Abstract
Anxiety disorders are among the most prevalent psychiatric disorders, yet much is unknown about the underlying mechanisms. The dorsal raphe (DR) is at the crux of the anxiety-inducing effects of uncontrollable stress, a key component of models of anxiety. Though DR serotonin (5-HT) neurons play a prominent role, anxiety-associated changes in the physiology of 5-HT neurons remain poorly understood. A 5-day social defeat model of anxiety produced a multifaceted, anxious phenotype in intruder mice that included increased avoidance behavior in the open field test, increased stress-evoked grooming, and increased bladder and heart weights when compared to control mice. Intruders were further compared to controls using electrophysiology recordings conducted in midbrain slices wherein recordings targeted 5-HT neurons of the ventromedial (vmDR) and lateral wing (lwDR) subfields of the DR. Though defining membrane characteristics of 5-HT neurons were unchanged, γ-aminobutyric-acid-mediated (GABAergic) synaptic regulation of 5-HT neurons was altered in a topographically specific way. In the vmDR of intruders, there was a decrease in the frequency and amplitude of GABAergic spontaneous inhibitory postsynaptic currents (sIPSCs). However, in the lwDR, there was an increase in the strength of inhibitory signals due to slower sIPSC kinetics. Synaptic changes were selective for GABAergic input, as glutamatergic synaptic input was unchanged in intruders. The distinct inhibitory regulation of DR subfields provides a mechanism for increased 5-HT output in vmDR target regions and decreased 5-HT output in lwDR target regions, divergent responses to uncontrollable stress that have been reported in the literature but were previously poorly understood.
Keywords: Anxiety, GABA, spontaneous inhibitory postsynaptic current, lateral wings of the dorsal raphe, resident-intruder paradigm, ventromedial dorsal raphe
Anxiety disorders are among the most prevalent mental diseases, yet the underlying mechanisms are poorly understood. Serotonin (5-HT) is thought to play a key role in anxiety disorders, and the dorsal raphe (DR) may be central to that role, as it is the major source of 5-HT projections to the forebrain.1,2 Several studies have highlighted DR 5-HT neurons as key elements in mediating the effects of anxiolytic drugs. For example, studies have suggested that GABAA receptors on DR 5-HT neurons are crucial mediators of the anxiolytic effects of benzodiazepines.3−7 Despite what is understood about anxiolytic drug mechanisms, it is unclear what changes occur in the 5-HT system in anxiety disorders.
Studies using animal models of anxiety have begun to elucidate the mechanisms that underlie anxiety disorders and highlight targets to guide novel approaches to pharmacotherapy. Increased DR 5-HT activity has been shown to be an essential component of the deleterious effects of “uncontrollable stress”, a common element of models that investigate anxious and depressive behavior (reviewed in ref (8)). Chronic social defeat models of varying severity and duration have been used in rats and mice to recapitulate many features of anxiety disorders and depression, although the phenotype depends on the type of paradigm used.9−12 Several studies have indicated that chronic social defeat activates neurons in the DR.10,13 However, because the majority of chronic defeat studies fail to account for the neurochemical identity and topographical location of activated DR neurons, defeat-induced changes in the 5-HT system remain unclear. Growing evidence suggests that the raphe should not be assessed as a unitary target, as many studies tend to do when only characterizing the dense cluster of 5-HT neurons in the ventromedial DR (vmDR). The efferent output of the vmDR is primarily to forebrain projection areas and includes the amygdala, ventral hippocampus, and medial prefrontal cortex (mPFC),14−17 areas known to be involved in the regulation of fear, emotion, and perception of controllability among several other behavioral states. However, there is growing evidence for a distinct role for 5-HT neurons of the lateral wings of the DR (lwDR), a region that is also referred to in the literature as the ventrolateral DR and the ventrolateral periaqueductal gray. In contrast to the vmDR, lwDR 5-HT neurons have subcortical efferent connections that include the dorsolateral periaqueductal gray (dlPAG) and the rostral ventrolateral medulla (RVLM), where 5-HT output dampens panic behaviors and sympathetic responses, respectively.15,18−21 Furthermore, compared to vmDR 5-HT neurons, lwDR 5-HT neurons are more likely to be activated by a stressor14,15,20 due to their distinct intrinsic physiology.22 Therefore, our hypothesis is that 5-HT neurons within these two raphe subfields are differentially regulated in a social defeat model of anxiety. Understanding the differential role for lwDR neurons is especially crucial for translational research, given that the highest abundance of 5-HT neurons in the DR of nonhuman primates and humans is not in the vmDR as it is in rodents, but rather in the lwDR.23,24 To our knowledge, lwDR 5-HT neuron physiology and synaptic regulation have never been assessed in a model of anxiety.
An important aspect of synaptic regulation that may play a role in anxiety is the inhibitory regulation of 5-HT cells by DR GABA neurons. As the major limbic input to the DR, afferent projections from the lateral habenula and medial prefrontal cortex (mPFC) primarily target DR GABA neurons that then inhibit vmDR 5-HT neurons.25−27In vivo studies have shown that the inhibitory circuit from the mPFC to DR 5-HT cells is needed to mitigate the deleterious effects of chronic uncontrollable stress.28 In addition, a wide range of stressors, including social defeat, activated non-5-HT, putatively GABAergic, DR cells.29−31 However, GABAergic input to DR subfields remains poorly understood. Namely, it is unknown whether local GABAergic input varies between the vmDR and lwDR and whether that input is differentially altered in anxiety.
To begin to fill the void in our understanding in the neurophysiology of anxiety disorders, we used a repeated social defeat model to produce an anxious phenotype in mice. Electrophysiology then allowed us to investigate underlying cellular mechanisms by targeting vmDR and lwDR 5-HT neurons. We assessed intrinsic membrane properties along with local glutamatergic and GABAergic synaptic input to determine whether these two subpopulations of 5-HT neurons were altered following repeated social defeat. Forebrain and hindbrain projections of DR 5-HT neurons affect a broader range of behaviors beyond those seen in anxiety disorders and various types of social defeat paradigms can be used to mimic the clinical syndromes of both anxiety and depression. Thus, defeat-induced dysregulation seen in the vmDR and lwDR components of the 5-HT system may have implications for other types of mental disorders.
Results and Discussion
Repeated Social Stress Induces an Anxiety-Like Syndrome Consisting of an Array of Physiological and Behavioral Changes
Many studies in untreated rodents have identified differences in lwDR and vmDR 5-HT neurons, differences that suggest there may be differential regulation of these subpopulations in stress-related disorders including anxiety.14,15,20,22,40 However, to the best of our knowledge, distinct regulation of these subpopulations has never been evaluated in a model of anxiety.
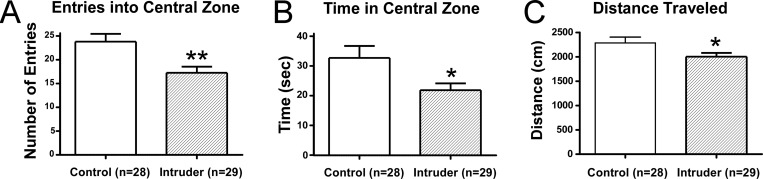
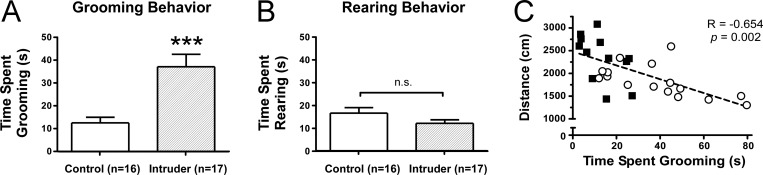
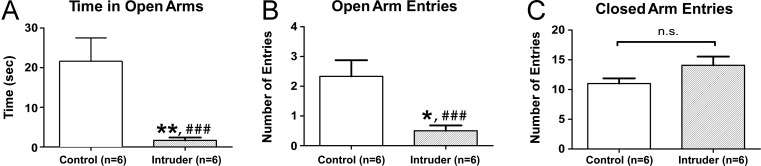
A 5-day social defeat paradigm was tested for its ability to produce anxiety-like behavior in adult male mice. Experimental mice, or “intruders”, underwent 5 daily episodes of social defeat and were compared to behavioral controls using the open field test on day 6 and electrophysiological recordings on day 7. Intruders showed a significant decrease in the number of entries into the central zone and a decrease in the time spent in the central zone (Figure 1). There was also a decrease in the total distance moved throughout the open field arena (Figure 1). Grooming is a displacement behavior seen in rodent models of anxiety32,33 with a compelling parallel to obsessive compulsive disorders. During the open field test, intruders demonstrated increased stressor-evoked grooming compared to controls (Figure 2) and grooming was negatively correlated with total distance traveled (Figure 2). This suggested that increased grooming behavior may help account for the decreased locomotor activity measured during the open field test. In a separate experiment, intruders demonstrated anxiety-like behavior in the elevated plus maze test (Figure 3), namely, a decrease in the number of entries into open arms and decreased time spent in open arms compared to controls.
Figure 1.
Repeated social defeat induces anxiety-like behavior in intruders. Following 5 days of social defeat, anxious behavior was assessed using the open field test. (A) Intruders demonstrated fewer entries into the central zone and (B) less time spent in the central zone. (C) Intruders also traveled less distance throughout the entire open field arena. Distance traveled was inversely correlated to increases in another stress-related behavior, grooming (see Figure 2). Error bars indicate mean ± SEM; *p < 0.05 Student’s t test, **p < 0.01 Student’s t test.
Figure 2.
Repeated social defeat increases stress-induced grooming. (A) During the open field test, intruders spend more time when compared to controls. (B) Rearing behavior during the open-field test was comparable between groups. (C) Increased time spent grooming was correlated to less distance traveled in the open field, suggesting grooming behavior may explain the decreased locomotor activity observed in the open field test. Locomotor activity in the elevated plus maze was comparable between groups (see Figure 3). Error bars indicate mean ± SEM; *p < 0.001 Student’s t test.
Figure 3.
Repeated social defeat induces anxiety-like behaviors in the elevated plus maze test. In a separate set of experiments, mice were subjected to 5 days of social stress or the control condition followed by the elevated plus maze test on day 6. (A) Intruders show increased anxiety-like avoidance behavior compared to controls, as indicated by decreased time spent in the open arms and (B) decreased number of entries into open arms. (C) The number of closed arm entries did not differ between intruders and controls, suggesting comparable degrees of locomotor activity in the elevated plus maze. Error bars indicate mean ± SEM; *p < 0.05 unpaired t test with Welch’s correction, **p < 0.01 unpaired t test with Welch’s correction, ###p < 0.001 Welch’s test for unequal variance.
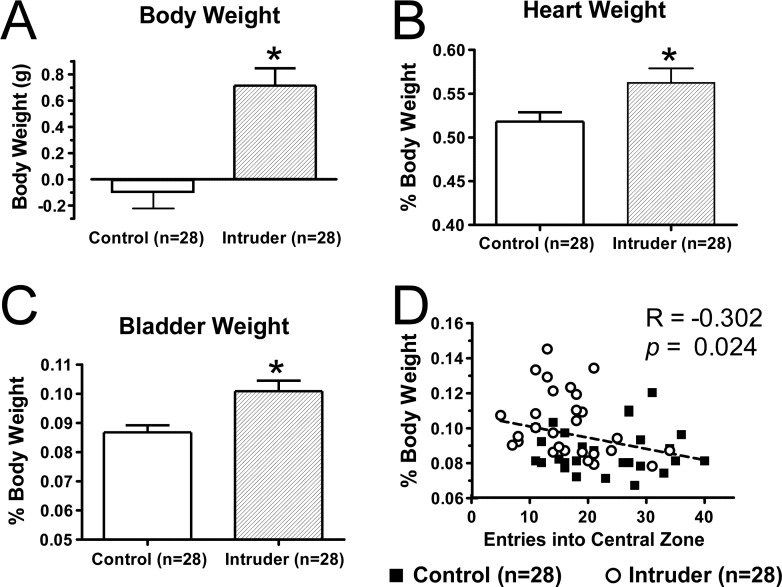
In addition to increases in anxiety-like behavior, intruders demonstrated an increase in body weight, bladder weight, and heart weight (Figure 4). Anxiety disorders are also characterized by an increased sympathetic tone that contributes to the heightened responses to innocuous stressors. Social stress-induced bladder wall hypertrophy, which has been linked to urinary retention in intruders,34 along with cardiac hypertrophy may be sequelae of prolonged activation of the sympathetic system in intruders. Though it lies beyond the scope of our studies, the mechanisms underlying the pathological changes in bladder and heart will likely lend insight as to the interaction between anxiety and urogenital tract pathology or cardiovascular disease, conditions that are known to be comorbid with anxiety in human patients.35,36 These changes also highlight the importance of understanding serotoninergic control of the sympathetic system, which includes the descending projections of lwDR 5-HT neurons along with other serotoninergic brainstem nuclei.
Figure 4.
Repeated social defeat induces an array of stress-associated changes in visceral organs. (A) The 5-day chronic social defeat paradigm induced an increase in the body weight of intruders compared to controls. In addition, the heart weight and bladder of intruders was increased, as measured by both raw weight (data not shown) and (B,C) percent of body weight. Bladder weight was correlated to other measures of anxiety seen in intruders including (D) fewer entries into the central zone of the open field and decreased average velocity (data not shown). Error bars indicate mean ± SEM.
Defining Characteristics of 5-HT Neurons Remain Unaltered by Social Stress
The 5-day social defeat paradigm was used to investigate neurophysiological changes in DR 5-HT neurons. A total of 57 mice underwent 5 days of social defeat or 5 days of the control condition, followed by an open field test on day 6 and electrophysiology recordings in raphe brain slices on day 7. Comparisons were made between recordings in intruders and recordings in controls, considering each subregion separately. The characteristic features of 5-HT neurons include high membrane resistance, a large after-hyperpolarization, and a 5-HT1AR-mediated response along with additional subfield-specific membrane properties.22,37−40 Membrane properties and 5-HT1A autoreceptor responses were measured in vmDR and lwDR neurons of brain slices obtained from 14 control mice and 16 intruders. There were no significant differences in membrane properties between controls and intruders in either subregion (Supporting Information Table S1). The 5-HT1AR-mediated response was assessed by measuring membrane hyperpolarization and changes in resistance upon bath application of 100 nM 5-carboxyamidotryptamine (5-CT). There was no difference between intruders and controls in 5-CT-induced membrane hyperpolarization or the 5-CT-induced decreases in membrane resistance in either subregion (Supporting Information Table S2).
Social Stress Has Differential Presynaptic Effects on Inhibitory Input to vmDR and lwDR 5-HT Neurons
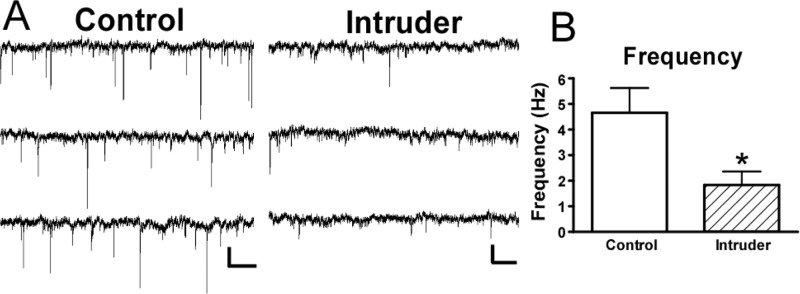
While GABAergic input to DR neurons has long been implicated in the mechanisms of anxiolytic drugs, to our knowledge, no one has examined changes in GABAergic input induced using a model of anxiety. Because local GABAergic input to DR subregions is poorly understood, an initial experiment was conducted in untreated mice to determine whether GABAergic input differs between the vmDR and the lwDR. Voltage clamp GABAA receptor-mediated spontaneous inhibitory postsynaptic current (sIPSC) recordings were performed in 26 neurons from a total of 10 group housed, untreated mice, revealing no differences in GABAergic input to vmDR compared to lwDR neurons (Supporting Information Table S3).
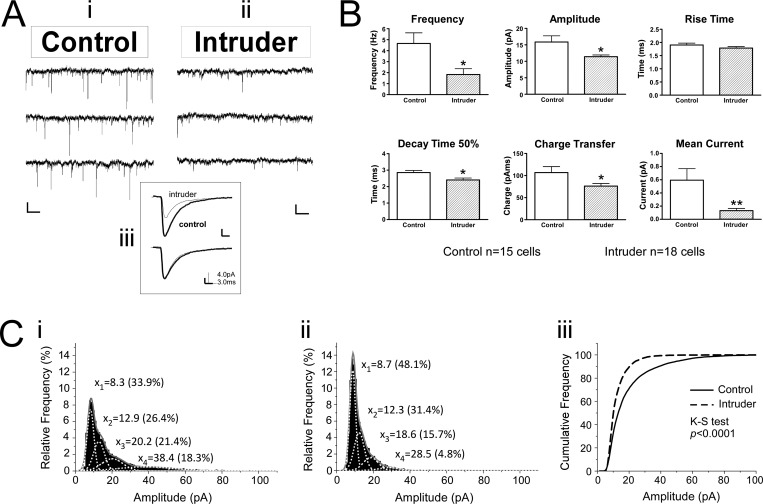
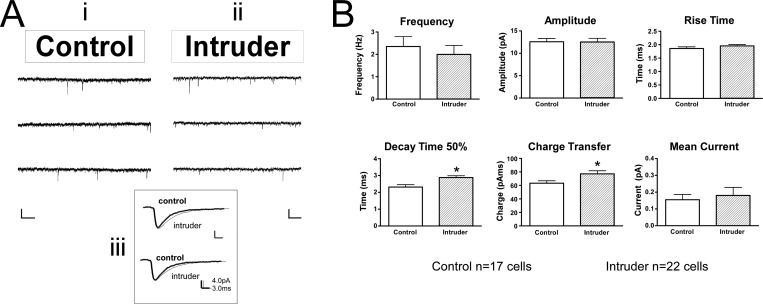
In a separate experiment, voltage clamp recordings were conducted in raphe slices from 10 control mice and 11 intruder mice, following the 5-day social defeat paradigm and open field test. Comparisons between intruders and controls revealed that GABAergic inputs were altered in a topographically specific way after social defeat. In the vmDR, the frequency of sIPSC events was significantly lower in intruders than in controls (Table 2, Figure 5A, B). This lower frequency of sIPSC input is suggestive of a presynaptic mechanism for decreased GABAergic input. However, the stress-induced changes in the lwDR were distinct from those seen in the vmDR, as the frequency of sIPSC events was comparable between intruders and controls in recordings targeting the lwDR (Table 2, Figure 6). These data suggest that the output of GABA neurons innervating vmDR 5-HT neurons was decreased following repeated social defeat, while a separate population of GABA neurons innervates lwDR 5-HT neurons and was not altered by repeated social defeat. This decreased inhibitory input to vmDR neurons could be due to a decrease in the activity of GABA neurons present in the slice. Alternatively, the decreased GABAergic input could have been due to stress-induced loss of GABA neurons in anxious mice. While further studies are underway to investigate this hypothesis, support for the latter is garnered from a genetic model of maladaptive stress responsivity wherein non-5-HT cells of the DR are lost due to apoptotic death following exposure to chronic mild stress,42 though it is unclear from that study whether the non-5-HT DR cells are GABAergic neurons.
Table 2. Results of sIPSC Recordings in lwDR Neurons after Social Defeata.
| lwDR | frequency (Hz) | weighted decay tau (ms) | decay time 50% (ms) | rise time (ms) | average amplitude (pA) | median amplitude (pA) | charge per EPSC (pAms) | mean phasic current (pA) |
|---|---|---|---|---|---|---|---|---|
| control (n = 18) | 2.4 ± 0.4 | 5.4 ± 1.4 | 2.3 ± 0.1 | 1.9 ± 0.06 | 12.6 ± 0.7 | 10.0 ± 0.6 | 63.3 ± 3.6 | 0.15 ± 0.03 |
| intruder (n = 23) | 2.0 ± 0.4 | 4.8 ± 0.4 | 2.9 ± 0.1** | 2.0 ± 0.05 | 12.5 ± 0.9 | 10.9 ± 0.7 | 77.1 ± 4.6* | 0.18 ± 0.05 |
| t test p value | 0.556 | 0.617 | 0.003 | 0.222 | 0.952 | 0.365 | 0.029 | 0.682 |
Values are mean ± SEM. *p < 0.05. **p < 0.01.
Figure 5.
Repeated social defeat leads to decreased sIPSC frequency and amplitude with faster sIPSC kinetics in vmDR 5-HT neurons. (A) Raw data traces from representative vmDR recordings show differences in sIPSC between (Ai) control and (Aii) intruder. Scale bar is 20 pA, 512 ms. The inset (Aiii) contains the averaged PSC from the traces in Ai and Aii, overlaid to enable comparison. The lower trace of the inset shows the average event with normalized amplitude to allow comparison of rise time and decay kinetics. (B) The mean values obtained from each treatment group demonstrate a decrease in the frequency and amplitude of sIPSC events in intruders, along with faster decay time 50%. This contributed to a decrease in charge transfer and a lower mean current. (C) Histograms of all recorded events in the controls (Ci) and intruders (Cii) were fit with a multiple peak Gaussian function. Of all the events recorded in controls, 18.3% were in the large amplitude population centered at 38.4 pA while only 4.8% of intruder events were centered at the largest amplitude peak at 28.5 pA. Thus, there was a loss of large-amplitude events in intruders. The cumulative probability plot in the third panel (Ciii) demonstrates the significant shift toward small events in vmDR recordings in intruders. Error bars indicate mean ± SEM; *p < 0.05 Student’s t test, **p < 0.01 Student’s t test.
Figure 6.
Repeated social defeat leads to slower sIPSC kinetics in lwDR 5-HT neurons. (A) Raw data traces from representative lwDR recordings. The inset (Aiii) demonstrates the averaged event from the respective traces in Ai and Aii. Scale bar is 20 pA, 512 ms. Note the differences in the shape, i.e., kinetics, of the two averaged events. (B) Comparison of the mean values of each treatment group demonstrate a slower decay time 50% and consequent increase in charge transfer in neurons recorded from intruders while the frequency, rise time, and amplitude remain comparable to controls. Error bars indicate mean ± SEM; *p < 0.05 Student’s t test.
These data provide the first evidence of differential inhibitory regulation of vmDR and lwDR 5-HT neurons, implicating different sources of GABAergic input for these distinct subpopulations. Because it remains unclear where the GABA neurons that innervate specific DR subregions reside within the midbrain slice, it is not yet possible to selectively stimulate GABAergic input to specific DR subregions. These data highlight the importance of raphe topography and the potential benefit of more detailed mapping of non-5-HT DR neurons toward our understanding of anxiety and other stress-related diseases. With this growing understanding of DR circuitry, techniques such as optogenetics may prove to be a useful tool for future studies.60
The Effect of Social Stress on Synaptic Input to 5-HT Neurons Is Selective for Inhibitory Inputs
It has been demonstrated in other brain regions that chronic stress can alter dendritic morphology and thereby alter potential synaptic contacts;43−46 this could be another possible mechanism for the stress-induced decreased sIPSC frequency observed in the vmDR. If social defeat results in global changes in dendrite morphology such that a loss of synaptic contacts underlies the decreased sIPSC frequency observed in the vmDR of intruders, we would also expect to see decreased frequency of other types of synaptic input to vmDR 5-HT neurons in intruders. In addition to inhibitory regulation by GABAergic input, the activity of DR 5-HT neurons is also modulated by glutamatergic input mediated by AMPA/kainate-receptors.41,47,48 To determine whether social defeat induced global changes in synaptic input, we measured glutamatergic synaptic excitatory postsynaptic currents (sEPSCs) in controls and intruders. Voltage clamp recordings of sEPSCs were conducted in slices from a total of 13 control mice and 18 intruder mice, yielding no significant differences between intruders and controls in either subregion (Supporting Information Table S4). This suggested that synaptic changes were selective for GABAergic inputs.
Repeated Social Stress Induces Distinct Postsynaptic Changes in vmDR and lwDR 5-HT Neurons
In addition to the frequency of sIPSC events, the size and shape of sIPSC events were evaluated to give insight as to whether social defeat might result in differences in postsynaptic changes in the inhibitory regulation of DR 5-HT neurons. Evaluation of sIPSC amplitude and event kinetics yielded insight into the properties of the ion channel pore of GABAA receptors expressed by 5-HT neurons targeted for recording. In the vmDR, there was a decrease in the average sIPSC amplitude (Table 1, Figure 5A, B). Gaussian fits of histograms of event amplitudes revealed a loss of large-amplitude events in intruders and a significant shift in the distribution toward smaller events (K–S test, p < 0.0001, Figure 5C, D). There was a small but significant decrease in decay time 50% in intruders where more narrow events indicated faster kinetics of events measured in intruders (Figure 5B). The faster event kinetics and decreased amplitude contributed to a smaller charge flux per event mediated by the ion channel pores of GABAA receptors, that is, decreased strength of GABAergic signals in intruders. Combined with the decreased frequency of GABAergic sIPSC events, this smaller charge flux resulted in a dramatic decrease in the mean inhibitory current (Table 1, Figure 5B).
Table 1. Results of sIPSC Recordings in vmDR Neurons after Social Defeata.
| vmDR | frequency (Hz) | weighted decay tau (ms) | decay time 50% (ms) | rise time (ms) | average amplitude (pA) | median amplitude (pA) | charge per EPSC (pAms) | mean phasic current (pA) |
|---|---|---|---|---|---|---|---|---|
| control (n = 16) | 4.7 ± 1.0 | 7.7 ± 1.1 | 2.9 ± 0.1 | 1.9 ± 0.07 | 15.9 ± 1.9 | 13.1 ± 1.4 | 106.8 ± 14.6 | 0.59 ± 0.17 |
| intruder (n = 20) | 1.8 ± 0.5* | 18.6 ± 5.7 | 2.4 ± 0.1* | 1.8 ± 0.06 | 11.4 ± 0.5* | 10.4 ± 0.4* | 76.2 ± 5.9* | 0.13 ± 0.03** |
| t test p value | 0.011 | 0.102 | 0.015 | 0.205 | 0.020 | 0.044 | 0.036 | 0.007 |
Values are mean ± SEM. *p < 0.05. **p < 0.01.
The frequency and amplitude of sIPSCs were comparable between intruders and controls in the lwDR (Table 2, Figure 6). However, the decay time 50% was significantly longer in lwDR neurons recorded from intruders, such that wider events had slower decay kinetics (Figure 6B). This change resulted in a larger charge flux per event (Figure 6B), that is, increased strength of GABAergic signals in intruders. In the lwDR, overall mean inhibitory current was comparable between intruders and controls (Table 2, Figure 6). Changes in the strength of the inhibitory signals measured in baseline experiments conducted in brain slices have important implications for stress-activated circuits in the intact brain. Several studies have shown that DR GABA neurons are activated above baseline in response to stressors ranging from mild handling to chronic social defeat.10,17,20,21,29,30 Thus, local GABAergic input to the DR is expected to increase in the intact brain in response to a variety of stressors, making even subtle post synaptic changes in the strength of GABAergic signals all the more relevant. Even though changes in sIPSC charge flux in the lwDR did not result in increased overall baseline inhibitory current (Table 2, Figure 6), this increased strength of lwDR GABAergic signaling likely has a substantial effect on intact brain circuits, particularly in response to stressors.
These data suggest that, despite the comparable sIPSC features in untreated mice (Supporting Information Table S3), there were subregion-specific changes in GABAA receptors following exposure to repeated social stress. Further investigation of the cellular and molecular distinctions between DR subregions in models of anxiety is ongoing. Evaluation of the intrinsic properties of vmDR and lwDR neurons in untreated mice (17) demonstrated subfield specific differences in the intrinsic excitability and after-hyperpolarization that follows action potentials. These data now add GABAA receptors to the list of ion channels and receptor subunits in 5-HT subpopulations that may provide a way to selectively target different components of the serotonin system.
Functional Consequences of the Neurophysiological Changes in the Anxious Brain
Because the DR subfields have diverging efferent projections, our data suggest that the 5-HT output in brain regions targeted by either subfield would be differentially affected in anxiety disorders. Regions that are preferentially targeted by midline DR neurons but not the lwDR, for example, the mPFC, amygdala, or ventral hippocampus,14−17,20 should receive increased 5-HT release due to decreased inhibitory tone on vmDR 5-HT neurons. Likewise, our data suggests that areas selectively targeted by the lwDR, such as the RVLM and the dlPAG18−20 should receive decreased 5-HT release. In fact, several in vivo studies49−51 have demonstrated findings to this effect, findings to which our data add a mechanistic understanding.
Seminal studies in the mechanisms underlying anxiety and depression have shown that controllability over a stressor mitigates learned helplessness.28 Uncontrollable stress fails to activate the mPFC and leads to increased activation of DR 5-HT neurons, resulting in an increase in 5-HT levels in amygdala, ventral hippocampus, and mPFC,49−51 regions that are linked to dysregulated emotional responses and fear processing. On the other hand, controllable stress activates the mPFC, leading to inhibition of DR 5-HT neurons.8 It is now known that mPFC projections primarily target DR GABA neurons that then inhibit vmDR 5-HT neurons.27,52 Our data suggest that, in the anxious brain, there is loss of GABA input to vmDR neurons that could lead to the increased 5-HT release in vmDR target regions. Thus, due to the anxiety-associated loss of the GABA mediator of mPFC feedback, even innocuous stressors previously perceived as controllable would now induce the effects of uncontrollable stressors.
Although uncontrollable stress increased 5-HT levels in amygdala, ventral hippocampus, and mPFC, it was shown to decrease 5-HT release in dlPAG for reasons that were previously unclear.49−51 Together with a better understanding of the microcircuitry of the DR and the distinct characteristics of the lwDR, our data provide an explanation for these and other in vivo findings. The RVLM and the dlPAG are targeted by lwDR 5-HT neurons that act as crucial inhibitory regulators of sympathomotor and behavioral panic responses, respectively.18−21 Our findings suggest that, in the anxious brain, lwDR 5-HT neurons would have more potent inhibitory feedback, decreased 5-HT release in lwDR target regions, and thus insufficient attenuation of panic responses. Studies using a rat model of panic disorder demonstrated consistent findings whereby decreased activation of lwDR 5-HT neurons was associated with increased sympathomotor responses and exaggerated panic.21 Insufficient lwDR output may have contributed to the increased sympathetic tone and the associated changes observed in the heart and bladder of anxious mice in our study. The changes in the physiology of lwDR cells and other descending 5-HT neurons therefore have implications for panic responses and the somatic pathology that is often comorbid with anxiety disorders.
Conclusions
In summary, repeated social defeat had effects on the 5-HT system that extended beyond 5-HT neurons themselves. The local GABAergic circuits that modulate DR 5-HT activity were selectively altered by social stress such that vmDR 5-HT cells demonstrated a loss of inhibitory input while lwDR 5-HT neurons demonstrated the potential for more potent inhibitory input. Our findings help explain divergent changes in 5-HT release in vmDR and lwDR target regions induced by uncontrollable stress and offer an intriguing mechanism for the behavioral and sympathetic dysregulation seen in anxiety. Our findings also highlight new avenues for the exploration of drug targets that may differentially affect subpopulations of 5-HT neurons and thus 5-HT output in specific regions of the brain. Although current approaches to pharmacotherapy regard the entire 5-HT system as a unitary target, this study underscores the potential benefit that targeting specific elements of the serotonin system may have for the understanding and treatment of specific components of anxiety disorders and visceral organ sequelae.
Methods
Animals
Adult male Pet-1::eYFP mice and wild-type littermates were used at 2–4 months of age. These mice were on a C57BL/6 background and contained 5-HT neurons that express yellow fluorescent protein (YFP) under the control of the 5-HT specific Pet-1 promoter53 and that are comparable to 5-HT cells in wild-type littermates.22 All mice were housed in a standard animal facility with lights on 06:00 to 18:00 h. Animal protocols were approved by the Institutional Animal Care and Use Committee and were conducted in accordance to the NIH Guide for the Care and Use of Laboratory Animals.
Repeated Social Defeat
The 5-day repeated social defeat paradigm was based on a paradigm used previously in rats9,54 and mice.55 This paradigm is sometimes referred to as a chronic, episodic resident-intruder paradigm. The first day of social defeat was staggered to generate 1–2 mice per day of alternating treatment groups for electrophysiology experiments. Mice were singly housed 1 day prior to the start of the repeated social defeat protocol. Behavioral procedures were conducted between 16:00 and 19:00 h in a fully lighted experimental room, after which all mice were returned to their home cages in a lighted home room. The lights in the home room were turned off after the return of all the mice. Control mice were not present in the experimental room during defeats. Control mice were placed in a clean, empty cage for 2 min and then behind a wire partition for the remainder of the 30 min session. “Intruders” were mice that were placed into the home cage of a larger, male “resident” mouse and allowed to interact until the resident initiated an attack or a threat leading to a defeat posture that is typical of mice.56 All defeats occurred within 2 min. The intruder was then placed behind a wire partition for the remainder of the 30 min session with continued exposure to the resident. Defeat sessions were repeated daily for 5 days; intruders encountered a different resident each day.
Singly housed “residents” were wild type males of various backgrounds. These mice were over 4 months old and were screened for aggressive behavior during the week preceding the defeat experiments. Only those residents that defeated intruders within 2 min were used for experiments. Intruders used to screen residents were not used in experiments.
Behavioral Tests of Anxiety-like Behavior
Mice underwent the 5-day social defeat paradigm, were subjected to the open field test on day 6, and were sacrificed on the morning of day 7. Behavioral tests were conducted in an experimental room between 18:00 and 20:00 h in the dark, under dim, red light. For the open field test, mice were placed facing the corner and behavior was videotaped for 5 min. Videotaped behavior was analyzed using Ethnovision software (Noldus Information Technology, Leesburg, VA). While observing animals in the open field test, it was noted that some mice stop to groom more than others. To determine whether stress-associated grooming may have contributed to the decrease in measured locomotor activity in the open field (Figure 1), videotaped open field behavior was analyzed for numbers of bouts and duration of bouts of grooming and rearing. The grooming observed was characterized by short, interrupted spurts, consistent with stress-evoked stereotypy rather than low-stress comfort grooming.57,58
Anxiety-like avoidance behavior was also tested in the elevated plus maze using adult male C57BL/6 mice from Taconic (Hudson, NY) at 2–3 months of age. Following the 5-day social defeat paradigm, intruders and behavioral controls were tested on day 6 for anxiety-like behavior in the elevated plus maze. Behavioral tests were conducted in an experimental room between 18:00 and 20:00 h in the dark, under dim, red light. Mice were placed in the center of the maze, facing an open arm, and behavior was videotaped for 5 min. Video was hand-scored for four-paw entries by two observers blinded to the treatment groups; scores were averaged for each mouse.
Organ Weights
Body weight was measured in the evening of day 1 following the first defeat session and in the evening of day 6 following the open field test to determine the change in body weight. On the morning of day 7, brains were dissected for slice preparation and the bladder and heart were dissected and their weights compared to day 7 body weight obtained just prior to euthanasia.
Whole-Cell Electrophysiology
Electrophysiology recordings were conducted as previously described.22,38,41 In brief, on the morning of day 7, mice were decapitated, brains were dissected, and 200 μm thick midbrain slices were cut. Slices were submerged in aCSF solution with 50 μM tryptophan bubbled with 95% O2/5% CO2 for 1 h at 36 °C and then at room temperature until use. For recordings, slices were perfused with 35 °C aCSF at a flow rate of 1.5–2.0 mL/min in a recording chamber and cells were visualized using IR/DIC and fluorescence microscopy with a Nikon E600 upright microscope and Nikon Elements software (Optical Apparatus, Ardmore, PA). YFP label was visualized to target vmDR and lwDR 5-HT neurons. Biocytin was included in the intracellular solution to enable immunohistochemical verification of TPH content. Whole-cell recording electrodes filled with a solution containing 130 mM Kgluconate, 5 mM NaCl, 10 mM Na-phosphocreatinine, 1 mM MgCl2, 0.02 mM EGTA, 10 mM HEPES, 2 MgATP, 0.5 Na2GTP, and 0.1% biocytin (pH 7.32) had an access resistance of 6–10 MΩ, and were used to obtain recordings in current clamp and voltage clamp modes using Digidata digitizer, Multiclamp 700B amplifier, and pClamp 9.0 software (Molecular Devices, Union City, CA). Electrodes for recording IPSCs were filled with 70 mM Kgluconate, 70 mM KCl, 2 mM NaCl, 10 mM Na phosphocreatine, 4 mM EGTA, 10 mM HEPES, 2 mM MgATP, 0.3 mM Na2GTP, and 0.1% Biocytin (pH 7.3) and had an access resistance of 3–7 MΩ. The high Cl– concentration altered the Cl– reversal potential so that the sIPSCs were larger, inward currents, which were further isolated by blocking EPSCs with 20 μM DNQX. Reported values do not incorporate a junction potential of approximately +15 mV, as calculated using Clampex software. After recordings, DR slices were fixed for 2–3 h with 4% paraformaldehyde and processed for immunohistochemistry.
Electrophysiology Data Analysis
Any cells that were immunonegative for TPH were excluded from analysis. Membrane properties and 5-HT1A receptor (5-HT1AR)-mediated responses were measured in current clamp recordings and analyzed using Clampfit 9.0 (Molecular Devices) as previously described.22,38 Voltage clamp data were analyzed using MiniAnalysis (Synaptosoft, Decatur, GA) as previously described.41 The reported decay tau value is the tau obtained from an exponential fit of the 10–90% decay of the averaged PSC. A single exponential fit was used for sEPSCs and a double exponential fit for sIPSCs. Using the equation for the double exponential fit y = (A1)exp(−x/T1) + (A2)exp(−x/T2), the weighted tau was calculated for the averaged sIPSC using the following formula: Tw= (A1T1 + A2T2)/(A1 + A2). The area under curve of the averaged PSC was the charge per PSC; this was multiplied by PSC frequency to obtain mean phasic current. Gaussian fits of event histograms were obtained using the peak analyzer function in OriginPro 8.1 software (Origin Lab Corporation, Northampton, MA); one to five peaks were chosen in order to minimize the reduced χ2 value. Group averages were obtained by compiling the mean or median values for each cell, as indicated. Histograms and cumulative probability plots describing events from all cells in each group were compared using the Kolmogorov–Smirnov test. Student’s t test was used to generate p values unless otherwise noted, and p < 0.05 was deemed significant. Additional statistical analysis was performed using Prism 5 (GraphPad Software, La Jolla, CA) and OriginPro 8.1.
Immunohistochemistry
Immunohistochemical identification of each neuron was completed as previously described after recording.38,40,41,59 In brief, a standard immunohistochemistry protocol was used on midbrain slices using mouse anti-TPH (1:200, Sigma, St. Louis, MO) along with secondary donkey anti-mouse Alexa Fluor 488 (1:200, Invitrogen, Carlsbad, CA) and streptavidin-conjugated Pacific Blue to visualize cells filled with biocytin during recording (1:100, Invitrogen). Images were captured using a Leica DMR fluorescent microscope (Leica Microsystems, Bannockburn, IL) and OpenLab software (Improvision, Lexington, MA) and then confirmed on a Leica DMIRE2 confocal microscope (Leica Microsystems) using Leica confocal software (Leica Microsystems).
Drugs
All chemicals for making the sucrose aCSF, aCSF, and intracellular electrolyte solution were purchased from Fisher Scientific (Pittsburgh, PA). 5-CT and all chemicals for the intracellular electrolyte solution were purchased from Sigma-Aldrich. DNQX was purchased from Tocris (Ellisville, MO).
Acknowledgments
Preliminary data were presented previously in abstract form at the 2009 Society for Neuroscience Annual Meeting, Chicago, IL (Program No. 161.3) and were part of the dissertation authored by L.K.C. The authors thank Dr. Evan Deneris for supplying the Pet-1::eYFP mice. The authors would also like to thank Adaure Akanwa, Zachary Spangler, Anna Raper, and Monisha Chakravarthy for technical support.
Glossary
Abbreviations
- 5-CT
5-carboxyamidotryptamine
- 5-HT
5-hydroxytryptamine (serotonin)
- 5-HT1AR
5-HT1A receptor
- aCSF
artificial cerebrospinal fluid
- dlPAG
dorsolateral periaqueductal gray
- DR
dorsal raphe
- GABA
γ-aminobutyric acid
- lwDR
lateral wings of the DR
- mPFC
medial prefrontal cortex
- RVLM
rostral ventrolateral medulla
- sEPSC
spontaneous excitatory postsynaptic current
- sIPSC
spontaneous inhibitory postsynaptic current
- TPH
tryptophan hydroxylase
- vmDR
ventromedial DR
Supporting Information Available
Table S1: Membrane properties of DR neurons after social defeat. Table S2: Autoreceptor-mediated responses of DR neurons after social defeat. Table S3: Results of sIPSC recordings in DR neurons of untreated mice. Table S4: Results of sEPSC recordings in DR neurons after social defeat. This material is available free of charge via the Internet at http://pubs.acs.org.http://pubs.acs.org.
Author Contributions
L.K.C. assisted in experimental design, conducted social defeat experiments, behavioral tests, electrophysiological recordings, analyzed data, and wrote the manuscript. S.F.R. assessed grooming behavior and analyzed data. S.G.B. assisted in experimental design, analysis, and manuscript preparation.
This research was supported by the following grants from the National Institute of Mental Health: R01 MH075047 (S.G.B.), RC MH089800 (S.G.B.), and MH082611 (L.K.C.).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Azmitia E. C.; Segal M. (1978) An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 179, 641–667. [DOI] [PubMed] [Google Scholar]
- Molliver M. E. (1987) Serotonergic neuronal systems: what their anatomic organization tells us about function. J. Clin. Psychopharmacol. 7, 3S–23S. [PubMed] [Google Scholar]
- Soubrie P.; Thiebot M. H.; Jobert A.; Hamon M. (1981) Serotoninergic control of punished behavior: effects of intra-raphe microinjections of chlordiazepoxide, GABA and 5-HT on behavioral suppression in rats. J. Physiol. (Paris) 77, 449–453. [PubMed] [Google Scholar]
- Thiebot M. H.; Hamon M.; Soubrie P. (1982) Attenuation of induced-anxiety in rats by chlordiazepoxide: role of raphe dorsalis benzodiazepine binding sites and serotoninergic neurons. Neuroscience 7, 2287–2294. [DOI] [PubMed] [Google Scholar]
- Maier S. F.; Kalman B. A.; Grahn R. E. (1994) Chlordiazepoxide microinjected into the region of the dorsal raphe nucleus eliminates the interference with escape responding produced by inescapable shock whether administered before inescapable shock or escape testing. Behav. Neurosci. 108, 121–130. [DOI] [PubMed] [Google Scholar]
- Gallager D. W.; Mallorga P.; Thomas J. W.; Tallman J. F. (1980) GABA-benzodiazepine interactions: physiological, pharmacological and developmental aspects. Fed. Proc. 39, 3043–3049. [PubMed] [Google Scholar]
- Gallager D. W. (1978) Benzodiazepines: potentiation of a GABA inhibitory response in the dorsal raphe nucleus. Eur. J. Pharmacol. 49, 133–143. [DOI] [PubMed] [Google Scholar]
- Maier S. F.; Watkins L. R. (2005) Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 29, 829–841. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S.; Vining C.; Iyer V.; Kinni V. (2006) Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J. Neuroendocrinol. 18, 13–24. [DOI] [PubMed] [Google Scholar]
- Martinez M.; Calvo-Torrent A.; Pico-Alfonso M. A. (1998) Social Defeat and Subordination as Models of Social Stress in Laboratory Rodents: A Review. Aggressive Behav. 24, 241–256. [Google Scholar]
- Miczek K. A.; de Wit H. (2008) Challenges for translational psychopharmacology research--some basic principles. Psychopharmacology (Berlin, Ger.) 199, 291–301. [DOI] [PubMed] [Google Scholar]
- Keeney A.; Jessop D. S.; Harbuz M. S.; Marsden C. A.; Hogg S.; Blackburn-Munro R. E. (2006) Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J. Neuroendocrinol. 18, 330–338. [DOI] [PubMed] [Google Scholar]
- Matsuda S.; Peng H.; Yoshimura H.; Wen T. C.; Fukuda T.; Sakanaka M. (1996) Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci. Res. 26, 157–170. [PubMed] [Google Scholar]
- Abrams J. K.; Johnson P. L.; Hollis J. H.; Lowry C. A. (2004) Anatomic and functional topography of the dorsal raphe nucleus. Ann. N.Y. Acad. Sci. 1018, 46–57. [DOI] [PubMed] [Google Scholar]
- Lowry C. A., Evans A. K., Gasser P. J., Hale M. W., Staub D. R., and Shekhar A. (2008) Topographic organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In Serotonin and Sleep: Molecular, Functional and Clinical Aspects (Monti J. M., Pandi-Perumai S. R., Jacobs B. L., and Nutt D. J., Eds.), Birkhauser Verlag AG, Basel, Switzerland. [Google Scholar]
- Meloni E. G.; Reedy C. L.; Cohen B. M.; Carlezon W. A. Jr. (2008) Activation of raphe efferents to the medial prefrontal cortex by corticotropin-releasing factor: correlation with anxiety-like behavior. Biol. Psychiatry 63, 832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale M. W.; Hay-Schmidt A.; Mikkelsen J. D.; Poulsen B.; Bouwknecht J. A.; Evans A. K.; Stamper C. E.; Shekhar A.; Lowry C. A. (2008) Exposure to an open-field arena increases c-Fos expression in a subpopulation of neurons in the dorsal raphe nucleus, including neurons projecting to the basolateral amygdaloid complex. Neuroscience 157, 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bago M.; Dean C. (2001) Sympathoinhibition from ventrolateral periaqueductal gray mediated by 5-HT(1A) receptors in the RVLM. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 280, R976–984. [DOI] [PubMed] [Google Scholar]
- Bago M.; Marson L.; Dean C. (2002) Serotonergic projections to the rostroventrolateral medulla from midbrain and raphe nuclei. Brain Res. 945, 249–258. [DOI] [PubMed] [Google Scholar]
- Johnson P. L.; Lightman S. L.; Lowry C. A. (2004) A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann. N.Y. Acad. Sci. 1018, 58–64. [DOI] [PubMed] [Google Scholar]
- Johnson P.; Lowry C.; Truitt W.; Shekhar A. (2008) Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. J. Psychopharmacol. 22, 642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. K.; Craige C. P.; Beck S. G. (2010) Increased intrinsic excitability of lateral wing serotonin neurons of the dorsal raphe: a mechanism for selective activation in stress circuits. J. Neurophysiol. 103, 2652–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A.; Parent A. (1998) Chemoarchitecture of the primate dorsal raphe nucleus. J. Chem. Neuroanat. 15, 111–127. [DOI] [PubMed] [Google Scholar]
- Austin M. C.; O’Donnell S. M. (1999) Regional distribution and cellular expression of tryptophan hydroxylase messenger RNA in postmortem human brainstem and pineal gland. J. Neurochem. 72, 2065–2073. [DOI] [PubMed] [Google Scholar]
- Ferraro G.; Montalbano M. E.; Sardo P.; La Grutta V. (1996) Lateral habenular influence on dorsal raphe neurons. Brain Res. Bull. 41, 47–52. [DOI] [PubMed] [Google Scholar]
- Allers K. A.; Sharp T. (2003) Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience 122, 193–204. [DOI] [PubMed] [Google Scholar]
- Celada P.; Puig M. V.; Casanovas J. M.; Guillazo G.; Artigas F. (2001) Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. J. Neurosci. 21, 9917–9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S. F.; Watkins L. R. (1998) Stressor controllability, anxiety, and serotonin. Cognit. Ther. Res. 22, 595–613. [Google Scholar]
- Gardner K. L.; Thrivikraman K. V.; Lightman S. L.; Plotsky P. M.; Lowry C. A. (2005) Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience 136, 181–191. [DOI] [PubMed] [Google Scholar]
- Berton O.; Covington H. E. 3rd; Ebner K.; Tsankova N. M.; Carle T. L.; Ulery P.; Bhonsle A.; Barrot M.; Krishnan V.; Singewald G. M.; Singewald N.; Birnbaum S.; Neve R. L.; Nestler E. J. (2007) Induction of deltaFosB in the periaqueductal gray by stress promotes active coping responses. Neuron 55, 289–300. [DOI] [PubMed] [Google Scholar]
- Paul E. D.; Hale M. W.; Lukkes J. L.; Valentine M. J.; Sarchet D. M.; Lowry C. A. (2011) Repeated social defeat increases reactive emotional coping behavior and alters functional responses in serotonergic neurons in the rat dorsal raphe nucleus. Physiol. Behav. 104, 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff A. V.; Tuohimaa P. (2004) Contrasting grooming phenotypes in C57Bl/6 and 129S1/SvImJ mice. Brain Res. 1028, 75–82. [DOI] [PubMed] [Google Scholar]
- Kalueff A. V.; Tuohimaa P. (2005) The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J. Neurosci. Methods 143, 169–177. [DOI] [PubMed] [Google Scholar]
- Chang A.; Butler S.; Sliwoski J.; Valentino R.; Canning D.; Zderic S. (2009) Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am. J. Physiol. Renal Physiology 297, F1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J. Q.; Brown S. O.; Calhoun E. A. (2008) Mental health diagnoses in patients with interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome: a case/control study. J. Urol. 180, 1378–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne P. P.; Davidson K. W.; Kessler R. C.; Asmundson G. J.; Goodwin R. D.; Kubzansky L.; Lydiard R. B.; Massie M. J.; Katon W.; Laden S. K.; Stein M. B. (2008) Anxiety disorders and comorbid medical illness. Gen. Hosp. Psychiatry 30, 208–225. [DOI] [PubMed] [Google Scholar]
- Kirby L. G.; Pernar L.; Valentino R. J.; Beck S. G. (2003) Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience 116, 669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S. G.; Pan Y. Z.; Akanwa A. C.; Kirby L. G. (2004) Median and dorsal raphe neurons are not electrophysiologically identical. J. Neurophysiol. 91, 994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermaelen C. P.; Aghajanian G. K. (1983) Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 289, 109–119. [DOI] [PubMed] [Google Scholar]
- Calizo L. H.; Akanwa A.; Ma X.; Pan Y. Z.; Lemos J. C.; Craige C.; Heemstra L. A.; Beck S. G. (2011) Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology 61, 524–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J. C.; Pan Y. Z.; Ma X.; Lamy C.; Akanwa A. C.; Beck S. G. (2006) Selective 5-HT receptor inhibition of glutamatergic and GABAergic synaptic activity in the rat dorsal and median raphe. Eur. J. Neurosci. 24, 3415–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEuen J. G.; Beck S. G.; Bale T. L. (2008) Failure to mount adaptive responses to stress results in dysregulation and cell death in the midbrain raphe. J. Neurosci. 28, 8169–8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Tellez R. I.; Hernandez-Torres E.; Gamboa C.; Flores G. (2009) Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse 63, 794–804. [DOI] [PubMed] [Google Scholar]
- Izquierdo A.; Wellman C. L.; Holmes A. (2006) Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 26, 5733–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. C.; Wellman C. L. (2004) Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 60, 236–248. [DOI] [PubMed] [Google Scholar]
- Liu R. J.; Aghajanian G. K. (2008) Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc. Natl. Acad. Sci. U.S.A. 105, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z. Z.; Williams J. T. (1989) GABA- and glutamate-mediated synaptic potentials in rat dorsal raphe neurons in vitro. J. Neurophysiol. 61, 719–726. [DOI] [PubMed] [Google Scholar]
- Adell A.; Celada P.; Abellan M. T.; Artigas F. (2002) Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res. Brain Res. Rev. 39, 154–180. [DOI] [PubMed] [Google Scholar]
- Amat J.; Matus-Amat P.; Watkins L. R.; Maier S. F. (1998) Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Res. 797, 12–22. [DOI] [PubMed] [Google Scholar]
- Amat J.; Matus-Amat P.; Watkins L. R.; Maier S. F. (1998) Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 812, 113–120. [DOI] [PubMed] [Google Scholar]
- Bland S. T.; Hargrave D.; Pepin J. L.; Amat J.; Watkins L. R.; Maier S. F. (2003) Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology 28, 1589–1596. [DOI] [PubMed] [Google Scholar]
- Jankowski M. P.; Sesack S. R. (2004) Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J. Comp. Neurol. 468, 518–529. [DOI] [PubMed] [Google Scholar]
- Scott M. M.; Wylie C. J.; Lerch J. K.; Murphy R.; Lobur K.; Herlitze S.; Jiang W.; Conlon R. A.; Strowbridge B. W.; Deneris E. S. (2005) A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc. Natl. Acad. Sci. U.S.A. 102, 16472–16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S.; Vining C. (2003) Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm. Behav. 43, 158–165. [DOI] [PubMed] [Google Scholar]
- Yap J. J.; Takase L. F.; Kochman L. J.; Fornal C. A.; Miczek K. A.; Jacobs B. L. (2006) Repeated brief social defeat episodes in mice: Effects on cell proliferation in the dentate gyrus. Behav. Brain Res. 172, 344–350. [DOI] [PubMed] [Google Scholar]
- Miczek K. A.; Thompson M. L.; Shuster L. (1982) Opioid-like analgesia in defeated mice. Science 215, 1520–1522. [DOI] [PubMed] [Google Scholar]
- Fentress J. C. (1977) Tonic hypothesis and patterning of behavior. Ann. N.Y. Acad. Sci. 290, 370–395. [DOI] [PubMed] [Google Scholar]
- Kalueff A. V.; Tuohimaa P. (2004) Grooming analysis algorithm for neurobehavioural stress research. Brain Res. Brain Res. Protoc. 13, 151–158. [DOI] [PubMed] [Google Scholar]
- Kirby L. G.; Freeman-Daniels E.; Lemos J. C.; Nunan J. D.; Lamy C.; Akanwa A.; Beck S. G. (2008) Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J. Neurosci. 28, 12927–12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden M. R.; Selimbeyoglu A.; Mirzabekov J. J.; Lo M.; Thompson K. R.; Kim S. Y.; Adhikari A.; Tye K. M.; Frank L. M.; Deisseroth K. (2012) A prefrontal cortex-brainstem neuronal projection that controls respoinse to behavioaral challenge. Nature 492, 428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.