Abstract
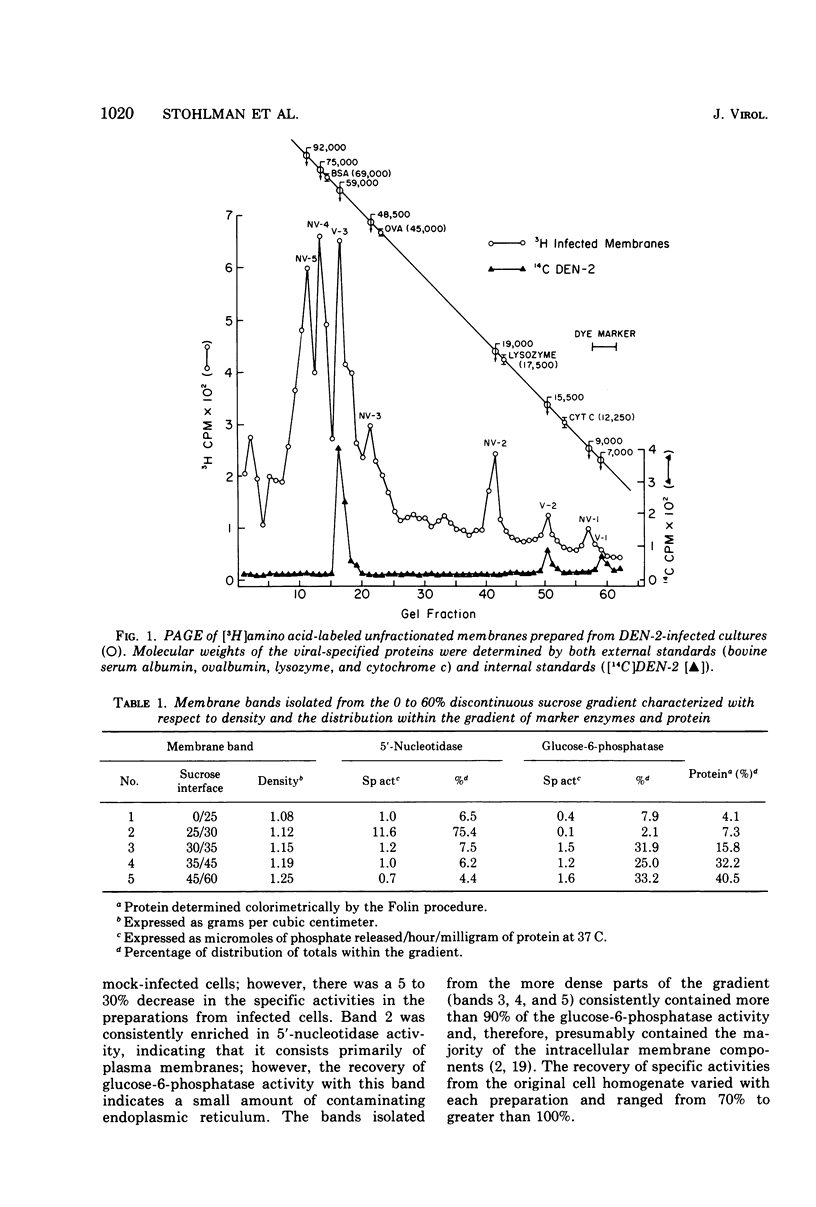
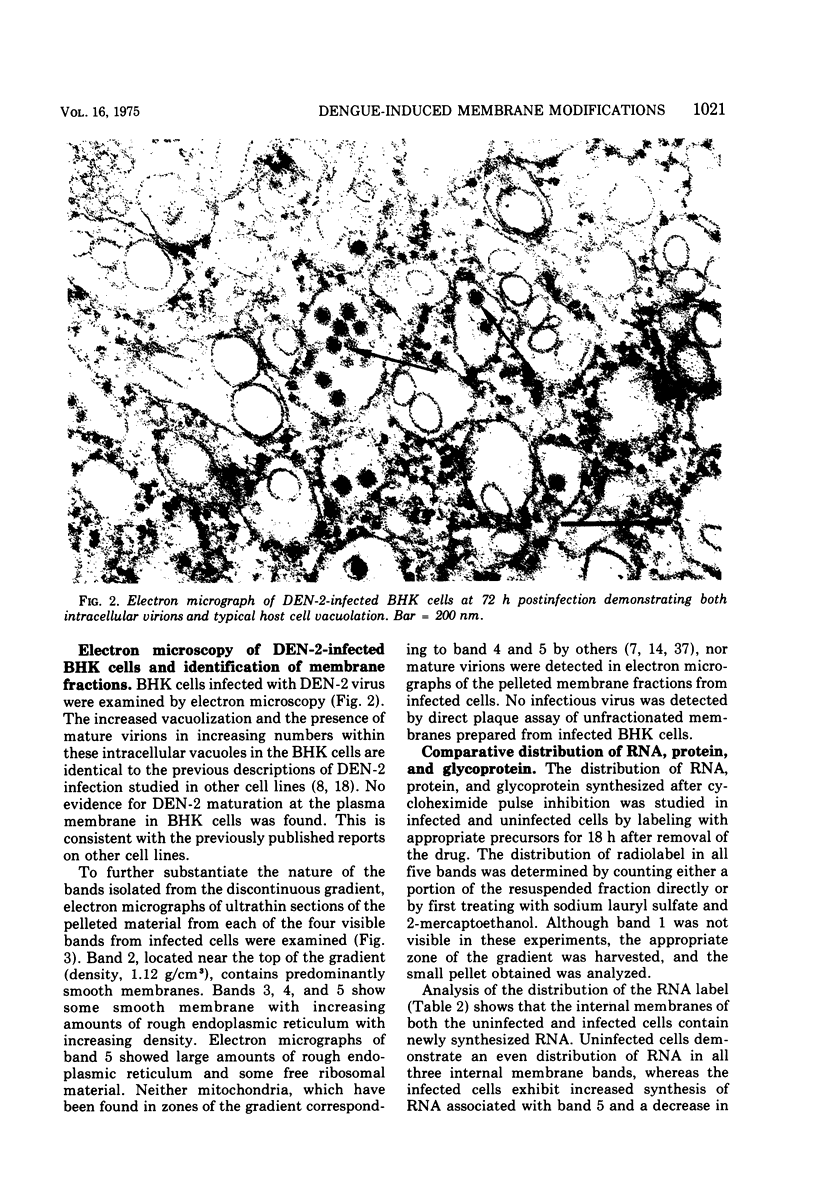
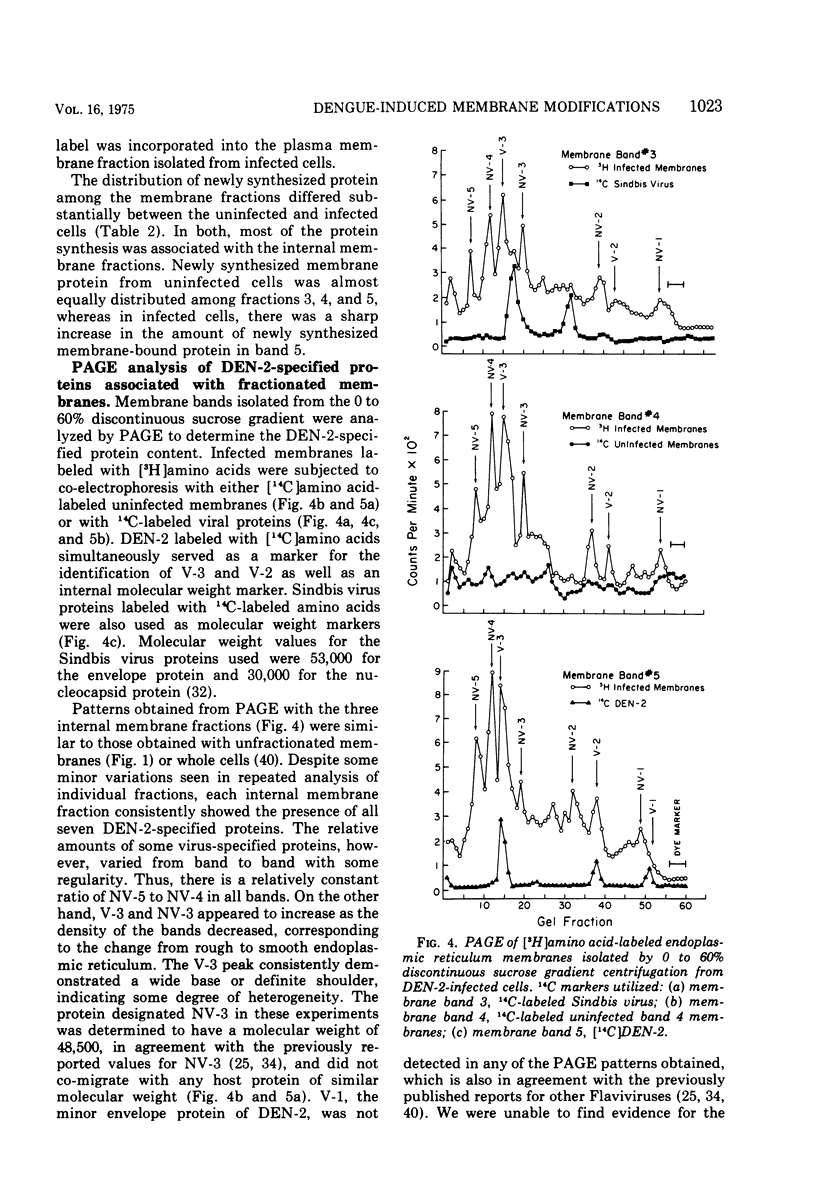
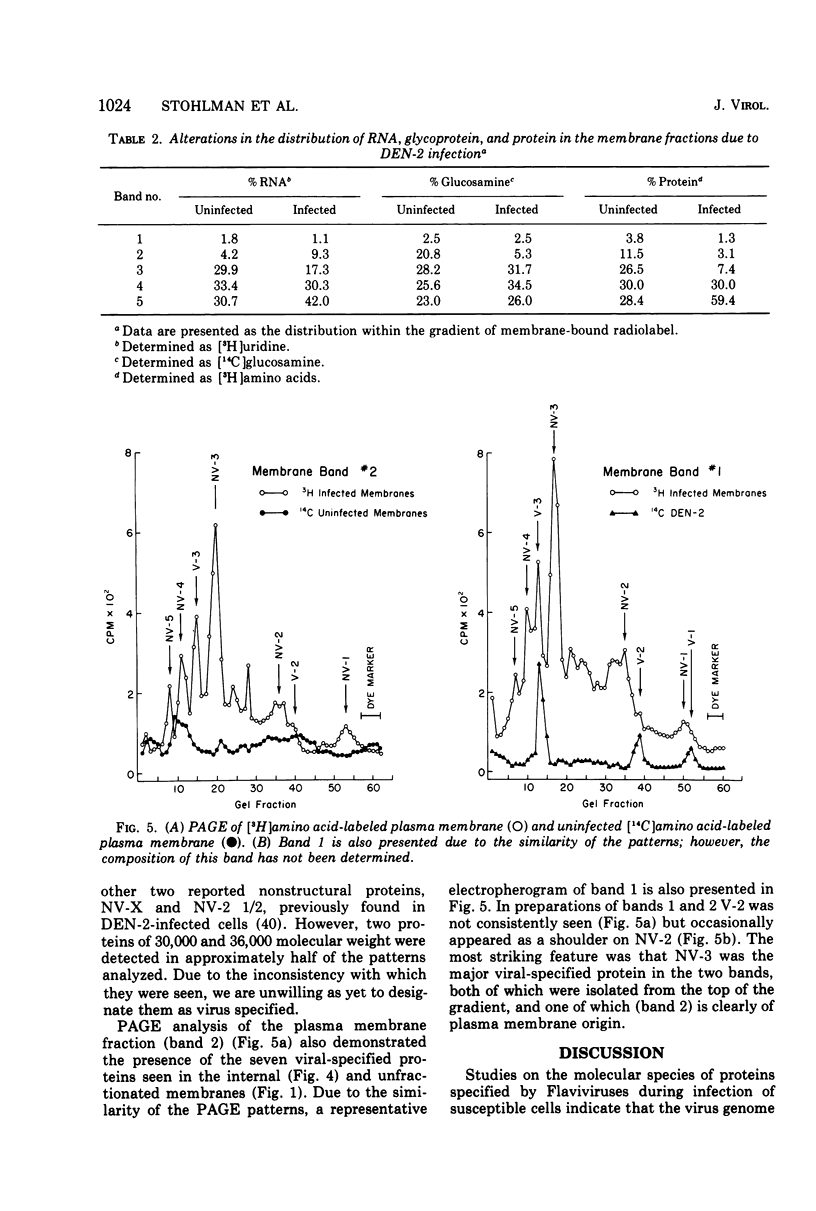
Enzymatic markers and electron microscopy were utilized to determine the cellular origin of the membrane types isolated from type 2 dengue virus-infected BHK cells by discontinuous sucrose gradient centrifugation. The results showed an apparent separation of plasma membrane, smooth and rough endoplasmic reticulum with increasing density. Virus-induced protein and RNA synthesis, as indicated by the incorporation of radiolabled precursors, was localized on the rough endoplasmic reticulum. Glycosylation, measured by the incorporation of radiolabeled glucosamine into membrane-associated proteins, was most active in the bands of intermediate and smooth endoplasmic reticulum. Polyacrylamide gel electrophoresis of isolated membrane bands, radiolabeled in the presence of actinomycin D, after pulse inhibition by cycloheximide, revealed seven virus-specific proteins associated with all membrane fractions. Viral structural protein V-3, and nonstructural proteins NV-3 and NV-2, increased with decreasing density, whereas NV-5 and NV-4 remained constant. The viral capsid protein V-2 was depleted in the intermediate and smooth endoplasmic reticulum, suggesting that these membranes may serve as the sites for viral maturation. NV-3 was the most prominent virus-specified protein found in the plasma membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Bose H. R., Brundige M. A. Selective association of Sindbis virion proteins with different membrane fractions of infected cells. J Virol. 1972 May;9(5):785–791. doi: 10.1128/jvi.9.5.785-791.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt W. E., Buescher E. L., Hetrick F. M. Production and characterization of arbovirus antibody in mouse ascitic fluid. Am J Trop Med Hyg. 1967 May;16(3):339–347. doi: 10.4269/ajtmh.1967.16.339. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D., Russ S. B., Brandt W. E., Russell P. K. Cytological localization of Dengue-2 antigens: an immunological study with ultrastructural correlation. Infect Immun. 1973 May;7(5):809–816. doi: 10.1128/iai.7.5.809-816.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro P. J., Brandt W. E., Hogrefe W. R., Russell P. K. Detection of dengue cell-surface antigens by peroxidase-labeled antibodies and immune cytolysis. Infect Immun. 1974 Aug;10(2):381–388. doi: 10.1128/iai.10.2.381-388.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W. Influenza virus proteins. II. Association with components of the cytoplasm. Virology. 1973 Jan;51(1):56–70. doi: 10.1016/0042-6822(73)90365-6. [DOI] [PubMed] [Google Scholar]
- Demsey A., Steere R. L., Brandt W. E., Veltri B. J. Morphology and development of dengue-2 virus employing the freeze-fracture and thin-section techniques. J Ultrastruct Res. 1974 Jan;46(1):103–116. doi: 10.1016/s0022-5320(74)80025-0. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc Soc Exp Biol Med. 1955 Jul;89(3):362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- Eylar O. R., Wisseman C. L., Jr Thermal inactivation of type 1 Dengue virus strains. Acta Virol. 1975 Apr;19(2):167–168. [PubMed] [Google Scholar]
- Filshie B. K., Rehacek J. Studies of the morphology of Murray Valley encephalitis and Japanese encephalitis viruses growing in cultured mosquito cells. Virology. 1968 Mar;34(3):435–443. doi: 10.1016/0042-6822(68)90063-9. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G. Proteins and glycoproteins of hamster kidney fibroblast (BHK21) plasma membranes and endoplasmic reticulum. Biochim Biophys Acta. 1971 Oct 12;249(1):81–95. doi: 10.1016/0005-2736(71)90085-x. [DOI] [PubMed] [Google Scholar]
- Hay A. J. Studies on the formation of the influenza virus envelope. Virology. 1974 Aug;60(2):398–418. doi: 10.1016/0042-6822(74)90335-3. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Wöllert W., Rott R., Scholtissek C. Association of influenza virus proteins with cytoplasmic fractions. Virology. 1974 Jan;57(1):28–41. doi: 10.1016/0042-6822(74)90105-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Compans R. W. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura T., Stollar V., Schlesinger R. W. Studies on the nature of dengue viruses. V. Structure and development of dengue virus in Vero cells. Virology. 1971 Nov;46(2):344–355. doi: 10.1016/0042-6822(71)90036-5. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- OTA Z. ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF JAPANESE B ENCEPHALITIS VIRUS IN PORCINE KIDNEY STABLE (PS) CELLS. Virology. 1965 Mar;25:372–378. doi: 10.1016/0042-6822(65)90057-7. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Quigley J. P. Virus-induced modification of cellular membranes related to viral structure. Annu Rev Microbiol. 1974;28(0):325–351. doi: 10.1146/annurev.mi.28.100174.001545. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Shapiro D., Brandt W. E., Cardiff R. D., Russell P. K. The proteins of Japanese encephalitis virus. Virology. 1971 Apr;44(1):108–124. doi: 10.1016/0042-6822(71)90158-9. [DOI] [PubMed] [Google Scholar]
- Shapiro D., Brandt W. E., Russell P. K. Change involving a viral membrane glycoprotein during morphogenesis of group B arboviruses. Virology. 1972 Dec;50(3):906–911. doi: 10.1016/0042-6822(72)90445-x. [DOI] [PubMed] [Google Scholar]
- Shapiro D., Kos K. A., Russel P. K. Japanese encephalitis virus glycoproteins. Virology. 1973 Nov;56(1):88–94. doi: 10.1016/0042-6822(73)90289-4. [DOI] [PubMed] [Google Scholar]
- Shapiro D., Kos K., Brandt W. E., Russell P. K. Membrane-bound proteins of Japanese encephalitis virus-infected chick embryo cells. Virology. 1972 May;48(2):360–372. doi: 10.1016/0042-6822(72)90046-3. [DOI] [PubMed] [Google Scholar]
- Singer S. J. Molecular biology of cellular membranes with applications to immunology. Adv Immunol. 1974;19(0):1–66. doi: 10.1016/s0065-2776(08)60251-5. [DOI] [PubMed] [Google Scholar]
- Stollar V., Stevens T. M., Schlesinger R. W. Studies on the nature of dengue viruses. II. Characterization of viral RNA and effects of inhibitors of RNA synthesis. Virology. 1966 Oct;30(2):303–312. doi: 10.1016/0042-6822(66)90105-x. [DOI] [PubMed] [Google Scholar]
- Stollar V. Studies on the nature of dengue viruses. IV. The structural proteins of type 2 dengue virus. Virology. 1969 Nov;39(3):426–438. doi: 10.1016/0042-6822(69)90091-9. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suitor E. C., Jr, Paul F. J. Syncytia formation of mosquito cell cultures mediated by type 2 dengue virus. Virology. 1969 Jul;38(3):482–485. doi: 10.1016/0042-6822(69)90162-7. [DOI] [PubMed] [Google Scholar]
- Trent D. W., Qureshi A. A. Structural and nonstructural proteins of Saint Louis encephalitis virus. J Virol. 1971 Mar;7(3):379–388. doi: 10.1128/jvi.7.3.379-388.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. Synthesis, transport, dynamics and fate of cell surface Ig and alloantigens in murine lymphocytes. Transplant Rev. 1973;14:50–75. doi: 10.1111/j.1600-065x.1973.tb00102.x. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Kiley M. P., Snyder R. M., Schnaitman C. A. Cytoplasmic compartmentalization of the protein and ribonucleic acid species of vesicular stomatitis virus. J Virol. 1972 Apr;9(4):672–683. doi: 10.1128/jvi.9.4.672-683.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. A., Boyle W. Purification of plasma membranes of rat liver. Application of zonal centrifugation to isolation of cell membranes. Biochim Biophys Acta. 1969 Apr;173(3):377–388. doi: 10.1016/0005-2736(69)90003-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Westaway E. G. Proteins specified by group B togaviruses in mammalian cells during productive infections. Virology. 1973 Feb;51(2):454–465. doi: 10.1016/0042-6822(73)90444-3. [DOI] [PubMed] [Google Scholar]




