Abstract
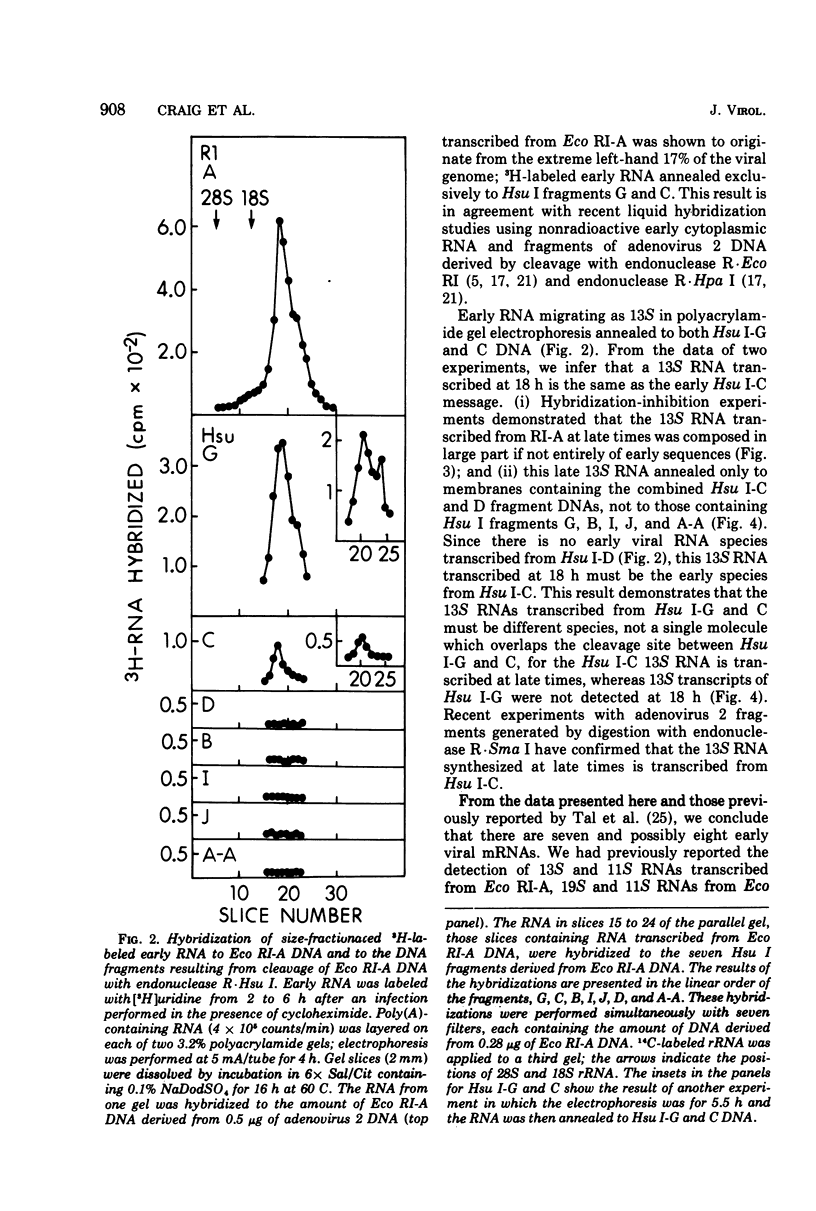
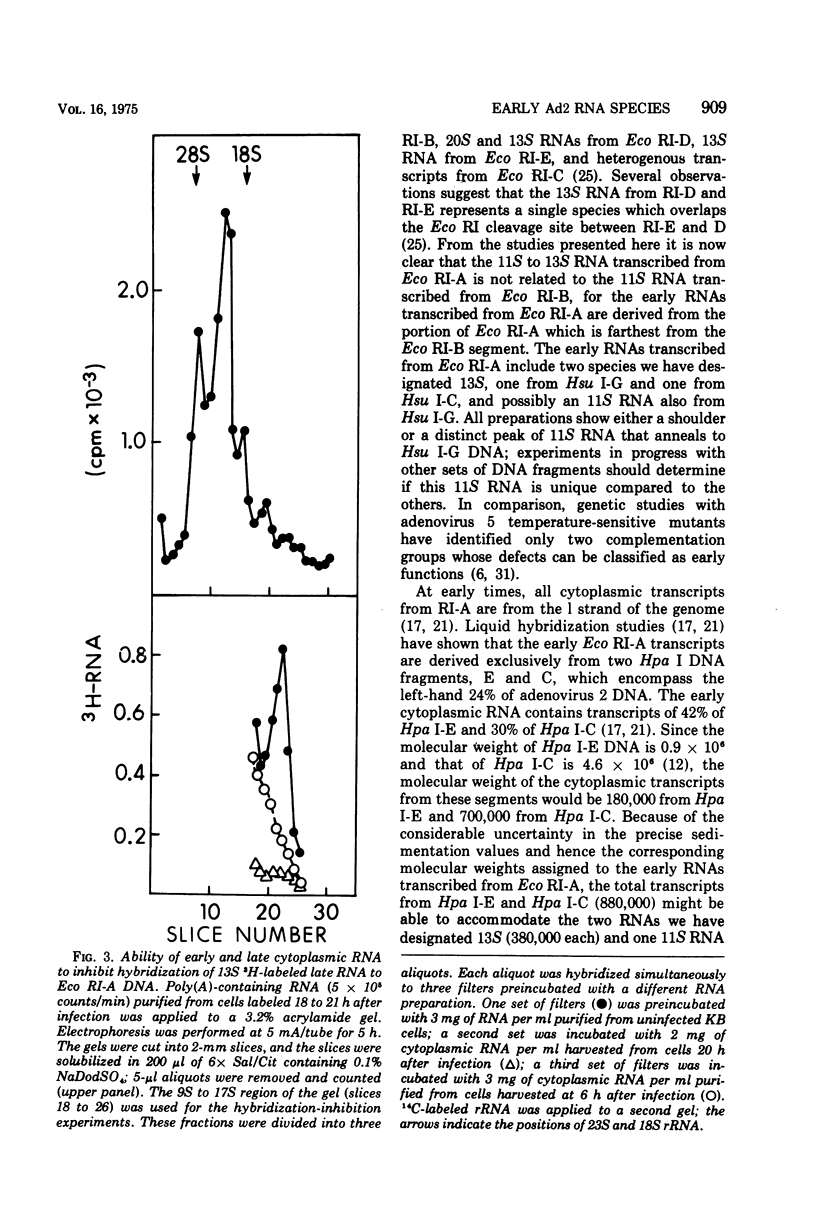
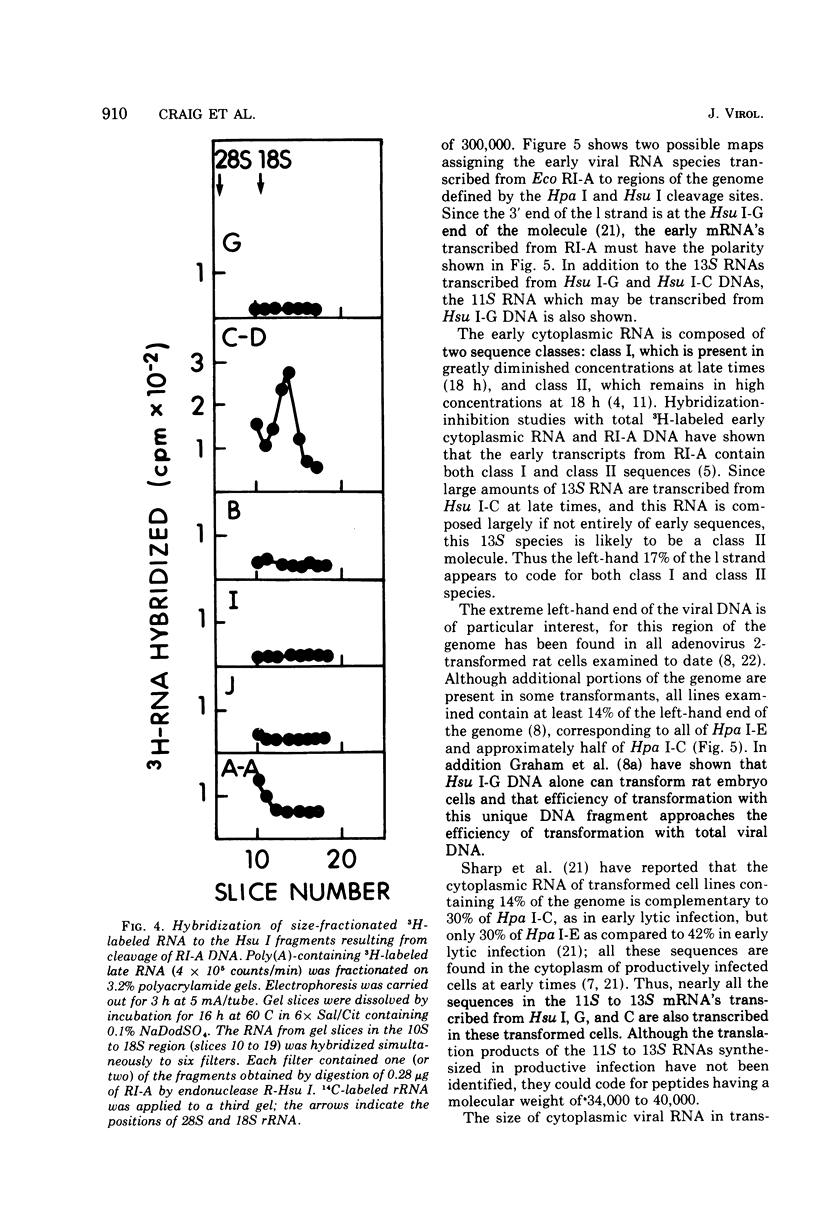
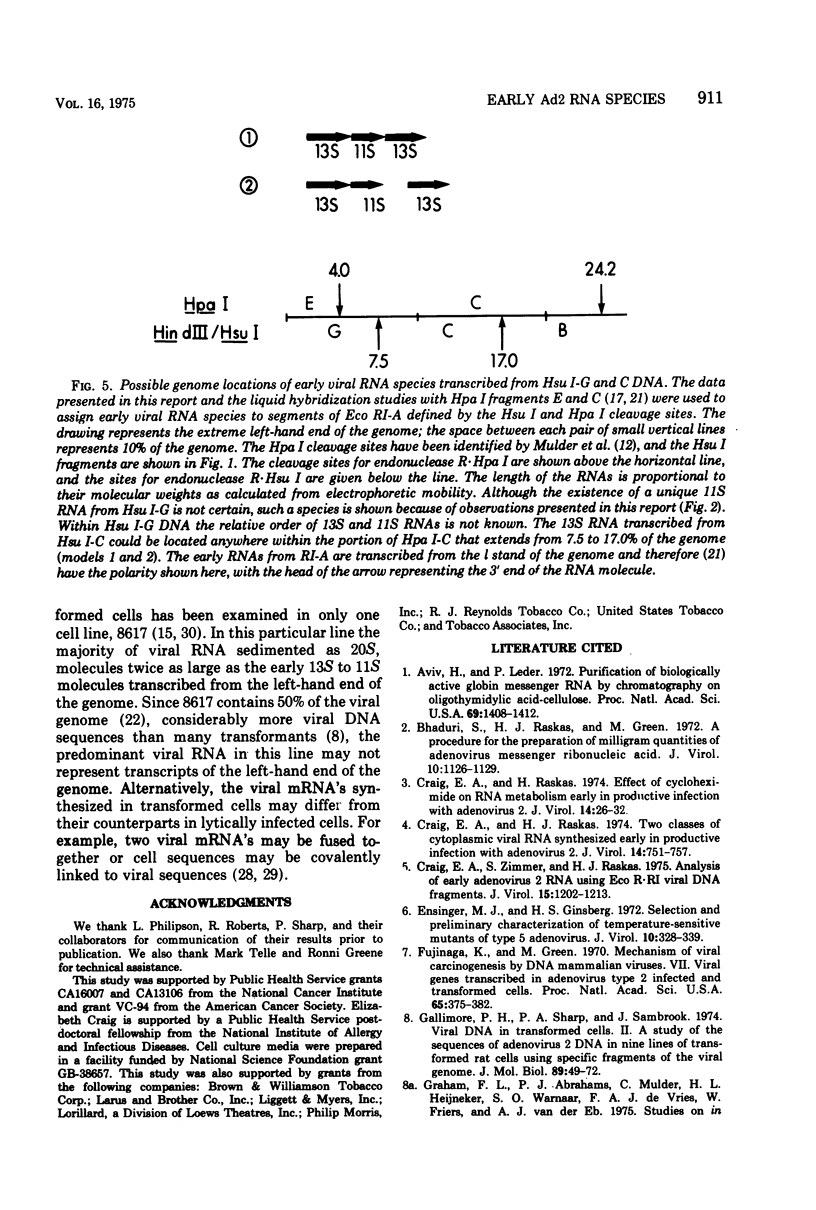
Unique fragments of adenovirus type 2 DNA generated by cleavage with endonuclease R-Eco RI or endonuclease R-Hsu I (Hin dIII) were used to map cytoplasmic viral RNAs transcribed early in productive infection. Radioactive early viral RNA was first fractionated by polyacrylamide gel electrophoresis. Eluted viral RNAs were then tested for hybrid formation with DNA fragments. The Eco RI DNA fragment (Eco RI-A) which contains the left-hand 58% of the genome hybridized 13S and 11S RNAs. More detailed mapping of these RNAs was achieved by hybridization to the seven Hsu I fragments of Eco RI-A. The early RNA annealed only to Hsu I-G and C, two fragments which comprise the extreme left-hand 17% of the genome. Viral RNA migrating as 13S and 11S annealed to Hsu I-G, and 13S RNA annealed to Hsu I-C. A 13S RNA is transcribed from Eco RI-A late in infection (18 h). Hybridization-inhibition studies with Eco RI-A DNA, early cytoplasmic RNA, and 3H-labeled 13S late RNA demonstrated that this RNA synthesized at late times is an early RNA species which continues to be synthesized in large amounts at 18 h. This 13S RNA synthesized at 18 h hybridized to Hsu I-C but not to Hsu I-G DNA. These results establish that the 13S RNAs transcribed from Hsu I-G and C at early times must be different species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaduri S., Raskas H. J., Green M. Procedure for the preparation of milligram quantities of adenovirus messenger ribonucleic acid. J Virol. 1972 Dec;10(6):1126–1129. doi: 10.1128/jvi.10.6.1126-1129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A. Analysis of early adenovirus 2 RNA using Eco R-R1 viral DNA fragments. J Virol. 1975 May;15(5):1202–1213. doi: 10.1128/jvi.15.5.1202-1213.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Effect of cycloheximide on RNA metabolism early in productive infection with adenovirus 2. J Virol. 1974 Jul;14(1):26–32. doi: 10.1128/jvi.14.1.26-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Two classes of cytoplasmic viral RNA synthesized early in productive infection with adenovirus 2. J Virol. 1974 Oct;14(4):751–757. doi: 10.1128/jvi.14.4.751-757.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by DNA mammalian viruses. VII. Viral genes transcribed in adenovirus type 2 infected and transformed cells. Proc Natl Acad Sci U S A. 1970 Feb;65(2):375–382. doi: 10.1073/pnas.65.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J Virol. 1971 Aug;8(2):203–214. doi: 10.1128/jvi.8.2.203-214.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Arrand J. R., Delius H., Keller W., Pettersson U., Roberts R. J., Sharp P. A. Cleavage maps of DNA from adenovirus types 2 and 5 by restriction endonucleases EcoRI and HpaI. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):397–400. doi: 10.1101/sqb.1974.039.01.051. [DOI] [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T., Gardner J., Green M. Biochemical studies on adenovirus multiplication, XIX. Resolution of late viral RNA species in the nucleus and cytoplasm. Proc Natl Acad Sci U S A. 1971 Mar;68(3):557–560. doi: 10.1073/pnas.68.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T., Green M. Biochemical studies on adenovirus multiplication. 18. Resolution of early virus-specific RNA species in Ad 2 infected and transformed cells. Virology. 1971 Jul;45(1):154–162. doi: 10.1016/0042-6822(71)90122-x. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U., Tibbetts C., Vennström B., Persson T. RNA synthesis and processing in adenovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):447–456. doi: 10.1101/sqb.1974.039.01.057. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Tal J., Craig E. A., Raskas H. J. Sequence relationships between adenovirus 2 early RNA and viral RNA size classes synthesized at 18 hours after infection. J Virol. 1975 Jan;15(1):137–144. doi: 10.1128/jvi.15.1.137-144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal J., Craig E. A., Zimmer S., Raskas H. J. Localization of adenovirus 2 messenger RNA's to segments of the viral genome defined by endonuclease R-R1. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4057–4061. doi: 10.1073/pnas.71.10.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C., Pettersson U., Johansson K., Philpson L. Relationship of mRNA from productively infected cells to the complementary strands of adenovirus type 2 DNA. J Virol. 1974 Feb;13(2):370–377. doi: 10.1128/jvi.13.2.370-377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuei D., Fujinaga K., Green M. The mechanism of viral carcinogenesis by DNA mammalian viruses: RNA transcripts containing viral and highly reiterated cellular base sequences in adenovirus-transformed cells (DNA-RNA hybridization-viral-cell mRNA). Proc Natl Acad Sci U S A. 1972 Feb;69(2):427–430. doi: 10.1073/pnas.69.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Darnell J. E. Presence of cell and virus specific sequences in the same molecules of nuclear RNA from virus transformed cells. Nat New Biol. 1971 Jul 21;232(29):73–76. doi: 10.1038/newbio232073a0. [DOI] [PubMed] [Google Scholar]
- Wall R., Weber J., Gage Z., Darnell J. E. Production of viral mRNA in adenovirus- transformed cells by the post- transcriptional processing of heterogeneous nuclear RNA containing viral and cell sequences. J Virol. 1973 Jun;11(6):953–960. doi: 10.1128/jvi.11.6.953-960.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Ustacelebi S., Williams J. F. Characterization of temperature-sensitive mutants of adenovirus type 5: nucleic acid synthesis. Virology. 1973 Feb;51(2):499–503. doi: 10.1016/0042-6822(73)90450-9. [DOI] [PubMed] [Google Scholar]


