Abstract
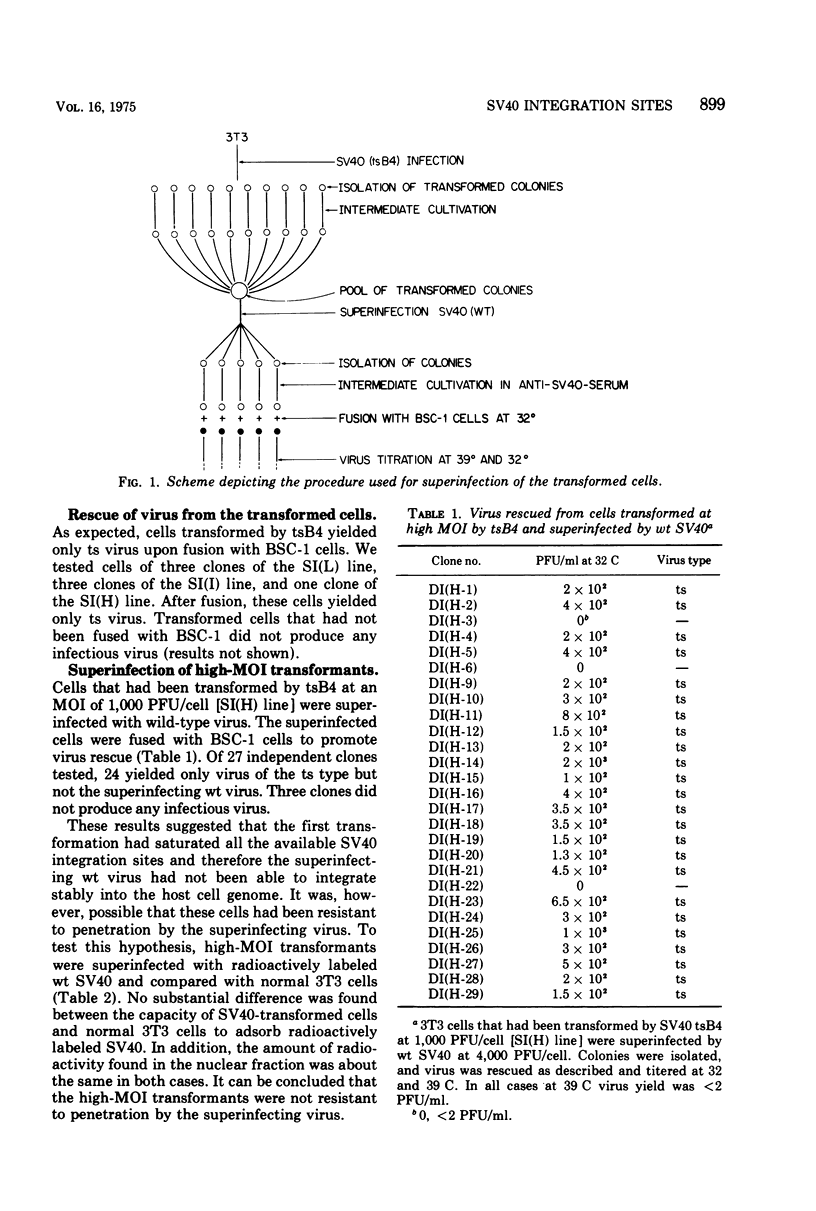
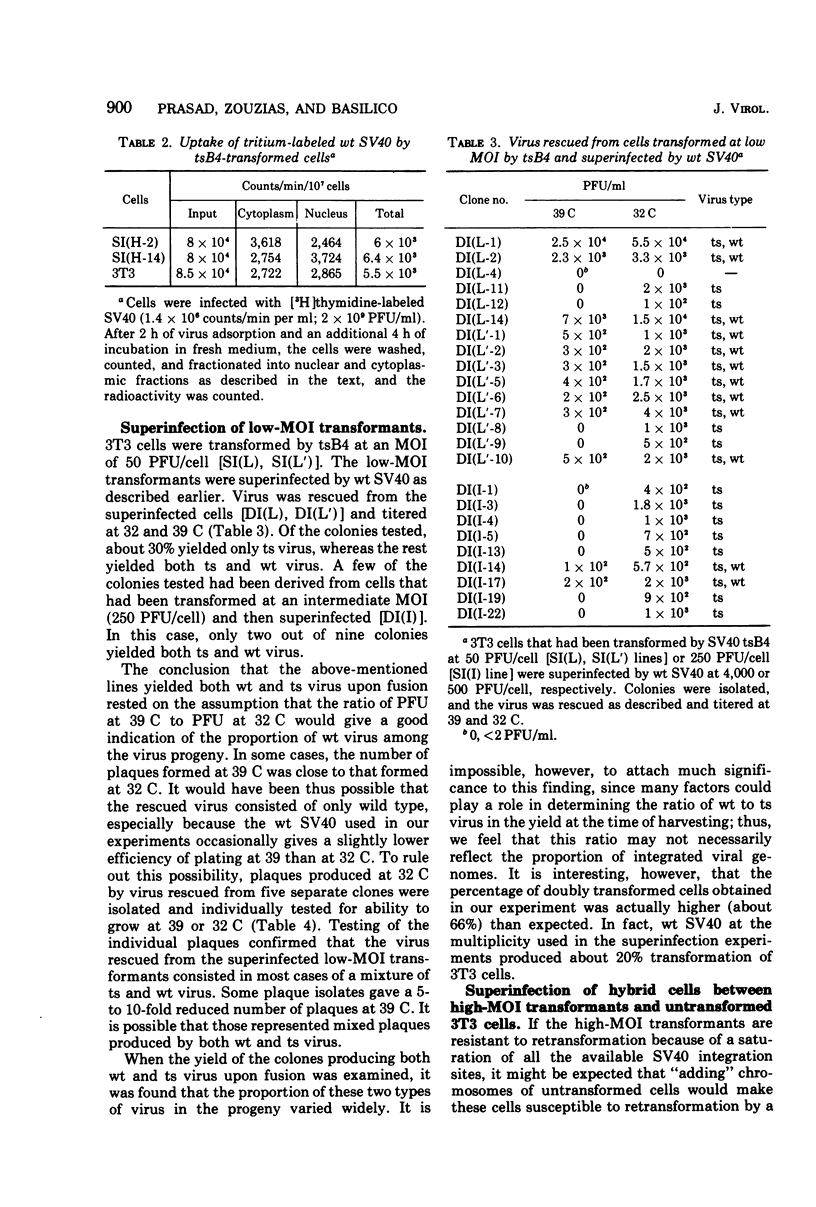
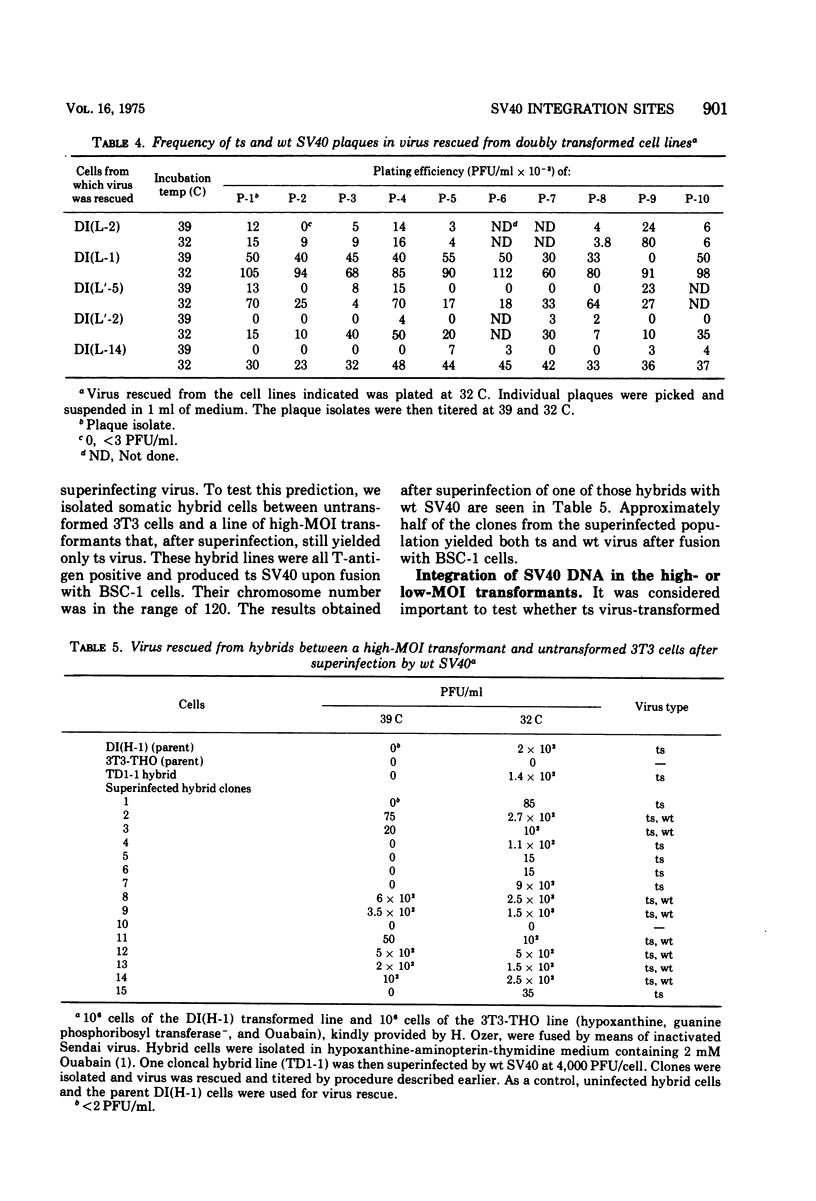
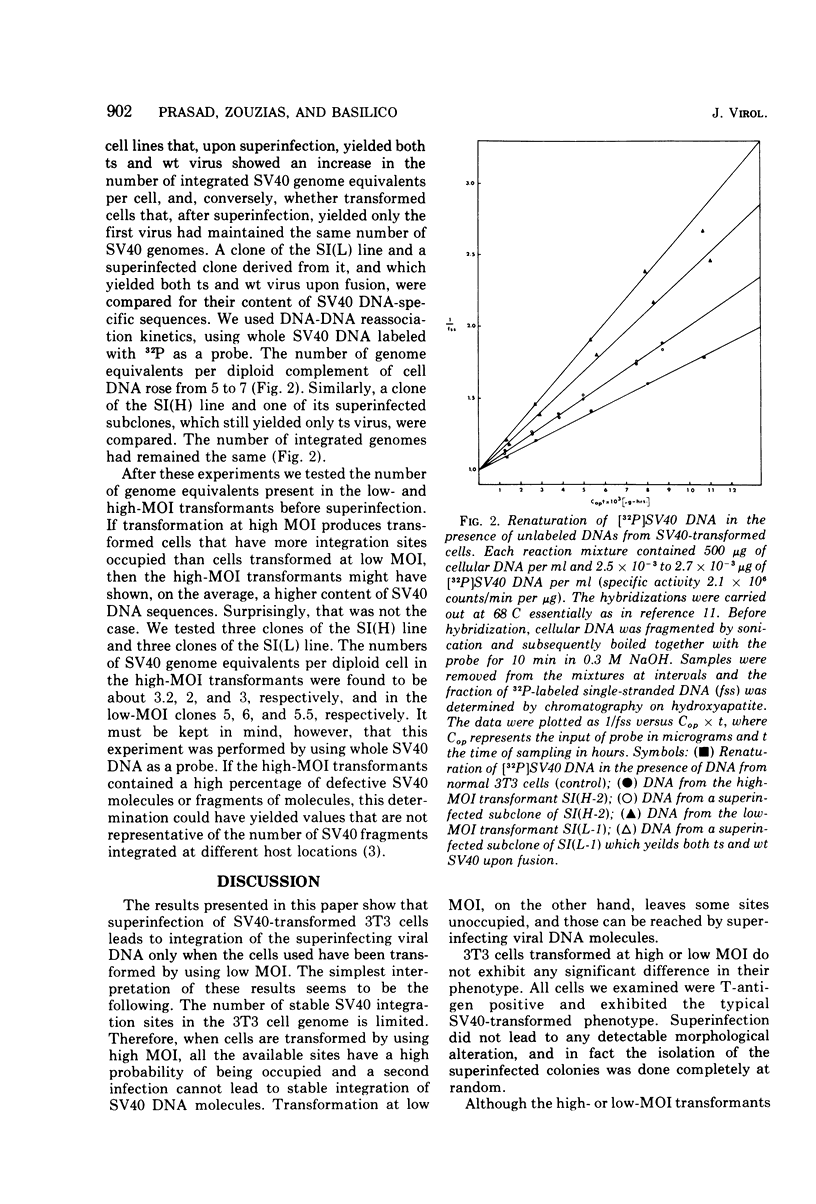
To gain information on the specificity of simian virus 40 (SV40) integration in the genome of transformed cells, mouse 3T3 cells were transformed by a temperature-sensitive (ts) SV40 mutant, using high multiplicity of infection (MOI). Transformed cells were superinfected with wild-type (wt) virus at high MOI. Clones were isolated and fused with permissive BSC-1 cells to promote virus rescue. All rescued viruses were of the ts type only. When the high-MOI transformants were infected with 3H-labeled wt SV40, the amount of radioactivity associated with their nuclear fraction was found to be similar to that of 3T3 cells. 3T3 cells were then transformed by ts SV40 at low MOI and superinfected by wt virus at high MOI. Upon fusion with BSC-1 cells, most clones produced both ts and wt virus. These results suggest that the number of stable SV40 integration sites in the 3T3 genome is limited, since they can be saturated by transformation at high MOI. When the MOI is low, the sites are not saturated and a subsequent infection can lead to integration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan M., McKenna G., Sharp P. A. Cleavage of mouse DNA by a restriction enzyme as a clue to the arrangement of genes. Cold Spring Harb Symp Quant Biol. 1974;38:383–395. doi: 10.1101/sqb.1974.038.01.041. [DOI] [PubMed] [Google Scholar]
- Botchan M., Ozanne B., Sugden B., Sharp P. A., Sambrook J. Viral DNA in transformed cells. III. The amounts of different regions of the SV40 genome present in a line of transformed mouse cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4183–4187. doi: 10.1073/pnas.71.10.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Girardi A. J., Koprowski H. Assignment of the T-antigen gene of simian virus 40 to human chromosome C-7. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3617–3620. doi: 10.1073/pnas.70.12.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbs D. R., Kit S. Isolation of double lysogens from 3T3 cells transformed by plaque morphology mutants of SV40. Proc Natl Acad Sci U S A. 1970 Mar;65(3):536–543. doi: 10.1073/pnas.65.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Martin M. A. Simian virus 40 DNA integration within the genome of virus-transformed mammalian cells. Virology. 1973 Feb;51(2):351–357. doi: 10.1016/0042-6822(73)90434-0. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Sharp P. A., Sambrook J. Transcription of simian virus 40. II. Hybridization of RNA extracted from different lines of transformed cells to the separated strands of simian virus 40 DNA. J Virol. 1973 Jul;12(1):90–98. doi: 10.1128/jvi.12.1.90-98.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger H. C., Basilico C. Mutation causing temperature-sensitive expression of cell transformation by a tumor virus (SV40-3T3 mouse cells-growth control). Proc Natl Acad Sci U S A. 1972 Jan;69(1):109–114. doi: 10.1073/pnas.69.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Takemoto K. K., Todaro G. J. The resistance of SV40-transformed 3T3 to supertransformation. Proc Soc Exp Biol Med. 1971 Mar;136(3):742–746. doi: 10.3181/00379727-136-35355. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A., Pollack R. Isolation and characterization of revertant cell lines. VI. Susceptibility of revertants to retransformation by simian virus 40 and murine sarcoma virus. J Virol. 1974 Dec;14(6):1404–1410. doi: 10.1128/jvi.14.6.1404-1410.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


