Abstract
Background: Growing evidence indicates that toxicity of fine particulate matter ≤ 2.5 μm in diameter (PM2.5) differs by chemical component. Exposure to components may differ by population.
Objectives: We investigated whether exposures to PM2.5 components differ by race/ethnicity, age, and socioeconomic status (SES).
Methods: Long-term exposures (2000 through 2006) were estimated for 215 U.S. census tracts for PM2.5 and for 14 PM2.5 components. Population-weighted exposures were combined to generate overall estimated exposures by race/ethnicity, education, poverty status, employment, age, and earnings. We compared population characteristics for tracts with and without PM2.5 component monitors.
Results: Larger disparities in estimated exposures were observed for components than for PM2.5 total mass. For race/ethnicity, whites generally had the lowest exposures. Non-Hispanic blacks had higher exposures than did whites for 13 of the 14 components. Hispanics generally had the highest exposures (e.g., 152% higher than whites for chlorine, 94% higher for aluminum). Young persons (0–19 years of age) had levels as high as or higher than other ages for all exposures except sulfate. Persons with lower SES had higher estimated exposures, with some exceptions. For example, a 10% increase in the proportion unemployed was associated with a 20.0% increase in vanadium and an 18.3% increase in elemental carbon. Census tracts with monitors had more non-Hispanic blacks, lower education and earnings, and higher unemployment and poverty than did tracts without monitors.
Conclusions: Exposures to PM2.5 components differed by race/ethnicity, age, and SES. If some components are more toxic than others, certain populations are likely to suffer higher health burdens. Demographics differed between populations covered and not covered by monitors.
Keywords: air pollution, chemical components, environmental justice, particulate matter, PM2.5, race, socioeconomic status
Concepts of environmental inequality and environmental justice refer to larger health burdens from environmental stressors for some populations than for others. The U.S. Environmental Protection Agency (EPA) uses “environmental justice” to refer to “fair treatment and meaningful involvement of all people regardless of race, color, national origin, or income with respect to the development, implementation, and enforcement of environmental laws, regulations, and policies,” (U.S. EPA 2010), and notes that such conditions reflect not only adverse consequences but also a lack of positive environmental, health, economic, or social benefits (U.S. EPA 2011b). The earliest studies of environmental justice focused on proximity to potentially harmful locations (e.g., incinerators) (Chavis and Lee 1987; U.S. General Accounting Office 1983).
In addition to more recent studies on proximity (Chakraborty et al. 2011; Maantay 2007; Maantay et al. 2009; Mohai and Saha 2007; Pastor et al. 2004), many other types of environmental justice issues have been researched (American Lung Association 2001; Brown 1995; Mohai et al. 2009; Waller et al. 1999). Procedural inequities could affect remediation of hazardous sites regarding priority for cleanup, time from identification of hazards to remediation, and degree of remediation or for regulatory actions, such as industry fines (Carruthers 2007; Lavelle and Coyle 1992). Adverse health outcomes may be used as a marker for environmental justice concerns, such as blood lead levels (Peters et al. 2011), which are higher for non-Hispanic black children than for non-Hispanic white children (Centers for Disease Control and Prevention 2005) or asthma, which in 1995 had a prevalence of 67.4 per 1,000 persons for African Americans and 56.2 per 1,000 persons for whites (National Institutes of Health 1999). Some populations may have a different health response to environmental conditions, meaning that a given level of exposure could have a larger impact on some groups than on others (Bell and Dominici 2008; Grineski et al. 2010; Zanobetti and Schwartz 2000). This effect modification could be related to genetics, baseline health status, access to health care, psychosocial hazards, or other factors (Bell et al. 2002; Clougherty 2010; Clougherty and Kubzansky 2009; Cory-Slechta et al. 2010; Couch and Coles 2011; Gee and Payne-Sturges 2004; Glass et al. 2009; McEwen and Tucker 2011; O’Neill et al. 2003; Ren et al. 2010; Samet and White, 2004; Son et al. 2012; Zanobetti et al. 2000).
Another type of environmental justice is whether some populations face higher exposures to contaminants than do other populations. In this article, we examine this type of environmental justice concern with respect to chemical components of airborne particulate matter (PM) with aerodynamic diameter ≤ 2.5μm (PM2.5). PM2.5 is associated with numerous adverse human health effects, especially cardiopulmonary responses (Pope and Dockery 2006). The majority of health studies on particles have estimated the effects of total PM2.5 mass without regard to chemical composition. In addition, the standard set by the U.S. EPA for particles is based on total mass. However, chemical structure varies widely, such as larger contributions to PM2.5 of nitrate in the western United States and sulfate in the eastern United States (Bell et al. 2007). Growing scientific evidence indicates that some PM2.5 components or sources are more harmful than others (e.g., Ito et al. 2011; Lippmann et al. 2006; Ostro et al. 2007, 2008; Peng et al. 2009). The true toxicity of different parts of the particulate mixture is unknown but is a critical research need (Health Effects Institute 2002; National Research Council 2004).
In a recent study, Miranda et al. (2011) reported that non-Hispanic blacks and persons > 64 years of age had higher PM2.5 exposures than did other U.S. population subgroups. Because the chemical structure of particles is likely to affect its toxicity, we investigated exposures to selected PM2.5 chemical components based on the hypothesis that exposures would differ by race/ethnicity, age, and socioeconomic indicators and that differences in exposures to PM2.5 components would be larger than differences in exposure to PM2.5 total mass.
Methods
We estimated population-level exposures for different groups (e.g., race/ethnicity) to PM2.5 and for the following 14 PM2.5 components measured by the U.S. EPA’s national monitoring network: sulfate (SO42–), nitrate (NO3–), ammonium (NH4+), organic carbon matter (OCM), elemental carbon (EC), sodium ion (Na+), aluminum (Al), calcium (Ca), chlorine (Cl), nickel (Ni), silicon (Si), titanium (Ti), vanadium (V), and zinc (Zn). These components were selected because they contribute ≥ 1% to total PM2.5 mass for yearly or seasonal averages, and/or have been associated with adverse health outcomes in previous studies including mortality, heart rate, heart rate variability, and low birth weight (Bell et al. 2007, 2009; Dominici et al. 2007; Franklin et al. 2008; Huang et al. 2012; Lippmann et al. 2006; Ostro et al. 2007, 2008; Rohr et al. 2011; Wilhelm et al. 2012).
Daily air pollution measures were obtained for 2000 through 2006 (U.S. EPA 2011a). Pollutant monitors were matched to U.S. census tracts, which are geographic units representing small subdivisions of a county and are the smallest spatial unit for which demographic variables of interest were available. Tracts from the 2000 Census (U.S. Census Bureau 2007) were designed to have an optimal population of 4,000 persons (range, 1,500–8,000) and to follow government boundaries (e.g., county), geographic features (e.g., rivers), or other identifiable features (e.g., roadways), where possible. The median land area of the 2000 census tracts in the continental United States was 5.06 km2.
Census tracts in the continental United States were included in our analysis if they had PM2.5 component monitors in operation for ≥ 3 years with ≥ 180 days of observations during the study period. Results were based on 219 monitors in 215 census tracts. Land use near monitors was 43% residential, 34% commercial, 8% industrial, 8% agricultural, and 4% forest.
We calculated long-term averages for each pollutant and 2000 census tract with a monitor for that pollutant. If multiple monitors were present for the same pollutant in a single tract, we averaged daily monitor values within a tract, and then averaged daily values to generate long-term averages. The population and area of census tracts varied. The mean (± SD) distance between a census tract’s centroid and monitor was 2.3 km ± 4.9 km (median 0.8 km; maximum 46.7 km).
For each census tract, we considered population characteristics (U.S. Census 2007):
Race: population self-identified as non-Hispanic white, non-Hispanic black or African American, non-Hispanic Asian, Hispanic, or other [SF1.P08 (Summary File 1, Table P8)]
Educational attainment: persons ≥ 25 years of age with less than a high school degree or equivalent, high school degree or equivalent, or some college (SF3.P37)
Poverty: persons in poverty using Census-defined poverty levels (SF3.P87)
Unemployment: persons ≥ 16 years of age who were unemployed, employed, or not job seekers (SF3.P43)
Age: 0–19, 20–64, or ≥ 65 years of age (SF1.P12)
Earnings: average annual earnings of those ≥ 16 years of age with earnings (SF3.P84)
Total population: (SF1.P08).
We excluded census tracts with populations ≤ 100 (n = 1; for tract with population = 1). For each population characteristic and category (e.g., race/ethnicity, Hispanic), we estimated the average exposure to each pollutant for that group in the United States as a whole by weighting levels in each census tract by the population as
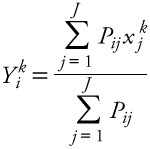 |
[1] |
where Yik is the national average estimated exposure to pollutant k for persons with characteristic i (e.g., Hispanic), j is the number of census tracts with pollutant data (J = 215), Pi,j is the number of persons with characteristic i in census tract j, and xjk is the concentration of pollutant k for census tract j. This provides an estimate of average exposure for each pollutant and population group, accounting for population size and pollutant levels in each census tract. In addition, we performed univariate regression to estimate differences in exposure to PM2.5 and for each component according to census tract characteristics (e.g., percentage of persons unemployed), which are expressed as the percent change in exposure compared with overall mean levels associated with a 10% increase in a given population characteristic.
Whereas the regression analysis investigated whether some groups had higher exposures than others among areas with monitors, we further contrasted population characteristics between census tracts with and without monitors for PM2.5 or its components. We calculated population characteristics for census tracts with and without monitors and performed univariate logistic regression to estimate the percent increase in the probability of a census tract having a monitor with a 10% increase in each population characteristic. This analysis investigated whether some populations are better covered by the existing monitoring network than others.
Results
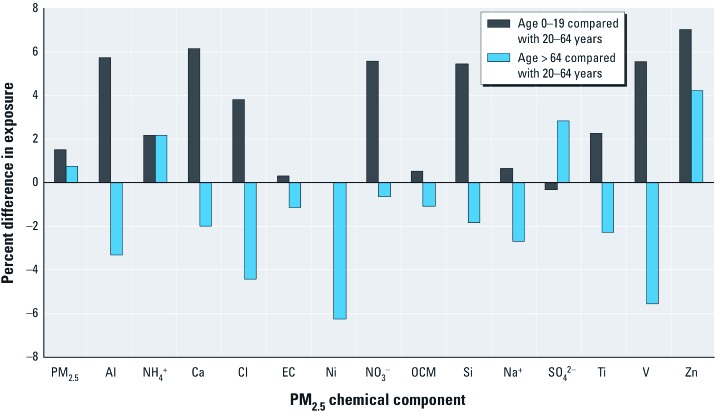
Exposures among children and young adults (0–19 years of age) were as high or higher than exposures among other age groups for PM2.5 and all components except SO42–, which was highest among adults ≥ 65 years of age [relative differences in exposures are presented in Figure 1; see also Supplemental Material, Table S1 (http://dx.doi.org/10.1289/ehp.1205201) for average exposure estimates according to age]. For example, those < 20 years of age had levels 7.0% higher than adults (20–64 years of age) for Zn and 6.2% higher for Ca. Older persons (≥ 65 years of age) had lower exposures than other adults (20–64 years of age) for most pollutants, with the exception of similar levels of PM2.5 (< 1% differences) and higher estimated exposures to NH4+, SO42–, and Zn.
Figure 1.
Percentage differences in exposure by age, comparing persons 0–19 or > 64 years of age with those 20–64 years of age.
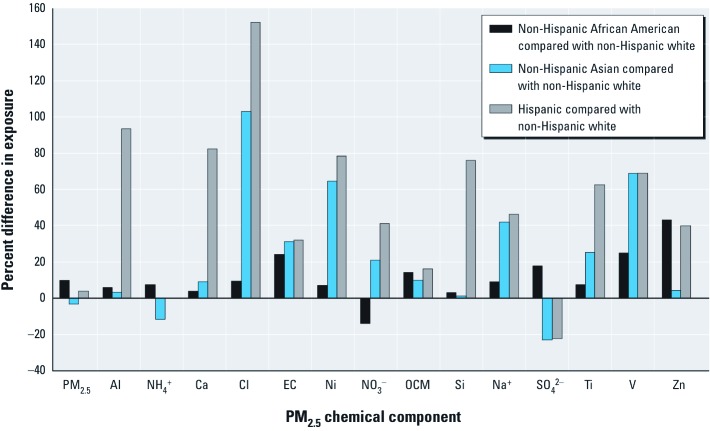
Non-Hispanic whites had the lowest estimated exposures for 11 of the 14 components [relative differences in exposures are presented in Figure 2; see also Supplemental Material, Table S2 (http://dx.doi.org/10.1289/ehp.1205201) for average exposure estimates according to race/ethnicity]. Hispanics had the highest estimated exposures for 10 of the 14 components and were tied with African Americans for the highest estimated exposure to V. Levels for Hispanics were higher than for non-Hispanic whites for 12 of the 14 components (e.g., 152% higher for Cl and 94% for Al). SO42– levels for Hispanics were 22% lower than for non-Hispanic whites. Estimated exposures were higher for African Americans than for whites for 13 of the 14 components (e.g., 43% higher for Zn, 25% for V). African Americans had the highest average exposure levels for NH4+, SO42–, and Zn and the lowest estimated exposure to NO3–. Asians had higher estimated exposures than whites for most of the components considered (e.g., 103% for Cl, 69% for V, 64% for Ni), but they had the lowest estimated exposures of any race/ethnicity group for PM2.5, NH4+, and SO42–.
Figure 2.
Percentage differences in exposure by race/ethnicity category, comparing non-Hispanic African American and non-Hispanic Asian to non-Hispanic white.
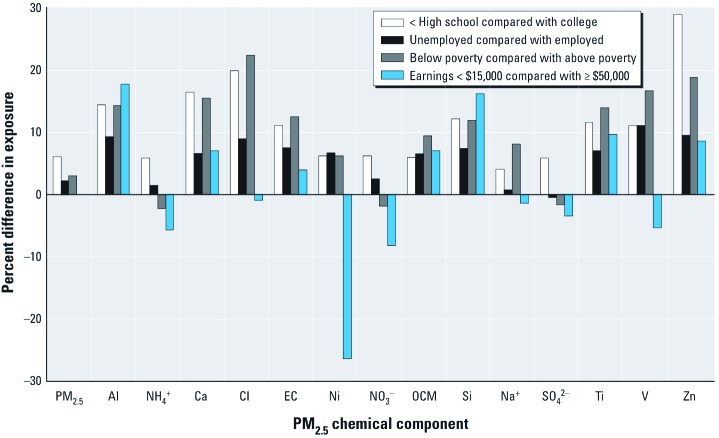
In general, persons with lower-socioeconomic status (SES) had higher estimated exposures, based on indicators of education, unemployment, poverty, and earnings [relative differences in exposures are presented in Figure 3; see also Supplemental Material, Tables S3 and S4 (http://dx.doi.org/10.1289/ehp.1205201) for average exposure estimates according to the SES indicator]. Persons with less than a high school education had higher estimated exposures to PM2.5 and all components than did those with a college education (e.g., 6.2% higher PM2.5, 29% higher Zn, 20% higher Cl), and higher estimated exposures than those with a high school degree for PM2.5 and all components except SO42–. Estimated exposures were ≥ 10% higher among persons without a high school education than among those with a college education for Al, Ca, Cl, EC, Si, Ti, V, and Zn.
Figure 3.
Percentage differences in exposure by category of socioeconomic indicators (education, unemployment, poverty, earnings).
PM2.5 exposures for unemployed persons were 2.3% higher than for employed persons [Figure 3 and Supplemental Material, Table S3 (http://dx.doi.org/10.1289/ehp.1205201)]. The unemployed had higher levels than employed persons for 13 of the 14 components (e.g., 11% higher for V, 9.5% for Zn). Persons in the poverty category had exposures 3.0% higher than those above the poverty line for PM2.5, and higher exposures for 11 of the 14 components, at ≥ 10% for Al, Ca, Cl, EC, Si, Ti, V, and Zn. Persons in the lowest earnings category had 18% higher Al and 16% higher Si exposures than did those in the highest earnings category but 26% lower levels of Ni.
Table 1 shows estimated percent differences from overall mean census-tract exposure levels with a 10% increase in individual population characteristics. For example, a 10% increase in the proportion of the Asian population was associated with 53.5% higher levels for Cl, 50.0% for V, and 45.0% for Ni. Census tracts with a higher percentage of Asians also had higher levels of EC and NO3– and lower levels of SO42–. A 10% increase in the proportion of Hispanics was associated with significantly higher levels of 11 of the components and lower levels of SO42–. For example, an additional 10% of the Hispanic population was associated with increases of 18.2%, 25.4%, and 21.3% in Al, Cl, and Ni, respectively. Increases in age, unemployment, education, poverty, and earnings at the census tract level also were associated with differences in exposures. For example, a 10% increase in the proportion of the population without a high school degree was associated with increases of 19.1% in Zn and 12.2% in V.
Table 1.
Percent increase in long-term average census tract exposure per an additional 10% increase in the population with that characteristic.
| Population | PM2.5 | Al | NH4+ | Ca | Cl | EC | Ni | NO3– | OCM | Si | Na+ | SO42– | Ti | V | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||||||||||
| < 20 | 2.69 | 16.0* | 6.47 | 19.0* | –0.82 | –1.37 | 7.50 | 17.7* | –0.84 | 12.3* | 0.28 | –1.46 | 7.27 | 5.00 | 19.5 | |
| 20–64 | –5.00* | –11.2 | –11.3* | –14.7* | 7.89 | 3.69 | 14.4 | –14.2* | 3.32 | –7.48 | 6.54 | –6.37 | –3.18 | 15.0 | –27.2* | |
| ≥ 65 | 3.07 | –11.9 | 6.24 | –11.8 | –11.3 | –3.41 | –38.1* | –10.2 | –3.81 | –10.9 | –11.1 | 13.1* | –8.64 | –33.9* | 7.47 | |
| Race/ethnicity | ||||||||||||||||
| White | –1.37* | –4.93* | –1.02 | –5.61* | –8.13* | –5.35* | –10.0* | –2.20 | –2.72* | –4.08* | –4.01* | –0.27 | –4.77* | –8.89* | –6.71* | |
| African American | 1.88* | –0.39 | 1.95* | 0.32 | –2.08 | 2.93* | 2.50 | –2.92 | 1.89* | –0.64 | 0.46 | 4.22* | 0.23 | 2.22 | 6.10 | |
| Asian | –2.92 | –5.76 | –8.40 | 4.97 | 53.5* | 20.3* | 45.0* | 19.8* | 4.69 | –5.47 | 29.9* | –18.3* | 16.8* | 50.0* | –5.41 | |
| Hispanic | 0.13 | 18.2* | –0.18 | 16.7* | 25.4* | 7.17* | 21.3* | 12.9* | 3.04* | 15.9* | 8.47* | –7.39* | 13.2* | 16.7* | 4.73 | |
| Other | –16.4* | 8.33 | –36.5* | 31.0 | 84.2* | 23.3* | 72.5* | 18.4 | 16.8 | 10.8 | 23.3 | –59.4* | 16.8 | 52.2* | 63.4 | |
| Education | ||||||||||||||||
| < High school | 4.69* | 8.30* | 5.26* | 13.3* | 11.5 | 9.55* | 7.50 | 5.66 | 5.14* | 8.04* | 3.64 | 4.31* | 9.77* | 12.2* | 19.1* | |
| High school | 2.34 | –11.1* | 7.34* | –15.7* | –19.8* | –5.38 | –26.9* | –7.43 | –4.66 | –10.4* | –12.8* | 12.5* | –13.9* | –20.6* | 5.07 | |
| College | –3.55* | –2.36 | –5.21* | –4.26 | –2.08 | –4.62* | 2.50 | –1.63 | –2.03 | –2.36 | 1.03 | –5.96* | –2.50 | –2.22 | –13.4* | |
| Unemployed | 4.63 | 13.5 | 3.10 | 13.9 | 22.4 | 18.3* | 16.9 | 10.3 | 12.0* | 12.6 | 3.72 | –2.52 | 15.2* | 20.0* | 22.0 | |
| Poverty | 2.16* | 7.31* | –0.69 | 9.89* | 9.39 | 8.78* | 11.3 | –2.14 | 7.15* | 6.19* | 3.24 | 0.17 | 7.95* | 8.89* | 11.2 | |
| Earnings ($US/year) | ||||||||||||||||
| < $15,000 | 0.07 | 10.4* | –3.73 | 10.1* | 3.63 | 3.12 | –8.13 | –2.45 | 5.30* | 10.4* | 0.09 | –3.77 | 8.64* | –1.67 | 5.14 | |
| $15,000–$29,999 | 2.61 | –3.37 | 4.59 | –6.61 | –8.65 | 3.81 | 8.13 | –2.74 | 1.24 | –3.82 | –1.30 | 7.15* | –2.50 | –4.44 | 6.99 | |
| $30,000–$49,999 | –1.55 | –17.2* | 4.49 | –18.8* | –8.4 | –10.5* | –15.0 | 2.43 | –11.7* | –17.0* | –4.05 | 5.04 | –18.0* | –7.78 | –7.88 | |
| ≥ $50,000 | –1.21 | –10.8 | 2.38 | –6.79 | 3.25 | –3.75 | 20.6 | 6.19 | –6.07 | –10.8 | 3.15 | 0.21 | –7.05 | 11.1 | –12.2 | |
| This table provides the percent increase in exposure level, evaluated at the mean for a 10% increase in population characteristic of a census tract. White, African American, and Asian refer to non-Hispanics. *p < 0.05. | ||||||||||||||||
Supplemental Material, Table S5 (http://dx.doi.org/10.1289/ehp.1205201) compares populations of the 215 census tracts with monitors used in this study and the 64,413 tracts without monitors. In addition, 286 tracts have component monitors that did meet our inclusion criteria (e.g., sampling duration). Tracts with monitors for components had higher percentages of non-Hispanic blacks (20.5%) than did tracts without monitors (13.5%). The tracts with monitors versus those without monitors had lower SES based on education (25.5% with < high school education vs. 20.8% and 44.3% with college vs. 50.1%), unemployment (8.64% vs. 6.47%), poverty (19.9% vs. 13.4%), and earnings (39.6% for < $50,000/year vs. 33.9%). Results from univariate logistic regression indicate that a 10% increase in the population that is non-Hispanic black is associated with a 10.3% increase in the probability that a census tract has a monitor [see Supplemental Material, Table S5 (http://dx.doi.org/10.1289/ehp.1205201)]. The same increase in the population (10%) for those who had less than a high school education, who were unemployed, who were in poverty, or who had earnings < $15,000/year was associated with a 22.6%, 41.2%, 39.2%, and 37.6%, respectively, increase in the probability of a census tract having a monitor.
Discussion
To our knowledge, this is the first study of how exposures to PM2.5 components may differ by population for race/ethnicity, age, and SES. In an earlier study, Marshall (2008) examined PM2.5 from diesel sources and hexavalent chromium based on individual-level exposure estimates in California and found higher exposures for persons who were younger (< 7 years vs. > 80 years of age), less educated (< high school vs. college), or nonwhite. Previous studies compared exposure levels of various populations for other pollutants, including PM2.5. U.S. counties in the lowest quantile of air quality had a higher fraction of non-Hispanic blacks and persons in poverty than did counties in the highest quantile of air quality for PM2.5 and ozone (Miranda et al. 2011). In the same study, the investigators found that 20% of the counties with the worst air quality for PM2.5 and for ozone had more persons > 64 years of age and more children < 5 years of age, respectively. Areas with parks or adjacent to parks in Los Angeles, California, had higher NO2 and PM2.5 levels in low SES or high minority neighborhoods (Su et al. 2011). In the United States, Hispanic, African-American, or Asian/Pacific Islander women had higher air pollution exposures during pregnancy than did white women after adjusting for education and other factors, based on an air pollution index that incorporated levels of PM with aerodynamic diameter ≤ 10 μm (PM10), ozone, carbon monoxide, NO2, and sulfur dioxide (Woodruff et al. 2003). In that study, Woodruff et al. (2003) found that lower education was associated with higher pollution levels, after adjustment for race/ethnicity. In Hamilton, Ontario, Canada, levels of total suspended particles (TSP) were higher in census tracts with more Latin Americans or fewer Asian Canadians, with no observable trends between TSP and black Canadians, after adjusting for SES (Buzzelli and Jerrett, 2004). In the same area, Jerrett et al. (2001) observed that TSP levels were higher in census tracts with higher dwelling values and lower income.
In Tampa, Florida, blacks, Hispanics, and persons in poverty resided in neighborhoods closer to toxic release inventory (TRI) sites, whereas whites lived closer to air pollutant monitors (Stuart et al. 2009). In California, census tracts within a mile of TRI facilities had higher fractions of minorities, especially Latinos, lower rates of home ownership, and lower incomes (Pastor et al. 2004). In Orange County, Florida, Chakraborty and Zandbergen (2007) reported that Hispanic or black children were more likely to live or attend school near TRI sources than were white children. In regions of West Virginia, Louisiana, and Maryland, African Americans lived closer to TRI sites than did whites (Perlin et al. 2001).
Our estimates are consistent with these overall trends, indicating the highest PM2.5 exposures for non-Hispanic blacks, the least educated, the unemployed, and those in poverty. However, overall differences were small in magnitude, with the largest difference at 9.9% higher for non-Hispanic blacks than for whites. We estimated larger disparities for exposures to PM2.5 components than to PM2.5. Whereas PM2.5 levels for those without a high school education were 6.2% higher than those with college, Zn levels were 29% higher. Unemployed persons had 2.3% higher PM2.5 than employed persons, but 11% higher levels for V. Similarly, estimated differences among race/ethnicity, earnings, or age categories were larger for many components than for PM2.5. The directions of the associations were different among components. For example, those in the lowest earnings category (< $15,000/year) had higher levels than those earning ≥ $50,000/year for seven components (18% higher for Al) and lower levels for seven components (26% lower for Ni).
We used community-level exposures for census tracts. More precise measures would incorporate spatial heterogeneity (Peng and Bell 2010), as well as daily activity patterns, indoor exposures (e.g., environmental tobacco smoke), inhalation rates, and occupational exposures at the individual level. Many of these factors (e.g., occupation) may differ by population. Exposures were estimated from ambient monitors, and thus do not reflect the personal exposures of all individuals within the census tract.
Our research does not disentangle demographic characteristics of race/ethnicity, education, unemployment, poverty, and earnings; and many population characteristics co-vary [see Supplemental Material, Table S6 (http://dx.doi.org/10.1289/ehp.1205201) for correlations]. For example, race, education, earnings, and poverty were correlated. Future work could examine patterns in population characteristics in relation to PM2.5 component exposures and patterns related to community factors, such as urbanicity and property values.
Only 215 census tracts had PM2.5 component monitors meeting the inclusion criteria, covering 0.3% of the population. The monitor coverage hinders ability to fully investigate equity issues, especially for rural populations, which likely have different characteristics. As population demographics and chemical composition of particles differ dramatically by region (Bell et al. 2007), the geographical distribution of monitors could affect results. In this study, 37% of monitors were in the South (defined by U.S. Census regions), 27% in the Midwest, 19% in the West, and 17% in the Northeast. Future research may consider alternative methods of estimating exposure, such as air quality modeling and satellite imagery (Anderson et al. 2012; Bell 2006; Boldo et al. 2011; Fann et al. 2012), to estimate exposures for a larger population.
Our results show that populations potentially at risk for higher exposures to components do not appear to be underrepresented in areas with monitors compared with areas without monitors. This contrasts with the study by Miranda et al. (2011) that found that U.S. counties without sufficient monitoring for PM2.5 and ozone had fewer non-Hispanic blacks, Hispanics, and persons < 5 years of age and a higher percentage of persons > 64 years of age. Our findings may differ because of the use of census tracts (median land area = 5.06 km2; SD = 571 km2) rather than counties (median land area = 1,582 km2; SD = 3,375 km2) and because of differences between monitoring networks for PM2.5 and PM2.5 components. Other studies also have shown links between population characteristics and monitoring networks. In São Paulo, Brazil, areas with higher SES were more likely to have PM10 and ozone monitors (Bravo and Bell 2010).
Additional challenges in this area of research include the choice and interpretation of SES indicators, because true SES relates to historical conditions, full sources of income, as well as access to resources beyond official earnings, neighborhood-level SES, insurance, access to health care, use of health care systems, and social networks (Bell et al. 2002; O’Neill et al. 2003). The interpretation of SES indicators can vary by region or subculture. Subjective measures of SES include factors such as satisfaction with position, comparison to peers, and perception of financial security. Perceived and actual SES may differ and can have different trends by population (Brown et al. 2008). Traditional measures of SES (e.g., income, education) can be supplemented with subjective social status measures, which in some cases may be more closely linked to health outcomes than to conventional measures (Dennis et al. 2012; Singh-Manoux et al. 2003).
The 14 PM2.5 chemical components investigated here were selected because they contribute ≥ 1% to PM2.5 total mass and/or were found to be potentially harmful to health in earlier studies. However, the full health impacts of various particle mixtures and the identities of the most harmful components or set of components are unknown. A further complication is that all components come from multiple sources, although some components are more strongly linked to some sources than to others (e.g., Ni and V from oil combustion, SO42– from coal combustion, Si from road dust).
A growing body of scientific literature, including epidemiological and toxicological studies, indicates health associations with various PM2.5 chemical components (U.S. EPA 2009). For example, results of toxicological studies using animal models and human-cell cultures suggest the possibility of adverse respiratory effects for Zn (Gerlofs-Nijland et al. 2007; Wu et al. 2003, 2004), Al (Graff et al. 2007), V (Veranth et al. 2007), SO42– (Riley et al. 2005), and NO3– (Huang et al. 2003). Animal models have shown associations with cardiovascular outcomes, such as for zinc (Bagaté et al. 2004, 2006). As additional information becomes available on which chemical components and related sources are most harmful, future studies could examine how such exposures differ by population.
Conclusions
Our estimates suggest differences among populations in PM2.5 component exposures. However, exposure differences may only partly determine whether health impacts from these pollutants are greater in some population groups than in others. The actual difference among groups for health burdens from PM2.5 or its components depends not only on the distribution of exposure, but whether effects are modified by population characteristics. In other words, although we show in this study that some populations have higher exposures than others, a separate issue is whether a given exposure results in the same health response across populations. Methods for risk assessment are needed to assess different effects of environmental exposures across populations and communities that incorporate temporal and spatial connections among risk factors in real-world settings (Schwartz et al. 2011).
Our findings highlight the need for additional research to understand health responses to complex pollutant mixtures, as opposed to effects of individual pollutants. Advances in this field of research are further complicated by inadequate data on multiple pollutants and limitations in statistical methods and exposure assessment (Dominici et al. 2010). However, our work takes a step toward that goal by providing information on differences in exposures that can be used to inform future studies investigating differential health impacts from PM2.5 components and the particulate mixture.
Supplemental Material
Footnotes
This work was funded by the U.S. Environmental Protection Agency through the Harvard University Clean Air Center (EPA RD-83479801) and by the National Institutes of Health (grants R01-ES019560 and R01-ES019587).
The authors declare they have no actual or potential competing financial interests.
References
- American Lung Association Urban air pollution and health inequities: a workshop report. Environ Health Perspect. 2001;109:S357–S374. [PMC free article] [PubMed] [Google Scholar]
- Anderson HR, Butland BK, van Donkelaar A, Brauer M, Strachan DP, Clayton T, et al. Satellite-based estimates of ambient air pollution and global variations in childhood asthma prevalence. Environ Health Perspect. 2012;120:1333–1339. doi: 10.1289/ehp.1104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagaté K, Meiring JJ, Cassee FR, Borm PJA. The effect of particulate matter on resistance and conductance vessels in the rat. Inhal Toxicol. 2004;16:431–436. doi: 10.1080/08958370490439588. [DOI] [PubMed] [Google Scholar]
- Bagaté K, Meiring JJ, Gerlofs-Nijland ME, Cassee FR, Wiegand H, Osornio-Vargas A, et al. Ambient particulate matter affects cardiac recovery in a Langendorff ischemia model. Inhal Toxicol. 2006;18:633–643. doi: 10.1080/08958370600742706. [DOI] [PubMed] [Google Scholar]
- Bell ML. The use of ambient air quality modeling to estimate individual and population exposure for human health research: a case study of ozone in the northern Georgia region of the United States. Environ Int. 2006;32:586–593. doi: 10.1016/j.envint.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Bell ML, Davis D, Cifuentes L, Cohen A, Gouveia N, Grant L, et al. International expert workshop on the analysis of the economic and public health impacts of air pollution: workshop summary. Environ Health Perspect. 2002;110:1163–1168. doi: 10.1289/ehp.021101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Dominici F. Effect modification by community characteristics on the short-term effects of ozone exposure and mortality in 98 U.S. communities. Am J Epidemiol. 2008;167:986–997. doi: 10.1093/aje/kwm396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115:989–995. doi: 10.1289/ehp.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med. 2009;179:1115–1120. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldo E, Linares C, Lumbreras J, Borge R, Narros A, García-Pérez J, et al. Health impact assessment of a reduction in ambient PM2.5 levels in Spain. Environ Int. 2011;37:342–348. doi: 10.1016/j.envint.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Bravo MA, Bell ML. Spatial heterogeneity of PM10 and O3 in São Paulo, Brazil, and implications for human health studies. J Air Waste Manag Assoc. 2010;61:69–77. doi: 10.3155/1047-3289.61.1.69. [DOI] [PubMed] [Google Scholar]
- Brown P. Race, class, and environmental health: a review and systematization of the literature. Environ Res. 1995;69:15–30. doi: 10.1006/enrs.1995.1021. [DOI] [PubMed] [Google Scholar]
- Brown RA, Adler NE, Worthman CM, Copeland WE, Costello EJ, Angold A. Cultural and community determinants of subjective social status among Cherokee and White youth. Ethn Health. 2008;13:289–303. doi: 10.1080/13557850701837302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzelli M, Jerrett M. Racial gradients of ambient air pollution exposure in Hamilton, Canada. Environ Plan A. 2004;36:1855–1876. [Google Scholar]
- Carruthers DV. Environmental justice and the politics of energy on the US–Mexico border. Environ Politics. 2007;16:394–413. [Google Scholar]
- Centers for Disease Control and Prevention. Blood Lead Levels—United States, 1999–2002. 2005. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5420a5.htm. [accessed 6 August 2012]
- Chakraborty J, Maantay JA, Brender JD. Disproportionate proximity to environmental health hazards: methods, models, and measurement. Am J Public Health. 2011;101:S27–S36. doi: 10.2105/AJPH.2010.300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty J, Zandbergen PA. Children at risk: measuring racial/ethnic disparities in potential exposure to air pollution at school and home. J Epidemiol Community Health. 2007;61:1074–1079. doi: 10.1136/jech.2006.054130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis BF, Lee CT. Toxic Wastes and Race in the United States: A National Report on the Racial and Socioeconomic Characteristics of Communities with Hazardous Waste Sites. New York:Commission for Racial Justice, United Church of Christ 1987 [Google Scholar]
- Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect. 2010;118:167–176. doi: 10.1289/ehp.0900994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty JE, Kubzansky LD. A framework for examining social stress and susceptibility to air pollution in respiratory health. Environ Health Perspect. 2009;117:1351–1358. doi: 10.1289/ehp.0900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Stern S, Weston D, Allen JL, Liu S. Enhanced learning deficits in female rats following lifetime Pb exposure combined with prenatal stress. Toxicol Sci. 2010;117:427–438. doi: 10.1093/toxsci/kfq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch SR, Coles CJ. Community stress, psychosocial hazards, and EPA decision-making in communities impacted by chronic technological disasters. Am J Public Health. 2011;101(suppl 1):S140–S148. doi: 10.2105/AJPH.2010.300039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EF, Webb DA, Lorch SA, Mathew L, Bloch JR, Culhane JF. Subjective social status and maternal health in a low-income urban population. Matern Child Health J. 2012;16:834–843. doi: 10.1007/s10995-011-0791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Barr CD, Bell ML. Protecting human health from air pollution: shifting from a single pollutant to a multipollutant approach. Epidemiology. 2010;21:187–194. doi: 10.1097/EDE.0b013e3181cc86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Ebisu K, Zeger SL, Samet JM, Bell ML. Does the effect of PM10 on mortality depend on PM nickel and vanadium content? A reanalysis of the NMMAPS data. Environ Health Perspect. 2007;115:1701–1703. doi: 10.1289/ehp.10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann N, Lamson AD, Anenberg SC, Wesson K, Risley D, Hubbell BJ. Estimating the national public health burden associated with exposure to ambient PM2.5 and ozone. Risk Anal. 2012;32:81–95. doi: 10.1111/j.1539-6924.2011.01630.x. [DOI] [PubMed] [Google Scholar]
- Franklin M, Koutrakis P, Schwartz J. The role of particle composition on the association between PM2.5 and mortality. Epidemiology. 2008;19:680–689. doi: 10.1097/ede.0b013e3181812bb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112:1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlofs-Nijland ME, Dormans JA, Bloemen HJ, Leseman DL, John A, Boere F, et al. Toxicity of coarse and fine particulate matter from sites with contrasting traffic profiles. Inhal Toxicol. 2007;19:1055–1069. doi: 10.1080/08958370701626261. [DOI] [PubMed] [Google Scholar]
- Glass TA, Bandeen-Roche K, McAtee M, Bolla K, Todd AC, Schwartz BS. Neighborhood psychosocial hazards and the association of cumulative lead dose with cognitive function in older adults. Am J Epidemiol. 2009;169:683–692. doi: 10.1093/aje/kwn390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff DW, Schmitt MT, Dailey LA, Duvall RM, Karoly ED, Devlin RB. Assessing the role of particulate matter size and composition on gene expression in pulmonary cells. Inhal Toxicol. 2007;19:S23–S28. doi: 10.1080/08958370701490551. [DOI] [PubMed] [Google Scholar]
- Grineski SE, Staniswalis JG, Peng Y, Atkinson-Palombo C. Children’s asthma hospitalizations and relative risk due to nitrogen dioxide (NO2): effect modification by race, ethnicity, and insurance status. Environ Res. 2010;110:178–188. doi: 10.1016/j.envres.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Effects Institute. Boston: Health Effects Institute; 2002. Understanding the Health Effects of Components of the Particulate Matter Mix: Progress and Next Steps. [Google Scholar]
- Huang W, Cao J, Tao Y, Dai L, Lu SE, Hou B, et al. Seasonal variation of chemical species associated with short-term mortality effects of PM2.5 in Xi’an, a Central City in China. Am J Epidemiol. 2012;175:556–566. doi: 10.1093/aje/kwr342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SL, Hsu MK, Chan CC. Effects of submicrometer particle compositions on cytokine production and lipid peroxidation of human bronchial epithelial cells. Environ Health Perspect. 2003;111:478–482. doi: 10.1289/ehp.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Mathes R, Ross Z, Nádas A, Thurston G, Matte T. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect. 2011;119:467–473. doi: 10.1289/ehp.1002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Kanaroglou P, Eyles J, Finkelstein N, Giovis C, et al. A GIS-environmental justice analysis of particulate air pollution in Hamilton, Canada. Environ Plan A. 2001;33:955–973. [Google Scholar]
- Lavelle M, Coyle M. Unequal protection: the racial divide in environmental law. Natl Law J. 1992;21:S1–S12. [Google Scholar]
- Lippmann M, Ito K, Hwang JS, Maciejczyk P, Chen LC. Cardiovascular effects of nickel in ambient air. Environ Health Perspect. 2006;114:1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maantay J. Asthma and air pollution in the Bronx: methdological and data considerations in using GIS for environmental justice and health research. Health Place. 2007;13:32–56. doi: 10.1016/j.healthplace.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Maantay JA, Tu J, Maroko AR. Loose-coupling an air dispersion model and a geographic information system (GIS) for studying air pollution and asthma in the Bronx, New York City. Int J Environ Health Res. 2009;19:59–79. doi: 10.1080/09603120802392868. [DOI] [PubMed] [Google Scholar]
- Marshall JD. Environmental inequality: air pollution exposures in California’s South Coast Air Basin. Atmos Environ. 2008;42:5499–5503. [Google Scholar]
- McEwen BS, Tucker P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am J Public Health. 2011;101:S131–S139. doi: 10.2105/AJPH.2011.300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda ML, Edwards SE, Keating MH, Paul CJ. Making the environmental justice grade: the relative burden of air pollution exposure in the United States. Int J Environ Res Public Health. 2011;8:1755–1771. doi: 10.3390/ijerph8061755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohai P, Pellow D, Roberts JT. Environmental justice. Ann Rev Environ Resources. 2009;34:405–430. [Google Scholar]
- Mohai P, Saha R. Racial inequality in the distribution of hazardous wates: a national-level reassessment. Social Problems. 2007;54:343–370. [Google Scholar]
- National Institutes of Health. Bethesda, MD: National Heart, Lung, and Blood Institute; 1999. Data Fact Sheet. Asthma Statistics. [Google Scholar]
- National Research Council. Washington, DC: National Academies Press; 2004. Research Priorities for Airborne Particulate Matter: Continuing Research Progress. [Google Scholar]
- O’Neill MS, Jerrett M, Kawachi I, Levy JI, Cohen AJ, Gouveia N, et al. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect. 2003;111:1861–1870. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Feng WY, Broadwin R, Green S, Lipsett M. The effects of components of fine particulate air pollution on mortality in California: results from CALFINE. Environ Health Perspect. 2007;115:13–19. doi: 10.1289/ehp.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro BD, Feng WY, Broadwin R, Malig BJ, Green RS, Lipsett MJ. The impact of components of fine particulate matter on cardiovascular mortality in susceptible subpopulations. Occup Environ Med. 2008;65:750–756. doi: 10.1136/oem.2007.036673. [DOI] [PubMed] [Google Scholar]
- Pastor M, Jr, Sadd JL, Morello-Frosch R. Waiting to inhale: the demographics of toxic air release facilities in 21st-century California. Soc Sci Quart. 2004;85:420–440. [Google Scholar]
- Peng RD, Bell ML. Spatial misalignment in time series studies of air pollution and health data. Biostatistics. 2010;11:720–740. doi: 10.1093/biostatistics/kxq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng RD, Bell ML, Geyh AS, McDermott A, Zeger SL, Samet JM, et al. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ Health Perspect. 2009;117:957–963. doi: 10.1289/ehp.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin SA, Wong D, Sexton K. Residential proximity to industrial sources of air pollution: interrelationships among race, poverty, and age. J Air Waste Manag Assoc. 2001;51:406–421. doi: 10.1080/10473289.2001.10464271. [DOI] [PubMed] [Google Scholar]
- Peters JL, Kubzansky LD, Ikeda A, Spiro A, Wright RO, Weisskopf MG, et al. Childhood and adult socioeconomic position, cumulative lead levels, and pessimism later in life. Am J Epidemiol. 2011;174:1345–1353. doi: 10.1093/aje/kwr269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Ren C, Vokonas PS, Suh H, Fang S, Christiani DC, Schwartz J.2010Effect modification of air pollution on urinary 8-hydroxy-2’-deoxyguanosine by genotypes: an application of the multiple testing procedure to identify significant SNP interactions. Environ Health 978; doi: 10.1186/1476-069X-9-78[Online 7 December 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley MR, Boesewetter DE, Turner RA, Kim AM, Collier JM, Hamilton A. Comparison of the sensitivity of three lung derived cell lines to metals from combustion derived particulate matter. Toxicol In Vitro. 2005;19:411–419. doi: 10.1016/j.tiv.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Rohr AC, Kamal A, Morishita M, Mukherjee B, Keeler GJ, Harkema JR, et al. Altered heart rate variability in spontaneously hypertensive rats is associated with specific particulate matter components in Detroit, Michigan. Environ Health Perspect. 2011;119:474–480. doi: 10.1289/ehp.1002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, White RH. Urban air pollution, health, and equity. J Epidemiol Community Health. 2004;58:3–5. doi: 10.1136/jech.58.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Bellinger D, Glass T. Exploring potential sources of differential vulnerability and susceptibility in risk from environmental hazards to expand the scope of risk assessment. Am J Public Health. 2001;101:S94–S101. doi: 10.2105/AJPH.2011.300272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: its determinants and its association with measures of ill-health in the Whitehall II study. Soc Sci Med. 2003;56:1321–1333. doi: 10.1016/s0277-9536(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Son JY, Lee JT, Kim H, Yi O, Bell ML. Susceptibility to air pollution effects on mortality in Seoul, Korea: a case-crossover analysis of individual-level effect modifiers. J Expo Sci Environ Epidemiol. 2012;22:227–234. doi: 10.1038/jes.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart AL, Mudhasakul S, Sriwatanapongse W. The social distribution of neighborhood-scale air pollution and monitoring protection. J Air Waste Manag Assoc. 2009;59:591–602. doi: 10.3155/1047-3289.59.5.591. [DOI] [PubMed] [Google Scholar]
- Su JG, Jerrett M, de Nazelle A, Wolch J. Does exposure to air pollution in urban parks have socioeconomic, racial, or ethnic gradients? Environ Res. 2011;111:319–328. doi: 10.1016/j.envres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2000 Census of Population and Housing. 2007. Available: http://www.census.gov/prod/cen2000/ [accessed 2 October 2012]
- U.S. EPA (U.S. Environmental Protection Agency) EPA/600/R-08/139-F. Research Triangle Park, NC:U.S. EPA, Office of Research and Development. 2009. Integrated Science Assessment for Particulate Matter. [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) EPA’s Action Development Process. Interim Guidance on Considering Environmental Justice During the Development of an Action Plan. 2010. Available: http://www.epa.gov/environmentaljustice/plan-ej/index.html [accessed 27 September 2012]
- U.S. EPA (U.S. Environmental Protection Agency) AirExplorer Homepage. 2011a. Available: http://www.epa.gov/airdata/ [accessed 19 December 2010]
- U.S. EPA (U.S. Environmental Protection Agency) EPA Plan EJ 2014. 2011b. Available: http://www.epa.gov/environmentaljustice/plan-ej/index.html [accessed 27 September 2012]
- U.S. General Accounting Office. Washington, DC: U.S. General Accounting Office; 1983. Siting of Hazardous Waste Landfills and Their Correlation with Racial and Economic Status of Surrounding Communities. [Google Scholar]
- Veranth J, Kaser E, Veranth M, Koch M, Yost G.2007Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part Fibre Toxicol 42; doi: 10.1186/1743-8977-4-2[Online 27 February 2007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller LA, Louis TA, Carlin BP. Environmental justice and statistical summaries of differences in exposure distributions. J Expo Anal Environ Epidemiol. 1999;9:56–65. doi: 10.1038/sj.jea.7500026. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ghosh J, Su J, Cockburn M, Jerrett M, Ritz B. Traffic-related air toxics and term low birth weight in Los Angeles County, California. Environ Health Perspect. 2012;120:132–138. doi: 10.1289/ehp.1103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Parker JD, Kyle AD, Schoendorf KC. Disparities in exposure to air pollution during pregnancy. Environ Health Perspect. 2003;111:942–946. doi: 10.1289/ehp.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Samet JM, Silbajoris R, Dailey LA, Sheppard D, Bromberg PA, et al. Heparin-binding epidermal growth factor cleavage mediates zinc-induced epidermal growth factor receptor phosphorylation. Am J Respir Cell Mol Biol. 2004;30:540–547. doi: 10.1165/rcmb.2003-0233OC. [DOI] [PubMed] [Google Scholar]
- Wu W, Wang X, Zhang W, Reed W, Samet JM, Whang YE, et al. Zinc-induced PTEN protein degradation through the proteasome pathway in human airway epithelial cells. J Biol Chem. 2003;278:28258–28263. doi: 10.1074/jbc.M303318200. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. Race, gender, and social status as modifiers of the effects of PM10 on mortality. J Occup Environ Med. 2000;42:469–474. doi: 10.1097/00043764-200005000-00002. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J, Gold D. Are there sensitive subgroups for effects of airborne particles? Environ Health Perspect. 2000;108:841–845. doi: 10.1289/ehp.00108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.