Abstract
Background
The risk of sudden cardiac death and the assessment of risk factors in prediction models have not been assessed in women with coronary artery disease (CAD). We sought to evaluate sudden cardiac death (SCD) incidence, risk factors and their predictive accuracy among a population of women with CAD.
Methods
The Hormone and Estrogen Replacement Study (HERS) evaluated the effects of hormone replacement therapy on cardiovascular events among 2,763 postmenopausal women with CAD. SCD was defined as death from cardiac origin that occurred within 1 hour of symptom onset. The associations between candidate predictor variables and SCD were evaluated in a Cox proportional hazards model. The C-index was used to compare the predictive value of the clinical risk factors with left ventricular ejection fraction alone and in combination. The net reclassification improvement (NRI) was also computed.
Results
Over a mean follow-up of 6.8 years, SCD comprised 136 of the 254 cardiac deaths. The annual SCD event rate was 0.79% (95% CI, 0.67–0.94%). The following variables were independently associated with SCD in the multivariate model: myocardial infarction, heart failure, estimated glomerular filtration rate (eGFR) <40ml/min/1.73m2, atrial fibrillation, physical inactivity and diabetes. The incidences of SCD among women with 0 (n=683), 1 (n=1224), 2 (n=610), and 3 plus (n=246) risk factors at baseline were 0.3, 0.5, 1.2 and 2.9% per year, respectively. The combination of clinical risk factors and LVEF (C-index 0.681) were better predictors of SCD than LVEF alone (C-index 0.600) and resulted in a NRI of 0.20 (p<0.001).
Conclusions
SCD comprised the majority of cardiac deaths among postmenopausal women with CAD. Independent predictors of SCD including myocardial infarction, heart failure, eGFR <40ml/min/1.73m2, atrial fibrillation, physical inactivity and diabetes improved SCD prediction when used in addition to LVEF.
Introduction
Sudden cardiac death (SCD) is an important clinical and public health problem with approximately 250,000 to 300,000 Americans dying annually from this condition.1 Several population-based studies have suggested that women who experience SCD are less likely to have a history of clinical cardiovascular disease,2, 3 evidence of structural heart disease, or left ventricular dysfunction than men with SCD.4 In the Nurses’ Health Study, greater than two-thirds of women with SCD events had no reported history of cardiac disease. Women with CAD are also reported to have a lower risk of ventricular arrhythmias and sudden cardiac death events compared to men.5, 6 These studies have also identified SCD risk factors that include both traditional ones associated with CAD2, 7 and nontraditional measures such as depression8 and dietary factors.9–11 Although these studies provide important insight into the epidemiology of SCD in women across the general population, very little data exist on the risk factors and predictors of SCD in women with CAD.
In clinical practice the only established predictor for SCD is severely decreased left ventricular systolic function.12 Recent studies, however, suggest that ejection fraction alone is unlikely to be sufficient for effective SCD risk prediction, because it lacks both sensitivity and specificity.13 Less than one third of all SCD cases have severely decreased LV ejection fraction that would have qualified them as candidates for an ICD.14–17 As a result of these studies, recent reports from the National Heart, Lung and Blood Institute (NHLBI) and Heart Rhythm Society (HRS) emphasize the need to identify risk factors early in the course of the SCD pathway before LV dysfunction develops and to assess their utility in prediction algorithms.18
We evaluated these research questions in a study of post-menopausal women with established CAD. Our first goal was to quantify SCD risk in this population in comparison with risks for mortality from other cardiac and non-cardiac etiologies. The second goal was to identify the most relevant risk factors for SCD among a large number of candidate variables using both traditional longitudinal methods and competing risk models. Finally, we determined the extent to which the identified risk factors could predict SCD risk and compared their incremental predictive value to prediction based on left ventricular ejection fraction alone.
Methods
Design
The trial design, methods, baseline findings and main outcomes of the Heart and Estrogen/Progestin Replacement Study (HERS) have been published previously.19, 20 All participants provided written informed consent before study entry. The trial was a randomized, double-blinded, placebo-controlled trial of the effect of 0.25 mg of conjugated estrogens plus 2.50 mg of medroxyprogesterone acetate daily versus placebo on coronary artery disease event rate among 2,763 postmenopausal women with documented CAD.20 Participants were postmenopausal women less than 80 years of age with no previous hysterectomy and a history of at least one of the following: myocardial infarction (MI), coronary artery bypass graft surgery, percutaneous coronary angioplasty (PTCA), or >50% angiographic narrowing of a coronary artery (hence CAD). Among the reasons for exclusion was decompensated heart failure equivalent to New York Heart Association class III or IV.19, 20
Study Population
A total of 2,763 postmenopausal women with CAD and an average age of 67 years were enrolled in HERS for a median follow-up of 4.1 years; 2,321 women (93% of those surviving) consented to follow-up in HERS II for a median additional follow-up of 2.7 years. There was no significant difference in the rates of primary CAD events or secondary cardiovascular events including SCD among women assigned to the hormone group compared with the placebo group in HERS, HERS II or overall.19, 21
Baseline characteristics
A series of baseline characteristics were evaluated in this analysis as potential risk factors for sudden cardiac death (SCD), non-sudden cardiac death, death from other causes, and survivors. Sociodemographic factors included age, race and education. Lifestyle factors included physical activity, cigarette smoking within 1 year of enrollment, regular alcohol use and body mass index. Physical activity was defined by participation in a regular exercise program at least three times per week such as cardiac rehabilitation, aerobics, or vigorous walking for at least 10 minutes per session. Additional candidate risk factors from baseline included diabetes, systolic blood pressure, high-density lipoprotein, low-density lipoprotein, triglycerides, and estimated glomerular filtration rate from creatinine (eGFR), categorized as >60, 40–60, and <40 ml/min/1.73m2. Estimated GFR was evaluated with the use of the 4-variable simplified Modification of Diet in Renal Disease equation.22 Heart disease severity was evaluated by the presence of left ventricular hypertrophy (LVH) on the 12 lead electrocardiogram, previous myocardial infarction, history of percutaneous coronary angioplasty, and prior congestive heart failure. Electrocardiographic abnormalities that were evaluated included left bundle branch block, corrected QT interval and atrial fibrillation. The presence of atrial fibrillation was assessed by standard 12-lead electrocardiograms obtained at study enrollment and at yearly follow-up visits. Finally, we reviewed 1,773 of the 2,763 participants’ (66% of the HERS cohort) medical records retrospectively to obtain echocardiographic data on the left ventricular ejection fraction (LVEF). Left ventricular function was modeled as normal (LVEF > 50%), mildly decreased (LVEF 40–50%), moderately decreased (LVEF 35 to 39%) and severely decreased (LVEF < 35%). We divided LVEF into multiple categories in order to optimize its ability to predict SCD. LVEF was not modeled as a linear variable because its association with SCD risk was found to be non-linear.
Outcome Variables
SCD was defined as death that occurred within 1 hour of the onset of symptoms. This definition, which matches the one proposed by the NHLBI and HRS working group on SCD,18 required the participant to have been observed alive within the previous hour and did not include fatal events that occurred during sleep. A central committee adjudicated all of the events. Data from all deaths, hospitalizations, and other suspected outcomes events were reviewed and classified according to pre-specified criteria by an independent morbidity and mortality subcommittee blinded to treatment assignment. Family members reported suspected outcome events within 24 hours to the HERS Coordinating Center, which had the primary responsibility for the outcome database. Clinics then obtained and sent specified documentation to the coordinating center that included hospital discharge summaries, electrocardiograms, cardiac enzyme levels and other test results.
Non-sudden cardiac deaths included documented fatal MI, unobserved death that occurred out of the hospital in the absence of other known cause, and death due to coronary revascularization or congestive heart failure.19 All other deaths were categorized as non-cardiac mortality events including deaths from pulmonary embolism, cancer and stroke.
Statistical Analysis
We estimated annual sudden cardiac, non-sudden cardiac, and non-cardiac death rates with 95% confidence intervals. We then compared these three groups to survivors using the chi-squared test for categorical variables and ANOVA for continuous variables. Time-dependent risk factors for SCD were screened using unadjusted Cox models, in which other causes of death, losses to follow-up, and the end of the study were treated as censoring. Variables associated with SCD at a p-value < 0.1 were considered for inclusion in a multivariate analysis. Backwards deletion with a retention criterion of p<0.2 was then used to select the final multivariate model. In addition, we repeated this analysis using the Fine-Gray model23 in which non-sudden cardiac death and non-cardiac death are treated as competing risks. In contrast to the Cox model, the Fine-Gray model is sensitive to indirect adverse or protective effects: in particular, a factor strongly associated with the competing risks could appear protective against SCD essentially by reducing the time at risk. We also repeated the assessment of risk factors for SCD in the sub-cohort with echocardiograms.
Our next series of methods evaluated baseline clinical characteristics as well as LVEF for SCD risk prediction. We first compared SCD event rates by the number of risk factors as a rough clinical guideline. Then, focusing on the subset of women with echocardiograms, we used the C-index, a measure of discrimination24, to evaluate risk prediction models using LVEF only, selected clinical characteristics (myocardial infarction, heart failure, low eGFR, atrial fibrillation, physical inactivity, diabetes, and alcohol use), and both. C-indices were estimated using 20 repetitions of 10-fold cross-validation. We assessed net reclassification improvement (NRI)25 to determine how well addition of clinical characteristics to an LVEF-only prediction model reclassifies SCD cases into higher risk strata and other women into lower categories. Finally, we calculated the C-index for non-SCD and non-cardiac death using the model with LVEF and clinical characteristics. To evaluate whether the risk model differentially predicted these endpoints, we used bootstrap resampling to estimate bias-corrected 95 percentile confidence intervals for the pairwise difference between the C-indices for SCD, non-SCD and non-cardiac death.
Results
Among the 2,763 women enrolled in HERS, there were 254 cardiac deaths and 246 non-cardiac deaths during the 6.8-year follow-up period. SCD comprised 54% (136 events) of the cardiac-related deaths and 27% of all deaths with an annual event rate of 0.79% (95% CI (0.67–0.94%)) per year (figure 1). In addition there were 118 non-sudden cardiac deaths (0.69%, 95% CI (0.57–0.82%) per year) and 246 non-cardiac deaths (1.43%, 95% CI (1.27–1.63%) per year). The baseline clinical characteristics and laboratory values of the study participants stratified by SCD, non-SCD, other causes of death, and survivors are presented in Table 1. There were no differences in most of the baseline characteristics across the different groups. In particular, electrocardiographic parameters including the corrected QT interval, history of left bundle branch block, and LVH did not differ significantly across the categories. Participants who died of cardiac causes (either sudden or non-sudden) had a higher prevalence of CHF and diabetes, a higher BMI, and a lower LDL than those who died of non-cardiac causes. In comparison between participants who had subsequent SCD (n=136) and non-SCD events (n=118), all table 1 characteristics were nonsignificant at a p-value ≤ 0.05.
Figure 1.
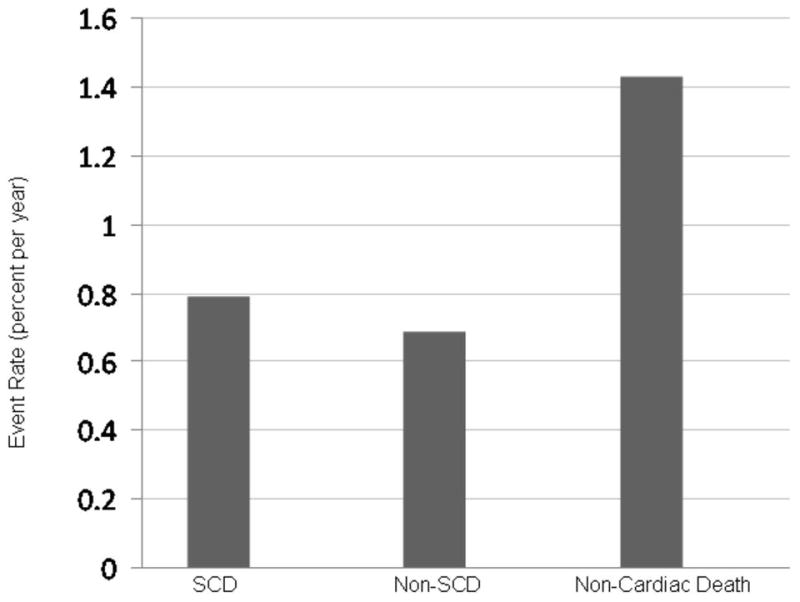
Event Rates of SCD, non-SCD and non-Cardiac Death
Table 1.
Baseline characteristics across categories of death
| Sudden cardiac death | Non-sudden cardiac death | Other Deaths | Survivors | ||
|---|---|---|---|---|---|
| p value | |||||
| N | 136 | 118 | 246 | 2263 | |
| Age (yr; mean ± SD) | 68 ± 6 | 68 ± 6 | 69 ± 6 | 66 ± 7 | 0.001 |
| White (%) | 85 | 79 | 87 | 90 | 0.003 |
| Education (yr; mean ± SD) | 12 ± 3 | 12 ± 3 | 13 ± 3 | 13 ± 3 | 0.001 |
| Current Smoker (%) | 16 | 12 | 19 | 12 | 0.02 |
| Any alcohol use (%) | 26 | 32 | 31 | 41 | 0.001 |
| Exercise (≥3 times/wk, %) | 29 | 28 | 35 | 40 | 0.001 |
| Prior CHF (%) | 34 | 29 | 18 | 10 | 0.001 |
| Numbers of prior MI | 0.001 | ||||
| 0 (%) | 41 | 42 | 44 | 50 | |
| 1 (%) | 49 | 45 | 50 | 45 | |
| >1 (%) | 10 | 13 | 7 | 4 | |
| History of PTCA (%) | 36 | 36 | 37 | 44 | 0.02 |
| Diabetes (%) | 43 | 53 | 34 | 23 | 0.001 |
| History of AF (%) | 2 | 4 | 3 | 1 | 0.001 |
| BMI (kg/m2; mean ± SD) | 29.3 ± 5.8 | 29.0 ± 6.2 | 27.7 ± 5.5 | 28.6 ± 5.5 | 0.02 |
| SBP (mmHg; mean ± SD) | 139 ± 20 | 143 ± 23 | 139 ± 20 | 1343 ± 18 | 0.001 |
| QT corrected interval (milliseconds, mean ± SD) | 453 ± 34 | 447 ± 31 | 448 ± 32 | 438 ± 29 | 0.001 |
| History of LBBB (%) | 10 | 14 | 12 | 7 | 0.001 |
| History of LVH (%) | 14 | 15 | 14 | 9 | 0.02 |
| Estimated GFR | 0.001 | ||||
| <40 (ml/min; %) | 26 | 23 | 20 | 8 | |
| 40–60 (ml/min; %) | 48 | 57 | 53 | 58 | |
| >60 (ml/min; %) | 26 | 20 | 27 | 34 | |
| HDL (mg/dl; mean ± SD) | 47 ± 14 | 49 ± 13 | 52 ± 15 | 50 ± 13 | 0.02 |
| LDL (mg/dl; mean ± SD) | 149 ± 40 | 150 ± 42 | 143 ± 36 | 145 ± 38 | 0.30 |
| Triglycerides (mg/dl; mean±SD) | 176 ± 71 | 182 ± 67 | 163 ± 65 | 165 ± 63 | 0.006 |
Clinical variables associated with SCD
In unadjusted models myocardial infarction, heart failure, eGFR<40 ml/min/1.73m2, atrial fibrillation, physical inactivity, diabetes, alcohol use, PTCA, left bundle branch block, and left ventricular hypertrophy were associated with SCD at a p-value < 0.1. Of these, myocardial infarction, heart failure, low eGFR, atrial fibrillation, physical inactivity, diabetes, and alcohol use were retained in the final multivariate model (table 2). Myocardial infarction, heart failure, and low eGFR were associated with an approximate 2-fold or higher risk for SCD after adjustment. Results using the competing risks analysis were essentially the same.
Table 2.
Risk Factors for Sudden Cardiac Death among Women with Coronary Artery Disease
| Complete HERS Cohort (N=2,763) | LVEF Sub-cohort (N=1,773) | |||||
|---|---|---|---|---|---|---|
| Cox Model | Fine-Gray Model | Cox Model | ||||
| RR (95%CI) | P value | RR (95%CI) | P value | RR (95%CI) | P value | |
| Non-use of alcohol | 1.42 (0.94–2.15) | 0.093 | 1.43 (0.93 – 2.17) | 0.101 | - | - |
| Prior Myocardial Infarctions | ||||||
| 1 | 1.13 (0.77 – 1.65) | 0.539 | 1.12 (0.77 –1.64) | 0.540 | 0.92 (0.56 – 1.50) | 0.735 |
| >1 | 2.13 (1.32 – 3.44) | 0.002 | 1.91 (1.18 – 3.08) | 0.008 | 1.75 (0.95 – 3.26) | 0.075 |
| Congestive Heart Failure | 2.15 (1.49 – 3.11) | <0.001 | 2.10 (1.47 – 3.00) | <0.001 | 2.49 (1.59 – 3.90) | <0.001 |
| Estimated GFR (eGFR) | ||||||
| <40 ml/min | 1.96 (1.18 – 3.23) | 0.009 | 1.79 (1.09 –2.90) | 0.020 | 1.99 (1.06 – 3.74) | 0.031 |
| 40–60 ml/min | 0.92 (0.59 – 1.45) | 0.722 | 0.91 (0.58 – 1.42) | 0.675 | 0.91 (0.52 – 1.61) | 0.754 |
| Atrial fibrillation | 1.92 (1.02 – 3.61) | 0.042 | 1.86 (0.94 – 3.67) | 0.059 | 4.49 (2.32 – 8.68) | <0.001 |
| Physical Inactivity | 1.61 (1.04 – 2.50) | 0.035 | 1.54 (0.98 – 2.38) | 0.060 | - | - |
| Diabetes | 1.52 (1.06 – 2.17) | 0.022 | 1.44 (1.00 – 2.06) | 0.049 | 1.60 (1.04 – 2.47) | 0.033 |
| LVEF | ||||||
| 40–50% | - | - | - | - | 1.22 (0.68 – 2.19) | 0.501 |
| 35–39% | - | - | - | - | 2.90 (1.59 – 5.29) | 0.001 |
| < 35% | - | - | - | - | 2.78 (1.48 – 5.23) | 0.001 |
| Left Bundle Branch Block | - | - | - | - | 1.54 (0.96 – 2.47) | 0.071 |
LVEF assessments were based upon clinical echocardiograms that were obtained prior to randomization (median 32 months, interquartile range 17 to 56 months). The LVEF was normal in 1,226 (69%), mildly decreased in 318 (18%), moderately decreased in 137 (8%), and severely decreased in 92 participants (5%). In the echocardiogram subcohort, 319 deaths occurred during follow-up: 90 SCD events (0.81%, 95% CI (0.66–1.00%) per year), 78 non-SCD events (0.71%, 95% CI (0.57–0.88%) per year), and 151 non-cardiac deaths (1.37%, 95% CI (1.16–1.60%) per year). Women with echocardiograms accounted for 90 of the 136 (66%) SCD cases. Myocardial infarction, heart failure, low eGFR, atrial fibrillation, diabetes, left bundle branch block and LVEF were retained in the final multivariate model selected using data for this sub-cohort (table 2).
Prediction of SCD Using Numbers of Clinical Risk Characteristics
Among 2763 women in the HERS cohort, 25% had none of the seven risk factors identified in the full cohort, 44% had one, 22% had two, and 9% had three or more risk factors at baseline. SCD risk increased almost 10-fold across the four groups (figure 2). Participants with no risk factors had an annualized SCD risk of 0.34%, compared to 2.9% for those with at least 3 risk factors.
Figure 2.
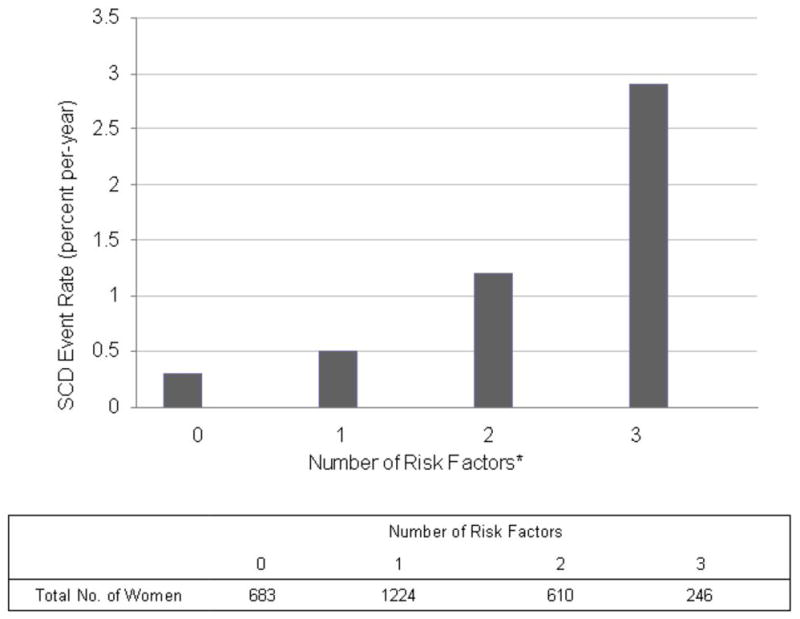
SCD Events according to the number of Risk Factors
*The risk factors evaluated in this analysis included the following: greater than 1 myocardial infarction, congestive heart failure, estimated GFR < 40ml/min/1.73m2, atrial fibrillation, physical inactivity, and diabetes.
Prediction of SCD Using LVEF and Clinical Risk Characteristics
C-indices for the models with LVEF alone, clinical characteristics alone, and both were 0.600, 0.666, and 0.681 respectively. Net reclassification for the addition of clinical characteristics to the LVEF-only model was 20% (p-value< 0.001; table 3) mostly due to reclassification of 24% of the women who died of SCD into a higher risk category.
Table 3.
Net Reclassification Improvement
| SCD Cases | 5-year SCD risk: LVEF + clinical characteristics | ||||
|---|---|---|---|---|---|
| <5% | 5–10% | >10% | Total | ||
| 5-year SCD risk: LVEF only | <5% | 41 | 11 | 6 | 59 |
| 5–10% | 2 | 4 | 10 | 16 | |
| >10% | 1 | 2 | 12 | 15 | |
| Total | 45 | 17 | 28 | 90 | |
| Case Net Reclassification Improvement: (11+6+10-2-1-2)/90 = 22/90 = 24% | |||||
| Controls | 5-year SCD risk: LVEF + clinical characteristics | ||||
| <5% | 5–10% | >10% | Total | ||
| 5-year SCD risk: LVEF only | <5% | 1,343 | 115 | 27 | 1,485 |
| 5–10% | 48 | 45 | 28 | 121 | |
| >10% | 24 | 26 | 27 | 77 | |
| Total | 1,415 | 186 | 82 | 1,683 | |
| Control Net Reclassification Improvement: (48+24+26-115-27-28)/1,683 = −72/1,683 = −4% | |||||
| Net Reclassification Improvement: 24% – 4% = 20% (95% CI 8%–33%, p-value = 0.002) | |||||
Reclassification across risk groups defined as <5 %, 5–10%, >10% for sudden cardiac death (SCD). SCD cases and controls are cross-classified by their SCD risk estimated using the LVEF-only model (rows) versus a model using clinical characteristics and LVEF (columns). Italicized numbers are the women who are reclassified into a higher (above the diagonal of the table) or lower (below the diagonal) risk category after addition of clinical characteristics to the LVEF-only model and contribute to the net reclassification calculations. Net reclassification improvement reflects the degree to which SCD cases are reclassified into a higher risk category, and controls are reclassified into a lower category.
Prediction of non-sudden cardiac death and non-cardiac death
The C-index for predicting non-sudden cardiac death in the model with both LVEF and clinical characteristics was 0.702 whereas the C-index for predicting non-cardiac death was substantially lower at 0.404. We found no significant difference in the model’s ability to discriminate risk for SCD and non-SCD (C-index pairwise difference: −0.021, 95% CI (−0.405, 0.10). The model predicted SCD significantly better than non-cardiac death (C-index pairwise difference: 0.277, 95% CI (0.116, 0.413).
Discussion
In this cohort study of postmenopausal women with CAD, SCD comprised the majority of cardiac-related deaths during the 6.8 year follow-up period. We found heart failure, reduced kidney function, atrial fibrillation and diabetes as independent risk factors for SCD in both the overall analysis and the echocardiographic subgroup. MI and physical inactivity were additional risk factors for SCD in the original analysis; however, these variables were not independent markers of risk when LVEF was added to the model. In a competing risks analysis, we confirmed that myocardial infarction, heart failure, reduced kidney function and diabetes were associated with SCD in the overall cohort. In developing a risk-stratification approach, we delineated nearly a 10-fold gradient in SCD risk by using the number of risk factors present at baseline. Clinical characteristics were better predictors of SCD risk than LVEF alone, and the combination did better still.
Our study quantifies the risk of SCD events in an ambulatory cohort of women with CAD. During the 6.8 year follow-up period, 136 of the 254 cardiovascular deaths were adjudicated as sudden, making SCD the leading cause of cardiovascular-related mortality among this group. Prior population-based studies have found approximately a 10-fold lower risk of SCD in women.2 Further, the annual rate of SCD among women in HERS is lower than SCD rates observed in populations with an established cardiomyopathy (e.g. participants in the ICD trials). In this context we have identified a group of well-functioning women at intermediate risk of SCD.
Most SCD cases occur in the general population or among individuals without advanced cardiovascular disease.14, 18, 26 With the exception of LVEF, other risk stratification variables are not utilized routinely in clinical practice. Our findings highlight that a simple assessment of clinical risk factors has better predictive value for SCD than LVEF alone. These findings complement prior studies in higher risk populations that suggest an improvement in SCD risk prediction when clinical risk factors are combined with ejection fraction.27, 28 Our final model, which consisted of both LVEF and clinical risk factors, also differentiated between SCD and non-cardiac deaths; however, it did not discriminate between sudden and non-sudden cardiac events. This latter finding remains an area of important investigation and will require the evaluation of unique risk factors associated with cardiac arrhythmias. From the standpoint of clinical relevance, our model improves SCD prediction compared to LVEF alone, which may impact the care of women with CAD given that SCD comprised the majority of cardiac-related deaths.
Among the identified risk factors, the strength of atrial fibrillation as an independent predictor of SCD was surprising. This finding suggests that a history of atrial arrhythmias increases the risk of ventricular tachyarrhythmias, which are the presumed cause of the majority of SCD events, especially in patients with structural heart disease. Electrical remodeling processes in the atria, characterized by shorter action potential duration and refractoriness, underlie the mechanisms implicated in atrial fibrillation.29 Similar changes may also occur in ventricular myocytes and subsequently increase the risk of ventricular tachycardia or ventricular fibrillation in susceptible patients.30 Also, the short-long-short sequences of ventricular conduction in atrial fibrillation may trigger ventricular arrhythmias. Alternatively, atrial fibrillation may be an additional marker of underlying structural heart disease. Regardless of the mechanism, our study indicates a need to evaluate atrial fibrillation further in another risk-based or predictive model for SCD events. This approach may require combining several cohorts of women with CAD.
Exercise and regular physical activity in women have been strongly associated with improved cardiovascular outcomes and lower all-cause mortality.31–34 Our findings suggest a strong association between regular exercise consisting of at least 3 sessions per week and a lower risk of SCD events. Given that very few noninvasive therapies protect against SCD, it is important to evaluate whether regular exercise reduces the incidence of SCD.
Diabetes and impaired glucose tolerance are other well-established risk factors for SCD in both men and women.35 Population-based studies including the Paris Prospective Study and the Honolulu Heart Program, which each enrolled approximately 8,000 men have demonstrated independent associations of diabetes and impaired glucose tolerance with SCD risk.36, 37 Similarly, the Nurses’ Health Study also identified diabetes as one of the strongest clinical risk factors for SCD in women.2
The echocardiographic subgroup analysis confirmed that many of the risk factors identified in the original analysis remained independent predictors of SCD risk after including LVEF. LVEF < 35% is currently considered the most clinically relevant risk factor for SCD risk stratification and identifies patients who may benefit from an implantable cardioverter-defibrillator (ICD).38, 39 Although we did not have LVEF measures in the entire cohort, our subgroup analysis demonstrated that heart failure, reduced kidney function, atrial fibrillation and diabetes remained independent risk factors for SCD after adjustment for LVEF. Prior MI and physical inactivity likely co-segregate with left ventricular function, as the inclusion of LVEF in the subgroup analysis precluded their addition to the model. Finally, although LVEF measures were not standardized in a core, imaging laboratory, their assessment via chart review mimics echocardiographic-based methods of risk stratification in clinical practice. In addition, echocardiograms were not obtained at the outset of the study unlike other predictors of SCD in our study; however, the application of a LVEF measure from earlier time-points also represents clinical practice.
Several limitations of our study should be considered. First, the limited number of SCD events may have resulted in a relatively small number of potential risk factors. In addition, we did not validate our prediction model in other cohorts. Future studies will require collaborations across multiple cohorts to refine further SCD risk discrimination and provide a means for both replication and cross-validation. The majority of women in this analysis were white; therefore, further assessment is required in post-menopausal women of other ethnicities. Left ventricular ejection fraction, a powerful predictor of risk for SCD, was not measured in all the participants of this study. Although we included a separate, subgroup analysis, our findings may be limited given the lower number of SCD events. Finally, the variables identified in our analysis also appeared to be associated with non-sudden cardiac death. This current limitation in risk prediction will require future studies to identify unique predictors of SCD.
In conclusion, SCD comprised the majority of cardiac-related deaths among post-menopausal women with coronary artery disease. We found that in addition to LVEF, MI, heart failure, an eGFR < 40 ml/min, atrial fibrillation, physical inactivity, and diabetes were independently associated with SCD in this large, multicenter sample of women with coronary artery disease. The risk factors served as better predictors of SCD than LVEF alone and enhanced risk discrimination when both were combined. A simple risk stratification based on the number of risk factors predicted a 10-fold gradient in the incidence of SCD. Future projects should focus on combining studies to allow more robust estimates for established risk factors and to identify additional markers that augment the predictive ability of a SCD risk model.
Acknowledgments
Acknowledgment/Funding
RD was supported by grants K23DK089118 from the NIH and KL2 RR024132 from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research. Additional grant support was obtained from a Kynett-FOCUS Junior Faculty Investigator Award for Research in Women’s Cardiovascular Health funded by the Edna G. Kynett Memorial Foundation at the University of Pennsylvania School of Medicine; ZHT is supported by R01 HL102090.
Footnotes
The authors do not have any conflicts of interest.
Contributor Information
Dr. Rajat Deo, Section of Electrophysiology, Division of Cardiovascular Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Dr. Eric Vittinghoff, Departments of Medicine, Epidemiology, and Biostatistics, University of California, San Francisco, California.
Ms. Feng Lin, Departments of Medicine, Epidemiology, and Biostatistics, University of California, San Francisco, California.
Dr. Zian H. Tseng, Cardiac Electrophysiology Section, Division of Cardiology, University of California, San Francisco, California.
Dr. Stephen B. Hulley, Departments of Medicine, Epidemiology, and Biostatistics, University of California, San Francisco, California.
Dr. Michael G. Shlipak, General Internal Medicine Section, Veterans Affairs Medical Center, San Francisco, California and Departments of Medicine, Epidemiology and Biostatistics, University of California, San Francisco, California.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Albert CM, Chae CU, Grodstein F, et al. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107(16):2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Wilson PW, D’Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. 1998;136(2):205–212. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 4.Chugh SS, Uy-Evanado A, Teodorescu C, et al. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The Ore-SUDS (Oregon Sudden Unexpected Death Study) J Am Coll Cardiol. 2009;54(22):2006–2011. doi: 10.1016/j.jacc.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Lampert R, McPherson CA, Clancy JF, Caulin-Glaser TL, Rosenfeld LE, Batsford WP. Gender differences in ventricular arrhythmia recurrence in patients with coronary artery disease and implantable cardioverter-defibrillators. J Am Coll Cardiol. 2004;43(12):2293–2299. doi: 10.1016/j.jacc.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 7.Deo R, Lin F, Vittinghoff E, Tseng ZH, Hulley SB, Shlipak MG. Kidney dysfunction and sudden cardiac death among women with coronary heart disease. Hypertension. 2008;51(6):1578–1582. doi: 10.1161/HYPERTENSIONAHA.107.103804. [DOI] [PubMed] [Google Scholar]
- 8.Whang W, Kubzansky LD, Kawachi I, et al. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’ Health Study. J Am Coll Cardiol. 2009;53(11):950–958. doi: 10.1016/j.jacc.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiuve SE, Korngold EC, Januzzi JL, Jr, Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr. 2011;93(2):253–260. doi: 10.3945/ajcn.110.002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiuve SE, Rimm EB, Mukamal KJ, et al. Light-to-moderate alcohol consumption and risk of sudden cardiac death in women. Heart Rhythm. 2010;7(10):1374–1380. doi: 10.1016/j.hrthm.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiuve SE, Rimm EB, Manson JE, et al. Intake of total trans, trans-18:1, and trans-18:2 fatty acids and risk of sudden cardiac death in women. Am Heart J. 2009;158(5):761–767. doi: 10.1016/j.ahj.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chugh SS, Reinier K, Teodorescu C, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51(3):213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehi A, Haas D, Fuster V. Primary prophylaxis with the implantable cardioverter-defibrillator: the need for improved risk stratification. JAMA. 2005;294(8):958–960. doi: 10.1001/jama.294.8.958. [DOI] [PubMed] [Google Scholar]
- 14.Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47(6):1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 15.Connolly SJ, Gent M, Roberts RS, et al. Canadian implantable defibrillator study (CIDS) : a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101(11):1297–1302. doi: 10.1161/01.cir.101.11.1297. [DOI] [PubMed] [Google Scholar]
- 16.Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest : the Cardiac Arrest Study Hamburg (CASH) Circulation. 2000;102(7):748–754. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 17.A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337(22):1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 18.Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention: report from a national heart, lung, and blood institute and heart rhythm society workshop. Circulation. 2010;122(22):2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 20.Grady D, Applegate W, Bush T, Furberg C, Riggs B, Hulley SB. Heart and Estrogen/progestin Replacement Study (HERS): design, methods, and baseline characteristics. Control Clin Trials. 1998;19(4):314–335. doi: 10.1016/s0197-2456(98)00010-5. [DOI] [PubMed] [Google Scholar]
- 21.Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288(1):49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 24.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 26.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345(20):1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 27.Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50(12):1150–1157. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 28.Atwater BD, Thompson VP, Vest RN, 3rd, et al. Usefulness of the Duke Sudden Cardiac Death risk score for predicting sudden cardiac death in patients with angiographic (>75% narrowing) coronary artery disease. Am J Cardiol. 2009;104(12):1624–1630. doi: 10.1016/j.amjcard.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 29.Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation. Time course and mechanisms. Circulation. 1996;94(11):2968–2974. doi: 10.1161/01.cir.94.11.2968. [DOI] [PubMed] [Google Scholar]
- 30.Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95(8):754–763. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- 31.Kushi LH, Fee RM, Folsom AR, Mink PJ, Anderson KE, Sellers TA. Physical activity and mortality in postmenopausal women. JAMA. 1997;277(16):1287–1292. [PubMed] [Google Scholar]
- 32.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 33.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341(9):650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 34.Whang W, Manson JE, Hu FB, et al. Physical exertion, exercise, and sudden cardiac death in women. JAMA. 2006;295(12):1399–1403. doi: 10.1001/jama.295.12.1399. [DOI] [PubMed] [Google Scholar]
- 35.Siscovick DS, Sotoodehnia N, Rea TD, Raghunathan TE, Jouven X, Lemaitre RN. Type 2 diabetes mellitus and the risk of sudden cardiac arrest in the community. Rev Endocr Metab Disord. 2010;11(1):53–59. doi: 10.1007/s11154-010-9133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99(15):1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 37.Curb JD, Rodriguez BL, Burchfiel CM, Abbott RD, Chiu D, Yano K. Sudden death, impaired glucose tolerance, and diabetes in Japanese American men. Circulation. 1995;91(10):2591–2595. doi: 10.1161/01.cir.91.10.2591. [DOI] [PubMed] [Google Scholar]
- 38.Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: executive summary. Heart Rhythm. 2008;5(6):934–955. doi: 10.1016/j.hrthm.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death--executive summary: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Eur Heart J. 2006;27(17):2099–2140. doi: 10.1093/eurheartj/ehl199. [DOI] [PubMed] [Google Scholar]


