Abstract
A link between T cell proliferation and the protein kinase C (PKC) family of serine/threonine kinases has been recognized for about 30 years. However, despite the wealth of information on PKC-mediated control of, T cell activation, understanding of the effects of PKCs on the cell cycle machinery in this cell type remains limited. Studies in other systems have revealed important cell cycle-specific effects of PKC signaling that can either positively or negatively impact proliferation. The outcome of PKC activation is highly context-dependent, with the precise cell cycle target(s) and overall effects determined by the specific isozyme involved, the timing of PKC activation, the cell type, and the signaling environment. Although PKCs can regulate all stages of the cell cycle, they appear to predominantly affect G0/G1 and G2. PKCs can modulate multiple cell cycle regulatory molecules, including cyclins, cyclin-dependent kinases (cdks), cdk inhibitors and cdc25 phosphatases; however, evidence points to Cip/Kip cdk inhibitors and D-type cyclins as key mediators of PKC-regulated cell cycle-specific effects. Several PKC isozymes can target Cip/Kip proteins to control G0/G1 → S and/or G2 → M transit, while effects on D-type cyclins regulate entry into and progression through G1. Analysis of PKC signaling in T cells has largely focused on its roles in T cell activation; thus, observed cell cycle effects are mainly positive. A prominent role is emerging for PKCθ, with non-redundant functions of other isozymes also described. Additional evidence points to PKCδ as a negative regulator of the cell cycle in these cells. As in other cell types, context-dependent effects of individual isozymes have been noted in T cells, and Cip/Kip cdk inhibitors and D-type cyclins appear to be major PKC targets. Future studies are anticipated to take advantage of the similarities between these various systems to enhance understanding of PKC-mediated cell cycle regulation in T cells.
Keywords: protein kinase C, signal transduction, T cell activation, cell cycle, cyclin, cyclin-dependent kinase, cyclin-dependent kinase inhibitor
An association between protein kinase C (PKC) signaling and T cell proliferation has been recognized for almost three decades. Over 30 years ago, it was determined that a combination of phorbol esters and elevated intracellular calcium potently induces proliferation of cells of the T cell lineage (e.g., Whitfield et al., 1973; Lyall et al., 1980; Sutherland et al., 1981). Shortly thereafter, it was determined that PKC, then recognized as a calcium-dependent enzyme, represented the major cellular receptor for phorbol esters (Castagna et al., 1982) and it was not long before the connection between these phenomena was made (Isakov et al., 1986; Isakov and Altman, 1987). Since then, a central role for PKC in T cell receptor (TCR) signaling has been firmly established. Despite the long association of PKC with T cell proliferation, details on how PKC signaling interacts with the cell cycle machinery in this cell type are only beginning to emerge. In this regard, our knowledge in T cells lags behind that in other cell types, including other hematopoietic lineages. In this review, we outline our current understanding of the proliferative effects of PKC signaling in T cells within the context of the broader knowledge that has been gained in other systems.
THE PROTEIN KINASE C FAMILY
Protein kinase C represents a family of serine/threonine kinases that belong to the AGC (cAMP-dependent, cGMP-dependent, and protein kinase C) superfamily of protein kinases (Nieto, 2007; Matsuoka et al., 2009; Black, 2010; Rosse et al., 2010; Ryu et al., 2010). PKC isozymes are lipid-dependent kinases (requiring phosphatidylserine binding for activity) and are grouped into three subfamilies based on their structure and requirement for additional co-factors and calcium. Physiological activation of classical PKCs (PKCα, PKCβI and PKCβII, which are splice variants of the prkcb gene, and PKCγ) is induced by the lipid second messenger diacylglycerol (DAG) and calcium, while activation of the novel PKCs (PKCδ, PKCε, PKCθ, and PKCη) requires only DAG. In contrast, the atypical PKCs (PKCζ and PKCι/λ) are not dependent on lipid second messengers or calcium for activity. Instead, their function is regulated by protein–protein interactions mediated by a PB1 domain as well as a carboxyl-terminal PDZ ligand motif. Engagement of growth factor or cytokine receptors leads to activation of phospholipase C (PLC) β or PLCγ, which cleave phosphatidylinositol 4,5-bisphosphate to generate DAG and the soluble second messenger inositol trisphosphate (which induces release of calcium from intracellular stores). The production of DAG recruits classical and novel PKCs to the plasma membrane, where they undergo a conformational change resulting in full activation. Unlike other AGC kinases, such as Akt, activation of PKCs does not require acute phosphorylation of the enzyme: phosphorylations necessary for catalytic competence occur shortly after synthesis and the enzyme is constitutively phosphorylated at these sites (Matsuoka et al., 2009; Rosse et al., 2010). As a result, changes in phosphorylation do not provide an indication of PKC activity; rather signaling-induced translocation of the enzyme to the membrane/particulate fraction represents the most reliable means of monitoring kinase activation. Reversal of signaling can occur by metabolism of DAG by DAG kinase and release of PKCs from the membrane, as well as by agonist-induced enzyme degradation or removal of priming phosphorylation with subsequent rapid degradation (Leontieva and Black, 2004; Newton, 2010). In addition to activation by growth factor signaling, classical and novel PKCs can be stimulated by a number of pharmacological agents that mimic the effects of DAG, such as phorbol esters and macrocyclic lactone bryostatins. However, in contrast to DAG, these agonists, which include phorbol 12-myristate 13-acetate [PMA; also known as 12-O-tetradecanoylphorbol-13-acetate (TPA)], phorbol 12,13-dibutyrate (PDBu), and bryostatin 1, are not rapidly metabolized and thus give a more sustained PKC activation.
Despite limitations related to their lack of specificity for individual PKC isozymes, their ability to promote PKC downregulation, and the existence of additional targets for these agents (Griner and Kazanietz, 2007), use of pharmacological agonists and membrane permeant DAG analogs has provided significant insight into the downstream effects of PKC activation. However, a complete understanding of PKC signaling will require defining the specific function(s) of individual PKC isozymes, and progress toward this goal has proved technically difficult. Understanding of the functions of atypical PKCs, PKCζ, and PKCι, lags behind that of other members of the PKC family, perhaps largely due to their insensitivity to pharmacological activators (e.g., phorbol esters and bryostatins) and synthetic DAGs. In the absence of isozyme-specific pharmacological PKC agonists and inhibitors, early studies relied on overexpression strategies to decipher the roles of individual isozymes, which can result in non-physiological levels of expression, activity, and regulation. RNA interference technology and genetically altered mice are helping to circumvent these problems, but are not without drawbacks of their own. Potential limitations include the need for a high level of silencing to sufficiently deplete enzyme activity (e.g.,>80%, Cameron et al., 2008; M. A. Pysz, A. R. Black, and J. D. Black, unpublished results), and the fact that knockdown of one PKC isozyme can affect accumulation of other members of the family (M. A. Pysz, A. R. Black, and J. D. Black, unpublished data). Overlapping roles of different isozymes means that multiple crosses of transgenic mice may be needed to observe phenotypes.
An additional source of confusion regarding the functions of individual PKC isozymes is the fact that many so-called PKC inhibitors are of questionable specificity (Griner and Kazanietz, 2007; Soltoff, 2007). As an example of particular relevance to T cell activation, special caution is needed when considering studies that have used rottlerin to infer effects of signaling from PKCθ. While this agent was originally considered to be a specific inhibitor of novel PKCs, recent studies have demonstrated that it does not inhibit PKCδ (Soltoff, 2007). In keeping with this finding, the IC50 for PKCθ inhibition by rottlerin in the presence of 100 μM ATP is>300 μM (Villalba et al., 1999), a concentration far in excess of that used in studies on its cellular effects. In contrast, rottlerin is a potent inhibitor of other kinases such as PRAK and MAPKAP-K2 (Soltoff, 2007); thus, any effects of this inhibitor cannot be ascribed to direct inhibition of PKCθ.
Despite these limitations, our knowledge of the roles of individual PKCs is emerging. Of note, in addition to the proliferative/cell cycle effects which are the subject of this review, PKC isozymes have been found to regulate multiple cellular processes of direct relevance to T cell development and function, including differentiation, migration, survival, apoptosis, endocytosis, and secretion/exocytosis (Reyland, 2009; Rosse et al., 2010).
THE MAMMALIAN CELL CYCLE
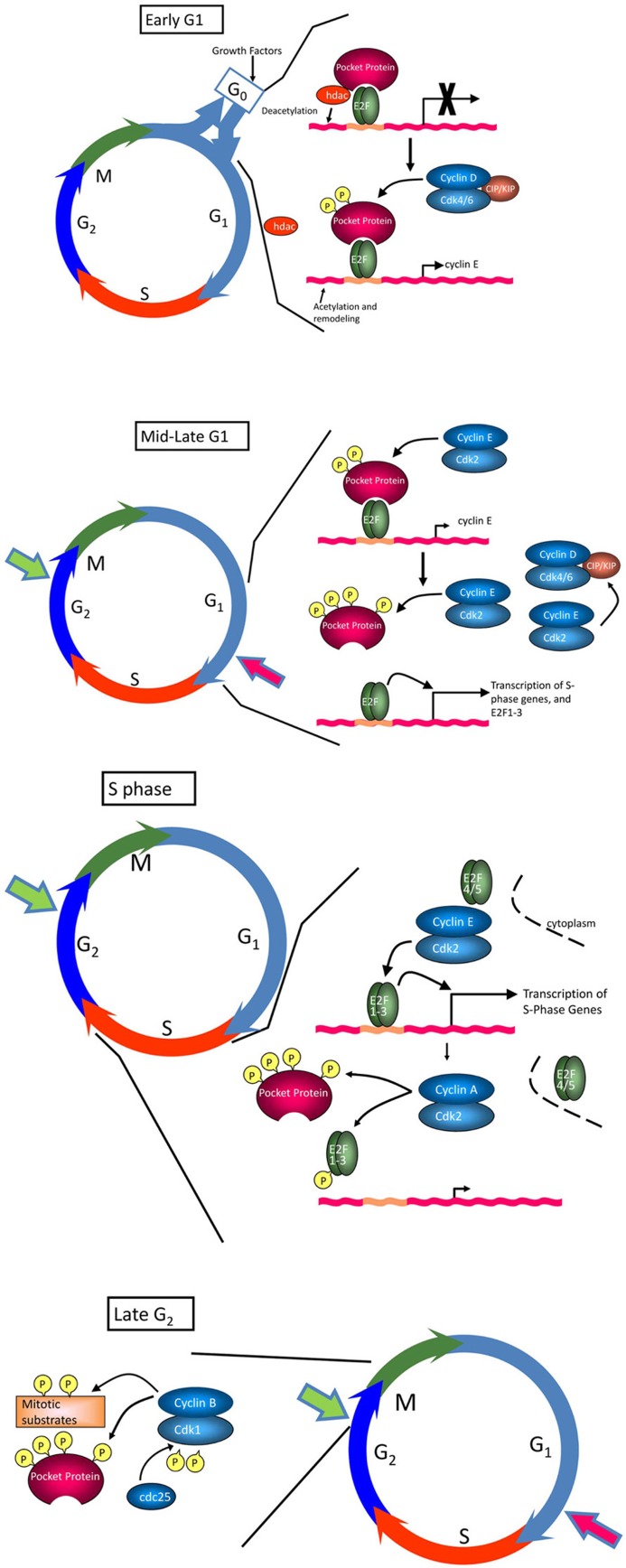
Several excellent reviews have been written on the regulation of the cell cycle (Sherr and Roberts, 2004; Cobrinik, 2005; Malumbres and Barbacid, 2005; Du and Pogoriler, 2006; Satyanarayana and Kaldis, 2009) and only a brief description will be given here. The cell cycle has been classically divided into four phases, G1 (or Gap 1 in which cells prepare for DNA synthesis), S phase (in which DNA is synthesized), G2 (in which cells prepare for division) and mitosis (or M phase, in which sister chromatids are separated and the cell divides; Figure 1). Transit through the cell cycle is regulated by four major classes of cyclins whose expression is strictly controlled and limited to particular cell cycle phases. Cyclins are the regulatory subunits for cyclin-dependent kinases (cdks), whose activity is absolutely dependent on association with specific cyclin partners. Entry of quiescent cells into the cell cycle and transit through early G1 is regulated by D-type cyclins, which complex with cdk4 and cdk6 (Musgrove et al., 2011). There are three D-type cyclins, D1, D2, and D3, which are expressed to varying degrees in different tissues; cyclins D2 and D3 appear to be the major players in T cells. Transit through late G1 and progression into S phase is regulated by cyclin E complexed with cdk2 (Hwang and Clurman, 2005; Malumbres and Barbacid, 2005). S phase transit and early G2 are regulated by cyclin A/cdk2 and cyclin A/cdk1 complexes, whereas cyclin B, complexed with cdk1, regulates progression into M phase (Malumbres and Barbacid, 2005; Sanchez and Dynlacht, 2005). In addition to being regulated by cyclin binding, the activity of cdks is under the control of Cip/Kip and Ink4 cdk inhibitor proteins (ckis; Sherr and Roberts, 1995). Members of the Cip/Kip family, including p21Cip1, p27Kip1, and p57Kip2, have a dual activity in cell cycle regulation. They negatively regulate cell cycle progression by binding to cyclin/cdk2 and cyclin/cdk1 complexes and inhibiting their enzymatic activity. Conversely, these proteins can promote progression by enhancing the association of cyclin D with cdk4 and cdk6 without inhibiting the activity of these complexes (Sherr and Roberts, 1999). The Ink4 ckis, which include p15Ink4b, p16Ink4a, p18Ink4c, and p19Ink4d, block the activity of cdk4 and cdk6 by preventing their association with cyclin D. Cdk activity is also regulated by phosphorylation: positive phosphorylation is mediated by cdk activating kinase (CAK or cdk7/cyclin H; Fisher and Morgan, 1994), while negative phosphorylation involves the kinases Wee1 and Myt1. Removal of inhibitory phosphorylation, by e.g., Cdc25 phosphatases, is necessary for full cdk activity.
FIGURE 1.
The cell cycle. The cell cycle consists of four phases, G1, S, G2, and M. In early G1, hypophosphorylated pRb binds the E2F transcription factor, and recruits histone deacetylase (HDAC) and other factors to actively repress transcription of E2F-regulated genes important for transition into S phase and DNA replication (e.g., PCNA, topoisomerase I, c-Myc, cyclin E, Cdc25c). Progression through early G1 is dependent on growth factors, which promote expression of D-type cyclins. Formation of cyclin D/cdk4 and cyclin D/cdk6 complexes, which is facilitated by Cip/Kip ckis, leads to phosphorylation of pRb at a subset of available sites and release of HDAC and other inhibitory factors, relieving repression of E2F and promoting upregulation of cyclin E. Cyclin E/cdk2 complexes, relieved from repression by Cip/Kip ckis by sequestration of these inhibitory molecules in cyclin D/cdk complexes, complete pocket protein phosphorylation in mid to late G1, enabling a wave of E2F-dependent transcriptional activity essential for S progression. Together, these events drive cells through the restriction point (large red arrow), which commits cells to the proliferative cycle. If conditions are not optimal to signal this transition, cells exit the cycle and enter G0 or quiescence, a reversible non-replicative state. Once cells enter S phase, cyclin E/cdk2 activity is inhibited by proteasomal degradation of cyclin E in the cytoplasm. Continued inactivation/hyperphosphorylation of pRb allows the transcription of cyclin A and cyclin B, required for subsequent phases of the cell cycle. Cyclin A/cdk2 complexes phosphorylate a number of proteins to facilitate S phase completion and transit into G2/M. Cyclin B is actively synthesized during G2 and associates with cdk1 to trigger mitosis. Cdk1 is maintained in an inactive state by the kinases, Wee1 and Myt1. As cells approach M phase, the phosphatase cdc25 is activated to remove inhibitory phosphates on Tyr14 and Thr15, driving the cells into mitosis. A checkpoint in late G2 (large green arrow) prevents cells from entering M phase if the genome is damaged. This DNA damage checkpoint ensures that cells do not initiate mitosis until they have repaired damaged DNA after replication.
While there are multiple checkpoints that allow cells to undergo cell cycle arrest in response to various stresses, the most relevant to normal tissue homeostasis and differentiation is that which directs entry and exit from the cell cycle in G1 (Prasad et al., 1994; Liu et al., 2012). The expression of D-type cyclins is acutely regulated by mitogenic signals. As such, these proteins are the main sensors for the growth environment of the cell, and are intimately involved in regulation of the entry of quiescent cells into the cell cycle. Major targets for cyclin D/cdk complexes include the retinoblastoma protein (pRb) and related pocket proteins, p107 and p130 (Cobrinik, 2005). In the hypophosphorylated state, pocket proteins bind to E2F transcription factors on the promoters of growth-related genes, where they act as transcriptional repressors and actively block expression of genes necessary for DNA replication (Trimarchi and Lees, 2002; Du and Pogoriler, 2006; Figure 1). Phosphorylation of pocket proteins relieves this repression, allowing for transcription of E2F-dependent genes, one of which is cyclin E. Cyclin E/cdk2 then completes phosphorylation of pocket proteins, leading to their release from E2F and robust transcription of growth-related genes. At early stages of G1, cells require mitogenic signals to support cyclin expression and cdk activity; however, once sufficient levels of cyclin E have accumulated to maintain its own expression, cells have passed the so-called “restriction point” and are able to proceed through to the next cell cycle without further mitogenic input. In the face of loss of mitogenic signals prior to the restriction point or of negative growth signals, cell cycle progression is halted and cells eventually withdraw into G0 phase and quiescence (Grana et al., 1998; Classon and Dyson, 2001).
PKC SIGNALING AND T CELL PROLIFERATION
T CELL DEVELOPMENT AND TCR SIGNALING
T lymphocytes arise from bone marrow-derived CD34+ stem cells, which seed the thymus and undergo multistage differentiation to become mature circulating cells (for references, see Koch and Radtke, 2011). An early event in this process involves VDJ recombination of the TCR-β chain which then complexes with pre-Tα to form the pre-TCR. Signaling from the pre-TCR leads to proliferation of pre-T cells and rearrangement of the TCR-α chain, which combines with the β chain and CD3 to form the TCR. Further differentiation, accompanied by negative and positive selection, eventually leads to the development of mature naïve T cells, including CD4+ helper T (Th), CD8+ cytotoxic T (Tc), and regulatory T (Treg) cells. These naïve cells exit the thymus and remain dormant as they circulate through secondary lymphoid organs until activated by antigen. These organs, which include the spleen, lymph nodes, and Peyer’s patches, transiently house naïve T cells and are the first line of defense against pathogens that traverse the skin or the epithelial lining of the respiratory, gastrointestinal, and urogenital tracts.
Activation of T cells requires interaction of the TCR with major histocompatibility complex (MHC) bound antigen on antigen presenting cells (APCs), such as dendritic cells, macrophages, and B cells (for references, see Marsland and Kopf, 2008; Smith-Garvin et al., 2009; Fooksman et al., 2010; Dustin and Depoil, 2011). The interface between the T cell and APC is marked by the formation of a structure, termed the immune synapse or supramolecular activation cluster (SMAC), which serves to regulate T cell signaling. Productive activation of T cells requires two signals. The first signal is provided by the MHC-bound TCR, while the second signal is provided by co-stimulatory molecules such as CD28 (which binds to B7 proteins on the APC). Additionally, cytokines such as IL-12 and tumor necrosis factor alpha (TNF-α) can provide a third signal that regulates the response to T cell activation. A number of experimental manipulations can activate T cells in the absence of APC interaction; these include crosslinking of the TCR and CD28 with insoluble antibodies and combined treatment of cells with phorbol ester and calcium ionophore.
T cell receptor co-activation leads to the engagement of multiple downstream signaling pathways including those involving phosphatidylinositol 3-kinase (PI-3K), tyrosine kinases such as Lck, and PLCγ (for references, see Altman et al., 2000; Marsland and Kopf, 2008; Smith-Garvin et al., 2009; Fooksman et al., 2010; Dustin and Depoil, 2011). Activation of PLCγ results in production of DAG, which recruits PKCθ to the immune synapse where it interacts indirectly with CD28 through binding to Lck (Kong et al., 2011; Isakov and Altman, 2012). PKCθ then phosphorylates CARMA1, leading to the assembly of the CARMA1–BCL10–MALT1 (CBM) signalosome. PLCγ-generated inositol trisphosphate releases calcium from intracellular stores. The combined action of downstream TCR signaling eventually leads to activation of NF-κB, AP1, and nuclear factor of activated T cells (NFAT) transcription factors (Marsland and Kopf, 2008; Smith-Garvin et al., 2009; Fooksman et al., 2010; Dustin and Depoil, 2011). Together, these events promote functional activation of T cells which is marked by cell proliferation/clonal expansion and cytokine secretion. While the majority of the T cells that arise from activation are eventually cleared from the circulation, a small number develop into memory T cells which are primed for activation upon subsequent antigen exposure.
Antigen-induced proliferation is a key aspect of both T cell differentiation and clonal expansion (Koch and Radtke, 2011). Thus, mechanisms underlying regulation of the T cell cycle machinery are of critical importance to immune function. As the signaling pathways involved in T cell activation are being deciphered, increasing evidence is pointing to the importance of the PKC family in mediating proliferative responses in these cells. The following section outlines our current understanding of the role of individual PKC isozymes in regulating proliferation in T cells within the context of knowledge gained from other systems.
PKCs AND THE CELL CYCLE
As our knowledge of the proliferative roles of the PKC family has developed, it has become increasingly apparent that the effects of these molecules are highly context-dependent. The fact that PKCs are activated by tumor promoting phorbol esters and are downstream of growth factor receptors initially led to the idea that they transduce positive mitogenic signals (Castagna et al., 1982; Kikkawa et al., 1983; Leach et al., 1983). Although a number of early studies supported this idea (Dicker and Rozengurt, 1978; Rozengurt, 1986; Takuwa et al., 1988), it soon became clear that PKCs can negatively and positively regulate cell cycle progression. Indeed, regulation of proliferation by the PKC enzyme system exhibits a high degree of complexity, with effects involving multiple cell cycle regulatory molecules, including cyclins, cdks, and ckis, and impacting various stages of the cell cycle (Black, 2010). Furthermore, individual isozymes can have opposing effects on cell cycle progression in different cell types and even within the same cell type, depending on the signaling environment. A single isozyme can target different cell cycle molecules in different cell types, can have opposite effects on a specific cell cycle target in different systems, and can modulate the same target to produce divergent cell cycle responses (for review, see Black, 2010). Thus, to gain a true understanding of the role of PKCs in regulation of proliferation in any given system, it is important to study the mechanisms by which individual isozymes affect specific cell cycle molecules in that system.
T lymphocytes express all members of the PKC family with the exception of PKCγ (Koretzky et al., 1989; Chen et al., 1994; Thuille et al., 2006). A role for PKC isozymes in cell cycle regulation in CD3+ T lymphocytes was suggested by the early recognition that phorbol esters, in conjunction with calcium ionophore, are potent mitogens for these cells (Altman et al., 1990). While studies have concentrated largely on the role of PKCθ in mediating signaling from the immune synapse, a role for other PKC isozymes is emerging. Notably, different isozymes can have pro-proliferative and/or anti-proliferative functions, arguing that, as in other cell types, PKC signaling can regulate entry into the cell cycle, transit through the various cell cycle phases, as well as cell cycle withdrawal in T cells. The following sections discuss current understanding of the growth regulatory functions of individual PKC family members, followed by a summary of the limited information available on cell cycle-specific effects of these isozymes in T cells.
PROLIFERATIVE EFFECTS OF INDIVIDUAL PKC FAMILY MEMBERS
PKCα
Use of selective pharmacological inhibitors, antisense technology, or siRNA has identified an anti-proliferative and differentiation-inducing role of PKCα in multiple cell types, e.g., intestinal epithelial cells, keratinocytes, mammary epithelial cells, and melanoma cells (Black, 2000, 2010). Anti-proliferative effects of PKCα affecting G1 → S transit include downregulation of cyclin D1 (Detjen et al., 2000; Hizli et al., 2006; Guan et al., 2007), as well as induction of p21Cip1 (Frey et al., 1997, 2000; Abraham et al., 1998; Slosberg et al., 1999; Black, 2000; Detjen et al., 2000; Tibudan et al., 2002; Clark et al., 2004; Matsumoto et al., 2006) and p27Kip1 (Frey et al., 1997, 2000; Detjen et al., 2000; Tibudan et al., 2002). Induction of p21Cip1 is also involved in the ability of this isozyme to delay S phase transit and induce G2/M arrest (Frey et al., 1997; Oliva et al., 2008). Our analysis in intestinal epithelial cells indicated that downregulation of cyclin D1 represents one of the earliest effects of PKCα signaling (Frey et al., 2004; Hizli et al., 2006): PKCα-induced loss of cyclin D1 results from translational and transcriptional inhibition, mediated by activation of the translational repressor 4E-BP1 and downregulation of the Id family of transcription factors, respectively (Clark et al., 2004; Hizli et al., 2006; Guan et al., 2007; Hao et al., 2011). Suppression of cyclin D1 expression by PKCα can involve different intermediate signaling events, including activation of the ERK/MAPK pathway (Clark et al., 2004; Hizli et al., 2006; Guan et al., 2007; Hao et al., 2011) and RORα-mediated suppression of Wnt/β-catenin signaling (Bird et al., 1998). Consistent with a role of PKCα in growth inhibition, activation/membrane association of this isozyme is detected in post-mitotic cells in the intestinal epithelium (Saxon et al., 1994; Frey et al., 2000) and epidermis (Tibudan et al., 2002) in vivo. Furthermore, PKCα knockout mice show increased proliferative activity within intestinal crypts, and the tumor suppressive activity of this isozyme in the intestine has been linked directly to its effects on the cell cycle machinery (Oster and Leitges, 2006; Pysz et al., 2009).
Growth-stimulatory effects of PKCα have been reported in glioma cells, osteoblasts, chick embryo hepatocytes, hepatocellular carcinoma cells, and myoblasts, among others (Black, 2000, 2010). Proliferative effects of PKCα on the cell cycle machinery include increased levels of cyclin D1 and cdk4, and enhanced cyclin/cdk2 complex activity (Zhou et al., 2002; Alisi et al., 2004; Wu et al., 2008; Lovatt and Bijlmakers, 2010). PKCα can also elicit a p21Cip1-dependent enhancement of proliferation as seen in glioma cells (Besson and Yong, 2000). The ability of PKCα to promote proliferation has been linked to signaling through the ERK/MAPK pathway (Schonwasser et al., 1998; Shatos et al., 2008).
Consistent with the cell cycle effects of PKCα described above, this isozyme is targeted by various physiological stimuli that elicit changes in proliferation (Bird et al., 1998; Black, 2000, 2010). Interestingly, PKCα can mediate opposing cell cycle-specific effects of these agents depending on context. For example, PKCα appears to mediate both proliferative (Buitrago et al., 2003) and growth-inhibitory (Chen et al., 1999; Bikle et al., 2001) effects of vitamin D in different systems. This dichotomy has even been observed in cells of the same tissue origin: decreased PKCα expression mediates all-trans retinoic acid (ATRA)-induced inhibition of G1 → S progression in SKRB-3 breast cancer cells (Nakagawa et al., 2003), whereas PKCα is required for ATRA-induced growth arrest in T-47D breast cancer cells (Cho et al., 1997).
A role for PKCα in positive regulation of proliferation in T cells was suggested by the finding that, unlike wild-type cells, T lymphocytes from transgenic mice overexpressing PKCα were able to proliferate in response to soluble anti-CD3 antibody (Iwamoto et al., 1992). This role was confirmed by studies of PKCα knockout mice: while PKCα was not required for differentiation of CD4+ and CD8+ cells or activation-induced IL-2 production, PKCα-/- T cells showed severe defects in TCR-induced proliferation and IFN-γ production (Pfeifhofer et al., 2006). These effects were specific to T cells since B cell proliferation was unaffected (Pfeifhofer et al., 2006; Gruber et al., 2009).
Interestingly, PKCα and PKCθ cooperate in regulation of T cell proliferation: while PKCα-/- and PKCθ-/- showed only a mild activation defect in a graft-versus-host model, double PKCα/PKCθ knockout mice had a severe defect in alloreactive T cell proliferation (Gruber et al., 2009). This effect is of direct physiological relevance since the double knockout mice had significantly improved transplant survival compared with single knockout and control animals (Gruber et al., 2009). These studies further indicated that the cooperative effects of PKCα and PKCθ are due to a combinatorial effect on NFAT activation. A role for this pathway in effects of PKCα is also supported by the fact that constitutively active PKCα can activate NFAT (and AP1) in T cells (Genot et al., 1995). While these studies indicate that PKCα and PKCθ have overlapping functions in regulation of the alloimmune response and NFAT activation, these isozymes clearly have non-redundant functions in T cells. PKCα-/- mice show a defect in Th1-dependent IgG2a/b switching, indicating that PKCα is particularly important in Th1 cells (Pfeifhofer et al., 2006), a role which contrasts with the more prominent function of PKCθ in Th2 function (Salek-Ardakani et al., 2004). These non-redundant actions of PKCα may reflect its recently identified role in phosphorylation of Akt on serine 473 in T cells (Yang et al., 2010). The relevance of this phosphorylation is supported by the finding that Akt links mTORC2 to Th1 cells whereas PKCθ regulates mTORC2-mediated Th2 differentiation (Lee et al., 2010).
PKCβ
The two major splice variants of the PKCβ gene (prkcb), PKCβI and PKCβII, have different functions; however, the fact that early studies did not always differentiate between these forms, and knockdown and knockout strategies can affect both isoforms, has complicated interpretation of their individual roles.
The cell cycle-specific effects of PKCβII, which have been noted in both G1 and G2/M phases, appear to be largely stimulatory (Black, 2010). Effects in G1 have been ascribed to the ability of PKCβII to enhance transcription of cyclin D1 (Li and Weinstein, 2006), promote pRb phosphorylation (Suzuma et al., 2002), or to stimulate CAK activity through direct phosphorylation (Acevedo-Duncan et al., 2002). Studies by Fields and colleagues have established that phosphorylation of lamins contributes to the effects of PKCβII on G2 → M transition (Goss et al., 1994; Walker et al., 1995; Thompson and Fields, 1996; Murray and Fields, 1998), while studies by Newton and colleagues (Chen et al., 2004) have also determined that PKCβII can affect M phase by regulation of cytokinesis through interaction with pericentrin. However, PKCβII can also inhibit proliferation and induce differentiation in some cell types, with induction of p21Cip1 and loss of Cdc25 potentially mediating this activity (Yoshida et al., 2003; Cejas et al., 2005). The PKCβI splice variant has been implicated in positive and negative regulation of proliferation in fibroblasts and colon cancer cells, respectively (Housey et al., 1988; Choi et al., 1990; Sauma et al., 1996); however, these findings relied exclusively on overexpression and further work will be required to determine the specific involvement of the PKCβI isozyme in these effects.
A number of studies indicate that PKCβI and/or PKCβII are involved in regulation of T cell proliferation. For example, antisense-mediated knockdown has implicated PKCβ isozyme(s) in IL-2 signaling (Gomez et al., 1995). Furthermore, PKCβ forms are likely involved in cytoskeletal changes following T cell activation. PKCβII localizes to a cytoskeletal aggregate that forms in close proximity to the microtubule organizing center following T cell activation (Black et al., 1988; Gregorio et al., 1992, 1994) and PKCβI has been shown to associate with microtubules in T cells and to play a role in T cell polarization (Volkov et al., 2001). Since cytoskeletal changes appear to be an important aspect of T cell activation (Repasky and Black, 1996; Martín-Cófreces et al.,2008; freces et al.,2008; ; Alarcónetal.,2011 2011), these observations are likely to be relevant to T cell signaling. This idea is supported by the finding that antisense-mediated knockdown of PKCβI reduced nuclear translocation of NFAT in TCR/CD28-stimulated Jurkat T lymphoma cells (Dreikhausen et al., 2003). However, PKCβ isozymes do not have an essential role in T cell function since PKCβ-/- mice have no appreciable T cell-related defects. This contrasts with a critical role for PKCβ in B cell receptor signaling (Thuille et al., 2006) and in dendritic cell differentiation (Farren et al., 2010). Thus, any role of PKCβI/II association with cytoskeletal elements is likely to be redundant. In this regard, it is noteworthy that T cell activation leads to translocation of PKCα and PKCθ to the same PKCβII-associated cytoskeletal aggregate described above (J. D. Black and E. A. Repasky, unpublished data; Wang et al., 1999).
PKCδ
PKCδ broadly inhibits cell cycle progression in G1 in response to pharmacological agonists and physiological activators such as ATRA, inositol hexaphosphate (IP6), interferons, and testosterone (Watanabe et al., 1992; Fukumoto et al., 1997; Ashton et al., 1999; Uddin et al., 2002; Kambhampati et al., 2003; Nakagawa et al., 2005; Vucenik et al., 2005; Cerda et al., 2006; Bowles et al., 2007). Effects on G1 → S phase progression are mediated by direct or indirect targeting of cyclin D1, cyclin E, cyclin A, p21Cip1, and/or p27Kip1 (Fukumoto et al., 1997; Vrana et al., 1998; Ashton et al., 1999; Nakagawa et al., 2005; Cerda et al., 2006; Afrasiabi et al., 2008). Cyclin D1 expression is downregulated by PKCδ in colon cancer cells (Cerda et al., 2006; Pysz et al., 2009), as well as in PKCδ overexpressing vascular smooth muscle cells (Fukumoto et al., 1997), primary bovine airway smooth muscle cells (Page et al., 2002), and NIH3T3 cells (Soh and Weinstein, 2003). Consistent with these findings, loss of PKCδ activity resulted in increased levels of cyclin D1 in colon cancer cells (Cerda et al., 2006) and bovine airway smooth muscle cells (Page et al., 2002). PKCδ has also been shown to inhibit mitosis in CHO cells and 3Y1 murine fibroblasts (Watanabe et al., 1992; Kitamura et al., 2003).
Although the majority of studies have detected a growth-inhibitory role for PKCδ, it can also act as a positive regulator of the cell cycle (Kitamura et al., 2003; Cho et al., 2004; Jackson and Foster, 2004; Czifra et al., 2006). PKCδ can enhance G1 → S transit through increased expression of cyclin D1, cyclin E, cyclin A, and/or cdk2 (Kitamura et al., 2003; Santiago-Walker et al., 2005; Grossoni et al., 2007), destabilization of p21Cip1 (Santiago-Walker et al., 2005; Walker et al., 2006), reduced nuclear localization of p21Cip1 (Sipeki et al., 2002; Ranta et al., 2011), and increased E2F promoter activity (Nakaigawa et al., 1996). In many cases, these effects are mediated by the ERK/MAPK pathway (Jackson and Foster, 2004; Grossoni et al., 2007). The opposing effects of PKCδ on cell cycle progression may be regulated by differential phosphorylation on Tyr155 (Acs et al., 2000; Steinberg, 2004).
T cells from PKCδ knockout mice are hyperproliferative and produce more IL-2 cytokine upon stimulation in response to allogeneic MHC. Thus, consistent with a predominant growth-inhibitory role of PKCδ in other systems, this isozyme appears to negatively regulate T cell proliferation, an effect that has been ascribed to attenuation of TCR/CD3-mediated signaling (Gruber et al., 2005a). A similar negative effect of PKCδ on proliferation is also seen in B cells (Miyamoto et al., 2002).
PKCε
PKCε generally mediates pro-proliferative responses, and its effects appear to be predominantly in G1/S rather than G2/M (Graham et al., 2000; Balciunaite and Kazlauskas, 2001). The enzyme has been implicated in mediating PDGF-induced G0/G1 → S progression (Balciunaite and Kazlauskas, 2001). Loss of PKCε activity in NSCLC cells is associated with induction of p21Cip1, prolonged G1 → S transition in response to serum, and reduced activation of cdk2 complexes (Bae et al., 2007), indicating that this isozyme suppresses p21Cip1 accumulation to facilitate cell cycle progression. PKCε can also induce cyclin D1 transcription and upregulate cyclin D1 and cyclin E protein (Soh and Weinstein, 2003; F. Hao, M. A Pysz, A. R. Black, and J. D. Black, unpublished data). Although PKCε is generally downregulated during differentiation (e.g., Yang et al., 2003), the enzyme promotes adipogenic commitment and is essential for terminal differentiation of 3T3-F442A preadipocytes (Webb et al., 2003). Its expression is also enhanced during myogenic differentiation, resulting in upregulation of cyclin D3 (Gaboardi et al., 2010).
The ability of constitutively active PKCε to activate NFAT and AP1 in Jurkat T lymphoma cells points to a role for this isozyme in T cell activation (Genot et al., 1995). Antisense-mediated knockdown has also implicated this isozyme in IL-2 signaling in T cells (Gomez et al., 1995). Furthermore, siRNA-mediated knockdown of PKCε in CD4+ T cells severely reduced proliferation in vitro and enhanced the growth-inhibitory effects of transforming growth factor beta (TGF-β; Mirandola et al., 2011). These findings support a predominantly growth-stimulatory role of PKCε in T cells, as seen in other systems (see above). However, PKCε-/- mice show no defects in T cell differentiation, proliferation or activation, indicating that the functions of this isotype may be in large part redundant, at least in the mouse (Gruber et al., 2005b). In contrast to this finding, analysis of Hashimoto thyroiditis patients points to a potential clinical relevance for proliferative effects of PKCε in T cells. These patients had significantly higher expression of PKCε in their T cells compared with healthy controls (Mirandola et al., 2011). Furthermore, while Hashimoto thyroiditis-derived T cells had diminished TGF-β responses compared with healthy controls, knockdown of PKCε in these cells restored normal responsiveness to TGF-β (Mirandola et al., 2011).
PKCη
PKCη has been associated with post-mitotic cells in a number of tissues including squamous epithelia (Kashiwagi et al., 2002; Breitkreutz et al., 2007), the epidermis (Breitkreutz et al., 2007), and the intestinal epithelium (Osada et al., 1993). Consistent with this localization, PKCη upregulated p21Cip1 and p27Kip1, decreased cdk2 kinase activity, and induced growth arrest in NIH3T3 cells and keratinocytes (Livneh et al., 1996; Ishino et al., 1998; Cabodi et al., 2000). However, this isozyme can also enhance proliferation as seen in MCF-7 breast cancer cells, where it upregulated cyclin D and cyclin E levels and promoted a redistribution of p21Cip1 and p27Kip1 from cdk2 to cdk4 complexes (Fima et al., 2001).
PKCη is recruited to the immune synapse, pointing to involvement of this isozyme in T cell activation (Fu and Gascoigne, 2012). This role was confirmed by the finding that PKCη-/- T cells have a defective proliferative response to anti-CD3 stimulation in vitro (Fu et al., 2011). A somewhat more severe proliferative defect was also observed in response to antigen presentation both in vitro and in vivo (Fu et al., 2011). Consistent with a role for PKCη in mediating TCR signaling, activated PKCη-/- T cells showed a reduction in calcium flux and NF-κB translocation (Fu et al., 2011). While these effects are largely redundant with PKCθ, specific effects of PKCη were seen in T cell homeostatic proliferation, which involves self-antigen recognition and IL-7 and IL-15 signaling (Fu and Gascoigne, 2012). Notably, no defect in homeostatic proliferation was seen in PKCθ-/- mice, indicating that this effect is largely specific to PKCη, although double knockouts did have a somewhat more severe phenotype.
PKCθ
PKCθ has been implicated as a positive regulator of proliferation in a number of cell types including gastrointestinal stromal tumor cells and breast cancer cells, where it represses expression of p21Cip1 and/or p27Kip1 (Belguise and Sonenshein, 2007; Ou et al., 2008), and in capillary endothelial cells, where it promotes G2/M progression (Tang et al., 1997).
A large body of evidence has emerged to support a critical role for PKCθ in T cell activation. The functions of this isozyme are the subject of several excellent reviews in this issue (e.g., Freeley and Long, 2012; Isakov and Altman, 2012; Wang et al., 2012) and will only be discussed briefly here. While PKCθ is dispensable for differentiation of CD4+ and CD8+ T cells, it is intimately involved in T cell activation and transduces pro-proliferative signals in multiple pathways, including those triggered by the TCR, CD28, and TNF-α (Altman et al., 2000; So and Croft, 2012). As mentioned above, PKCθ is recruited to the immune synapse early in T cell activation, where it is required for formation of the CBM complex, which plays a central role in mediating downstream signaling during T cell activation (Rawlings et al., 2006). In keeping with this role, PKCθ signaling activates a number of transcription factors that regulate T cell activation and proliferation, including Ap1, NF-κB, and NFAT (Pfeifhofer et al., 2003). Studies using prkcq knockout mice have determined that PKCθ plays a central role in mediating proliferative responses during T cell activation. PKCθ-deficient T cells lose the ability to proliferate in response to TCR/CD28 activation in vitro (Sun et al., 2000; Pfeifhofer et al., 2003). A role for PKCθ in T cell expansion in vivo was also apparent from the defective proliferation seen in PKCθ-/- mice during allergic asthmatic reactions and in response to bacterial infection (Salek-Ardakani et al., 2004; Sakowicz-Burkiewicz et al., 2008).
As seen with PKC isozymes in other cell types, the action of PKCθ in proliferation appears to be highly context-dependent. For example, while a clear role for this isozyme in regulation of Th2 cell proliferation in vivo is seen in the allergic asthmatic response, this was not the case for Th1 cells (Salek-Ardakani et al., 2004). Furthermore, PKCθ-deficiency does not affect T cell proliferation in response to viral infection (Giannoni et al., 2005) and can mediate growth-inhibitory effects of cytokine withdrawal (Li et al., 2006b). Notably, while PKCθ generally plays a positive role in proliferation of effector T cells, it has the opposite effect in Treg cells, where it is sequestered from the immune synapse and promotes growth inhibition (Zanin-Zhorov et al., 2010).
A recent study has given insight into possible explanations for divergent functions of PKCθ (Kong et al., 2011). PKCδ and PKCθ are highly homologous; yet, as noted above, PKCδ is growth inhibitory in T cells. In keeping with these differences, PKCδ is not targeted to the immune synapse, disrupts signalosome assembly and cannot substitute for PKCθ in T cell function. These differences are due to a proline-rich motif in the V3 region of PKCθ that mediates indirect interaction with CD28 through Lck. Mutation of this sequence blocks localization of PKCθ to the immune synapse; conversely, a PKCδ mutant containing this sequence was targeted to the immune synapse and could substitute for PKCθ in T cell signaling (Kong et al., 2011; Isakov and Altman, 2012). These findings point to the importance of alterations in protein–protein interactions and localization in dictating the effects of PKC signaling, and offer a mechanism for the divergent roles of PKC isozymes in different cell types and in different signaling environments.
Atypical PKC isozymes
While analysis of the functions of atypical PKCs is less advanced than that of other PKC isotypes, PKCι and PKCζ generally appear to promote cell cycle progression. Consistent with a cell cycle stimulatory role of PKCζ, keratin-induced blockade of HaCaT cell cycle progression involved inhibition of PKCζ activity, a reduction in cyclin D1 and cyclin E levels, and pRb hypophosphorylation (Paramio et al., 2001). PKCζ can mediate transcriptional activation of cyclin D1 downstream of Ras (Kampfer et al., 2001), and can induce phosphorylation and proteasome-dependent degradation of p21Cip1 downstream of PI-3K (Scott et al., 2002). The ability of PKCζ to modulate the subcellular distribution of p27Kip1 during cell cycle reentry of quiescent MCF7 cells is also downstream of PI-3K (Castoria et al., 2004). PKCζ may also enhance cdc25 activity to promote G2/M transit in A549 lung epithelial cells, an effect associated with changes in cdk2 activity (Lee et al., 2011; Kang et al., 2012). Exciting studies by Murray, Fields and colleagues have recently identified PKCι as an oncogene which is required for the transformed growth of various human cancer cell types (Fields and Regala, 2007; Murray et al., 2011). Consistent with these findings, PKCι is upstream of PKCζ in Ras-related upregulation of cyclin D1 (Kampfer et al., 2001). PKCι also phosphorylates and activates CAK in response to PI-3K signaling in glioma and neuroblastoma cells (Acevedo-Duncan et al., 2002; Pillai et al., 2011; Desai et al., 2012) and may target cyclin E in ovarian cancer (Eder et al., 2005).
In contrast to PKCα and PKCε, constitutively active PKCζ had no effect on AP1 and NFAT in Jurkat cells (Genot et al., 1995). However, work of Gruber et al. (2008) points to a role for atypical PKCs in PKCθ-mediated pro-proliferative signaling in T cells. These studies found that PKCζ physically interacts with PKCθ in a yeast two-hybrid screen and that PKCζ is a substrate for PKCθ. This physical interaction likely occurs in vivo since PKCζ and PKCι are constitutively localized in lipid rafts to which PKCθ is recruited following activation of primary T cells and Jurkat cells. Use of dominant negative mutant proteins further implicated the atypical isozymes in NF-κB induction by PKCθ. In keeping with their common localization and structure, it appears that PKCι and PKCζ can substitute for each other in most T cell functions. Nonetheless, PKCζ function appears to be particularly important for activation of Th2 cells (Martin et al., 2005): while PKCζ knockout did not result in proliferative or signaling defects in naïve T cells, it dramatically inhibited activation of Th2 cells. This effect was reflected in disruption of STAT6, NFAT, and NF-κB activation following stimulation with anti-CD3. The dramatic upregulation of PKCζ noted during Th2 cell differentiation may account for the inability of PKCι to compensate for loss of PKCζ in these cells (Martin et al., 2005; Gruber et al., 2008). The physiological relevance of PKCζ signaling in Th2 cells is seen in the impaired allergic asthmatic response in PKCζ-/- mice (Martin et al., 2005).
Summary and discussion
From the above discussion, it is apparent that PKC signaling plays an important role in regulation of cell proliferation in a broad spectrum of cell types including T cells. PKC activation can either promote or inhibit transit through multiple stages of the cell cycle. The precise effect of PKCs on the cell cycle is highly context-dependent, and is influenced by the specific isozyme involved, the timing and duration of PKC activation, the cell type, and the signaling environment to which the cell is exposed; however, some themes are beginning to emerge. With regard to individual PKC family members, accumulating evidence indicates that PKCα can exert context-dependent inhibitory or stimulatory effects. While PKCδ can have positive effects on cell cycle progression, its effects are generally inhibitory. On the other hand, effects of PKCβII, PKCε, and atypical PKCs appear to be mainly pro-proliferative, while those of PKCη are generally inhibitory.
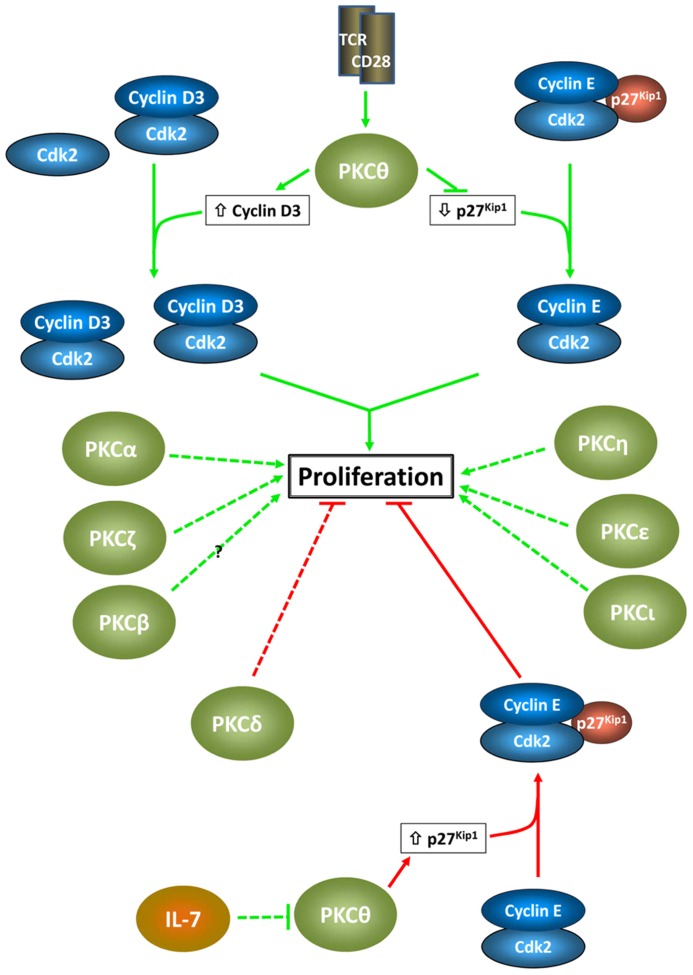
In T cells, multiple PKC isozymes mediate proliferative signals associated with TCR/CD28 engagement (Figure 2). These effects, which directly impact immune function, involve both redundant and non-redundant functions of individual PKC family members, and a high degree of cooperation between different PKC isozymes is becoming apparent. As in other systems, the effects of PKC signaling are highly context-dependent, with the reliance on individual isozymes differing between T cell subtypes. While the majority of the characterized effects of PKC signaling in T cells have been pro-proliferative, negative effects are also seen: PKCδ appears to play a predominantly inhibitory role and PKCθ can have negative proliferative effects dependent on the signaling environment and cell type.
FIGURE 2.
Proliferative effects of PKC isozymes in T cells. Positive and negative proliferative effects of individual PKC isozymes are indicated by green arrows and red barred lines, respectively. Proposed cell cycle targets in the growth-inhibitory and growth-stimulatory effects of PKCθ are shown. These targets reflect the importance of D-type cyclins and Cip/Kip ckis as targets for PKC signaling, as seen in other systems (note that in pre-T cells, cyclin D1 appears to be the target for PKCθ). The dashed lines indicate the lack of knowledge of specific cell cycle targets for other PKC isozymes in T cells.
Although effects of PKC signaling have been noted in all stages of the cell cycle, the predominant actions of PKC isozymes are in G1 and G2 phases. Similarly, while PKCs can modulate the activity of multiple cell cycle regulatory molecules, consistent with effects in G1 and G2, D-type cyclins and Cip/Kip cdk inhibitors (p21Cip1 and p27Kip1) are emerging as important targets of PKC control. In keeping with the involvement of these proteins in regulation of quiescence, accumulating evidence indicates that controlling cell cycle entry and exit is an important role for PKC signaling. The ability of PKCs to promote G0 → G1 progression has been noted in several cell types (Chiu et al., 2002, 2003; Santiago-Walker et al., 2005). PKC signaling has also been shown to promote cell cycle exit in a number of systems, including intestinal epithelial cells, keratinocytes, PKC-overexpressing fibroblasts, and leukemic cell lines (Black, 2000, 2010). Studies in leukemia cells (Zhang and Chellappan, 1996; Vrana et al., 1998; Wang et al., 1998), non-transformed intestinal epithelial cells (Frey et al., 2000), pancreatic cancer cells (Detjen et al., 2000), and keratinocytes (Tibudan et al., 2002) indicate that PKC family members are capable of activating a complete program of cell cycle withdrawal, which can include downregulation of cyclin D1, upregulation of p21Cip1 and p27Kip1, alterations in the expression and phosphorylation of the pocket proteins p107, pRb, and p130, and changes in E2F expression and complex formation (Zhang and Chellappan, 1996; Saunders et al., 1998). While the ability of PKC signaling to promote exit from quiescence following TCR/CD28 and pre-TCR engagement is established, further studies are required to define its role in promoting cell cycle exit during T cell development and the establishment of quiescent memory T cells.
SPECIFIC CELL CYCLE TARGETS OF PKC SIGNALING IN T CELLS
Antigen-induced proliferation is a key aspect of both T cell differentiation and clonal expansion (Koch and Radtke, 2011). Thus, the mechanisms underlying PKC isozyme-specific effects on the cell cycle machinery in T cells are of critical importance to immune function. As noted above, the cell cycle is tightly regulated by coordinated actions of cyclins, cdks and ckis, which modulate the activity of the retinoblastoma family and thus expression of E2F-dependent genes (Figure 1). Proliferative T cell signaling affects multiple members of this control network. For example, proliferation induced by TCR/CD28 costimulation is associated with increased pRb phosphorylation by cyclin D2/3 and cyclin E, and enhanced transcription of E2F-dependent genes such as cyclins E and A (Colombetti et al., 2006). Analysis of mechanisms underlying these changes has pointed to a particularly important role for cyclin D3, cdk6, and p27Kip1 in regulation of T cell proliferation. For example, cyclin D3 and cdk6 knockout mice show defects in T cell proliferation, whereas cdk4 and cdk2 knockout mice do not (Sicinska et al., 2003; Hu et al., 2009), and p27Kip1 null T cells show reduced mitogen requirements and are resistant to anergy (Mohapatra et al., 2001; Rowell et al., 2005; Li et al., 2006a).
While PKC activation mediates TCR signaling to NF-κB, NFAT, and Ap1, transcription factors that have been shown to have a direct role in regulation of the cell cycle machinery in T cells, the function of specific PKCs in these effects remains largely unexplored. However, limited information is emerging to indicate that, as in other cell types, D-type cyclins and Cip/Kip proteins are important targets of PKC in these cells. In keeping with the greater attention that has been paid to PKCθ, this evidence primarily concerns the effects of this isozyme. For example, saikosaponins inhibit PKCθ translocation and cause a G0/G1 arrest in activated T cells through downregulation of cdk6 and cyclin D3 and upregulation of p27Kip1 protein levels (Leung et al., 2005; Sun et al., 2009). A link to p27Kip1 is also supported by the finding that PKCθ loss leads to anergy (Deenick et al., 2010), a process that involves upregulation of this cki (Li et al., 2006a; Wells, 2007, 2009). Through its role in assembly of the CBM signalosome, PKCθ has also been implicated in regulation of cyclin E stability in T cells (Srivastava et al., 2012).
Evidence also points to an ability of PKCθ to regulate cyclin D3 and p27Kip1 in pre-T cells. These molecules are downstream of the pre-TCR and PKCθ is an important mediator of signaling from this receptor (Felli et al., 2004; Aifantis et al., 2006; Talora et al., 2006). Pre-TCR activation of PKCθ cooperates with Notch3 to induce cyclin D1 in lymphomagenesis, indicating that this cyclin can also be a target for PKCθ in these cells.
Surprisingly, p27Kip1 also appears to be involved in PKC-mediated cell cycle arrest following cytokine withdrawal in T cells. IL-7 withdrawal from the D1 thymocyte cell line results in G1 arrest due to upregulation of p27Kip1 (Li et al., 2006b). Notably, PKCθ is activated by IL-7 withdrawal in these cells and the upregulation of p27Kip1 could be blocked by a general PKC inhibitor. While these studies do not exclude other PKCs, p27Kip1 upregulation was not blocked by the classical PKC inhibitor Gö6976, indicating that the effect was mediated by novel or atypical isozyme(s) (Li et al., 2006b).
SIGNALING DOWNSTREAM OF PKC IN REGULATION OF THE CELL CYCLE
While cell cycle-specific effects of PKCs can involve direct phosphorylation of cell cycle regulatory molecules (Goss et al., 1994; Acevedo-Duncan et al., 2002; Scott et al., 2002), the effect of PKCs on the cell cycle is generally indirect and involves downstream signaling cascades. Several signaling pathways, including those involving PI-3K/Akt (e.g., Belguise and Sonenshein, 2007; Bakker et al., 2008; Ou et al., 2008) and Wnt-β-catenin (e.g., Gwak et al., 2009; Murray et al., 2009), have been implicated in PKC proliferative signaling. However, analysis of multiple systems has highlighted the Ras/Raf/MEK/Erk pathway as a particularly important mediator of proliferative effects of PKCs. Most members of the PKC family, including PKCα, PKCβ, PKCλ, PKCδ, PKCε, PKCζ, and PKCθ, can target this pathway in many cell types (Kampfer et al., 2001; Chiles, 2004; Clark et al., 2004; Jackson and Foster, 2004; Koike et al., 2006; Bakker et al., 2008). Activation can occur at multiple steps in the Ras–Raf–MEK–Erk cascade. For example, PKCα can intersect the pathway at the level of Ras (Clark et al., 2004) or downstream of Ras through direct phosphorylation of Raf (Kolch et al., 1993). Erk activation mediates the effects of PKC signaling on several cell cycle regulatory molecules, including D-type cyclins and Cip/Kip ckis (Kampfer et al., 2001; Clark et al., 2004; Koike et al., 2006; Matsumoto et al., 2006; Black, 2010; Ranta et al., 2011). Interestingly, Erk signaling can facilitate both positive and negative effects of PKC on cell cycle targets and cell proliferation, and can mediate divergent effects on individual cell cycle molecules even within a single cell type. For example, our analysis has determined that Erk signaling is required for both PKCα-induced cyclin D1 downregulation and PKCε-induced cyclin D1 upregulation in intestinal epithelial cells (Clark et al., 2004; F. Hao, M. A Pysz, A. R. Black and J. D. Black, unpublished data). Thus, in keeping with the complexity associated with the proliferative consequences of PKC activation in general, the effects mediated by Erk signaling are highly context-dependent. While it has been proposed that the duration of activation dictates the proliferative outcome of Erk signaling (cf. Yasuda and Kurosaki, 2008), the anti-proliferative effects of PKCα and the pro-proliferative effects of PKCε both require prolonged Erk activation, with differences in the localization of activated Erk providing a possible explanation for the divergent effects (Clark et al., 2004).
Erk signaling is important for pre-T cell and T cell proliferation (Yasuda and Kurosaki, 2008), pointing to the possible role of a PKC–Erk signaling axis in these cells. It has been proposed that Sos and RasGRP1 cooperate to regulate the sensitivity, duration, and amplitude of Erk signaling in T cells (Yasuda and Kurosaki, 2008). Although analysis of the roles of PKC isozymes in Erk activation in this system is complicated by the fact that RasGRP1 is also a DAG/phorbol ester activated protein (Yasuda and Kurosaki, 2008), siRNA-based analysis has led to the suggestion that PKC may mediate RasGRP1-independent Erk activation in T lymphocytes (Warnecke et al., 2012). This idea opens the possibility that the proliferative response in T cells may be regulated by the coordinated effects of PKC isozymes, Sos-GRB2 and RasGRP1 on Erk activation.
SUMMARY AND PERSPECTIVES
Although understanding of the impact of PKC signaling on the cell cycle machinery in T cells remains limited, several similarities with other cell types are beginning to emerge (Figure 2). As in other cell types, D-type cyclins and Cip/Kip ckis appear to be major targets of PKC signaling in T cells, pointing to effects in G1 and G2. To date, the majority of findings have indicated positive effects of PKCs on cell cycle progression in T cells. However, it should be noted that this may largely reflect a focus on the consequences of T cell activation, which would bias findings in that direction. Evidence for anti-proliferative effects of PKC signaling is indeed accumulating, with PKCδ emerging as a negative regulator. Further analysis is required to identify cell cycle targets which mediate these inhibitory effects. The context-dependence of PKC isozyme-mediated cell cycle regulation observed in other systems has also been noted in T cells, exemplified by the ability of PKCθ to both promote and inhibit T cell proliferation/cell cycle progression. Despite these advances, it is clear that understanding of the cell cycle-specific effects of individual PKC isozymes in T cells is still in its infancy. In addition to delineation of the cell cycle roles of individual PKC isozymes and identification of specific cell cycle targets, issues that remain to be addressed include (a) how the different signaling environments in T cell subsets affect PKC cell cycle signaling, (b) whether PKC signaling plays a role in maintenance of quiescence in T cells and in control of quiescence-related regulators such as FOXO and Krüppel-like transcription factors (Black et al., 2001; Wu and Lingrel, 2004; Vucenik et al., 2005; Hart et al., 2012; Warnecke et al., 2012), and (c) what mechanisms underlie the differential involvement of individual PKCs in T cell proliferation in vitro and in vivo. Given the emerging importance of mTOR in immune function (Powell et al., 2012), an area of particular interest is the interplay between PKC and mTOR signaling in control of T cell proliferation under the metabolic conditions in which activation occurs in vivo. Other areas that remain to be addressed are the relative contribution of direct activation by TCR/CD28 and of activation by secreted cytokines to PKC-mediated proliferative responses, as well as the role of cell survival in the proliferative effects of PKC manipulation, especially in vivo. With increasing knowledge of TCR and cytokine signaling and the availability of mouse models for analysis of PKC isozyme function in vivo, it is anticipated that a link between PKC and growth-inhibitory signaling in T cells will be confirmed, and that the molecular details underlying the effects of individual PKC isozymes on the cell cycle in T cell subsets will be elucidated in the near future.
Conflict of Interest Statement
The authors declare that there search was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank past and present members of the laboratory for their contributions to the study of PKC and control of cell cycle progression. We also thank Dr. Sharon S. Evans for critical reading of the manuscript and helpful suggestions. We apologize to many colleagues whose work was not cited due to space limitations. Support from NIH grants DK54909, DK60632, CA6056, and CA036727 is gratefully acknowledged.
REFERENCES
- Abraham C., Scaglione-Sewell B., Skarosi S. F., Qin W., Bissonnette M., Brasitus T. A. (1998). Protein kinase C alpha modulates growth and differentiation in Caco-2 cells. Gastroenterology 114 503–509 [DOI] [PubMed] [Google Scholar]
- Acevedo-Duncan M., Patel R., Whelan S., Bicaku E. (2002). Human glioma PKC-iota and PKC-betaII phosphorylate cyclin-dependent kinase activating kinase during the cell cycle. Cell Prolif. 35 23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acs P., Beheshti M., Szallasi Z., Li L., Yuspa S. H., Blumberg P. M. (2000). Effect of a tyrosine 155 to phenylalanine mutation of protein kinase cdelta on the proliferative and tumorigenic properties of NIH 3T3 fibroblasts. Carcinogenesis 21 887–891 [DOI] [PubMed] [Google Scholar]
- Afrasiabi E., Ahlgren J., Bergelin N., Tornquist K. (2008). Phorbol 12-myristate 13-acetate inhibits FRO anaplastic human thyroid cancer cell proliferation by inducing cell cycle arrest in G1/S phase: evidence for an effect mediated by PKCdelta. Mol. Cell. Endocrinol. 292 26–35 [DOI] [PubMed] [Google Scholar]
- Aifantis I., Mandal M., Sawai K., Ferrando A., Vilimas T. (2006). Regulation of T-cell progenitor survival and cell-cycle entry by the pre-T-cell receptor. Immunol. Rev. 209 159–169 [DOI] [PubMed] [Google Scholar]
- Alarcón B., Mestre D, Martínez-Martín N. (2011). The immunological synapse: a cause or consequence of T-cell receptor triggering? Immunology 133 420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alisi A., Spagnuolo S., Napoletano S., Spaziani A., Leoni S. (2004). Thyroid hormones regulate DNA-synthesis and cell-cycle proteins by activation of PKCalpha and p42/44 MAPK in chick embryo hepatocytes. J. Cell. Physiol. 201 259–265 [DOI] [PubMed] [Google Scholar]
- Altman A., Isakov N., Baier G. (2000). Protein kinase Ctheta: a new essential superstar on the T-cell stage. Immunol. Today 21 567–573 [DOI] [PubMed] [Google Scholar]
- Altman A., Mustelin T., Coggeshall K. M. (1990). T lymphocyte activation: a biological model of signal transduction. Crit. Rev. Immunol. 10 347–391 [PubMed] [Google Scholar]
- Ashton A. W., Watanabe G., Albanese C., Harrington E. O., Ware J. A., Pestell R. G. (1999). Protein kinase Cdelta inhibition of S-phase transition in capillary endothelial cells involves the cyclin-dependent kinase inhibitor p27(Kip1). J. Biol. Chem. 274 20805–20811 [DOI] [PubMed] [Google Scholar]
- Bae K. M., Wang H., Jiang G., Chen M. G., Lu L., Xiao L. (2007). Protein kinase C epsilon is overexpressed in primary human non-small cell lung cancers and functionally required for proliferation of non-small cell lung cancer cells in a p21/Cip1-dependent manner. Cancer Res. 67 6053–6063 [DOI] [PubMed] [Google Scholar]
- Bakker W., Sipkema P., Stehouwer C. D. A., Serne E. H., Smulders Y. M., Van Hinsbergh V. W. M., et al. (2008). Protein kinase C θ activation induces insulin-mediated constriction of muscle resistance arteries. Diabetes 57 706–713 [DOI] [PubMed] [Google Scholar]
- Balciunaite E., Kazlauskas A. (2001). Early phosphoinositide 3-kinase activity is required for late activation of protein kinase Cepsilon in platelet-derived-growth-factor-stimulated cells: evidence for signalling across a large temporal gap. Biochem. J. 358 281–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belguise K., Sonenshein G. E. (2007). PKCθ promotes c-Rel-driven mammary tumorigenesis in mice and humans by repressing estrogen receptor α synthesis. J. Clin. Invest. 117 4009–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A., Yong V. W. (2000). Involvement of p21(Waf1/Cip1) in protein kinase C alpha-induced cell cycle progression. Mol. Cell. Biol. 20 4580–4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D., Ng D., Tu C. L., Oda Y., Xie Z. (2001). Calcium- and vitamin D-regulated keratinocyte differentiation. Mol. Cell. Endocrinol. 177 161–171 [DOI] [PubMed] [Google Scholar]
- Bird J. J., Brown D. R., Mullen A. C., Moskowitz N. H., Mahowald M. A., Sider J. R., et al. (1998). Helper T cell differentiation is controlled by the cell cycle. Immunity 9 229–237 [DOI] [PubMed] [Google Scholar]
- Black A. R., Black J. D., Azizkhan-Clifford J. (2001). Sp1 and krüppel-like factor family of transcription factors in cell growth regulation and cancer. J. Cell. Physiol. 188 143–160 [DOI] [PubMed] [Google Scholar]
- Black J. D. (2000). Protein kinase C-mediated regulation of the cell cycle. Front. Biosci. 5 D406–D423 [DOI] [PubMed] [Google Scholar]
- Black J. D. (2010). “PKC and control of the cell cycle,” in Protein Kinase C in Cancer Signaling and Therapy, edKazanietz M. G. (New York: Humana Press; ) 155–188 [Google Scholar]
- Black J. D., Koury S. T., Bankert R. B., Repasky E. A. (1988). Heterogeneity in lymphocyte spectrin distribution: ultrastructural identification of a new spectrin-rich cytoplasmic structure. J. Cell Biol. 106 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. K., Maddali K. K., Dhulipala V. C., Korzick D. H. (2007). PKCdelta mediates anti-proliferative, pro-apoptic effects of testosterone on coronary smooth muscle. Am. J. Physiol. Cell Physiol. 293 C805–C813 [DOI] [PubMed] [Google Scholar]
- Breitkreutz D., Braiman-Wiksman L., Daum N., Denning M. F., Tennenbaum T. (2007). Protein kinase C family: on the crossroads of cell signaling in skin and tumor epithelium. J. Cancer Res. Clin. Oncol. 133 793–808 [DOI] [PubMed] [Google Scholar]
- Buitrago C. G., Pardo V. G., De Boland A. R., Boland R. (2003). Activation of RAF-1 through Ras and protein kinase Calpha mediates 1alpha,25(OH)2-vitamin D3 regulation of the mitogen-activated protein kinase pathway in muscle cells. J. Biol. Chem. 278 2199–2205 [DOI] [PubMed] [Google Scholar]
- Cabodi S., Calautti E., Talora C., Kuroki T., Stein P. L., Dotto G. P. (2000). A PKC-eta/Fyn-dependent pathway leading to keratinocyte growth arrest and differentiation. Mol. Cell 6 1121–1129 [DOI] [PubMed] [Google Scholar]
- Cameron A. J., Procyk K. J., Leitges M., Parker P. J. (2008). PKC alpha protein but not kinase activity is critical for glioma cell proliferation and survival. Int. J. Cancer 123 769–779 [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. (1982). Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J. Biol. Chem. 257 7847–7851 [PubMed] [Google Scholar]
- Castoria G., Migliaccio A., Di Domenico M., Lombardi M., De Falco A., Varricchio L., et al. (2004). Role of atypical protein kinase C in estradiol-triggered G1/S progression of MCF-7 cells. Mol. Cell. Biol. 24 7643–7653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejas P. J., Carlson L. M., Zhang J., Padmanabhan S., Kolonias D., Lindner I., et al. (2005). Protein kinase C beta II plays an essential role in dendritic cell differentiation and autoregulates its own expression. J. Biol. Chem. 280 28412–28423 [DOI] [PubMed] [Google Scholar]
- Cerda S. R., Mustafi R., Little H., Cohen G., Khare S., Moore C., et al. (2006). Protein kinase C delta inhibits Caco-2 cell proliferation by selective changes in cell cycle and cell death regulators. Oncogene 25 3123–3138 [DOI] [PubMed] [Google Scholar]
- Chen A., Davis B. H., Bissonnette M., Scaglione-Sewell B., Brasitus T. A. (1999). 1,25-Dihydroxyvitamin D(3) stimulates activator protein-1-dependent Caco-2 cell differentiation. J. Biol. Chem. 274 35505–35513 [DOI] [PubMed] [Google Scholar]
- Chen D., Purohit A., Halilovic E., Doxsey S. J., Newton A. C. (2004). Centrosomal anchoring of protein kinase C betaII by pericentrin controls microtubule organization, spindle function, and cytokinesis. J. Biol. Chem. 279 4829–4839 [DOI] [PubMed] [Google Scholar]
- Chen W., Schweins E., Chen X., Finn O. J., Cheever M. A. (1994). Retroviral transduction of protein kinase C-gamma into tumor-specific T cells allows antigen-independent long-term growth in IL-2 with retention of functional specificity in vitro and ability to mediate tumor therapy in vivo. J. Immunol. 153 3630–3638 [PubMed] [Google Scholar]
- Chiles T. C. (2004). Regulation and function of cyclin D2 in B lymphocyte subsets. J. Immunol. 173 2901–2907 [DOI] [PubMed] [Google Scholar]
- Chiu T., Santiskulvong C., Rozengurt E. (2003). ANG II stimulates PKC-dependent ERK activation, DNA synthesis, and cell division in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 285 G1–G11 [DOI] [PubMed] [Google Scholar]
- Chiu T., Wu S. S., Santiskulvong C., Tangkijvanich P., Yee H. F., Jr., Rozengurt E. (2002). Vasopressin-mediated mitogenic signaling in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 282 C434–C450 [DOI] [PubMed] [Google Scholar]
- Cho C. H., Lee C. S., Chang M., Jang I. H., Kim S. J., Hwang I., et al. (2004). Localization of VEGFR-2 and PLD2 in endothelial caveolae is involved in VEGF-induced phosphorylation of MEK and ERK. Am. J. Physiol. Heart Circ. Physiol. 286 H1881–H1888 [DOI] [PubMed] [Google Scholar]
- Cho Y., Tighe A. P., Talmage D. A. (1997). Retinoic acid induced growth arrest of human breast carcinoma cells requires protein kinase C alpha expression and activity. J. Cell. Physiol. 172 306–313 [DOI] [PubMed] [Google Scholar]
- Choi P. M., Tchou-Wong K. M., Weinstein I. B. (1990). Overexpression of protein kinase C in HT29 colon cancer cells causes growth inhibition and tumor suppression. Mol. Cell. Biol. 10 4650–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. A., Black A. R., Leontieva O. V., Frey M. R., Pysz M. A., Kunneva L., et al. (2004). Involvement of the ERK signaling cascade in protein kinase C-mediated cell cycle arrest in intestinal epithelial cells. J. Biol. Chem. 279 9233–9247 [DOI] [PubMed] [Google Scholar]
- Classon M., Dyson N. (2001). p107 and p130: versatile proteins with interesting pockets. Exp. Cell Res. 264 135–147 [DOI] [PubMed] [Google Scholar]
- Cobrinik D. (2005). Pocket proteins and cell cycle control. Oncogene 24 2796–2809 [DOI] [PubMed] [Google Scholar]
- Colombetti S., Basso V., Mueller D. L., Mondino A. (2006). Prolonged TCR/CD28 engagement drives IL-2-independent T cell clonal expansion through signaling mediated by the mammalian target of rapamycin. J. Immunol. 176 2730–2738 [DOI] [PubMed] [Google Scholar]
- Czifra G., Toth I. B., Marincsak R., Juhasz I., Kovacs I., Acs P., et al. (2006). Insulin-like growth factor-I-coupled mitogenic signaling in primary cultured human skeletal muscle cells and in C2C12 myoblasts. A central role of protein kinase Cdelta. Cell. Signal. 18 1461–1472 [DOI] [PubMed] [Google Scholar]
- Deenick E. K., Po L., Chapatte L., Murakami K., Lu Y.-C., Elford A. R., et al. (2010). c-Rel phenocopies PKCθ but not Bcl-10 in regulating CD8+ T-cell activation versus tolerance. Eur. J. Immunol. 40 867–877 [DOI] [PubMed] [Google Scholar]
- Desai S. R., Pillai P. P., Patel R. S., Mccray A. N., Win-Piazza H. Y., Acevedo-Duncan M. E. (2012). Regulation of Cdk7 activity through a phosphatidylinositol (3)-kinase/PKC-I-mediated signaling cascade in glioblastoma. Carcinogenesis 33 10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detjen K. M., Brembeck F. H., Welzel M., Kaiser A., Haller H., Wiedenmann B., et al. (2000). Activation of protein kinase Calpha inhibits growth of pancreatic cancer cells via p21(cip)-mediated G(1) arrest. J. Cell Sci. 113 (Pt17) 3025–3035 [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. (1978). Stimulation of DNA synthesis by tumour promoter and pure mitogenic factors. Nature 276 723–726 [DOI] [PubMed] [Google Scholar]
- Dreikhausen U. E., Gorf K., Resch K., Szamel M. (2003). Protein kinase Cbeta1, a major regulator of TCR-CD28-activated signal transduction leading to IL-2 gene transcription and secretion. Int. Immunol. 15 1089–1098 [DOI] [PubMed] [Google Scholar]
- Du W., Pogoriler J. (2006). Retinoblastoma family genes. Oncogene 25 5190–5200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Depoil D. (2011). New insights into the T cell synapse from single molecule techniques. Nat. Rev. Immunol. 11 672–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder A. M., Sui X., Rosen D. G., Nolden L. K., Cheng K. W., Lahad J. P., et al. (2005). Atypical PKCι contributes to poor prognosis through loss of apical–basal polarity and Cyclin E overexpression in ovarian cancer. Proc. Natl. Acad. Sci. U.S.A. 102 12519–12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farren M., Carlson L., Lee K. (2010). Tumor-mediated inhibition of dendritic cell differentiation is mediated by down regulation of protein kinase C beta II expression. Immunol. Res. 46 165–176 [DOI] [PubMed] [Google Scholar]
- Felli M. P., Vacca A., Calce A., Bellavia D., Campese A. F., Grillo R., et al. (2004). PKC[theta] mediates pre-TCR signaling and contributes to Notch3-induced T-cell leukemia. Oncogene 24 992–1000 [DOI] [PubMed] [Google Scholar]
- Fields A. P., Regala R. P. (2007). Protein kinase C iota: human oncogene, prognostic marker and therapeutic target. Pharmacol. Res. 55 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fima E., Shtutman M., Libros P., Missel A., Shahaf G., Kahana G., et al. (2001). PKCeta enhances cell cycle progression, the expression of G1 cyclins and p21 in MCF-7 cells. Oncogene 20 6794–6804 [DOI] [PubMed] [Google Scholar]
- Fisher R. P., Morgan D. O. (1994). A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell 78 713–724 [DOI] [PubMed] [Google Scholar]
- Fooksman D. R., Vardhana S., Vasiliver-Shamis G., Liese J., Blair D. A., Waite J., et al. (2010). Functional anatomy of T cell activation and synapse formation. Annu. Rev. Immunol. 28 79–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeley M., Long A. (2012). Regulating the regulator: phosphorylation of PKC theta in T cells. Front. Immunol. 3:227 10.3389/fimmu.2012.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey M. R., Clark J. A., Bateman N. W., Kazanietz M. G., Black A. R., Black J. D. (2004). Cell cycle- and protein kinase C-specific effects of resiniferatoxin and resiniferonol 9,13,14-ortho-phenylacetate in intestinal epithelial cells. Biochem. Pharmacol. 67 1873–1886 [DOI] [PubMed] [Google Scholar]
- Frey M. R., Clark J. A., Leontieva O., Uronis J. M., Black A. R., Black J. D. (2000). Protein kinase C signaling mediates a program of cell cycle withdrawal in the intestinal epithelium. J. Cell Biol. 151 763–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey M. R., Saxon M. L., Zhao X., Rollins A., Evans S. S., Black J. D. (1997). Protein kinase C isozyme-mediated cell cycle arrest involves induction of p21(waf1/cip1) and p27(kip1) and hypophosphorylation of the retinoblastoma protein in intestinal epithelial cells. J. Biol. Chem. 272 9424–9435 [DOI] [PubMed] [Google Scholar]
- Fu G., Gascoigne N. R. (2012). The role of protein kinase Cη in T cell biology. Front. Immunol. 3:177 10.3389/fimmu.2012.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G., Hu J., Niederberger-Magnenat N., Rybakin V., Casas J., Yachi P. P., et al. (2011). Protein kinase C eta is required for T cell activation and homeostatic proliferation. Sci. Signal. 4 ra84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S., Nishizawa Y., Hosoi M., Koyama H., Yamakawa K., Ohno S., et al. (1997). Protein kinase C delta inhibits the proliferation of vascular smooth muscle cells by suppressing G1 cyclin expression. J. Biol. Chem. 272 13816–13822 [DOI] [PubMed] [Google Scholar]
- Gaboardi G. C., Ramazzotti G., Bavelloni A., Piazzi M., Fiume R., Billi A. M., et al. (2010). A role for PKCε during C2C12 myogenic differentiation. Cell. Signal. 22 629–635 [DOI] [PubMed] [Google Scholar]
- Genot E. M., Parker P. J., Cantrell D. A. (1995). Analysis of the role of protein kinase C-α, -ε, and -ϖ in T cell activation. J. Biol. Chem. 270 9833–9839 [DOI] [PubMed] [Google Scholar]
- Giannoni F., Lyon A. B., Wareing M. D., Dias P. B., Sarawar S. R. (2005). Protein kinase C theta is not essential for T-cell-mediated clearance of murine gammaherpesvirus 68. J. Virol. 79 6808–6813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez J., Pitton C., Garcia A., Martinez De Aragon A., Silva A., Rebollo A. (1995). The zeta isoform of protein kinase C controls interleukin-2-mediated proliferation in a murine T cell line: evidence for an additional role of protein kinase C epsilon and beta. Exp. Cell Res. 218 105–113 [DOI] [PubMed] [Google Scholar]
- Goss V. L., Hocevar B. A., Thompson L. J., Stratton C. A., Burns D. J., Fields A. P. (1994). Identification of nuclear beta II protein kinase C as a mitotic lamin kinase. J. Biol. Chem. 269 19074–19080 [PubMed] [Google Scholar]
- Graham M. A., Rawe I., Dartt D. A., Joyce N. C. (2000). Protein kinase C regulation of corneal endothelial cell proliferation and cell cycle. Invest. Ophthalmol. Vis. Sci. 41 4124–4132 [PubMed] [Google Scholar]
- Grana X., Garriga J., Mayol X. (1998). Role of the retinoblastoma protein family, pRB, p107 and p130 in the negative control of cell growth. Oncogene 17 3365–3383 [DOI] [PubMed] [Google Scholar]
- Gregorio C. C., Kubo R. T., Bankert R. B., Repasky E. A. (1992). Translocation of spectrin and protein kinase C to a cytoplasmic aggregate upon lymphocyte activation. Proc. Natl. Acad. Sci. U.S.A. 89 4947–4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio C. C., Repasky E. A., Fowler V. M., Black J. D. (1994). Dynamic properties of ankyrin in T lymphocytes: colocalization with spectrin and protein kinase C beta. J. Cell Biol. 125 345–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griner E. M., Kazanietz M. G. (2007). Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer 7 281–294 [DOI] [PubMed] [Google Scholar]
- Grossoni V. C., Falbo K. B., Kazanietz M. G., De Kier Joffe E. D., Urtreger A. J. (2007). Protein kinase C delta enhances proliferation and survival of murine mammary cells. Mol. Carcinog. 46 381–390 [DOI] [PubMed] [Google Scholar]
- Gruber T., Barsig J., Pfeifhofer C., Ghaffari-Tabrizi N., Tinhofer I., Leitges M., et al. (2005a). PKCdelta is involved in signal attenuation in CD3+ T cells. Immunol. Lett. 96 291–293 [DOI] [PubMed] [Google Scholar]
- Gruber T., Thuille N., Hermann-Kleiter N., Leitges M., Baier G. (2005b). Protein kinase Cepsilon is dispensable for TCR/CD3-signaling. Mol. Immunol. 42 305–310 [DOI] [PubMed] [Google Scholar]
- Gruber T., Fresser F., Jenny M., Uberall F., Leitges M., Baier G. (2008). PKCtheta cooperates with atypical PKCzeta and PKCiota in NF-kappaB transactivation of T lymphocytes. Mol. Immunol. 45 117–126 [DOI] [PubMed] [Google Scholar]
- Gruber T., Hermann-Kleiter N., Pfeifhofer-Obermair C., Lutz-Nicoladoni C., Thuille N., Letschka T., et al. (2009). PKCθ cooperates with PKCα in alloimmune responses of T cells in vivo. Mol. Immunol. 46 2071–2079 [DOI] [PubMed] [Google Scholar]
- Guan L., Song K., Pysz M. A., Curry K. J., Hizli A. A., Danielpour D., et al. (2007). Protein kinase C-mediated down-regulation of cyclin D1 involves activation of the translational repressor 4E-BP1 via a phosphoinositide 3-kinase/Akt-independent, protein phosphatase 2A-dependent mechanism in intestinal epithelial cells. J. Biol. Chem. 282 14213–14225 [DOI] [PubMed] [Google Scholar]
- Gwak J., Jung S. J., Kang D. I., Kim E. Y., Kim D. E., Chung Y. H., et al. (2009). Stimulation of protein kinase C-alpha suppresses colon cancer cell proliferation by down-regulation of beta-catenin. J. Cell. Mol. Med. 13 2171–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao F., Pysz M. A., Curry K. J., Haas K. N., Seedhouse S. J., Black A. R., et al. (2011). Protein kinase Calpha signaling regulates inhibitor of DNA binding 1 in the intestinal epithelium. J. Biol. Chem. 286 18104–18117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. T., Hogquist K. A., Jameson S. C. (2012). Krüppel-like factors in lymphocyte biology. J. Immunol. 188 521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizli A. A., Black A. R., Pysz M. A., Black J. D. (2006). Protein kinase C alpha signaling inhibits cyclin D1 translation in intestinal epithelial cells. J. Biol. Chem. 281 14596–14603 [DOI] [PubMed] [Google Scholar]
- Housey G. M., Johnson M. D., Hsiao W. L., O’Brian C. A., Murphy J. P., Kirschmeier P., et al. (1988). Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell 52 343–354 [DOI] [PubMed] [Google Scholar]
- Hu M. G., Deshpande A., Enos M., Mao D., Hinds E. A., Hu G.-F., et al. (2009). A requirement for cyclin-dependent kinase 6 in thymocyte development and tumorigenesis. Cancer Res. 69 810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H. C., Clurman B. E. (2005). Cyclin E in normal and neoplastic cell cycles. Oncogene 24 2776–2786 [DOI] [PubMed] [Google Scholar]
- Isakov N., Altman A. (1987). Human T lymphocyte activation by tumor promoters: role of protein kinase C. J. Immunol. 138 3100–3107 [PubMed] [Google Scholar]
- Isakov N., Altman A. (2012). PKC-theta-mediated signal delivery from the TCR/CD28 surface receptors. Front. Immunol. 3:273 10.3389/fimmu.2012.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakov N., Scholz W., Altman A. (1986). Signal transduction and intracellular events in T-lymphocyte activation. Immunol. Today 7 271–277 [DOI] [PubMed] [Google Scholar]
- Ishino K., Ohba M., Kashiwagi M., Kawabe S., Chida K., Kuroki T. (1998). Phorbol ester-induced G1 arrest in BALB/MK-2 mouse keratinocytes is mediated by delta and eta isoforms of protein kinase C. Jpn. J. Cancer Res. 89 1126–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T., Hagiwara M., Hidaka H., Isomura T., Kioussis D., Nakashima I. (1992). Accelerated proliferation and interleukin-2 production of thymocytes by stimulation of soluble anti-CD3 monoclonal antibody in transgenic mice carrying a rabbit protein kinase C alpha. J. Biol. Chem. 267 18644–18648 [PubMed] [Google Scholar]
- Jackson D. N., Foster D. A. (2004). The enigmatic protein kinase Cdelta: complex roles in cell proliferation and survival. FASEB J. 18 627–636 [DOI] [PubMed] [Google Scholar]
- Kambhampati S., Li Y., Verma A., Sassano A., Majchrzak B., Deb D. K., et al. (2003). Activation of protein kinase C delta by all-trans-retinoic acid. J. Biol. Chem. 278 32544–32551 [DOI] [PubMed] [Google Scholar]
- Kampfer S., Windegger M., Hochholdinger F., Schwaiger W., Pestell R. G., Baier G., et al. (2001). Protein kinase C isoforms involved in the transcriptional activation of cyclin D1 by transforming Ha-Ras. J. Biol. Chem. 276 42834–42842 [DOI] [PubMed] [Google Scholar]
- Kang S. J., Lee Y. J., Lee E.-K., Kwak M.-K. (2012). Silver nanoparticles-mediated G2/M cycle arrest of renal epithelial cells is associated with NRF2-GSH signaling. Toxicol. Lett. 211 334–341 [DOI] [PubMed] [Google Scholar]
- Kashiwagi M., Ohba M., Chida K., Kuroki T. (2002). Protein kinase C eta (PKC eta): its involvement in keratinocyte differentiation. J. Biochem. 132 853–857 [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. (1983). Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J. Biol. Chem. 258 11442–11445 [PubMed] [Google Scholar]
- Kitamura K., Mizuno K., Etoh A., Akita Y., Miyamoto A., Nakayama K., et al. (2003). The second phase activation of protein kinase C delta at late G1 is required for DNA synthesis in serum-induced cell cycle progression. Genes Cells 8 311–324 [DOI] [PubMed] [Google Scholar]
- Koch U., Radtke F. (2011). Mechanisms of T cell development and transformation. Annu. Rev. Cell Dev. Biol. 27 539–562 [DOI] [PubMed] [Google Scholar]
- Koike K., Fujii T., Nakamura A. M., Yokoyama G., Yamana H., Kuwano M., et al. (2006). Activation of protein kinase C delta induces growth arrest in NPA thyroid cancer cells through extracellular signal-regulated kinase mitogen-activated protein kinase. Thyroid 16 333–341 [DOI] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., et al. (1993). Protein kinase C[alpha] activates RAF-1 by direct phosphorylation. Nature 364 249–252 [DOI] [PubMed] [Google Scholar]
- Kong K. F., Yokosuka T., Canonigo-Balancio A. J., Isakov N., Saito T., Altman A. (2011). A motif in the V3 domain of the kinase PKC-theta determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat. Immunol. 12 1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretzky G. A., Wahi M., Newton M. E., Weiss A. (1989). Heterogeneity of protein kinase C isoenzyme gene expression in human T cell lines. Protein kinase C-beta is not required for several T cell functions. J. Immunol. 143 1692–1695 [PubMed] [Google Scholar]
- Leach K. L., James M. L., Blumberg P. M. (1983). Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc. Natl. Acad. Sci. U.S.A. 80 4208–4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Gudapati P., Dragovic S., Spencer C., Joyce S., Killeen N., et al. (2010). Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 32 743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kim D., Oh J., Yoon S., Choi M., Lee S., et al. (2011). Silver nanoparticles induce apoptosis and G2/M arrest via PKCϖ -dependent signaling in A549 lung cells. Arch. Toxicol. 85 1529–1540 [DOI] [PubMed] [Google Scholar]
- Leontieva O. V., Black J. D. (2004). Identification of two distinct pathways of protein kinase Calpha down-regulation in intestinal epithelial cells. J. Biol. Chem. 279 5788–5801 [DOI] [PubMed] [Google Scholar]
- Leung C. Y., Liu L., Wong R. N. S., Zeng Y. Y., Li M., Zhou H. (2005). Saikosaponin-d inhibits T cell activation through the modulation of PKCθ, JNK, and NF-κB transcription factor. Biochem. Biophys. Res. Commun. 338 1920–1927 [DOI] [PubMed] [Google Scholar]
- Li H., Weinstein I. B. (2006). Protein kinase C beta enhances growth and expression of cyclin D1 in human breast cancer cells. Cancer Res. 66 11399–11408 [DOI] [PubMed] [Google Scholar]
- Li L., Iwamoto Y., Berezovskaya A., Boussiotis V. A. (2006a). A pathway regulated by cell cycle inhibitor p27Kip1 and checkpoint inhibitor Smad3 is involved in the induction of T cell tolerance. Nat. Immunol. 7 1157–1165 [DOI] [PubMed] [Google Scholar]
- Li W. Q., Jiang Q., Aleem E., Kaldis P., Khaled A. R., Durum S. K. (2006b). IL-7 promotes T cell proliferation through destabilization of p27Kip1. J. Exp. Med. 203 573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Song R., Gao Y., Li Y., Wang S., Liu H.-Y., et al. (2012). Protein kinase C-δ negatively regulates T cell receptor-induced NF-κ B activation by inhibiting the assembly of CARMA1 signalosome. J. Biol. Chem. 287 20081–20087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh E., Shimon T., Bechor E., Doki Y., Schieren I., Weinstein I. B. (1996). Linking protein kinase C to the cell cycle: ectopic expression of PKC eta in NIH3T3 cells alters the expression of cyclins and Cdk inhibitors and induces adipogenesis. Oncogene 12 1545–1555 [PubMed] [Google Scholar]
- Lovatt M., Bijlmakers M.-J. (2010). Stabilisation of β -catenin downstream of T cell receptor signalling. PLoS ONE 5:e12794 10.1371/journal.pone.0012794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall R. M., Dubois J. H., Crumpton M. J. (1980). Ionomycin stimulates T-lymphocytes to grow. Biochem. Soc. Trans. 8 720–721 [DOI] [PubMed] [Google Scholar]
- Malumbres M., Barbacid M. (2005). Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 30 630–641 [DOI] [PubMed] [Google Scholar]
- Marsland B. J., Kopf M. (2008). T-cell fate and function: PKC-θ and beyond. Trends Immunol. 29 179–185 [DOI] [PubMed] [Google Scholar]
- Martín-Cófreces N. B., Robles-Valero J., Cabrero J. R., Mittelbrunn M., Gordón-Alonso M., Sung C.-H., et al. (2008). MTOC translocation modulates IS formation and controls sustained T cell signaling. J. Cell. Biol. 182 951–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Villares R., Rodriguez-Mascarenhas S., Zaballos A., Leitges M., Kovac J., et al. (2005). Control of T helper 2 cell function and allergic airway inflammation by PKCzeta. Proc. Natl. Acad. Sci. U.S.A. 102 9866–9871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto E., Hatanaka M., Bohgaki M., Maeda S. (2006). PKC pathway and ERK/MAPK pathway are required for induction of cyclin D1 and p21Waf1 during 12-o-tetradecanoylphorbol 13-acetate-induced differentiation of myeloleukemia cells. Kobe J. Med. Sci. 52 181–194 [PubMed] [Google Scholar]
- Matsuoka H., Tsubaki M., Yamazoe Y., Ogaki M., Satou T., Itoh T., et al. (2009). Tamoxifen inhibits tumor cell invasion and metastasis in mouse melanoma through suppression of PKC/MEK/ERK and PKC/PI3K/Akt pathways. Exp. Cell Res. 315 2022–2032 [DOI] [PubMed] [Google Scholar]
- Mirandola P., Gobbi G., Masselli E., Micheloni C., Di Marcantonio D., Queirolo V., et al. (2011). Protein kinase Cε regulates proliferation and cell sensitivity to TGF-1β of CD4+ T lymphocytes: implications for hashimoto thyroiditis. J. Immunol. 187 4721–4732 [DOI] [PubMed] [Google Scholar]
- Miyamoto A., Nakayama K., Imaki H., Hirose S., Jiang Y., Abe M., et al. (2002). Increased proliferation of B cells and auto-immunity in mice lacking protein kinase C[delta]. Nature 416 865–869 [DOI] [PubMed] [Google Scholar]
- Mohapatra S., Agrawal D., Pledger W. J. (2001). p27Kip1 regulates T cell proliferation. J. Biol. Chem. 276 21976–21983 [DOI] [PubMed] [Google Scholar]
- Murray N. R., Fields A. P. (1998). Phosphatidylglycerol is a physiologic activator of nuclear protein kinase C. J. Biol. Chem. 273 11514–11520 [DOI] [PubMed] [Google Scholar]
- Murray N. R., Kalari K. R., Fields A. P. (2011). Protein kinase Cι expression and oncogenic signaling mechanisms in cancer. J. Cell. Physiol. 226 879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. R., Weems J., Braun U., Leitges M., Fields A. P. (2009). Protein kinase C betaII and PKCiota/lambda: collaborating partners in colon cancer promotion and progression. Cancer Res. 69 656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove E. A., Caldon C. E., Barraclough J., Stone A., Sutherland R. L. (2011). Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 11 558–572 [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Oliva J. L., Kothapalli D., Fournier A., Assoian R. K., Kazanietz M. G. (2005). Phorbol ester-induced G1 phase arrest selectively mediated by protein kinase Cdelta-dependent induction of p21. J. Biol. Chem. 280 33926–33934 [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Fujii T., Yokoyama G., Kazanietz M. G., Yamana H., Shirouzu K. (2003). Cell growth inhibition by all-trans retinoic acid in SKBR-3 breast cancer cells: involvement of protein kinase Calpha and extracellular signal-regulated kinase mitogen-activated protein kinase. Mol. Carcinog. 38 106–116 [DOI] [PubMed] [Google Scholar]
- Nakaigawa N., Hirai S., Mizuno K., Shuin T., Hosaka M., Ohno S. (1996). Differential effects of overexpression of PKC alpha and PKC delta/epsilon on cellular E2F activity in late G1 phase. Biochem. Biophys. Res. Commun. 222 95–100 [DOI] [PubMed] [Google Scholar]
- Newton A. C. (2010). Protein kinase C: poised to signal. Am. J. Physiol. Endocrinol. Metab. 298 E395–E402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto N. (2007). Ethanol and fish oil induce NFκB transactivation of the collagen α2(I) promoter through lipid peroxidation – driven activation of the PKC–PI3K–Akt pathway. Hepatology 45 1433–1445 [DOI] [PubMed] [Google Scholar]
- Oliva J. L., Caino M. C., Senderowicz A. M., Kazanietz M. G. (2008). S-phase-specific activation of PKC alpha induces senescence in non-small cell lung cancer cells. J. Biol. Chem. 283 5466–5476 [DOI] [PubMed] [Google Scholar]
- Osada S., Hashimoto Y., Nomura S., Kohno Y., Chida K., Tajima O., et al. (1993). Predominant expression of nPKC eta, a Ca(2+)-independent isoform of protein kinase C in epithelial tissues, in association with epithelial differentiation. Cell Growth Differ. 4 167–175 [PubMed] [Google Scholar]
- Oster H., Leitges M. (2006). Protein kinase C alpha but not PKCzeta suppresses intestinal tumor formation in ApcMin/+ mice. Cancer Res. 66 6955–6963 [DOI] [PubMed] [Google Scholar]
- Ou W. B., Zhu M. J., Demetri G. D., Fletcher C. D. M., Fletcher J. A. (2008). Protein kinase C-[theta] regulates KIT expression and proliferation in gastrointestinal stromal tumors. Oncogene 27 5624–5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K., Li J., Corbit K. C., Rumilla K. M., Soh J. W., Weinstein I. B., et al. (2002). Regulation of airway smooth muscle cyclin D1 transcription by protein kinase C-delta. Am. J. Respir. Cell Mol. Biol. 27 204–213 [DOI] [PubMed] [Google Scholar]
- Paramio J. M., Segrelles C., Ruiz S., Jorcano J. L. (2001). Inhibition of protein kinase B (PKB) and PKCzeta mediates keratin K10-induced cell cycle arrest. Mol. Cell. Biol. 21 7449–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifhofer C., Gruber T., Letschka T., Thuille N., Lutz-Nicoladoni C., Hermann-Kleiter N., et al. (2006). Defective IgG2a/2b class switching in PKCα-/- mice. J. Immunol. 176 6004–6011 [DOI] [PubMed] [Google Scholar]
- Pfeifhofer C., Kofler K., Gruber T., Tabrizi N. G., Lutz C., Maly K., et al. (2003). Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J. Exp. Med. 197 1525–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai P., Desai S., Patel R., Sajan M., Farese R., Ostrov D., et al. (2011). A novel PKC-ι inhibitor abrogates cell proliferation and induces apoptosis in neuroblastoma. Int. J. Biochem. Cell Biol. 43 784–794 [DOI] [PubMed] [Google Scholar]
- Powell J. D., Pollizzi K. N., Heikamp E. B., Horton M. R. (2012). Regulation of immune responses by mTOR. Annu. Rev. Immunol. 30 39–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. V., Cai Y. C., Raab M., Duckworth B., Cantley L., Shoelson S. E., et al. (1994). T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc. Natl. Acad. Sci. U.S.A. 91 2834–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pysz M. A., Leontieva O. V., Bateman N. W., Uronis J. M., Curry K. J., Threadgill D. W., et al. (2009). PKCα tumor suppression in the intestine is associated with transcriptional and translational inhibition of cyclin D1. Exp. Cell Res. 315 1415–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranta F., Leveringhaus J., Theilig D., Schulz-Raffelt G., Hennige A. M., Hildebrand D. G., et al. (2011). Protein kinase C delta (PKCδ ) affects proliferation of insulin-secreting cells by promoting nuclear extrusion of the cell cycle inhibitor p21Cip1/WAF1. PLoS ONE 6:e28828 10.1371/journal.pone.0028828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings D. J., Sommer K., Moreno-Garcia M. E. (2006). The CARMA1 signalosome links the signalling machinery of adaptive and innate immunity in lymphocytes. Nat. Rev. Immunol. 6 799–812 [DOI] [PubMed] [Google Scholar]
- Repasky E. A., Black J. D. (1996). “Dynamic properties of the lymphocyte membrane-cytoskeleton: relationship to lymphocyte activation status, signal transduction, and protein kinase C,” in Current Topics in Membranes, ed. Nelson W. J. (San Diego: Academic Press; ) 313–343 [Google Scholar]
- Reyland M. E. (2009). Protein kinase C isoforms: multi-functional regulators of cell life and death. Front. Biosci. 14 2386–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse C., Linch M., Kermorgant S., Cameron A. J. M., Boeckeler K., Parker P. J. (2010). PKC and the control of localized signal dynamics. Nat. Rev. Mol. Cell Biol. 11 103–112 [DOI] [PubMed] [Google Scholar]
- Rowell E. A., Walsh M. C., Wells A. D. (2005). Opposing roles for the cyclin-dependent kinase inhibitor p27kip1 in the control of CD4+ T cell proliferation and effector function. J. Immunol. 174 3359–3368 [DOI] [PubMed] [Google Scholar]
- Rozengurt E. (1986). Early signals in the mitogenic response. Science 234 161–166 [DOI] [PubMed] [Google Scholar]
- Ryu J. M., Lee M. Y., Yun S. P., Han H. J. (2010). High glucose regulates cyclin D1/E of human mesenchymal stem cells through TGF-β1 expression via Ca2+/PKC/MAPKs and PI3K/Akt/mTOR signal pathways. J. Cell. Physiol. 224 59–70 [DOI] [PubMed] [Google Scholar]
- Sakowicz-Burkiewicz M., Nishanth G., Helmuth U., Drögemüller K., Busch D. H., Utermöhlen O., et al. (2008). Protein kinase C-theta critically regulates the proliferation and survival of pathogen-specific T cells in murine listeriosis. J. Immunol. 180 5601–5612 [DOI] [PubMed] [Google Scholar]
- Salek-Ardakani S., So T., Halteman B. S., Altman A., Croft M. (2004). Differential regulation of Th2 and Th1 lung inflammatory responses by protein kinase C theta. J. Immunol. 173 6440–6447 [DOI] [PubMed] [Google Scholar]
- Sanchez I., Dynlacht B. D. (2005). New insights into cyclins, CDKs, and cell cycle control. Semin. Cell Dev. Biol. 16 311–321 [DOI] [PubMed] [Google Scholar]
- Santiago-Walker A. E., Fikaris A. J., Kao G. D., Brown E. J., Kazanietz M. G., Meinkoth J. L. (2005). Protein kinase C delta stimulates apoptosis by initiating G1 phase cell cycle progression and S phase arrest. J. Biol. Chem. 280 32107–32114 [DOI] [PubMed] [Google Scholar]
- Satyanarayana A., Kaldis P. (2009). Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 28 2925–2939 [DOI] [PubMed] [Google Scholar]
- Sauma S., Yan Z., Ohno S., Friedman E. (1996). Protein kinase C beta 1 and protein kinase C beta 2 activate p57 mitogen-activated protein kinase and block differentiation in colon carcinoma cells. Cell Growth Differ 7 587–594 [PubMed] [Google Scholar]
- Saunders N. A., Dicker A. J., Jones S. J., Dahler A. L. (1998). E2F1 messenger RNA is destabilized in response to a growth inhibitor in normal human keratinocytes but not in a squamous carcinoma cell line. Cancer Res. 58 1646–1649 [PubMed] [Google Scholar]
- Saxon M. L., Zhao X., Black J. D. (1994). Activation of protein kinase C isozymes is associated with post-mitotic events in intestinal epithelial cells in situ. J. Cell Biol. 126 747–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonwasser D. C., Marais R. M., Marshall C. J., Parker P. J. (1998). Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol. Cell. Biol. 18 790–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. T., Ingram A., Ball K. L. (2002). PDK1-dependent activation of atypical PKC leads to degradation of the p21 tumour modifier protein. EMBO J. 21 6771–6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatos M. A., Hodges R. R., Bair J. A., Lashkari K., Dartt D. A. (2008). PKC{alpha} stimulates conjunctival goblet cell proliferation using extracellular regulated kinase 1/2. Invest. Ophthalmol. Vis. Sci. 50 1619–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. (1995). Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9 1149–1163 [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. (1999). CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13 1501–1512 [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. (2004). Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18 2699–2711 [DOI] [PubMed] [Google Scholar]
- Sicinska E., Aifantis I., Le Cam L., Swat W., Borowski C., Yu Q., et al. (2003). Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 4 451–461 [DOI] [PubMed] [Google Scholar]
- Sipeki S., Bander E., Ways D. K, Faragó A. (2002). Activation of Erk1/Erk2 and transiently increased p53 levels together may account for p21 expression associated with phorbol ester-induced transient growth inhibition in HepG2 cells. Cell. Signal. 14 115–121 [DOI] [PubMed] [Google Scholar]
- Slosberg E. D., Klein M. G., Yao Y., Han E. K., Schieren I, Weinstein I. B. (1999). The alpha isoform of protein kinase C mediates phorbol ester-induced growth inhibition and p21cip1 induction in HC11 mammary epithelial cells. Oncogene 18 6658–6666 [DOI] [PubMed] [Google Scholar]
- Smith-Garvin J. E., Koretzky G. A., Jordan M. S. (2009). T cell activation. Annu. Rev. Immunol. 27 591–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- So T., Croft M. (2012). Regulation of the PKCtheta-NF-kappaB axis in T lymphocytes by the tumor necrosis factor receptor family member OX40. Front. Immunol. 3:133 10.3389/fimmu.2012.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh J. W., Weinstein I. B. (2003). Roles of specific isoforms of protein kinase C in the transcriptional control of cyclin D1 and related genes. J. Biol. Chem. 278 34709–34716 [DOI] [PubMed] [Google Scholar]
- Soltoff S. P. (2007). Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol. Sci. 28 453–458 [DOI] [PubMed] [Google Scholar]
- Srivastava R., Burbach B. J., Mitchell J. S., Pagán A. J., Shimizu Y. (2012). ADAP regulates cell cycle progression of T cells via control of cyclin E and cdk2 expression through two distinct CARMA1-dependent signaling pathways. Mol. Cell. Biol. 32 1908–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S. F. (2004). Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem. J. 384 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Cai T.-T., Zhou X.-B., Xu Q. (2009). Saikosaponin a inhibits the proliferation and activation of T cells through cell cycle arrest and induction of apoptosis. Int. Immunopharmacol. 9 978–983 [DOI] [PubMed] [Google Scholar]
- Sun Z., Arendt C. W., Ellmeier W., Schaeffer E. M., Sunshine M. J., Gandhi L., et al. (2000). PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 404 402–407 [DOI] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. (1981). Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc. Natl. Acad. Sci. U.S.A. 78 4515–4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuma K., Takahara N., Suzuma I., Isshiki K., Ueki K., Leitges M., et al. (2002). Characterization of protein kinase C beta isoform’s action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc. Natl. Acad. Sci. U.S.A. 99 721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Rasmussen H. (1988). Stimulation of mitogenesis and glucose transport by 1-monooleoylglycerol in Swiss 3T3 fibroblasts. J. Biol. Chem. 263 9738–9745 [PubMed] [Google Scholar]
- Talora C., Cialfi S., Oliviero C., Palermo R., Pascucci M., Frati L., et al. (2006). Cross talk among Notch3, pre-TCR, and Tal1 in T-cell development and leukemogenesis. Blood 107 3313–3320 [DOI] [PubMed] [Google Scholar]
- Tang S., Morgan K. G., Parker C., Ware J. A. (1997). Requirement for protein kinase C θ for cell cycle progression and formation of actin stress fibers and filopodia in vascular endothelial cells. J. Biol. Chem. 272 28704–28711 [DOI] [PubMed] [Google Scholar]
- Thompson L. J., Fields A. P. (1996). betaII protein kinase C is required for the G2/M phase transition of cell cycle. J. Biol. Chem. 271 15045–15053 [DOI] [PubMed] [Google Scholar]
- Thuille N., Gruber T., Pfeifhofer C., Hermann-Kleiter N., Lutz-Nicoladoni C., Letschka T., et al. (2006). Physiological and non-redundant functions of PKC isotypes in T lymphocytes. Curr. Immunol. Rev. 2 143–156 [Google Scholar]
- Tibudan S. S., Wang Y., Denning M. F. (2002). Activation of protein kinase C triggers irreversible cell cycle withdrawal in human keratinocytes. J. Invest. Dermatol. 119 1282–1289 [DOI] [PubMed] [Google Scholar]
- Trimarchi J. M., Lees J. A. (2002). Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell Biol. 3 11–20 [DOI] [PubMed] [Google Scholar]
- Uddin S., Sassano A., Deb D. K., Verma A., Majchrzak B., Rahman A., et al. (2002). Protein kinase C-delta (PKC-delta ) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J. Biol. Chem. 277 14408–14416 [DOI] [PubMed] [Google Scholar]
- Villalba M., Kasibhatla S., Genestier L., Mahboubi A., Green D. R., Altman A. (1999). Protein kinase Cθ cooperates with calcineurin to induce fas ligand expression during activation-induced T cell death. J. Immunol. 163 5813–5819 [PubMed] [Google Scholar]
- Volkov Y., Long A., Mcgrath S., Ni Eidhin D., Kelleher D. (2001). Crucial importance of PKC-beta(I) in LFA-1-mediated locomotion of activated T cells. Nat. Immunol. 2 508–514 [DOI] [PubMed] [Google Scholar]
- Vrana J. A., Saunders A. M., Chellappan S. P., Grant S. (1998). Divergent effects of bryostatin 1 and phorbol myristate acetate on cell cycle arrest and maturation in human myelomonocytic leukemia cells (U937). Differentiation 63 33–42 [DOI] [PubMed] [Google Scholar]
- Vucenik I., Ramakrishna G., Tantivejkul K., Anderson L. M., Ramljak D. (2005). Inositol hexaphosphate (IP6) blocks proliferation of human breast cancer cells through a PKCdelta-dependent increase in p27Kip1 and decrease in retinoblastoma protein (pRb) phosphorylation. Breast Cancer Res. Treat. 91 35–45 [DOI] [PubMed] [Google Scholar]
- Walker J. L., Castagnino P., Chung B. M., Kazanietz M. G., Assoian R. K. (2006). Post-transcriptional destabilization of p21cip1 by protein kinase C in fibroblasts. J. Biol. Chem. 281 38127–38132 [DOI] [PubMed] [Google Scholar]
- Walker S. D., Murray N. R., Burns D. J., Fields A. P. (1995). Protein kinase C chimeras: catalytic domains of alpha and beta II protein kinase C contain determinants for isotype-specific function. Proc. Natl. Acad. Sci. U.S.A. 92 9156–9160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chuang H. C., Li J. P., Tan T. H. (2012). Regulation of PKC-theta function by phosphorylation in T cell receptor signaling. Front. Immunol. 3:197 10.3389/fimmu.2012.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Y., Ostberg J. R., Repasky E. A. (1999). Effect of fever-like whole-body hyperthermia on lymphocyte spectrin distribution, protein kinase C activity, and uropod formation. J. Immunol. 162 3378–3387 [PubMed] [Google Scholar]
- Wang Z., Su Z. Z., Fisher P. B., Wang S., Vantuyle G., Grant S. (1998). Evidence of a functional role for the cyclin-dependent kinase inhibitor p21(WAF1/CIP1/MDA6) in the reciprocal regulation of PKC activator-induced apoptosis and differentation in human myelomonocytic leukemia cells. Exp. Cell Res. 244 105–116 [DOI] [PubMed] [Google Scholar]
- Warnecke N., Poltorak M., Kowtharapu B. S., Arndt B., Stone J. C., Schraven B., et al. (2012). TCR-mediated Erk activation does not depend on Sos and Grb2 in peripheral human T cells. EMBO Rep. 13 386–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Ono Y., Taniyama Y., Hazama K., Igarashi K., Ogita K., et al. (1992). Cell division arrest induced by phorbol ester in CHO cells overexpressing protein kinase C-delta subspecies. Proc. Natl. Acad. Sci. U.S.A. 89 10159–10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb P. R., Doyle C., Anderson N. G. (2003). Protein kinase C-epsilon promotes adipogenic commitment and is essential for terminal differentiation of 3T3-F442A preadipocytes. Cell Mol. Life Sci. 60 1504–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A. D. (2007). Cyclin-dependent kinases: molecular switches controlling anergy and potential therapeutic targets for tolerance. Semin. Immunol. 19 173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A. D. (2009). New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J. Immunol. 182 7331–7341 [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., Macmanus J. P., Gillan D. J. (1973). Calcium-dependent stimulation by a phorbol ester (PMA) of thymic lymphoblast DNA synthesis and proliferation. J. Cell. Physiol. 82 151–156 [DOI] [PubMed] [Google Scholar]
- Wu J., Lingrel J. B. (2004). KLF2 inhibits Jurkat T leukemia cell growth via upregulation of cyclin-dependent kinase inhibitor p21WAF1//CIP1. Oncogene 23 8088–8096 [DOI] [PubMed] [Google Scholar]
- Wu T. T., Hsieh Y. H., Hsieh Y. S., Liu J. Y. (2008). Reduction of PKC alpha decreases cell proliferation, migration, and invasion of human malignant hepatocellular carcinoma. J. Cell. Biochem. 103 9–20 [DOI] [PubMed] [Google Scholar]
- Yang L., Qiao G., Ying H., Zhang J., Yin F. (2010). TCR-induced Akt serine 473 phosphorylation is regulated by protein kinase C-alpha. Biochem. Biophys. Res. Commun. 400 16–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. C., Ng D. C., Bikle D. D. (2003). Role of protein kinase C alpha in calcium induced keratinocyte differentiation: defective regulation in squamous cell carcinoma. J. Cell. Physiol. 195 249–259 [DOI] [PubMed] [Google Scholar]
- Yasuda T., Kurosaki T. (2008). Regulation of lymphocyte fate by Ras/ERK signals. Cell Cycle 7 3634–3640 [DOI] [PubMed] [Google Scholar]
- Yoshida M., Heike Y., Ohno S., Ikekawa T., Wakasugi H. (2003). Involvement of PKC betaII in anti-proliferating action of a new antitumor compound gnidimacrin. Int. J. Cancer 105 601–606 [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A., Ding Y., Kumari S., Attur M., Hippen K. L., Brown M., et al. (2010). Protein kinase C-θ mediates negative feedback on regulatory T cell function. Science 328 372–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chellappan S. P. (1996). Transcriptional activation and expression of DP transcription factors during cell cycle and TPA-induced U937 differentiation. Mol. Cell. Differ. 4 297–316 [Google Scholar]
- Zhou X.-Y., Yashiro-Ohtani Y., Nakahira M., Park W. R., Abe R., Hamaoka T., et al. (2002). Molecular mechanisms underlying differential contribution of CD28 versus non-CD28 costimulatory molecules to IL-2 promoter activation. J. Immunol. 168 3847–3854 [DOI] [PubMed] [Google Scholar]