Abstract
Recently, we have shown that the antiangiogenic pigment epithelium-derived factor (PEDF) can bind the catalytic β-subunit of F1-ATP synthase and inhibit endothelial cell surface ATP synthase activity. This factor can additionally restrict tumor growth, invasion and metastasis, and can directly induce death on several tumor cell types. Active cell surface ATP synthase is also present in certain tumor cells and its ATP product is considered a stimulus for tumor growth. The present study aimed to elucidate the biological implications of the interactions between the extracellular PEDF and tumor cell surface ATP synthase. Incubation of T24 human urinary bladder carcinoma cells in media containing human recombinant PEDF protein for 48–96 h dramatically decreased cell viability in a concentration-dependent fashion as monitored by real-time cell impedance with a microelectronic system, microscopic imaging and biomarkers of live cells. Intact tumor cells exhibited cell surface ATP synthesis activity, which was inhibited by piceatannol, a specific inhibitor of F1/F0-ATP synthase. Immunoblotting revealed that the β subunit of F1-ATP synthase was present in plasma membrane fractions of these cells. Interestingly, pre-incubation of tumor cells with PEDF inhibited the activity of cell surface ATP synthase in a concentration-dependent fashion. The PEDF-derived peptide 34-mer decreased tumor cell viability and inhibited extracellular ATP synthesis to the same extent as full-length PEDF. Moreover, ATP additions attenuated both the PEDF-mediated decrease in tumor cell viability and the inhibition of endothelial cell tube formation. The results lead to conclude that PEDF is a novel inhibitor of tumor cell surface ATP synthase activity that exhibits a cytotoxic effect on tumor cells, and that the structural determinants for these properties are within the peptide region 34-mer of the PEDF polypeptide. The data strongly suggest a role for the interaction between the 34-mer region of PEDF and tumor cell-surface ATP synthase in promoting tumor cell death.
Keywords: PEDF, ATP synthase, tumor cell, cell viability, ATP, ATP synthesis
Introduction
Adenosine-5′-triphosphate (ATP), the main intracellular energy transfer molecule, is also a ubiquitous extracellular signaling molecule. It is present in the tumor interstitium in the hundreds micromolar range, while its extracellular levels remain undetectable in healthy tumor-free tissues (1). This implies that extracellular ATP is crucial as a stimulus for tumor growth. The presence of ATP synthase activity on the surface of endothelial and tumor cells can contribute to extracellular accumulation of the nucleotide. In this regard F1/F0 ATP synthase, traditionally studied in the mitochondria where it generates most cellular ATP, is detected on plasma membranes of several human tumor cell types (2). It is an active enzyme present on the cell surface not only of many tumor cell lines (3), but also normal cells such as endothelial cells (4), adipocytes (5), keratinocytes (6), and hepatic cells (7).
The ecto-F1-ATP synthase complex enzyme can serve as a multi-ligand receptor to regulate surface ATP levels and related to different biological effects, such as metastasis, proliferation and differentiation in tumor and endothelial cells, as well as in lipid metabolism and immune responses in other cells (8–10). Moreover, small inhibitor molecules of the mitochondrial F1/ F0 ATP synthase, such as piceatannol and resveratrol, inhibit endothelial tube formation, migration, and proliferation of endothelial cells, pointing towards a role for ecto-F1-ATP synthase in these processes (11,12). Angiostatin, an antiangiogenic and antitumorigenic protein, is a ligand that binds and inhibits tumor and endothelial cell surface ATP synthase (3,12). There is also evidence for antibodies directed against the catalytic β subunit of F1-ATP synthase that inhibit the activity of the synthase (3) and also bind the surface of tumor lung cells to prevent their proliferation (13). Therefore, targeting of cell surface ATP synthase in clinical cancer therapy is of interest (9).
Recently, we reported that pigment epithelium-derived factor (PEDF), an extracellular protein of 50 kDa, binds purified recombinant yeast F1-ATP synthase and mammalian cell surface ATP synthase β subunit (14). We also showed that PEDF inhibits endothelial cell surface ATP synthase activity. It has been established that PEDF is an antiangiogenic and neurotrophic factor, and has anti-inflammatory, and anti-oxidative properties (15–18). Crawford et al (19) first reported the role of PEDF as an anti-tumor factor. Since then it has been studied in multiple malignancies such as lung, breast, prostate, ovarian and pancreatic carcinomas, melanoma, glioma and osteosarcoma (20). As an anti-tumor agent, PEDF works both directly through pro-differentiation and anti-proliferation, and indirectly through its antiangiogenic and anti-metastatic properties (21,22). Doll et al (23) identified PEDF as a key inhibitor of stromal vasculature and epithelial tissue growth in mouse prostate and pancreas, and showed that exogenous PEDF can induce tumor epithelial apoptosis in vitro and limited in vivo tumor xenograft growth, triggering endothelial apoptosis.
The multifunctional PEDF protein is secreted by most cell types and is present in blood, the interphotoreceptor matrix, vitreous humor, aqueous humor, and cerebrospinal fluid (15). PEDF belongs to the serpin superfamily of proteins that share a common protein conformation (24). Although most members of the serpin superfamily display serine protease inhibition properties, PEDF, as other members (e.g., maspin, ovalbumin) does not have a demonstrable inhibitory activity against proteases. It exerts its diverse functions from the extracellular compartment via interactions with cell surface receptors, some of which have been identified. PEDF binds PEDF-R - a membrane-linked protein with phospholipase activity - (25), laminin receptor (26), cell surface ATP synthase (14), and LRP6 - a Wnt co-receptor - (27). It also has affinity for several extracellular matrix components such as heparin sulfate, collagen and hyaluronan (28–30). Structure-function studies have demonstrated that PEDF does not require the serpin-exposed loop region toward its carboxy-end for antiangiogenic and antitumorigenic activities, and that a region toward its amino-end located at position 44–77 (human sequence) termed 34-mer is sufficient to confer such activities (31–33). In contrast the 44-mer peptide (positions 78–121) lacks these properties but exhibits instead neurotrophic ones (34,35).
The overall aim of this study was to explore the possible association between PEDF and cell surface ATP synthase in tumor cells for mechanistic and structure-function studies. We used highly purified human recombinant PEDF protein and synthetic PEDF-derived peptides to evaluate their effects on bladder tumor (T24) cells. We performed enzymatic cell surface ATP synthase activity and cell viability assays. We also investigated the effects of exogenous extracellular ATP additions on PEDF-mediated biological activities. Our results link PEDF-mediated tumor cell death and cell surface ATP synthase inhibition. We discuss PEDF as a novel inhibitor of cell surface ATP synthase activity in tumorigenesis.
Materials and methods
Proteins and peptides
Human recombinant PEDF was obtained as described by Stratikos et al (36). PEDF protein was purified by cation exchange followed by anion exchange column chromatography. Briefly, cation exchange column chromatography was performed using a POROS S resin connected to a BioCAD 700E perfusion chromatography system, with buffer S (20 mM Na phosphate, pH 6.5, 50 mM NaCl) and elutions were with a linear gradient of 50–500 mM NaCl in buffer S. PEDF-containing fractions were pooled, dialyzed against buffer Q (50 mM Tris-HCl, pH 8.0) and subjected to POROS Q column chromatography in buffer Q and elutions were with a linear gradient of 100–300 mM NaCl in buffer Q. The PEDF eluted from the anion exchange column chromatography in two major peaks. PEDF protein from the second peak (fractions with conductivity values of 21–22 mS) was used in the present studies. Fractions were pooled, concentrated and the buffer exchanged to PBS using ultrafiltration devices (Centricon-30 or Amicon Ultra-30, Millipore). Storage of the final samples was at −80°C. PEDF-derived peptides 34-mer and 44-mer were chemically synthesized and purified (BioSynthesis, Inc.) as described before (34).
Cell lines
Human bladder and prostate carcinoma cell lines T24, DU145 and PC-3 were cultured in RPMI-1640, and human breast cancer MDA-MB-231 cells and the mouse 4T1 mammary carcinoma cell line were cultured in DMEM. For these cell lines, the media were supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (penicillin/streptomycin). Human microvascular endothelial cells immortalized with telomerase (HMVECs) were a generous gift of R. Shao, and were cultured as described previously (37). All cell lines were maintained at 37°C in a humidified incubator with 5% CO2.
Real-time CES assay
Real-time electrical cell impedance, a direct index of cell density, was monitored using the RT-CES™ system from ACEA Biosciences (San Diego, CA) (www.aceabio.com). Cells were seeded in 16-well sensor plates at 6,000 cells per well and cultured in complete medium for 18 h at 37°C in humidified incubator with 5% CO2. Cells were then washed with PBS and continued to be incubated in serum-free medium for 8 h. Then, PEDF was added to a final concentration of 0, 1, 10 or 100 nM. Cell impedance was monitored every hour for 96 h for each well via automatic calculation of cell index, a measure of cell impedance. Real-time data were acquired by the RT-CES software. Data from two replicates were averaged. The IC50 was calculated using the ACEA software.
Cell viability assays
Cell viability was measured by determining the relative levels of two biomarkers for live cells: intracellular ATP and mitochondrial dehydrogenase activity. The first determination was performed in duplicates upon completion of the real-time CES assay using a luciferase-coupled ATP quantification assay kit (CellTiter-Glo, Promega, Madison, WI) following manufacturer’s instructions. After 30 min of incubation at room temperature, the incubation solution in each well was transferred into a 96-well microtiter plate. Luminescence intensity in each well was measured using an Envision automated plate reader (Perkin Elmer, MA). The second determination was performed in parallel experiments using the Cell Counting kit-8 system (CCK-8, Dojindo, MD), following the manufacturer’s instructions. Cells were plated in triplicate in 24-well plates and grown to 90% confluency before they were treated with PEDF as above. Cells in each well were incubated with 50 μl of CCK-8 solution diluted 1:25 and incubated for 4 h at 37°C. Absorbance of each well was measured at 450 nm using the Envision automated plate reader. In all the cases, the absorbance reading for background was subtracted from the readings of samples. Data from replicates were averaged and statistical analysis was performed by a t-test. A p-value of <0.05 was taken as significant.
Membrane protein extraction
Confluent cells (90%) were harvested and separation of cytosolic and membrane fractions was obtained by differential ultracentrifugation at 80,000 × g. Membrane protein extraction was performed in phosphate buffer containing detergent (0.5% CHAPS) as described previously (14). Protein concentration was determined with Protein BCA assay (Pierce).
Immunoblot
Protein samples were resolved using NuPAGE 4–12% polyacrylamide gel in Bis-Tris buffer with NuPAGE MOPS-SDS as running buffer (Invitrogen). Pre-stained markers were from Bio-Rad (cat. no. 161–0305). After electrophoresis, proteins from the gel were transferred onto a nitrocellulose membrane using the iBlot Gel Transfer system (Invitrogen). Membranes were blocked with 1% BSA in Tris buffered saline containing 0.1% Tween-20 (TBS-T) for 1 h at room temperature. Primary antibodies were anti-F1-ATP synthase β-subunit diluted at 1:4,000 (Invitrogen, cat. no. A21351), anti-PEDF-R diluted at 1:500 (R&D Systems, cat. no. AF5365), or anti-Na+/K+ATPase diluted at 1:10,000 (Upstate, cat. no. 05369) in 1% BSA/TBS-T. Secondary antibodies were HRP-conjugated goat anti-mouse IgG diluted at 1:100,000 (Invitrogen), donkey anti-sheep IgG diluted at 1:20,000 (Sigma, St. Louis, MO) or goat anti-mouse IgG diluted at 1:2000, respectively, in 1% BSA/TBS-T. Washes between primary and secondary antibody incubations were 10 min each and 3 times with TBS-T. For immunodetection, SuperSignal West Dura Extended Duration Substrate (Pierce) was used following the manufacturer’s protocol. The membrane was then exposed to an X-ray film to visualize the chemiluminescence signal and the film was subsequently scanned on a flat base scanner to obtain digitalized images.
Cell surface ATP synthesis assay
The activity of cell surface ATP synthase was assessed using a method modified from Chi and Pizzo (3). Cells were seeded into 24-well plates and cultured to 80–90% confluency for analysis. Cells were washed with PBS, and incubated in 400 μl of fresh medium at room temperature for about 30 min. Then they were pre-incubated in triplicate with different concentrations of PEDF (0, 1, 10, 100 nM) for various times up to 48 h, before the addition of 100 μM ADP as substrate to start the reactions. Incubations were maintained at room temperature for the indicated periods of time (5, 15, 30, 60, or 120 sec). The inorganic phosphate required as second substrate for the ATP synthesis reaction was provided by the medium, which served as reaction buffer. Control reactions without inorganic phosphate were performed following the methods described (11). Piceatannol (Sigma), a specific inhibitor of F1/F0 ATP synthase, was used as a control inhibitor. An aliquot (100 μl) of the media was removed and subjected to centrifugation to separate cells and debris. A total of 50 μl of this solution was then assayed for the presence of ATP using the CellTiterGlo luminescence assay (Promega) and the Envision automated plate reader (Perkin Elmer). The intracellular pools of ATP were also analyzed in a similar manner, and did not change with treatments. Each assay point was averaged from triplicates. The ATP synthesized was calculated as percentage relative to that of controls without PEDF.
Tube formation assay
Endothelial tube formation by HMVECs was assessed using BD BioCoat™ Angiogenesis System- Endothelial Cell Tube Formation in vitro assay system (BD Biosciences, San Jose, CA, USA). Briefly, a 96-well plate coated with 50 μl of Matrigel per well was thawed overnight at 4°C and allowed to solidify at 37°C for 30 min. HMVECs (105 cells per well) were seeded into the wells and treated with effectors (ATP ranging between 5–500 nM) or inhibitors (PEDF ranging between 0.1–100 nM, or 2 μM piceatannol) in serum-free medium incubated at 37°C for 24 h. Endothelial tube formation in the wells was evaluated using an inverted microscope (Nikon Eclipse 2000) in brightfield. Fields in wells were photographed using a 4X objective in high resolution, and composites of different fields were made to include the whole wells using Adobe Photoshop. The area or pixels of the tubes were quantified from the digitalized images using NIH image J program (http://imagej.nih.gov/ij/) (38).
Results
PEDF decreases tumor cell viability
To examine the effects of PEDF on tumor cells, T24 cell cultures were incubated with serum-free medium containing increasing concentrations of human recombinant PEDF protein, and monitored, in parallel, in real-time for cell impedance using a microelectronic system and under a microscope. Cells treated without PEDF increased the electrical impedance represented as cell index value with time up to 100 h. However, the cell index decreased in those treated with PEDF in a concentration-dependent fashion with an estimated half maximal inhibition of 5.3 nM PEDF (Fig. 1A). These results suggested that PEDF treatment lowered the cell number. Examination under the microscope showed a decline in cell number with PEDF additions (Fig. 1B). Quantification of relative cell numbers using two different biomarkers for live cells, intracellular ATP content and mitochondrial dehydrogenase activity, corroborated the observation that PEDF decreased the viability of T24 cells (Fig. 1C). Similar results were obtained with human prostate cancer cell lines DU-145 and PC-3, and breast cancer cell lines MDA-MB-231 (human) and 4T1 (mouse) (data not shown). These results demonstrated a direct negative effect of PEDF on tumor cell viability.
Figure 1.
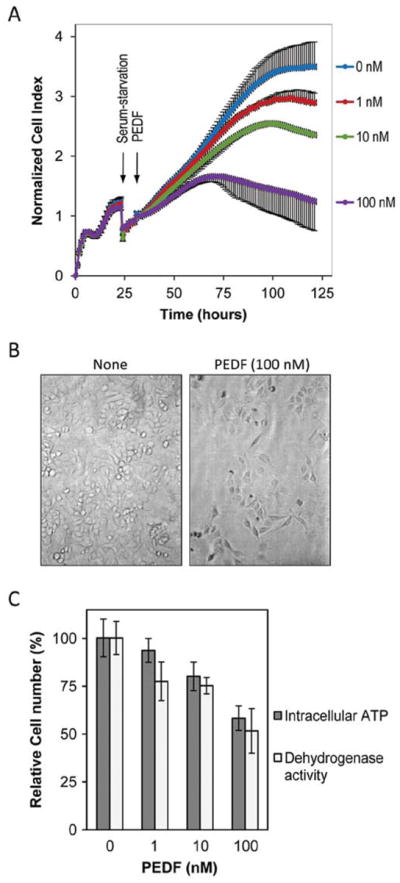
Effects of PEDF on viability of T24 cells in culture. T24 cells were cultured in complete media to subconfluency and then media were replaced with fresh media without serum containing indicated concentrations of recombinant human PEDF protein. Two sets of cultures were performed in parallel: one for cell impedance monitoring, and another for examining cells under the microscope. At end point (120 h) cell viability was determined. (A) Cell proliferation determination by real-time cell impedance monitoring of cells in media containing the indicated concentrations of PEDF. Cell index was normalized relative to the cell numbers at the time of PEDF addition. (B) Brightfield images of cells cultured in media without and with 100 nM PEDF at the 120-h end point. (C) Effect of PEDF on cell viability at the treatment end point. Plot of relative cell numbers quantified based on intracellular ATP content and mitochondrial dehydrogenase activity as biomarkers of live cells. Cell viability was expressed as percentage of luminescence values relative to untreated controls, and percentage of absorbance at 490 nm relative to untreated controls, respectively. Each point is the average of triplicate assays. Error bars indicate average ± SD.
Tumor cell surface exhibits ATP synthase activity
Cell surface ATP synthase activity assays of tumor cells showed a dramatic increase in extracellular ATP production within the first seconds after the reaction had started. Fig. 2A shows that the activity of T24 cells rose and peaked by 5 sec and then decreased slightly, but lasting for 60 sec. Similar observations were obtained with PC-3, 4T1 and MDA-MB-231 cell lines. Treatments of T24 cells with piceatannol, a specific inhibitor of the F1/F0 ATP synthase, decreased the cell surface ATP synthesis activity in a dose-dependent fashion with an estimated half-maximal inhibition concentration of ~0.02 μM piceatannol (Fig. 2B). However, the intracellular ATP levels remained constant throughout the preincubation and reaction time (M.D., unpublished data). These observations suggested that a piceatannol-sensitive ATP synthase was present in plasma membranes of these cells. Immunoblotting showed that plasma membrane extracts from T24 cells contained the β subunit of F1/F0 ATP synthase (Fig. 2C). Similar results were obtained with DU-145, PC-3, MDA-MB-231 and 4T1 cell lines. These results provide evidence for the presence of the β subunit of ATP synthase and an active ATP synthase in the surface of the tumor cells tested.
Figure 2.
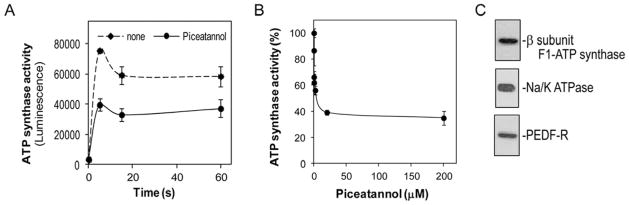
Cell surface ATP synthase in bladder tumor cells. (A) Activity of cell surface ATP synthase was assessed in intact T24 cells by measuring the extracellular ATP synthesized after addition of ADP substrate in the presence of inorganic phosphate. Cells were preincubated with piceatannol (20 μM) or medium alone (none) for 30 min before starting the reactions. ATP production is shown as a function of reaction time. Each point is the average of triplicate assays. Error bars indicate average ± SD. (B) Cell surface ATP synthase activity in T24 cells treated with various concentrations of piceatannol was measured 20 sec after starting the reaction, and was plotted as percentage of extracellular ATP production relative to controls without piceatannol. Each point is the average of triplicate assays. Error bars indicate average ± SD. (C) Immunoblot of F1-ATP synthase β subunit in T24 cell extracts enriched in plasma membrane proteins. A total of 6 μg of protein was loaded to the gel. Immunostaining with antibodies against the β subunit F1-ATP synthase, PEDF-R and Na/K ATPase (a plasma membrane marker) probed sequentially on the same blot is shown.
PEDF inhibited tumor cell surface ATP synthesis
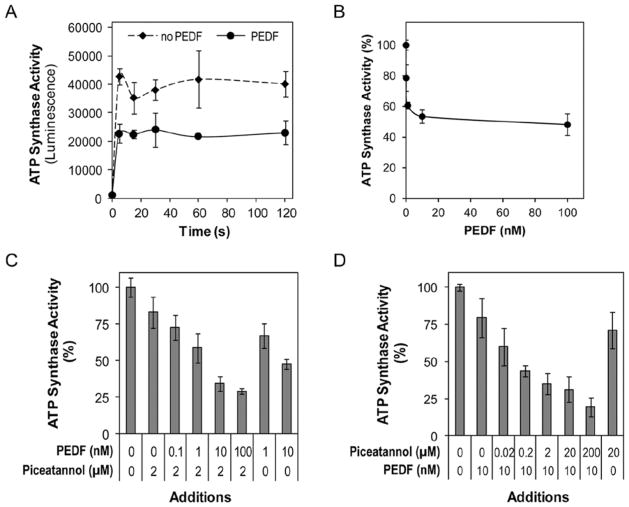
To determine the effects of PEDF on tumor cell surface ATP synthesis, T24 cells were preincubated with 100 nM PEDF for 5 min, 30 min, 1 h, 24 h or 48 h at 25°C to allow interactions with cell surface ATP synthase. The reaction was started by addition of 100 μM ADP in serum-free medium in the presence of PEDF and incubations were at 25°C for varying periods of time (5, 15, 30, 60 and 120 sec) before assaying for the ATP product. Preincubation of tumor cells with PEDF as short as 5 min inhibited the activity of cell surface ATP synthase. Fig. 3A shows a time curve of inhibition of ATP synthase with 100 nM PEDF and 30 min of preincubation. In another set of experiments, preincubations of 30 min and reactions in the presence of PEDF at 0.01, 0.1, 1, 10, and 100 nM were performed (Fig. 3B). All treatment conditions with PEDF led to a decrease in the extracellular ATP synthesis in a concentration-dependent fashion with a half maximal inhibition of ~1 nM PEDF. The intracellular ATP levels remained constant in all the experiments (M.D., unpublished data). Similar experiments performed with PC-3 and 4T1 cell lines led to the same conclusions. In a third set of experiments, cells were treated with combinations of PEDF and piceatannol. In the presence of 2 μM piceatannol and increasing concentrations of PEDF tumor cells surface ATP synthase decreased in a concentration-dependent fashion (Fig. 3C). In the presence of 10 nM PEDF and increasing concentrations of piceatannol, the inhibition was also observed (Fig. 3D). Although even in the presence of the highest concentration of PEDF and piceatannol tested the cell surface ATP synthase activity was not abolished completely, the results indicated that PEDF and piceatannol added together were more potent than either of them added alone.
Figure 3.
Effects of PEDF on extracellular ATP synthesis by intact T24 cells. (A) Activity of cell surface ATP synthase was assessed by measuring the amount of extracellular ATP synthesized after the addition of ADP in the presence of inorganic phosphate. Cells were preincubated with PEDF (100 nM) or medium alone (no PEDF) for 30 min before the addition of ADP to start the reaction. Extracellular ATP formation is shown as a function of time. (B) Concentration curve for inhibition of cell surface ATP synthase activity by PEDF. Cells were pre-incubated for 1 h with increasing concentrations of PEDF (as indicated) and then the reaction was started by addition of ADP to the medium followed by incubation at 25°C for 15 sec. Percentage of ATP production relative to controls (no PEDF) are shown. (C) Effects of combinations of piceatannol (2 μM) and increasing concentrations of PEDF on cell surface ATP synthase activity of T24 cells. Preincubations of cells with the indicated inhibitors were for 60 min and the incubations of the reactions after additions of substrate were for 20 sec. (D) Effects of combinations of PEDF (10 nM) and increasing concentrations of piceatannol on cell surface ATP synthase activity of T24 cells. Preincubations of cells with the indicated inhibitors were for 60 min and the reactions after additions of substrate were for 20 sec. Percentage of ATP production relative to controls without inhibitors is shown. Each point is the average of triplicate assays. Error bars indicate average ± SD.
The antiangiogenic PEDF-derived peptide 34-mer decreases tumor cell viability and cell surface ATP synthase activity
A synthetic 34-mer peptide, derived from human PEDF amino acids at positions Asp44-Asn77 has antiangiogenic activity (31–33), whereas another synthetic 44-mer peptide (positions Val78-Thr121) exhibits neuronal differentiating and neuroprotective activities of the full length PEDF (34,35), but is not antiangiogenic. To explore the effect of these PEDF peptide regions on tumor cells, viability assays were performed testing varying concentrations of 34-mer and 44-mer peptides ranging between 1–100 nM. Real-time cell impedance monitoring showed that the 34-mer peptide decreased the cell index while the 44-mer peptide did not, relative to control cells without peptides (M.D., unpublished data). Viability assays performed after 5 days of treatment showed that only the 34-mer peptide significantly lowered tumor cell numbers (Fig. 4A). Similar results were observed when experiments were performed with MDA-MB-231 cell lines.
Figure 4.
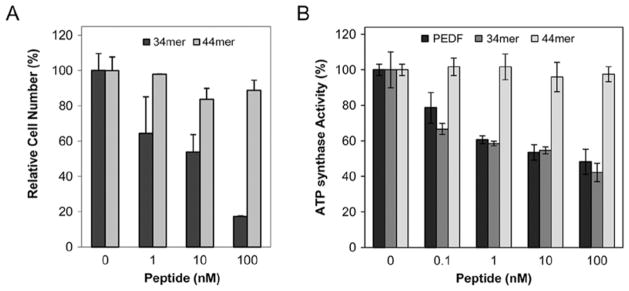
Effects of PEDF-derived 34-mer and 44-mer peptides on T24 cell number. (A) Cells were treated with peptides as indicated for 5 days and the cell numbers were quantified using intracellular ATP as biomarker. Cell viability was expressed as percentage of luminescence values relative to untreated controls. Each point is the average of triplicate assays. Error bars indicate average ± SD. (B) Effects of PEDF, 34-mer and 44-mer peptides on extracellular ATP synthesis by T24 cells. Assays were with 60 min preincubation and 15 sec of reaction incubation time. Percentage of ATP production relative to controls are shown. Each point is the average of triplicate assays. Error bars indicate average ± SD.
The effect of the 34-mer and 44-mer peptides on the activity of tumor cell surface ATP synthase was also tested. Cells were treated with increasing concentrations ranging between 0.1 and 100 nM of either peptide, and then extracellular ATP synthesis assays were performed. Similar experiments were performed using full-length PEDF protein as a control. Interestingly, the 34-mer was highly potent in attenuating the cell surface ATP synthase activity similar to PEDF (Fig. 4B), and picetannol (20 μM), whereas the 44-mer did not have a significant effect (Fig. 4B). Similar results were obtained when experiments were performed with PC-3, MDA-MB-231, and 4T1 cell lines. These results demonstrated that the PEDF-derived peptide 34-mer decreased cell viability and extracellular ATP synthesis in intact tumor cells, in the same way as the full length PEDF protein.
Exogenous ATP addition reverses the effects of PEDF on tumor cells
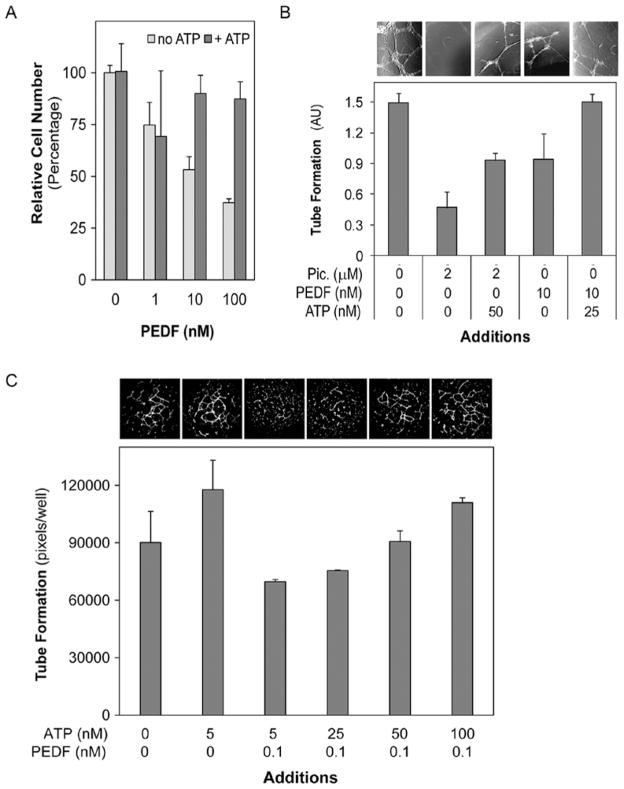
The above results suggested that tumor cell death by PEDF was associated with the inhibition of tumor cell surface ATP synthesis, which in turn decreases extracellular ATP levels. We proposed that exogenous additions of ATP restoring the depleted product may reverse the biological effects mediated by PEDF. The effects of exogenous ATP addition were explored using two biological assays previously used to study PEDF functions. A reversal effect of exogenous additions of ATP was observed in PEDF-mediated tumor cell death. Fig. 5A shows that 100 nM ATP additions reversed the effects of PEDF on attenuating tumor cell viability. Endothelial tube formation assays were also performed to test the role of ATP supplementation on PEDF-induced antiangiogenesis (Fig. 5B and C). Control cells showed a network of tubes after 24 h, and while the number of tubes in cells treated with 2 μM piceatannol or 10 nM PEDF decreased, cells treated in the same way but supplemented with exogenous ATP had higher numbers of tube networks than their unsupplemented counterparts (Fig. 5B). Endothelial tube formation in cultures treated with 0.1 nM PEDF increased in a dose-dependent fashion with additions of ATP between 5–100 nM (Fig. 5C). These results show that exogenous additions of ATP reversed the biological effects of PEDF on tumor and endothelial cells.
Figure 5.
Effects of supplementation of ATP on PEDF-mediated biological activities. (A) T24 cells were treated with PEDF (0–100 nM) without and with 100 nM ATP. Cell numbers were determined by cell viability assays and expressed as percentage relative to untreated controls. Each point is the average of duplicate assays. (B and C) Endothelial tube formation of HMVECs was followed in the presence of cell surface ATP synthase inhibitors PEDF or piceatannol, with or without ATP supplementation. Representative brightfield images of endothelial tube formation and plots of tube formation quantification are shown. Images and bars are aligned per treatment with corresponding additions of inhibitors and ATP indicated at the X-axis. Treatments were performed in triplicate. In (B) HMVECs tube formation was performed in the presence of 2 μM piceatannol (Pic.), 10 nM PEDF, and/or ATP (25–50 nM). Quantification was performed from photographs of selected fields from each well and the average area of tubes formed per treatment is shown in arbitrary area units. In (C) HMVECs tube formation was performed in the presence of 0.1 nM PEDF and increasing concentrations of exogenously added ATP (5–100 nM). Quantification of the tube formation was performed from photographs of the entire well. Plot of average of triplicates ± SD per treatment are shown.
Discussion
This study shows that PEDF is a novel inhibitor of tumor cell surface ATP synthesis that exhibits a cytotoxic effect on tumor cells. It also shows that a region (34-mer) within the PEDF polypeptide contains structural determinants for these properties. These conclusions are based on the negative effect on tumor cell viability and the inhibition of extracellular ATP synthase by exogenous additions of human recombinant PEDF protein and the 34-mer peptide. Furthermore, the PEDF-mediated inhibition of endothelial tube formation and decreased tumor cell viability are reversed by the addition of ATP, implying an inverse association between PEDF and extracellular levels of ATP. The fact that PEDF has affinity for the α and β subunits of F1-ATP synthase (14) and that these subunits are found in tumor cell surfaces, point to the role of PEDF as a ligand of the ecto-ATP synthase. Given that PEDF binds to several cell surface molecules, including heparan sulfate proteoglycans (28), PEDF-R (25), laminin receptor (26), cell surface ATP synthase (14), and LRP6 (27), the degree to which its effects on tumor cells are due to binding ATP synthase has been unclear. However, the present data provide evidence for a role for the interaction between the 34-mer region of the PEDF protein and tumor cell surface ATP synthase to regulate extracellular levels of ATP and in promoting anti-tumorigenic activity.
The role of F1-ATP synthase activity in extracellular ATP production was examined. When inorganic phosphate was omitted in the reactions, extracellular ATP synthesis was not observed with T24 cells (M.D., unpublished data) or endothelial cells (14), discarding the possibility that the ecto-adenylate kinase activity described before in endothelial cells (39) may be responsible for the observed ATP formation. The fact that piceatannol, a specific inhibitor of F1F0-ATP synthase activity, inhibited the extracellular ATP synthesis in intact tumor cells (Fig. 2) and in endothelial cells (14) points to the enzyme as responsible for this activity. Thus, the F1-ATP synthase activity could account for the observed extracellular ATP synthesis in tumor cells.
We compared PEDF to agents that bind and inhibit the ATP synthase and also are cytotoxic to tumor cells. Several small inhibitors of ATP synthase have demonstrable cytotoxic effects on tumor cells. For example, piceatannol, resveratrol and efrapeptins reduce tumor and endothelial cell proliferation rate (11,40,41), and inhibit surface ecto-ATP synthase activity, without affecting the intracellular ATP levels (11). Aurovertin B, an ATP synthase inhibitor that binds the β subunit of F1, inhibits strongly the proliferation of several breast cancer cell lines, but has little influence on normal cell lines (42,43). Piceatannol, resveratrol and aurovertins bind to a hydrophobic pocket between the γ subunit and the catalytic β subunit of F1 (44).
Another group of inhibitors are the α-helical basic peptide inhibitors that bind to F1 and block ATP hydrolysis, e.g., bacterial/chloroplast ε subunit, inhibitor F1 (IF1) and melittin (44). These inhibitors include α-helical structures containing basic residues, which appear to be crucial for their inhibitory activities through interactions with an acidic region containing the β-DELSEED motif in the catalytic β subunit of F1. In this regard, the 34-mer peptide contains the complete α-helix A of the 3-D structure of the PEDF protein, and has a theoretical pI of 9.7. This suggests that the 34-mer shares characteristics with α-helical basic peptide inhibitors for binding the β subunit of F1. Angiostatin and antibodies directed against the catalytic β-subunit of the F1 complex of ATP synthase induce endothelial and tumor cell death, and they bind the β subunit of F1 and inhibit cell surface ATP synthase (3,45). Of note, competition between PEDF and angiostatin for binding isolated yeast F1 revealed that the two antiangiogenic/antitumorigenic factors share binding sites on F1 (14). These observations lead us to conclude that blocking of the extracellular ATP synthase activity in tumor cells is likely a result of direct electrostatic interactions between the basic residues in the αhelix A of PEDF and the β-DELSEED motif of β subunit of the ecto-F1-ATP synthase.
Our results have biological implications. The interactions of extracellular PEDF ligands with cell surface F1F0-ATP synthase molecules regulate the levels of ATP, which in turn may affect the behavior of tumor cells, e.g., PEDF may interfere with ATP:P2X receptor-mediated signaling pathways by regulating the availability of the ATP ligands. In this way, blocking the ATP synthase activity by targeting the F1 catalytic domain with PEDF can trigger signal transduction to mediate tumor cell death, and inhibit endothelial tube formation necessary for tumorigenesis and angiogenesis. In summary, the features described here make cell surface ATP synthase an excellent candidate for mediating the anti-tumorigenic activity of PEDF. These findings imply that PEDF may act as a ligand of cell surface F1F0-ATP synthase on the surface of tumor cells, and that it behaves as an inhibitor of its extra-mitochondrial ATP synthesis activity. The PEDF:ATP synthase interaction may be a critical biochemical step for the anti-tumorigenic effects exerted by PEDF on different tumor types.
Acknowledgments
This work was supported in part by National Institutes of Health NEI Intramural Research Program and by the National Cancer Institute Grant no. CA134727 (to V.N.).
References
- 1.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PloS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das B, Mondragon MO, Sadeghian M, Hatcher VB, Norin AJ. A novel ligand in lymphocyte-mediated cytotoxicity: expression of the beta subunit of H+ transporting ATP synthase on the surface of tumor cell lines. J Exp Med. 1994;180:273–281. doi: 10.1084/jem.180.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi SL, Pizzo SV. Angiostatin is directly cytotoxic to tumor cells at low extracellular pH: a mechanism dependent on cell surface-associated ATP synthase. Cancer Res. 2006;66:875–882. doi: 10.1158/0008-5472.CAN-05-2806. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto K, Shimizu N, Obi S, et al. Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1646–H1653. doi: 10.1152/ajpheart.01385.2006. [DOI] [PubMed] [Google Scholar]
- 5.Kim BW, Choo HJ, Lee JW, Kim JH, Ko YG. Extracellular ATP is generated by ATP synthase complex in adipocyte lipid rafts. Exp Mol Med. 2004;36:476–485. doi: 10.1038/emm.2004.60. [DOI] [PubMed] [Google Scholar]
- 6.Burrell HE, Wlodarski B, Foster BJ, et al. Human keratinocytes release ATP and utilize three mechanisms for nucleotide interconversion at the cell surface. J Biol Chem. 2005;280:29667–29676. doi: 10.1074/jbc.M505381200. [DOI] [PubMed] [Google Scholar]
- 7.Martinez LO, Jacquet S, Esteve JP, et al. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75–79. doi: 10.1038/nature01250. [DOI] [PubMed] [Google Scholar]
- 8.Champagne E, Martinez LO, Collet X, Barbaras R. Ecto-F1Fo ATP synthase/F1 ATPase: metabolic and immunological functions. Curr Opin Lipidol. 2006;17:279–284. doi: 10.1097/01.mol.0000226120.27931.76. [DOI] [PubMed] [Google Scholar]
- 9.Mowery YM, Pizzo SV. Targeting cell surface F1F0 ATP synthase in cancer therapy. Cancer Biol Ther. 2008;7:1836–1838. doi: 10.4161/cbt.7.11.7155. [DOI] [PubMed] [Google Scholar]
- 10.Vantourout P, Radojkovic C, Lichtenstein L, Pons V, Champagne E, Martinez LO. Ecto-F1-ATPase: a moonlighting protein complex and an unexpected apoA-I receptor. World J Gastroenterol. 2010;16:5925–5935. doi: 10.3748/wjg.v16.i47.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arakaki N, Nagao T, Niki R, et al. Possible role of cell surface H+-ATP synthase in the extracellular ATP synthesis and proliferation of human umbilical vein endothelial cells. Mol Cancer Res. 2003;1:931–939. [PubMed] [Google Scholar]
- 12.Moser TL, Kenan DJ, Ashley TA, et al. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci USA. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu ZJ, Song QF, Jiang SS, et al. Identification of ATP synthase beta subunit (ATPB) on the cell surface as a non-small cell lung cancer (NSCLC) associated antigen. BMC Cancer. 2009;9:16. doi: 10.1186/1471-2407-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Notari L, Arakaki N, Mueller D, Meier S, Amaral J, Becerra SP. Pigment epithelium-derived factor binds to cell-surface F(1)-ATP synthase. FEBS J. 2010;277:2192–2205. doi: 10.1111/j.1742-4658.2010.07641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becerra SP. Focus on molecules: Pigment epithelium-derived factor (PEDF) Exp Eye Res. 2006;82:739–740. doi: 10.1016/j.exer.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Bouck N. PEDF: anti-angiogenic guardian of ocular function. Trends Mol Med. 2002;8:330–334. doi: 10.1016/s1471-4914(02)02362-6. [DOI] [PubMed] [Google Scholar]
- 17.Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: a multi-functional serpin family protein. J Cell Biochem. 2009;106:769–775. doi: 10.1002/jcb.22072. [DOI] [PubMed] [Google Scholar]
- 18.Yabe T, Sanagi T, Yamada H. The neuroprotective role of PEDF: implication for the therapy of neurological disorders. Curr Mol Med. 2010;10:259–266. doi: 10.2174/156652410791065354. [DOI] [PubMed] [Google Scholar]
- 19.Crawford SE, Stellmach V, Ranalli M, et al. Pigment epithelium-derived factor (PEDF) in neuroblastoma: a multifunctional mediator of Schwann cell antitumor activity. J Cell Sci. 2001;114:4421–4428. doi: 10.1242/jcs.114.24.4421. [DOI] [PubMed] [Google Scholar]
- 20.Broadhead ML, Dass CR, Choong PF. In vitro and in vivo biological activity of PEDF against a range of tumors. Expert Opin Ther Targets. 2009;13:1429–1438. doi: 10.1517/14728220903307475. [DOI] [PubMed] [Google Scholar]
- 21.Ek ET, Dass CR, Choong PF. PEDF: a potential molecular therapeutic target with multiple anti-cancer activities. Trends Mol Med. 2006;12:497–502. doi: 10.1016/j.molmed.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Hoshina D, Abe R, Yamagishi SI, Shimizu H. The role of PEDF in tumor growth and metastasis. Curr Mol Med. 2010;10:292–295. doi: 10.2174/156652410791065327. [DOI] [PubMed] [Google Scholar]
- 23.Doll JA, Stellmach VM, Bouck NP, et al. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nat Med. 2003;9:774–780. doi: 10.1038/nm870. [DOI] [PubMed] [Google Scholar]
- 24.Becerra SP, Sagasti A, Spinella P, Notario V. Pigment epithelium-derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. J Biol Chem. 1995;270:25992–25999. doi: 10.1074/jbc.270.43.25992. [DOI] [PubMed] [Google Scholar]
- 25.Notari L, Baladron V, Aroca-Aguilar JD, et al. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J Biol Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- 26.Bernard A, Gao-Li J, Franco CA, Bouceba T, Huet A, Li Z. Laminin receptor involvement in the anti-angiogenic activity of pigment epithelium-derived factor. J Biol Chem. 2009;284:10480–10490. doi: 10.1074/jbc.M809259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park K, Lee K, Zhang B, et al. Identification of a novel inhibitor of the canonical Wnt pathway. Mol Cell Biol. 2011;31:3038–3051. doi: 10.1128/MCB.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberdi E, Hyde CC, Becerra SP. Pigment epithelium-derived factor (PEDF) binds to glycosaminoglycans: analysis of the binding site. Biochemistry. 1998;37:10643–10652. doi: 10.1021/bi9802317. [DOI] [PubMed] [Google Scholar]
- 29.Becerra SP, Perez-Mediavilla LA, Weldon JE, et al. Pigment epithelium-derived factor binds to hyaluronan. Mapping of a hyaluronan binding site. J Biol Chem. 2008;283:33310–33320. doi: 10.1074/jbc.M801287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer C, Notari L, Becerra SP. Mapping the type I collagen-binding site on pigment epithelium-derived factor. Implications for its antiangiogenic activity. J Biol Chem. 2002;277:45400–45407. doi: 10.1074/jbc.M208339200. [DOI] [PubMed] [Google Scholar]
- 31.Amaral J, Becerra SP. Effects of human recombinant PEDF protein and PEDF-derived peptide 34-mer on choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51:1318–1326. doi: 10.1167/iovs.09-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filleur S, Volz K, Nelius T, et al. Two functional epitopes of pigment epithelial-derived factor block angiogenesis and induce differentiation in prostate cancer. Cancer Res. 2005;65:5144–5152. doi: 10.1158/0008-5472.CAN-04-3744. [DOI] [PubMed] [Google Scholar]
- 33.Mirochnik Y, Aurora A, Schulze-Hoepfner FT, et al. Short pigment epithelial-derived factor-derived peptide inhibits angiogenesis and tumor growth. Clin Cancer Res. 2009;15:1655–1663. doi: 10.1158/1078-0432.CCR-08-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alberdi E, Aymerich MS, Becerra SP. Binding of pigment epithelium-derived factor (PEDF) to retinoblastoma cells and cerebellar granule neurons. Evidence for a PEDF receptor. J Biol Chem. 1999;274:31605–31612. doi: 10.1074/jbc.274.44.31605. [DOI] [PubMed] [Google Scholar]
- 35.Bilak MM, Becerra SP, Vincent AM, Moss BH, Aymerich MS, Kuncl RW. Identification of the neuroprotective molecular region of pigment epithelium-derived factor and its binding sites on motor neurons. J Neurosci. 2002;22:9378–9386. doi: 10.1523/JNEUROSCI.22-21-09378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stratikos E, Alberdi E, Gettins PG, Becerra SP. Recombinant human pigment epithelium-derived factor (PEDF): characterization of PEDF overexpressed and secreted by eukaryotic cells. Protein Sci. 1996;5:2575–2582. doi: 10.1002/pro.5560051220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao R, Guo X. Human microvascular endothelial cells immortalized with human telomerase catalytic protein: a model for the study of in vitro angiogenesis. Biochem Biophys Res Commun. 2004;321:788–794. doi: 10.1016/j.bbrc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 38.Rasband WS. Image Processing with ImageJ. Biophotonics Int. 1997;11:36–42. [Google Scholar]
- 39.Quillen EE, Haslam GC, Samra HS, et al. Ectoadenylate kinase and plasma membrane ATP synthase activities of human vascular endothelial cells. J Cell Biochem. 2006;281:20728–20737. doi: 10.1074/jbc.M513042200. [DOI] [PubMed] [Google Scholar]
- 40.Chowdhury SA, Kishino K, Satoh R, et al. Tumor-specificity and apoptosis-inducing activity of stilbenes and flavonoids. Anticancer Res. 2005;25:2055–2063. [PubMed] [Google Scholar]
- 41.Wolter F, Clausnitzer A, Akoglu B, Stein J. Piceatannol, a natural analog of resveratrol, inhibits progression through the S phase of the cell cycle in colorectal cancer cell lines. J Nutr. 2002;132:298–302. doi: 10.1093/jn/132.2.298. [DOI] [PubMed] [Google Scholar]
- 42.Huang TC, Chang HY, Hsu CH, Kuo WH, Chang KJ, Juan HF. Targeting therapy for breast carcinoma by ATP synthase inhibitor aurovertin B. J Proteome Res. 2008;7:1433–1444. doi: 10.1021/pr700742h. [DOI] [PubMed] [Google Scholar]
- 43.van Raaij MJ, Abrahams JP, Leslie AG, Walker JE. The structure of bovine F1-ATPase complexed with the antibiotic inhibitor aurovertin B. Proc Natl Acad Sci USA. 1996;93:6913–6917. doi: 10.1073/pnas.93.14.6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong S, Pedersen PL. ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas. Microbiol Mol Biol Rev. 2008;72:590–641. doi: 10.1128/MMBR.00016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Gao F, Yu LL, et al. Dual functions of a monoclonal antibody against cell surface F1F0 ATP synthase on both HUVEC and tumor cells. Acta Pharmacol Sin. 2008;29:942–950. doi: 10.1111/j.1745-7254.2008.00830.x. [DOI] [PubMed] [Google Scholar]