Abstract
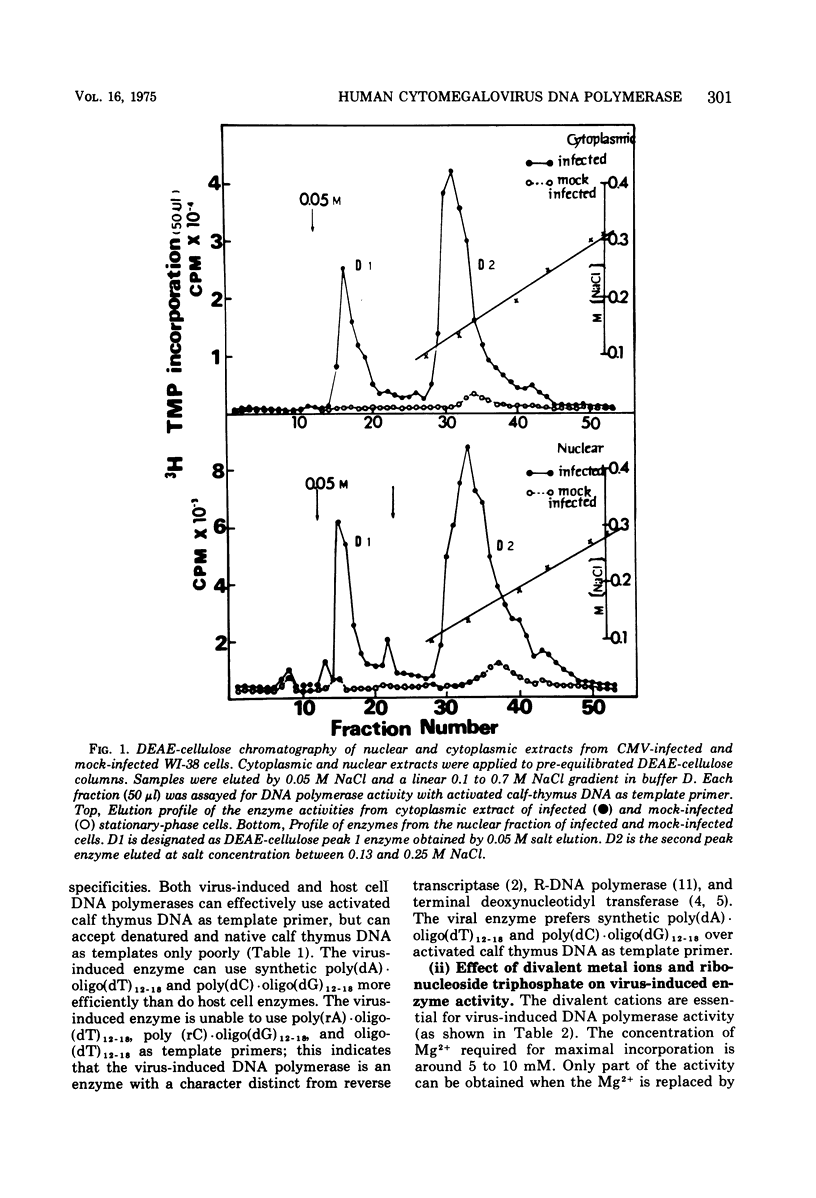
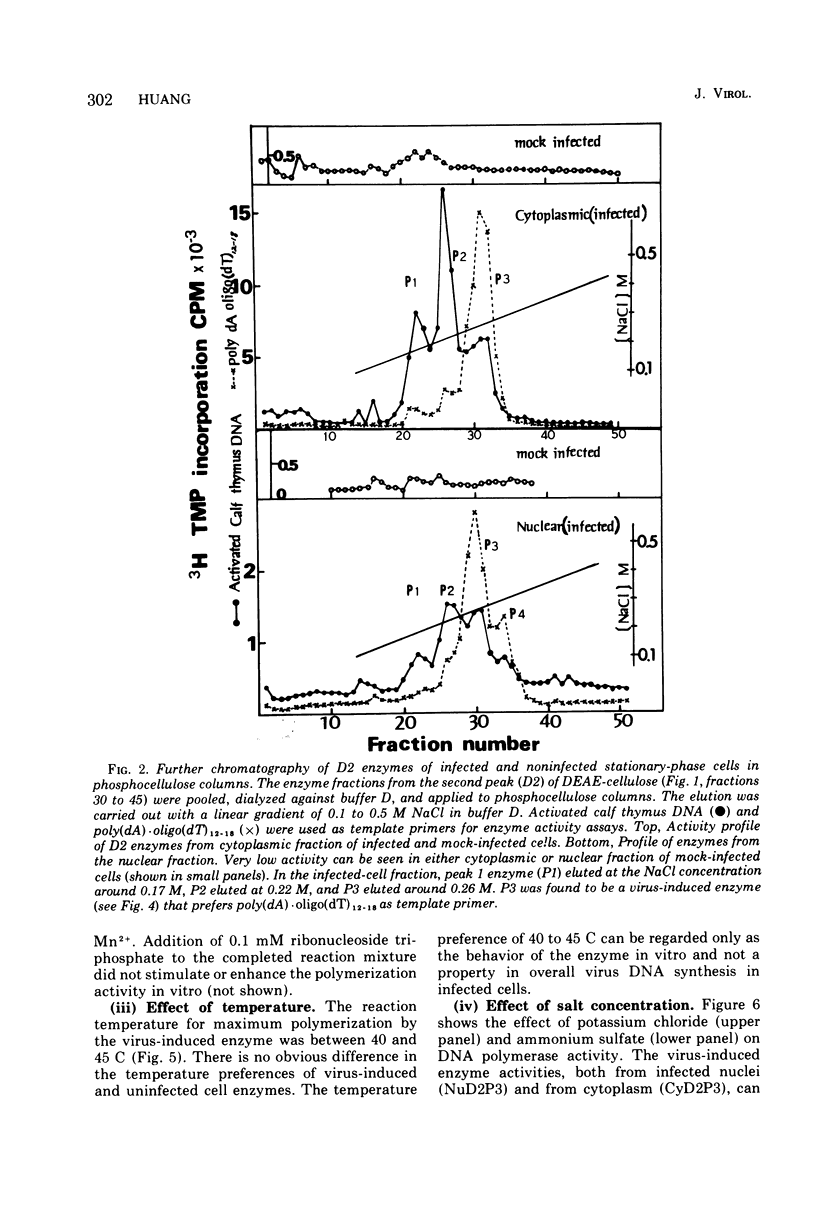
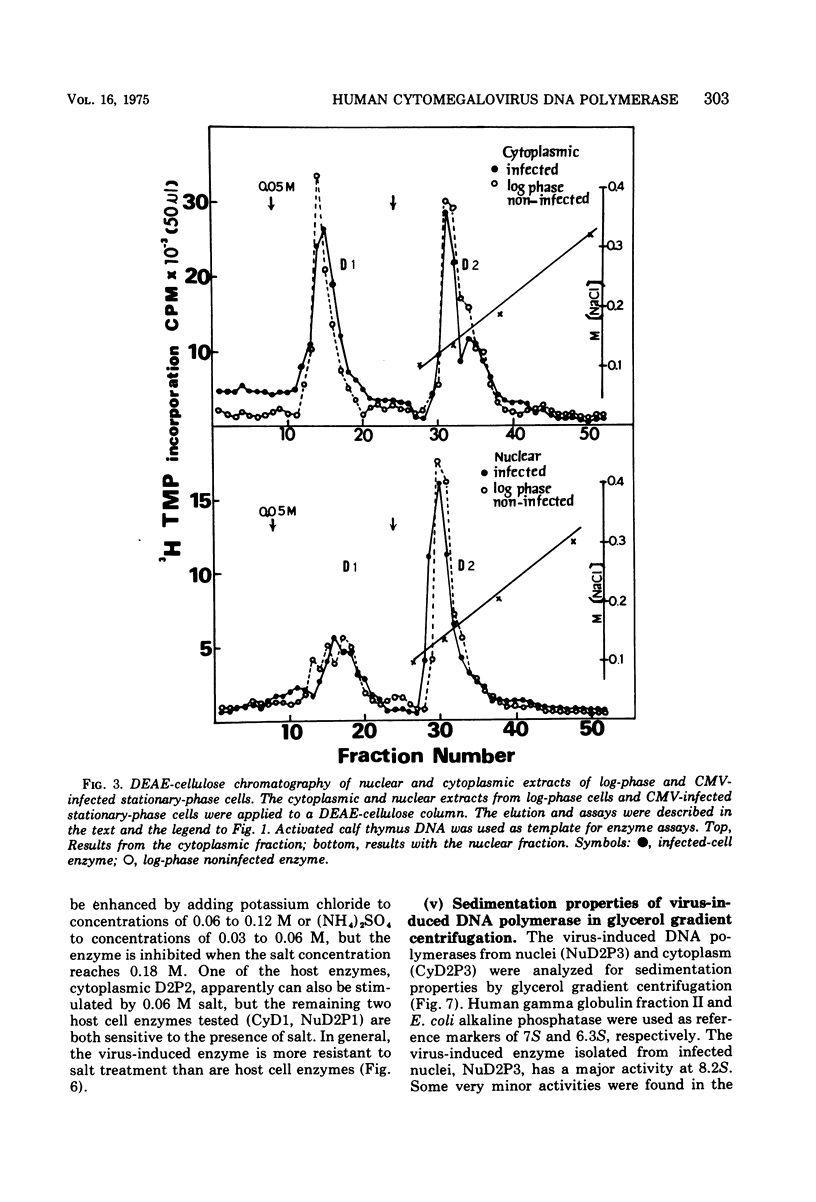
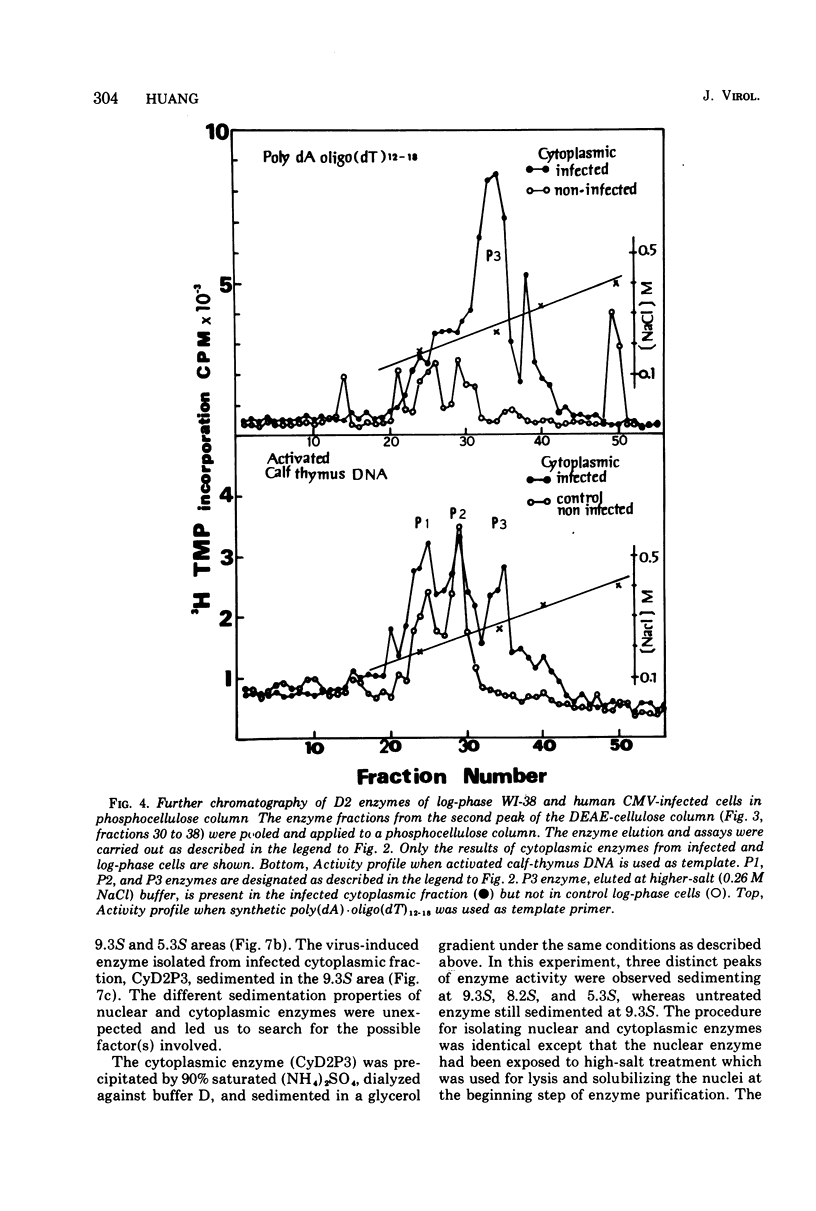
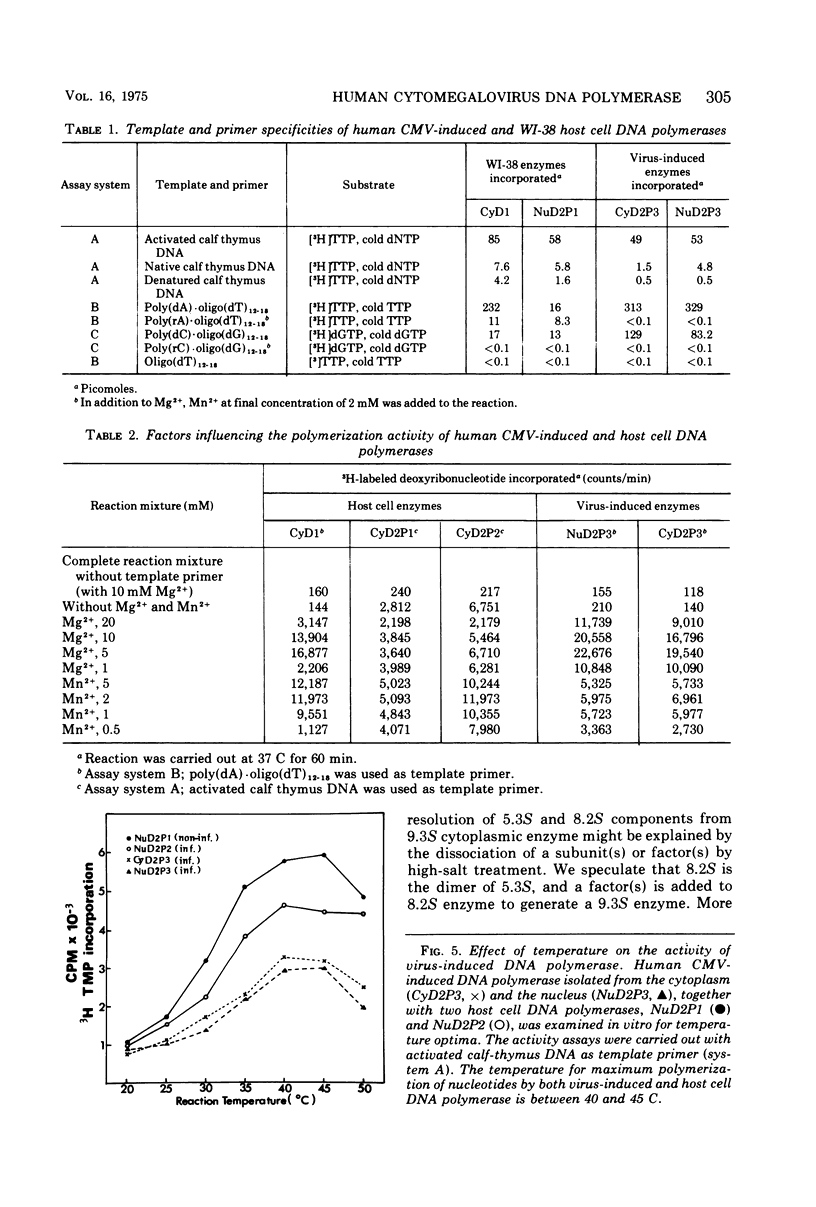
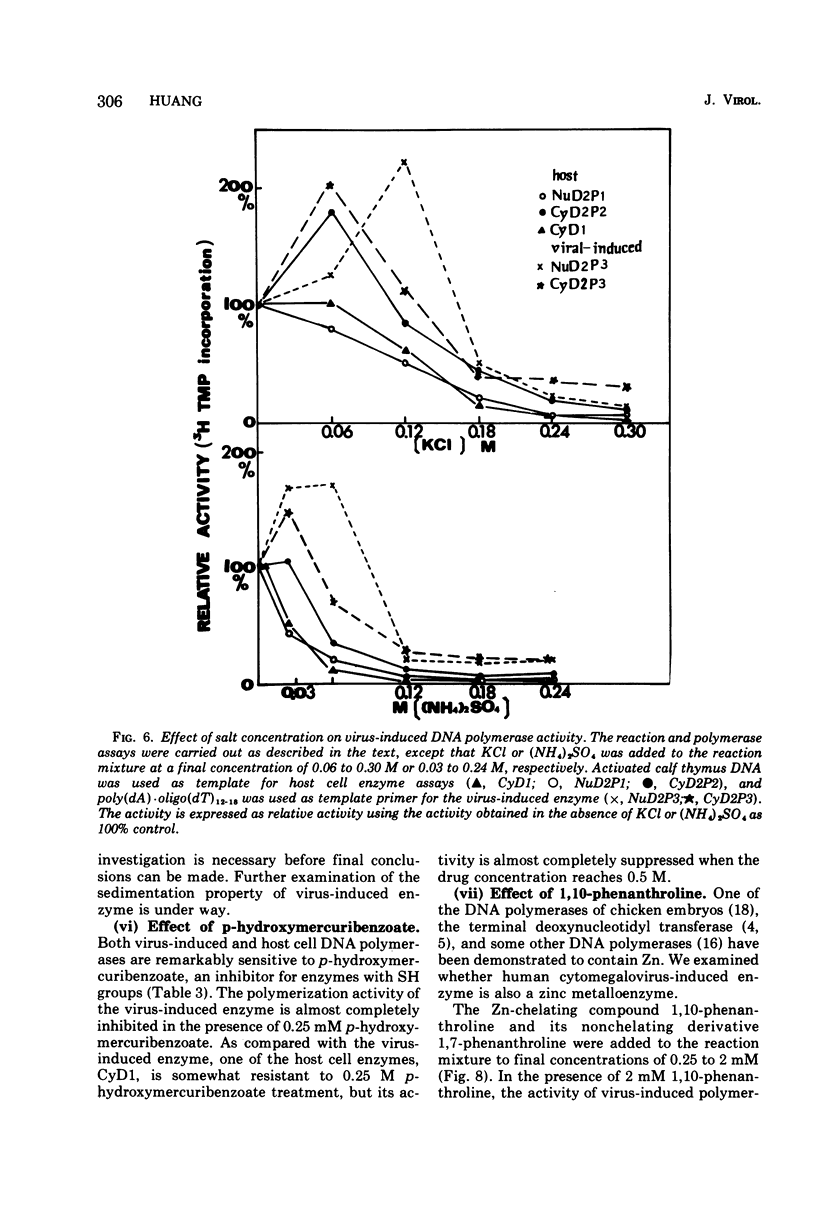
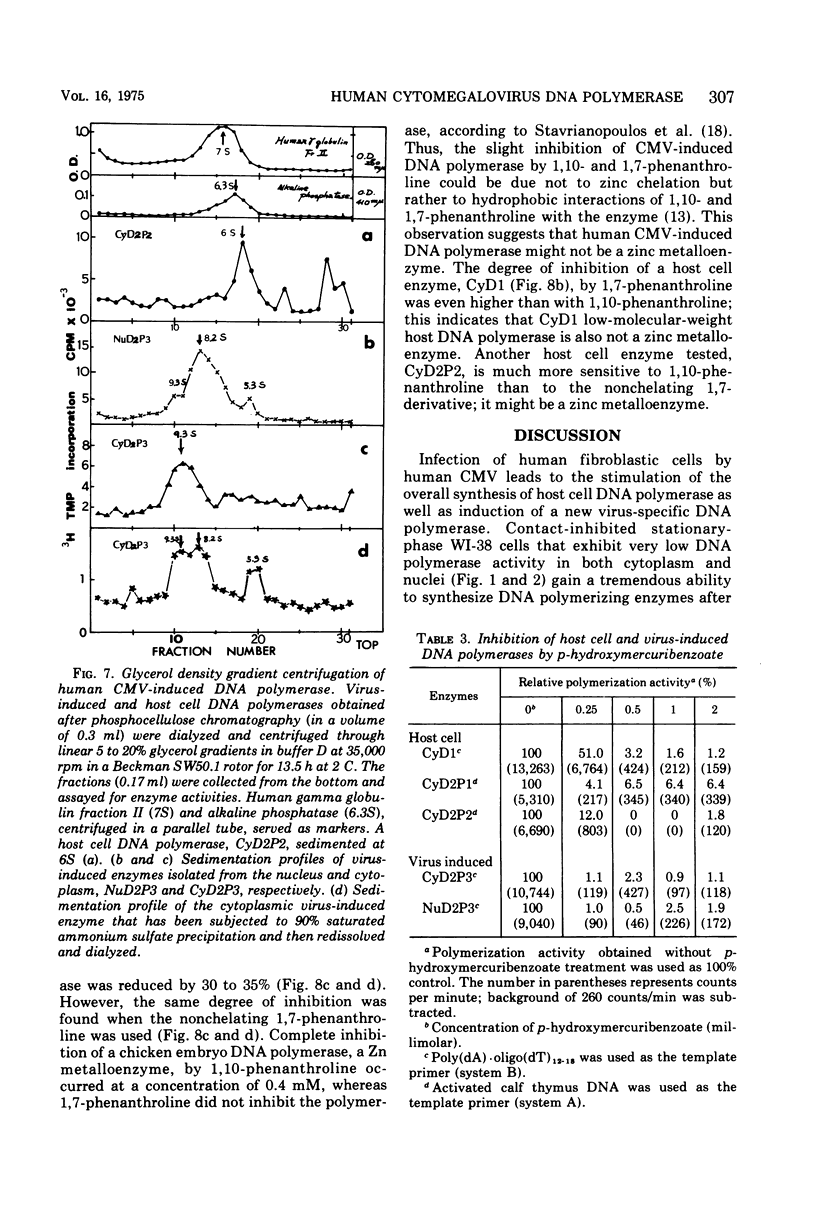
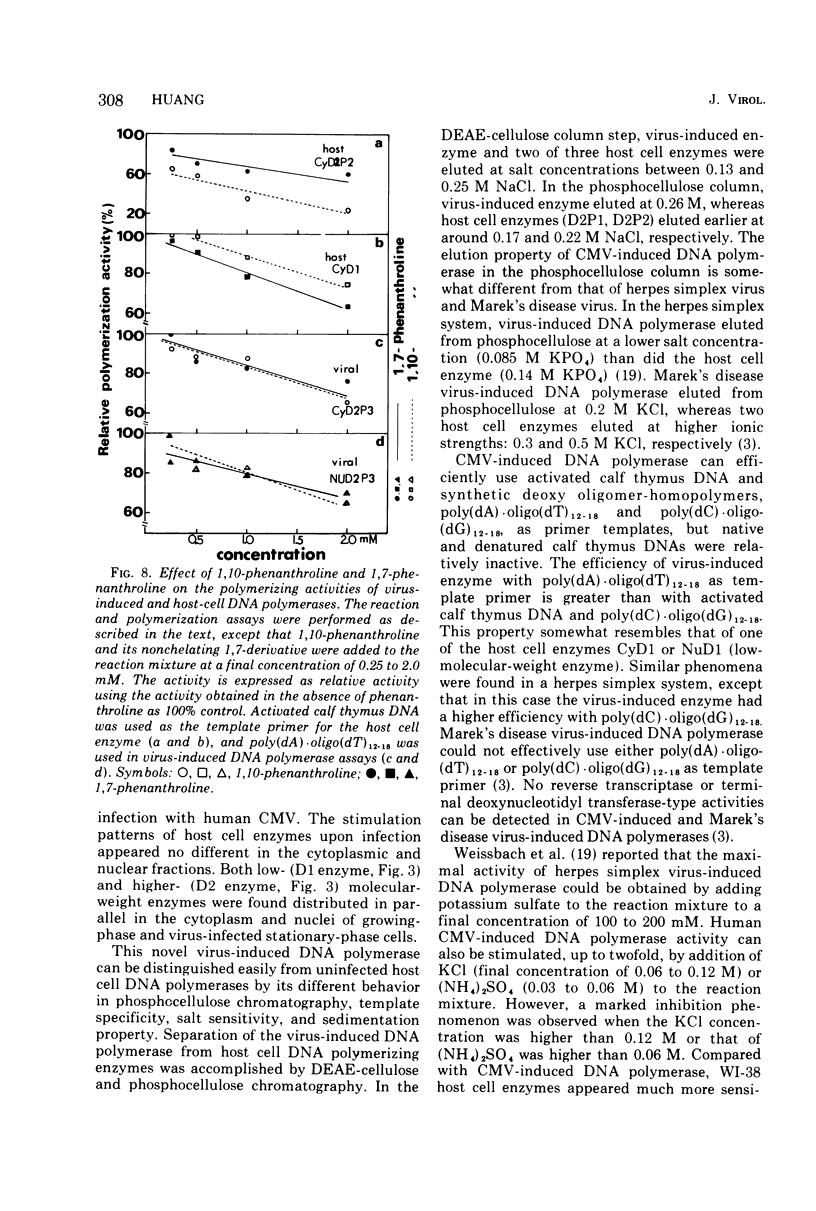
Infection of WI-38 human fibroblasts with human cytomegalovirus (CMV) led to the stimulation of host cell DNA polymerase synthesis and induction of a novel virus-specific DNA polymerase. This cytomegalovirus-induced DNA polymerase was purified and separated from host cell enzymes by DEAE-cellulose and phosphocellulose column chromatographies. It can be distinguished from host cell enzymes by chromatographic behavior, template primer specificity, sedimentation property, and the requirement of salt for maximal activity. This virus-induced enzyme has a sedimentation coefficient of 9.2S and is found in both the nuclei and cytoplasm of virus-infected cells, but not in uninfected cells. This enzyme could efficiently use activated calf-thymus DNA, oly(dA)-oligo(dT)12-18, and poly(dC)-oligo(dG)12-18 as template primers, especially poly(dA)-oligo(dT)12-18, but it could not use poly(rA)-oligo(dT)12-18, poly(rC)-oligo(dG)12-18, or oligo(dT)12-18. The enzyme requires Mg2+ for maximal activity, is sensitive to p-hydroxymercuribenzoate, and is not a zinc metalloenzyme. In addition, the cytomegalovirus-induced DNA polymerase activity can be enhanced by adding 0.06 to 0.12 M NaCl or 0.03 to 0.06 M (NH4)2SO4 to the reaction mixture.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht T., Rapp F. Malignant transformation of hamster embryo fibroblasts following exposure to ultraviolet-irradiated human cytomegalovirus. Virology. 1973 Sep;55(1):53–61. doi: 10.1016/s0042-6822(73)81007-4. [DOI] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Doxynucleotide-polymerizing enzymes of calf thymus gland. IV. Inhibition of terminal deoxynucleotidyl transferase by metal ligands. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1041–1048. doi: 10.1073/pnas.65.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Low molecular weight deoxyribonucleic acid polymerase in mammalian cells. J Biol Chem. 1971 Sep 25;246(18):5835–5837. [PubMed] [Google Scholar]
- Chang L. M., Brown M., Bollum F. J. Induction of DNA polymerase in mouse L cells. J Mol Biol. 1973 Feb 15;74(1):1–8. doi: 10.1016/0022-2836(73)90349-5. [DOI] [PubMed] [Google Scholar]
- Chang L. M. Development of terminal deoxynucleotidyl transferase activity in embryonic calf thymus gland. Biochem Biophys Res Commun. 1971 Jul 2;44(1):124–131. doi: 10.1016/s0006-291x(71)80167-5. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Pagano J. S. Human cytomegalovirus. II. Lack of relatedness to DNA of herpes simples I and II, Epstein-Barr virus, and nonhuman strains of cytomegalovirus. J Virol. 1974 Mar;13(3):642–645. doi: 10.1128/jvi.13.3.642-645.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. F., Boezi J. A., Blakesley R. W., Koenig M., Towle H. C. Marek's disease herpesvirus-induced DNA polymerase. J Virol. 1974 Nov;14(5):1209–1219. doi: 10.1128/jvi.14.5.1209-1219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B. J., Abrell J. W., Smith R. G., Gallo R. C. Human DNA polymerase 3 (R-DNA polymerase): distinction from DNA polymerase I and reverse transcriptase. Science. 1974 Mar 1;183(4127):867–869. doi: 10.1126/science.183.4127.867. [DOI] [PubMed] [Google Scholar]
- Mathewson P. R., Yost F. J., Jr, Harrison J. H. The absence of zinc in the mitochondrial and supernatant forms of malate dehydrogenase. Biochim Biophys Acta. 1973 Oct 10;321(2):413–422. doi: 10.1016/0005-2744(73)90182-4. [DOI] [PubMed] [Google Scholar]
- McCaffrey R., Smoler D. F., Baltimore D. Terminal deoxynucleotidyl transferase in a case of childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1973 Feb;70(2):521–525. doi: 10.1073/pnas.70.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., WATERMAN S., TURNER H. C., HUEBNER R. J. Cytopathogenic agent resembling human salivary gland virus recovered from tissue cultures of human adenoids. Proc Soc Exp Biol Med. 1956 Jun;92(2):418–424. [PubMed] [Google Scholar]
- Rifkind D., Goodman N., Hill R. B., Jr The clinical significance of cytomegalovirus infection in renal transplant recipients. Ann Intern Med. 1967 Jun;66(6):1116–1128. doi: 10.7326/0003-4819-66-6-1116. [DOI] [PubMed] [Google Scholar]
- Slater J. P., Mildvan A. S., Loeb L. A. Zinc in DNA polymerases. Biochem Biophys Res Commun. 1971 Jul 2;44(1):37–43. doi: 10.1016/s0006-291x(71)80155-9. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I. Deoxynucleotide-polymerizing enzymes in normal and malignant human cells. Cancer Res. 1974 May;34(5):1015–1026. [PubMed] [Google Scholar]
- Stavrianopoulos J. G., Karkas J. D., Chargaff E. DNA polymerase of chicken embryo: purification and properties. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1781–1785. doi: 10.1073/pnas.69.7.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]
- Weller T. H. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N Engl J Med. 1971 Jul 22;285(4):203–214. doi: 10.1056/NEJM197107222850406. [DOI] [PubMed] [Google Scholar]


