Abstract
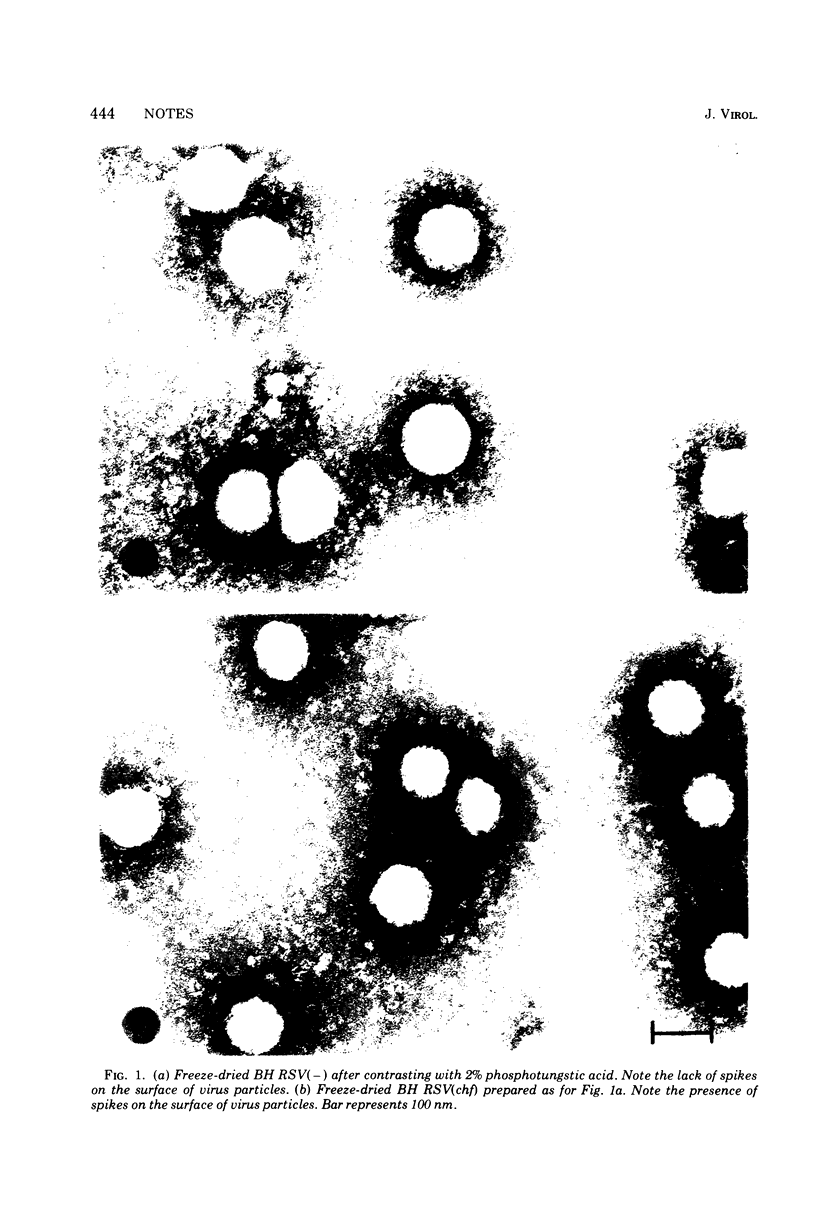
Electron microscopy observations of purified Bryan high-titer Rous sarcoma virus (BH RSV) using the freeze-drying technique showed that progeny made in the absence of a helper virus lacked visible surface projections or spikes. Phenotypic mixing experiments employing BH RSV and a thermolabile mutant of vesicular stomatitis virus, tl 17, yielded no evidence of pseudotype formation. Since tl 17 is known to be defective for an envelope glycoprotein, the lack of successful phenotypic mixing with BH RSV is consistent with the observed absence of viral spikes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolognesi D. P., Bauer H., Gelderblom H., Hüper G. Polypeptides of avian RNA tumor viruses. IV. Components of the viral envelope. Virology. 1972 Mar;47(3):551–566. doi: 10.1016/0042-6822(72)90545-4. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Abortive infection of Japanese quail cells with avian sarcoma viruses. Virology. 1972 Dec;50(3):701–712. doi: 10.1016/0042-6822(72)90424-2. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Inactivation of avian sarcoma viruses with UV light: a difference between helper-dependent and helper-independent strains. Virology. 1971 Feb;43(2):521–523. doi: 10.1016/0042-6822(71)90328-x. [DOI] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. ANALYSIS OF THE DEFECTIVENESS OF ROUS SARCOMA VIRUS, II. SPECIFICATION OF RSV ANTIGENICITY BY HELPER VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:41–48. doi: 10.1073/pnas.51.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. The defectiveness of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:572–580. doi: 10.1073/pnas.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Miyamoto T., Hanafusa T. A cell-associated factor essential for formation of an infectious form of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):314–321. doi: 10.1073/pnas.66.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D. N., Weiss R. A. Pseudotypes of vesicular stomatitis virus determined by exogenous and endogenous avian RNA tumor viruses. Virology. 1974 Jan;57(1):271–278. doi: 10.1016/0042-6822(74)90127-5. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Rifkin D., Compans R. W. Identification of the spike proteins of Rous sarcoma virus. Virology. 1971 Nov;46(2):485–489. doi: 10.1016/0042-6822(71)90049-3. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Electrophoretic analysis of the RNA of avian tumor viruses. Virology. 1972 Dec;50(3):753–764. doi: 10.1016/0042-6822(72)90429-1. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Tozawa H., Bauer H., Graf T., Gelderblom H. Strain-specific antigen of the avian leukosis sarcoma virus group. I. Isolation and immunological characterization. Virology. 1970 Mar;40(3):530–539. doi: 10.1016/0042-6822(70)90196-0. [DOI] [PubMed] [Google Scholar]
- Závada J. Pseudotypes of vesicular stomatitis virus with the coat of murine leukaemia and of avian myeloblastosis viruses. J Gen Virol. 1972 Jun;15(3):183–191. doi: 10.1099/0022-1317-15-3-183. [DOI] [PubMed] [Google Scholar]
- Závada J. VSV pseudotype particles with the coat of avian myeloblastosis virus. Nat New Biol. 1972 Nov 22;240(99):122–124. doi: 10.1038/newbio240122a0. [DOI] [PubMed] [Google Scholar]
- Závada J., Závodská E. Complementation and phenotypic stabilization of vesicular stomatitis virus temperature-sensitive and thermolabile mutants by avian myeloblastosis virus. Intervirology. 1974;2(1):25–32. doi: 10.1159/000149401. [DOI] [PubMed] [Google Scholar]