Abstract
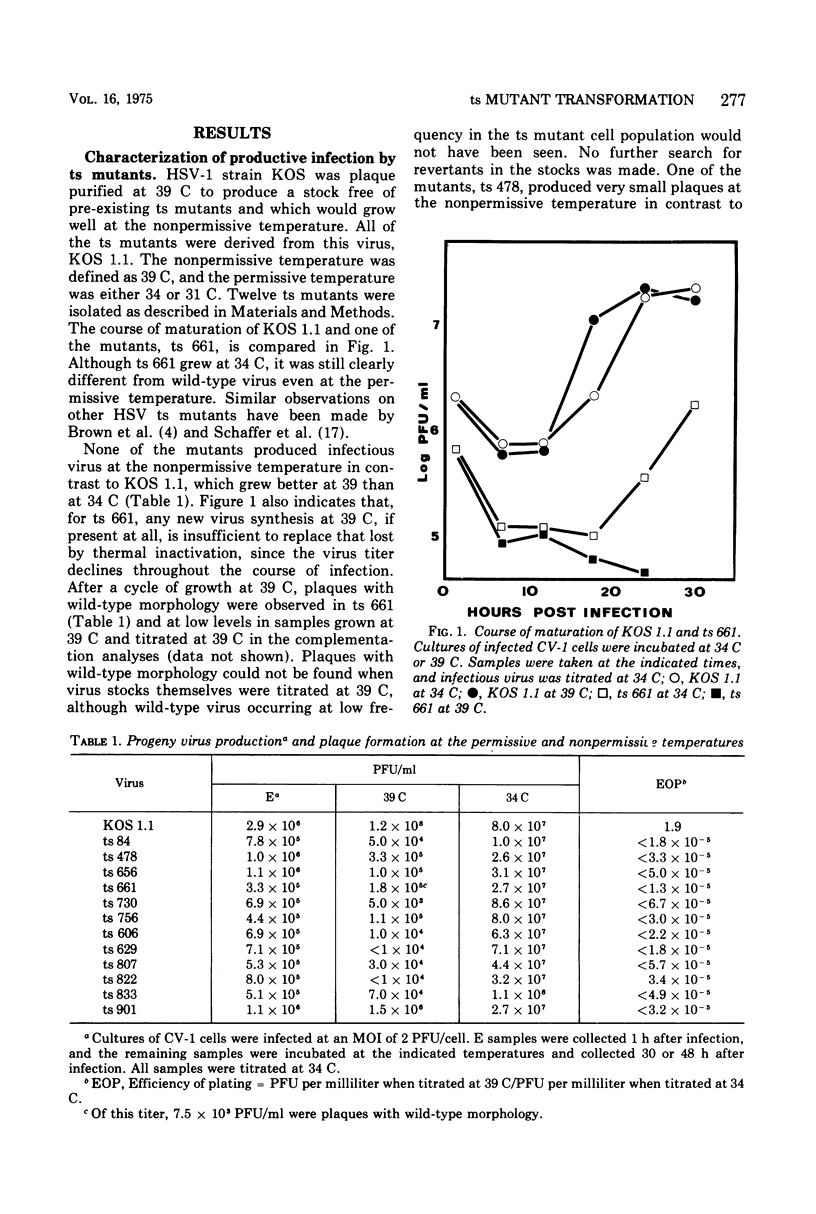
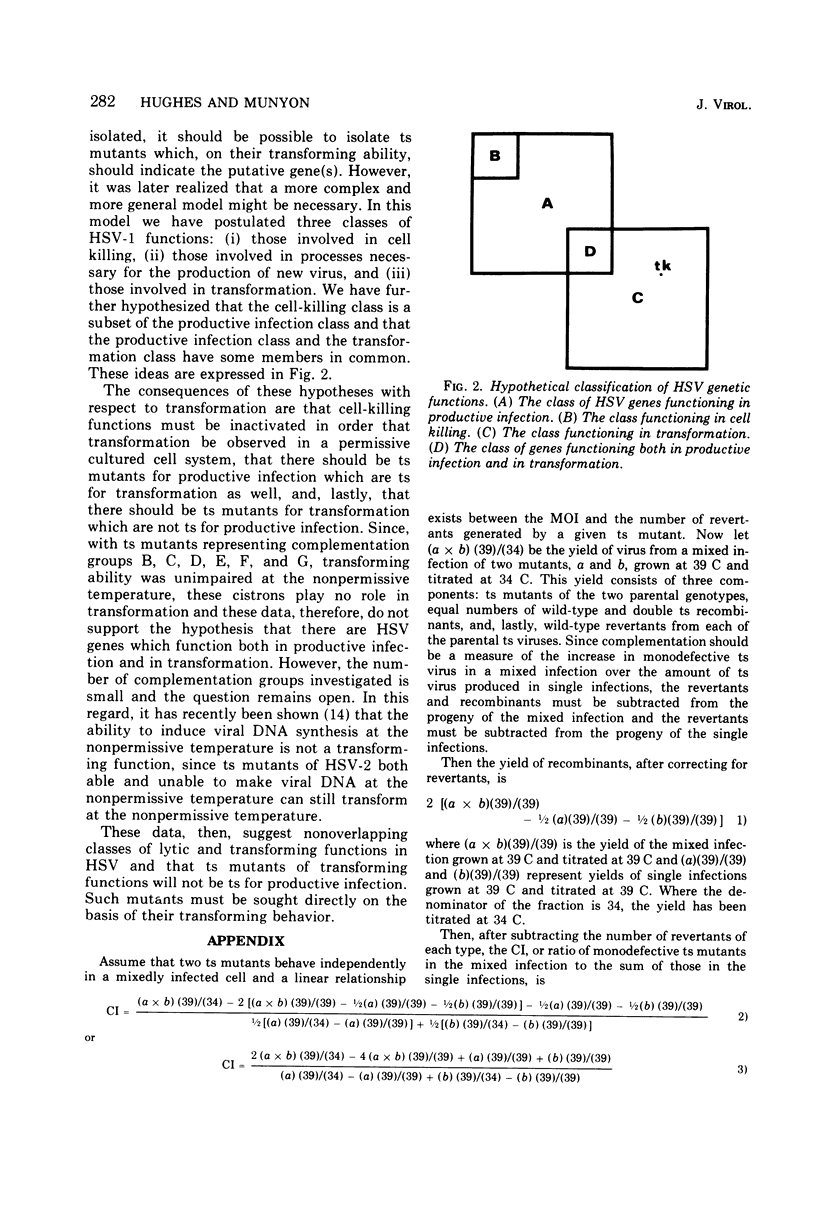
Twelve temperature-sensitive (ts) mutants of herpes simplex virus type 1 (HSV-1), representing seven complementation groups, were isolated subsequent to 5-bromodeoxyuridine mutagenesis. These mutants were identified by their inability to replicate in a line of monkey (CV-1) cells at 39 C. Seven of these mutants, representing six complementation groups, induced thymidine kinase (tk) and transformed Ltk- cells, a line of mouse L cells lacking tk, to a tk+ phenotype at both the permissive (34 C) and nonpermissive (39 C) temperatures. Thus, the defective cistrons in these six complementation groups, although necessary for lysis, have no essential function in this transformation system. Transformation by these 12 mutants was dependent on prior UV irradiation. Infection of cells with unirradiated virus under conditions which did not permit virus replication was not sufficient to allow cell transformation. Five mutants, representing two complementation groups, were tk- and were incapable of causing the tk--to-tk+ transformation at either 34 C of 39 C. The tk defects in these mutants are probably unrelated to the ts defects, since one of these complementation groups contains a tk+ member. Therefore, transformation of Ltk- cells to a tk+ phenotype by HSV-1 requires an active viral tk gene. One complementation group was represented by a single tk- member. The role of this cistron in transformation remains undetermined since the primary block to transformation is presumed to be the tk- phenotype. Mutants representing the seven complementation groups were unable to replicate at 39 C in two lines of HSV-1-transformed cells, indicating that the activities of resident wild-type copies of the defective cistrons, if present, could not be detected by complementation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron G. M., Schaffer P. A., Courtney R. J., Benyesh-Melnick M., Kit S. Thymidine kinase activity of herpes simplex virus temperature-sensitive mutants. Intervirology. 1973;1(2):96–109. doi: 10.1159/000148836. [DOI] [PubMed] [Google Scholar]
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Bastian F. O., Rabson A. S., Yee C. L., Tralka T. S. Herpesvirus hominis: isolation from human trigeminal ganglion. Science. 1972 Oct 20;178(4058):306–307. doi: 10.1126/science.178.4058.306. [DOI] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Complementation analysis of simian virus 40 mutants. J Virol. 1974 May;13(5):1101–1109. doi: 10.1128/jvi.13.5.1101-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Darai G., Munk K. Human embryonic lung cells abortively infected with Herpes virus hominis type 2 show some properties of cell transformation. Nat New Biol. 1973 Feb 28;241(113):268–269. doi: 10.1038/newbio241268a0. [DOI] [PubMed] [Google Scholar]
- Davis D. B., Munyon W., Buchsbaum R., Chawda R. Virus type-specific thymidine kinase in cells biochemically transformed by herpes simplex virus types 1 and 2. J Virol. 1974 Jan;13(1):140–145. doi: 10.1128/jvi.13.1.140-145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EDGAR R. S., DENHARDT G. H., EPSTEIN R. H. A COMPARATIVE GENETIC STUDY OF CONDITIONAL LETHAL MUTATIONS OF BACTERIOPHAGE T4D. Genetics. 1964 Apr;49:635–648. doi: 10.1093/genetics/49.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkle B., McAuslan B. R. Transformation of cultured mammalian cells by viable herpes simplex virus subtypes 1 and 2. Proc Natl Acad Sci U S A. 1974 Jan;71(1):220–224. doi: 10.1073/pnas.71.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macnab J. C. Transformation of rat embryo cells by temperature-sensitive mutants of herpes simplex virus. J Gen Virol. 1974 Jul;24(1):143–153. doi: 10.1099/0022-1317-24-1-143. [DOI] [PubMed] [Google Scholar]
- Munyon W., Buchsbaum R., Paoletti E., Mann J., Kraiselburd E., Davis D. Electrophoresis of thymidine kinase activity synthesized by cells transformed by herpes simplex virus. Virology. 1972 Sep;49(3):683–689. doi: 10.1016/0042-6822(72)90525-9. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Aron G. M., Biswal N., Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973 Mar;52(1):57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- Schaffer P., Vonka V., Lewis R., Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus. Virology. 1970 Dec;42(4):1144–1146. doi: 10.1016/0042-6822(70)90364-8. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent infections induced by herpes simplex viruses. Cancer Res. 1973 Jun;33(6):1399–1401. [PubMed] [Google Scholar]
- Takahashi M., Yamanishi K. Transformation of hamster embryo and human embryo cells by temperature sensitive mutants of herpes simplex virus type 2. Virology. 1974 Sep;61(1):306–311. doi: 10.1016/0042-6822(74)90267-0. [DOI] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Notkins A. L. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neurectomy. Science. 1974 Jun 14;184(4142):1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]


