed via a yes/no question followed by, “If yes, please write what you drank and how much.” Examples of responses included ‘1 beer,’ which were converted into servings based upon standard serving sizes (1 beer = 1 shot liquor = 1 glass wine). Participants were provided an open-ended question asking if anything unusual happened during the day that affected their sleep that night. Each night participants documented the number and reasons (also open-ended) for perceived nighttime awakenings as well as who slept in bed with them (e.g., boyfriend, baby).
Data Analysis
Numerical data were entered into SPSS 19 and analyzed using descriptive statistics. Data from open-ended questions were organized and analyzed into categories using quantitative content analysis (Polit & Hungler, 1999).
Results
Factors Related to Sleep Hygiene
Caffeine and alcohol
Consumption of caffeinated drinks was common. At 4 and 8 weeks, 84% and 86% of participants, respectively, reported caffeine use during the 3 days sleep diaries were completed. Of those who drank caffeinated drinks, participants drank on average 1.7 (SD = 1.27, range 0.33 – 7.0) drinks at 4 weeks and 1.7 (SD = 1.31, range 0 – 6.3) drinks at 8 weeks. The values for caffeine and alcohol use were averaged over the 3 days at each time point. Therefore, a value of 0.3 would equate to 1 drink in the 3 days measured. Similarly, a value of 1.0 would indicate 1 drink per day for each of the 3 days. Participants reported drinking a wide variety of caffeinated drinks including soda, coffee, tea, and energy drinks.
Eleven percent of participants reported drinking an alcoholic beverage anytime during the 3 measurement days at 4 weeks postpartum and 14% reported any use at 8 weeks postpartum. Of those who drank at 4 weeks over the 3 days measured, an average of 0.7 (SD = 0.43, range 0.3 – 1.3 drinks was reported. Of those who drank at 8 weeks, an average of 0.7 (SD = 0.57, range 0.3 – 1.7) drinks was reported over the 3 days. No more than 3 drinks were reported on a given day at either time point except for a single participant who reported consuming 5 alcoholic drinks on day 2 of the week 8 data period.
Nicotine
At 2 weeks, 22% of participants were smokers who smoked on average 6.6 (4.55) cigarettes per day with a range of 2 to 20 cigarettes (20 cigarettes = 1 pack). At 4 and 8 weeks postpartum, 26% (n = 131) and 24% (n = 123) of participants respectively, reported smoking. Of those who smoked, at 4 weeks participants smoked on average 5 (SD = 3.2, range 0.3 – 16.7) cigarettes over the 3 days. This increased at 8 weeks to a mean of 6 (SD = 4.6, range 0.3 – 19.3).
Physical and Social Sleep Environment
Household size and sleep location of mother and infant
Including the participant and infant, the mean number of people living in the household at 2 weeks postpartum was 4.9 (± 1.4) with a range of 3 to 11. When asked at 2 weeks where participants slept most nights, 89% reported sleeping in bed, 6% reported sleeping on a couch or sofa, and 5% said they slept in multiple locations (e.g., combination of bed, couch, recliner, etc.). Similarly, when asked about the infant’s sleep location, participants could mark multiple locations. Of the 143 responses, mother reported that infants slept in the following locations: In bed with mom (22%); in a crib or bassinet in the same room as mom (82%), in crib or bassinet in another room (16%) or in an ‘other’ location (7%, n = 11). The written responses for the ‘other’ locations were: car seat (n = 4), swing (n = 2), bouncer (n = 2), on couch in the living room (n = 1), on a Boppy pillow placed on the couch (n = 1), and a bassinet in the living room (n = 1). One participant whose twins slept with her wrote in the ‘other’ response, “Babies don’t have a bed.”
Bed sharing practices
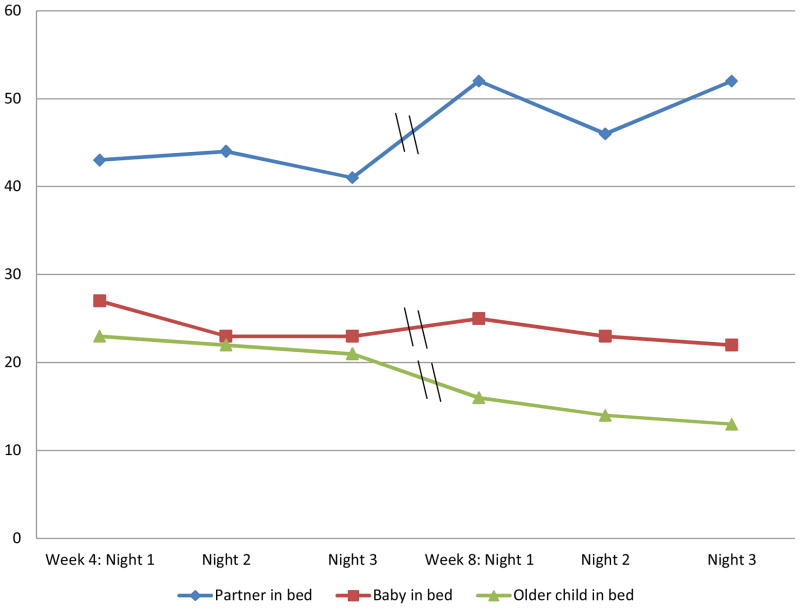
When asked at 2 weeks postpartum, “Who sleeps in bed with you most nights?,” participants (n = 142) reported sleeping with a male partner (38%, n = 54), with the infant (19%, n = 27), with 1 older child (29%, n = 41), with 2 older children (8%, n = 11), and alone (25%, n = 36). No one reported sleeping with more than 2 older children. Sleep diaries at 4 and 8 weeks asked who slept in bed with participants on each of the nights that sleep data were collected (see Figure 3). Between 22 and 27 percent of participants reported bed sharing with the infant on a given night at 4 and 8 weeks. Apart from the infant, from 4 to 8 weeks, participants tended to increase bed sharing with their male partners and decrease bed sharing with older children.
Figure 3.
Bed sharing at night as reported in sleep diaries as a percent of total sample (N = 142).
Nocturnal television use
At 2 weeks, participants were asked if they routinely slept with a television or radio on at night. Affirmative responses were; television (52%, n = 76), radio (2%, n = 3), both (1%, n = 2) and neither (45%, n = 66). Of those who slept with a television, 69% reported the device regularly remained on all night. Of the 31% who said the device did not stay on all night, on average, the television turned off either manually or automatically approximately 120 minutes after the participant retired. Incidentally, when filling out the 2-week survey, several participants anecdotally stated to data collectors that the television served to attenuate environmental sounds that were likely to wake them.
Reasons for nighttime awakenings at two weeks postpartum
At 2 weeks, participants were asked what sounds routinely awakened them at night. Responses were grouped into categories (see Table 2). The infant was the most common factor that woke participants. Besides the infant, sounds of other people in the home and sounds within the building were the most common causes for night awakenings. A number of participants chose to answer this open-ended question by saying ‘everything’ indicating there were multiple sources of sounds that would disrupt their sleep.
Table 2.
Nighttime Sounds that Woke Multiparous Women at Two Weeks Postpartum
| Type of Sound | (n = 142) |
|---|---|
| Infant crying | 56% |
| Other people or pets within the home | 21% |
| Noises within the building or neighbors | 10% |
| “Everything” | 13% |
| Traffic or car noise | 7% |
| Non-traffic noises outside | 6% |
Reasons for nighttime awakenings at 4 and 8 weeks postpartum
Based upon the sleep diary data, at 4 weeks postpartum, participants reported waking from sleep at night an average of 2.5 (SD = 1.44, range 0 – 8.7) times per night. At 8 weeks women on average woke 2.0 (SD = 1.82, range 0 – 15.7) times. Women were asked how many times these awakenings were to care for the infant versus awakenings for other reasons such as environmental sounds, to respond to other children, or for personal reasons (e.g., urination). Participants attributed the majority of their awakenings to the infant, which they were awakened for on average 2.2 (1.3) times per night at 4 weeks and 1.7 (1.3) times per night at 8 weeks.
Open-ended data on any unusual circumstances or factors that participants wrote in their sleep diaries were combined into 235 days of data (subtracting days when participants forgot to complete the sleep diary). Ninety-five responses were written by 77 different participants (some participants wrote more than one comment over the 6 days of data collection). Responses were subjected to content analysis to cluster responses into categories to gain insight into the reasons and contexts that adversely affected participant sleep. Of the 95 responses, the most common factors (32%, n = 30) were related to the infant including the infant crying for large parts of the night or having slept a large portion of the day and then not sleeping that night (n = 23), having spent the night in the emergency room with the infant (n = 4), and the infant’s reaction to immunizations (n = 3, noted only at the 8-week time point). The second most common factor that interfered with sleep was categorized as “family and social issues” (24%, n = 23). Included in this category were a wide variety of reasons such as children or family interfering with sleep (n = 9), losing sleep due to socializing with friends (n = 7), conflict with a past partner (n = 4), and family member sickness (n = 2) or death (n = 1). The third most common grouping were participant health issues (16%, n = 15) including physical (n = 11, common cold, new medication, headache, menstruation) and mental (n = 3; stress, anger, depressed) conditions that interfered with sleep.
Discussion
This study described the physical and social environment of sleep self-management in a sample of socioeconomically disadvantaged postpartum women. Overall, multiple factors occur within the physical and social sleep environment that may amplify the poor sleep these mothers obtain during this period of their lifespan. Results showed several factors (frequent caffeine use, smoking, difficulty sleeping due to emotional distress relating to family and relationship issues, television use, and environmental sounds) that were important and relevant to sleep quality and quantity, but are likely to be common to the sleep hygiene and sleep environments of the general population of low-income persons. Conversely, results also showed factors that were unique to the postpartum period (infant as a main source of sleep disruption, bed sharing practices).
Factors Common to the General Population
Exploring how the frequency of nocturnal awakenings changed over time and the reasons for nocturnal awakenings can help nurses understand how these common factors are manifested specifically in postpartum women. From 4 to 8 weeks postpartum, the number of perceived nighttime awakenings decreased from 2.5 to 2.0 awakenings, which suggests that maternal sleep was less fragmented at 8 weeks than at 4 weeks, as would be expected (Montgomery-Downs, Insana, Clegg-Kraynok, & Mancini, 2010). Maternal sleep fragmentation should decrease over time as infant sleep becomes more consolidated (Sheldon, 2006), but there was a small, yet potentially important difference between the number of awakenings due to the infant versus those not due to the infant. In other words, the infant was not the only factor that disturbed maternal sleep, which has implications for research and practice. Moreover, that half the sample was living in deep poverty and faced a multitude of stressors on a daily basis is also important context for interpreting these results. Participant sleep was disrupted by difficulty managing relationships with partners (current and former), family, and friends as well as their own physical and mental health issues amidst ongoing chronic sleep deprivation in the weeks after childbirth.
Furthermore, sleep-disrupting environmental sounds were problematic. Multiple sources of environmental sounds that affected either the initiation or maintenance of maternal sleep from sources within the bedroom, within the house or building, and within the neighborhood were identified (Mezick et al., 2008). This study’s findings in this area reinforce the call by Meznick and colleagues (2008) to better understand relationships between environmental and emotional factors in relation to sleep within disadvantaged populations.
Another finding from this study that might be found across populations is that over half of the women in the sample slept with a television on in the bedroom for part or all of the night. The use of the television to mask environmental noise outside the bedroom perhaps may be why environmental noise was not a common reason for nocturnal awakening in the sleep diaries. However, this is conjecture based upon anecdotal evidence the 2-week data collection point, since nighttime television use was not documented in sleep diaries. While sleeping with the television may be common today, the effects of nighttime television use on maternal sleep as well as anyone else sleeping in the room (infant, partner, other children) presents a unique issue for consideration by nurses and researchers. Researchers investigating sleep and circadian rhythms in infants and children should include documentation of sound and light related to television use during periods of sleep to further explore effects as these factors are problematic for sleep and health (Johnson, Cohen, First, & Brook, 2004; Sisson, Broyles, Newton, Baker, & Chernausek, 2011).
Furthermore, although sleep hygiene principles recommend that sleep occur in a quiet and dark or low light environment (Hauri, 1998), interventions that recommend or require turning off televisions entirely may not be viewed as feasible or realistic by women living in contexts where combinations of internal and external environmental sounds frequently disrupt sleep. The awakenings caused by home and neighborhood noises may be perceived as more problematic than the negative effects that nighttime light exposure has on sleep. Moreover, television may also serve another purpose of giving the women something else to think about besides daily stress and difficulties when attempting to initiate sleep.
Factors Unique to Postpartum Women
This study also revealed several factors within the physical and social sleep environment that may be unique to the postpartum period and should be included in research and practice interventions. Overall, great variation in women’s physical and social sleep environments occurred, which suggests that interventions should take care not to assume anything without first assessing that factor. Most notably was the finding that night-to-night bed sharing practices may vary considerably more than current surveys accommodate. The assumption that a mother consistently sleeps alone or only with a partner may be false given this study’s findings that bed sharing with multiple family members (including the baby and older children) was common and that who mothers shared the bed with changed over days and weeks. Similarly, this study’s findings revealed that a postpartum mother’s sleep context apart from bed sharing practices is also dynamic. The effects of ever-changing sleep contexts on the quality and quantity of maternal sleep and subsequent health outcomes needs further recognition within the design and measures of research studies investigating maternal sleep.
In another finding unique to postpartum mothers, the high proportion (85%) of caffeine use suggests that mothers may be using caffeine to cope with postpartum fatigue as has been found in previous research (Runquist, 2007). Caffeine assessment should include both the amount and timing of intake to help mothers maximize wakefulness, yet limit delays in sleep initiation. Similarly, renewed efforts to prevent postpartum smoking relapse in this population are warranted given that 25 percent of the sample reported smoking by 2 weeks postpartum.
Implications
Results from this study may be used to inform the development of theory-driven sleep promotion interventions. Specifically, the IFSMT (see Figure 1) is one such theory that can guide interventions. As one category of the risk and protective factors that is salient to the self-management process, the physical and social sleep environment is essential to address with individuals in a manner that integrates the elements of sleep hygiene and the sleep environment that are common to any population as well as those elements that are population specific. A thorough understanding of a woman’s physical and social sleep environment is necessary to populate intervention components to address those factors that are most relevant to the self-management of sleep for the individual. This recommendation is even more important given many of the factors investigated (e.g., caffeine intake, noise and light attenuation) can be altered with little expense and life disruption, and for women of low socioeconomic status, may lead to clinically meaningful improvements in sleep quality and quantity (Lee & Gay, 2011).
This descriptive study was limited by use of self-report to collect sensitive and personal data such as infant bed sharing and alcohol use. For example, it is possible that this self-report of infant bed sharing was low, since bed sharing is highly discouraged by health care providers (Task Force on Sudden Infant Death Syndrome, 2011). Another limitation was the lack of timing of caffeine and alcohol intake and smoking, which prevented this study from examining relationships between variables and is recommended in future studies seeking to measure the effects of these factors on sleep outcomes. Future studies should also measure temperature, sound, and light during periods of sleep with more sophistication (Missildine, 2008).
Conclusions
This study described factors within the physical and social environment that may affect maternal sleep in the first two months postpartum within socioeconomic disadvantage. Results from this study serve as groundwork for further studies and interventions to quantify and qualify how these factors influence sleep. Further descriptions of human physical and social sleep environments utilizing qualitative and quantitative methods are needed to support theory development explaining how individuals and families interface with sleep environments across the human lifespan. To date, evidence-based practice in this area of science remains underdeveloped in both nursing and sleep medicine. Interventions to modify sleep environments remain underappreciated for the impact interventions could have for directly improving sleep, and indirectly, improving health, safety, and productivity. Likewise, the lack of theory-driven approaches to sleep hygiene education may account for some of the inconclusive findings about this construct and limits the potential that could perhaps be realized (Yang, Lin, Hsu, & Cheng, 2010).
The wide variations found in the physical and social sleep environments of postpartum women in this study suggest that development of this science will require interdisciplinary collaborations between disciplines who can quantify the diverse components of the sleep environment including researchers and practitioners from nursing, medicine, engineering (noise, light, temperature, materials), architecture, anthropology, psychology, and others. Given evidence that socioeconomically disadvantaged women may benefit from even small improvements (Lee & Gay, 2011), nursing practice and research efforts should be directed towards increasing the baseline quality of sleep environments in this vulnerable population.
Acknowledgments
Funding provided by a Nurse Faculty Scholar grant from the Robert Wood Johnson Foundation and by the National Institute of Nursing Research Grant #P20NR010674.
Footnotes
Disclosure
The author reports no conflict of interest or relevant financial relationships.
References
- Chianese J, Ploof D, Trovato C, Chang JC. Inner-city caregivers’ perspectives on bed sharing with their infants. Academic Pediatrics. 2009;9(1):26–32. doi: 10.1016/j.acap.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Coleman-Phox K, Odouli R, Li DK. Use of a fan during sleep and the risk of sudden infant death syndrome. Archives of Pediatric & Adolescent Medicine. 2008;162(10):963–968. doi: 10.1001/archpedi.162.10.963. [DOI] [PubMed] [Google Scholar]
- Dorheim SK, Bondevik GT, Eberhard-Gran M, Bjorvatn B. Sleep and depression in postpartum women: A population-based study. Sleep. 2009;32(7):847–855. doi: 10.1093/sleep/32.7.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury DA, Ferguson SA, Thomas MJ. Restricted sleep and negative affective states in commercial pilots during short haul operations. Accident Analysis & Prevention. 2012;45(Supplement):80–84. doi: 10.1016/j.aap.2011.09.031. http://dx.doi.org/10.1016/j.bbr.2011.03.031. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Love GD, Rosenkranz MA, Urry HL, Davidson RJ, Singer BH, Ryff CD. Socioeconomic status predicts objective and subjective sleep quality in aging women. Psychosomatic Medicine. 2007;69(7):682–691. doi: 10.1097/PSY.0b013e31814ceada. [DOI] [PubMed] [Google Scholar]
- Fu LY, Moon RY, Hauck FR. Bed sharing among black infants and sudden infant death syndrome: Interactions with other known risk factors. Academic Pediatrics. 2010;10(6):376–382. doi: 10.1016/j.acap.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Geoghegan P, O’Donovan MT, Lawlor BA. Investigation of the effects of alcohol on sleep using actigraphy. Alcohol and Alcoholism. 2012 May 17; doi: 10.1093/alcalc/ags054. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Goyal D, Gay C, Lee KA. How much does low socioeconomic status increase the risk of prenatal and postpartum depressive symptoms in first-time mothers? Women’s Health Issues. 2010;20(2):96–104. doi: 10.1016/j.whi.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins R. The second most important issue: Effects of welfare reform on family income and poverty. In: Blank R, Haskins R, editors. The new world of welfare. Washington, DC: Brookings Institution; 2001. pp. 103–136. [Google Scholar]
- Hauri PJ. Insomnia. Clinics in Chest Medicine. 1998;19(1):157–168. doi: 10.1016/s0272-5231(05)70439-1. [DOI] [PubMed] [Google Scholar]
- Hiscock H, Bayer J, Gold L, Hampton A, Ukoumunne OC, Wake M. Improving infant sleep and maternal mental health: A cluster randomised trial. Archives of Disease In Childhood. 2007;92(11):952–958. doi: 10.1136/adc.2006.099812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, SK, First MB, Brook JS. Association between television viewing and sleep problems during adolescence and early adulthood. Archives of Pediatric & Adolescent Medicine. 2004;158(6):562–568. doi: 10.1001/archpedi.158.6.562. [DOI] [PubMed] [Google Scholar]
- Joyner BL, Oden RP, Ajao TI, Moon RY. Where should my baby sleep: A qualitative study of African American infant sleep location decisions. Journal of the National Medical Association. 2010;102(10):881–889. doi: 10.1016/s0027-9684(15)30706-9. [DOI] [PubMed] [Google Scholar]
- Kawada T. Noise and health - Sleep disturbance in adults. Journal of Occupational Health. 2011;53(6):413–416. doi: 10.1539/joh.11-0071-RA. [DOI] [PubMed] [Google Scholar]
- Kim M, Chun D, Han J. The research on bedroom environment and sleep quality in Korea. Paper presented at the 2nd International Conference on Sustainable Healthy Buildings; Seoul, Korea. 2009. Oct, Retrieved from http://www.sustainablehealthybuildings.org/PDF/2nd/Chungyoon%20Chun.pdf. [Google Scholar]
- Lee KA, Baker FC, Newton KM, Ancoli-Israel S. The influence of reproductive status and age on women’s sleep. Journal of Women’s Health. 2008;17(7):1209–1214. doi: 10.1089/jwh.2007.0562. [DOI] [PubMed] [Google Scholar]
- Lee KA, Gay CL. Can modifications to the bedroom environment improve the sleep of new parents? Two randomized controlled trials. Research in Nursing & Health. 2011;34(1):7–19. doi: 10.1002/nur.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexcen FJ, Kicks RA. Does cigarette smoking increase sleep problems? Perceptual and Motor Skills. 1993;77(1):16–18. doi: 10.2466/pms.1993.77.1.16. [DOI] [PubMed] [Google Scholar]
- Mastin DF, Bryson J, Corwyn R. Assessment of sleep hygiene using the sleep hygiene index. Journal of Behavioral Medicine. 2006;29(3):223–227. doi: 10.1007/s10865-006-9047-6. [DOI] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M, Strollo PJ, Buysse DJ, Kamarck TW, Reis SE. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE Project. Psychosomatic Medicine. 2008;70:410–416. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missildine K. Sleep and the sleep environment of older adults in acute care settings. Journal of Gerontological Nursing. 2008;34(6):15–21. doi: 10.3928/00989134-20080601-06. [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, Insana SP, Clegg-Kraynok MM, Mancini LM. Normative longitudinal maternal sleep: The first 4 postpartum months. American Journal of Obstetrics and Gynecology. 2010;203(5):465. e461–465. e467. doi: 10.1016/j.ajog.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Redline S. Correlates of adolescent sleep time and variability in sleep time: The role of individual and health related characteristics. Sleep Medicine. 2011;12(3):239–245. doi: 10.1016/j.sleep.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PJ, Adler NE, Williams DR, Jackson JS. Socioeconomic status and health: The role of sleep. Psychosomatic Medicine. 2002;64(2):337–344. doi: 10.1097/00006842-200203000-00018. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. The sleep environment. 2011 Retrieved from http://www.sleepfoundation.org/article/how-sleep-works/the-sleep-environment.
- Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive behavioral treatment for insomnia. New York, NY: Springer; 2005. [Google Scholar]
- Polit DF, Hungler BP. Nursing research: Principles and methods. 6. Philadelphia, PA: Lippincott; 1999. [Google Scholar]
- Quillin SIM, Glenn LL. Interaction between feeding method and co-sleeping on maternal-newborn sleep. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2004;33(5):580–588. doi: 10.1177/0884217504269013. [DOI] [PubMed] [Google Scholar]
- Rosekind MR, Gregory KB, Mallis MM, Brandt SL, Seal B, Lerner D. The cost of poor sleep: Workplace productivity loss and associated costs. Journal of Occupational and Environmental Medicine. 2011;52(1):91–98. doi: 10.1097/JOM.0b013e3181c78c30. [DOI] [PubMed] [Google Scholar]
- Runquist JJ. Persevering through postpartum fatigue. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2007;36(1):28–37. doi: 10.1111/j.1552-6909.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- Ryan P, Sawin KJ. The Individual and Family Self-management Theory: Background and perspectives on content, process, and outcomes. Nursing Outlook. 2009;57(4):217–225. doi: 10.1016/j.outlook.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Mendoza CK, Kimball M, Tomashek KM, Anderson RN, Blanding S. U.S. infant mortality trends attributable to accidental suffocation and strangulation in bed from 1984 through 2004: Are rates increasing? Pediatrics. 2009;123(2):533–539. doi: 10.1542/peds.2007-3746. [DOI] [PubMed] [Google Scholar]
- Sheldon SH. Sleep in infants and children. In: Lee-Chiong T, editor. Sleep: A comprehensive handbook. Hoboken, NJ: Wiley; 2006. pp. 507–510. [Google Scholar]
- Sisson SB, Broyles ST, Newton RL, Baker BL, Chernausek SD. TVs in the bedrooms of children: Does it impact health and behavior? Preventive Medicine. 2011;52:104–108. doi: 10.1016/j.ypmed.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Smith A. Effects of caffeine on human behavior. Food and Chemical Toxicology. 2002;40(9):1243–1255. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- Spilsbury JC, Storfer-Isser A, Kirchner HL, Nelson L, Rosen CL, Drotar D, Redline S. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. The Journal of Pediatrics. 2006;149(3):342–347. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- Stremler R, Hodnett E, Lee KA, MacMillan S, Mill C, Ongcangco L, Willan A. A behavioral-educational intervention to promote maternal and infant sleep: A pilot randomized, controlled trial. Sleep. 2006;29(12):1609–1615. doi: 10.1093/sleep/29.12.1609. [DOI] [PubMed] [Google Scholar]
- Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: Expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128(5):e1341–e1367. doi: 10.1542/peds.2011-2285. [DOI] [PubMed] [Google Scholar]
- Troxel WM, Robles TF, Hall M, Buysse DJ. Marital quality and the marital bed: Examining the covariation between relationship quality and sleep. Sleep Medicine Reviews. 2007;11(5):389–404. doi: 10.1016/j.smrv.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Wisconsin Milwaukee Self-Management Science Center Working Group. Individual and family self-management theory: Definition of self-management. 2011 Retrieved from http://www4.uwm.edu/smsc/framework/
- U.S. Department of Health and Human Services. The 2009 HHS poverty guidelines. 2009 Retrieved from http://aspe.hhs.gov/poverty/09poverty.shtml.
- Wade AG. The societal costs of insomnia. Neuropsychiatric Disease and Treatment. 2011;7:1–18. doi: 10.2147/NDT.S15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JK, Engelhardt CL. The direct economic costs of insomnia in the United States for 1995. Sleep. 1999;22(Suppl 2):S386–S393. [PubMed] [Google Scholar]
- Yang CM, Lin SC, Hsu SC, Cheng CP. Maladaptive sleep hygiene practices in good sleepers and patients with insomnia. Journal of Health Psychology. 2010;15(1):147–155. doi: 10.1177/1359105309346342. [DOI] [PubMed] [Google Scholar]