Abstract
Traumatic brain injury (TBI) remains a leading cause of mortality and morbidity worldwide. No effective pharmacological treatments are available for TBI because all Phase II/III TBI clinical trials have failed. This highlights a compelling need to develop effective treatments for TBI. Endogenous neurorestoration occurs in the brain after TBI, including angiogenesis, neurogenesis, synaptogenesis, oligodendrogenesis and axonal remodeling, which may be associated with spontaneous functional recovery after TBI. However, the endogenous neurorestoration following TBI is limited. Treatments amplifying these neurorestorative processes may promote functional recovery after TBI. Thymosin beta4 (Tβ4) is the major G-actin-sequestering molecule in eukaryotic cells. In addition, Tβ4 has other properties including anti-apoptosis and anti-inflammation, promotion of angiogenesis, wound healing, stem/progenitor cell differentiation, and cell migration and survival, which provide the scientific foundation for the corneal, dermal, and cardiac wound repair multicenter clinical trials. Here, we describe Tβ4 as a neuroprotective and neurorestorative candidate for treatment of TBI.
Keywords: thymosin beta4, traumatic brain injury, rat, neuroprotection, neurorestoration
Introduction
An estimated 1.4 million people sustain traumatic brain injury (TBI) each year in the United States, and more than 5 million people are coping with disabilities from TBI at an annual cost of more than $56 billion.1 There are no commercially-available pharmacological treatment options available for TBI because all clinical trial strategies have failed.2,3 The disappointing clinical trial results may be due to variability in treatment approaches and heterogeneity of the population of TBI patients.4-9 Another important aspect is that most clinical trial strategies have used drugs that target a single pathophysiological mechanism, although many mechanisms are involved in secondary injury after TBI.4 Neuroprotection approaches have historically been dominated by targeting neuron-based injury mechanisms as the primary or even exclusive focus of the neuroprotective strategy.3 In the vast majority of preclinical studies, the treatment compounds are administered early and, frequently, even before TBI.10,11 Clinically, the administration of a compound early may be problematic because of the difficulty in obtaining informed consent.12
Recent preclinical studies by us and others have revealed that endogenous neurorestoration is present after TBI, including neurogenesis, axonal sprouting, synaptogenesis, and angiogenesis, which may contribute to the spontaneous functional recovery.13-18 In addition, treatments that promote these neurorestorative processes have been demonstrated to improve functional recovery after brain injury.19,20 However, clinical trials in TBI have primarily targeted neuroprotection, and trials directed specifically at neurorestoration have not been conducted. The essential difference between neuroprotective and neurorestorative treatments is that the former target the lesion that is still not irreversibly injured and the latter treat the intact tissue.19 Thus, neurorestorative treatments can be made available for a larger number of TBI patients.
Tβ4 is a multifunctional regenerative small peptide containing 43-amino acids, and it is the major G-actin-sequestering molecule in eukaryotic cells.21 Tβ4 has pro-survival and pro-angiogenic properties, protects tissue against damage, and promotes tissue regeneration.22,23 It also plays a key role in corneal, epidermal and cardiac wound healing.21 Tβ4 participates in axonal path-finding, neurite formation, cell proliferation, and neuronal survival.24-26 Our previous studies show that Tβ4 reduces inflammation and stimulates remyelination and improves functional recovery in animal models of experimental autoimmune encephalomyelitis (EAE) and stroke.25,27 In summary, these pleiotropic properties make Tβ4 an ideal candidate for treatment of TBI.
Early (6 hours post injury) Tβ4 treatment reduces cortical lesion volume and improves functional recovery after TBI in rats
TBI patients frequently suffer from long-term deficits in cognitive and motor performance. No single animal model can adequately mimic all aspects of human TBI owing to the heterogeneity of clinical TBI.11 Some features of cognitive and motor function in humans have been successfully demonstrated in experimental brain trauma models.28-30 The controlled cortical impact (CCI) model is one of the most widely used TBI models. The CCI-TBI model has many clinically relevant features in that CCI causes not only cortical damage but also selective neuronal death in the hippocampus in rodents, leading to sensorimotor dysfunction and spatial learning and memory deficits, respectively.18,31-33
We have evaluated the efficacy of early Tβ4 treatment on spatial learning and sensorimotor functional recovery in rats after TBI induced by unilateral CCI.34 In brief, TBI rats received Tβ4 at a dose of either 6 or 30 mg/kg (RegeneRx Biopharmaceuticals Inc, Rockville, MD) or a vehicle control (saline) administered i.p. starting at 6 hours after injury and then at 24 and 48 hours. Spatial learning was performed during the last five days (31-35 days post injury) using the modified Morris water maze (MWM) test, which is extremely sensitive to the hippocampal injury.35-37 Tβ4-treated TBI rats showed significant improvement in spatial learning when compared to the saline-treated TBI rats. Tβ4 treatment also significantly reduced the swim latency to reach the hidden platform by rats post TBI compared to saline treatment. Using the modified Neurological Severity Score (mNSS) test, our data show that significantly improved scores were observed after TBI in the Tβ4-treated group compared to the saline-treated group. Our data also show that Tβ4 reduced the incidence of both right forelimb and hindlimb footfaults in TBI rats.34 Histological data show that early Tβ4 treatment reduced cortical lesion volume by 20% and 30% for 6 mg/kg and 30 mg/kg, respectively, and reduced hippocampal cell loss. These findings suggest that TB4 provides neuroprotection even when the treatment was initiated 6 hours post injury. In addition, 6-hour Tβ4 treatment promotes neurogenesis in the dentate gyrus (DG) of the hippocampus,38 which may contribute to improvement in spatial learning.
Delayed (24 hours post injury) Tβ4 treatment does not alter cortical lesion volume but improves functional recovery after TBI in rats
The CCI model we used causes cortical tissue loss. Traditionally, the target for neuroprotective treatment of TBI is to reduce the lesion volume.39,40 A major limitation of neuroprotection strategies is the short time window between injury and treatment. In the vast majority of preclinical TBI studies, the treatment compounds provide neuroprotection only when administered early (usually several hours after brain injury).11 The administration of a compound early in the clinical setting is not practical.41 The neuroprotective effects demonstrated in rodents may diminish if the treatment compounds are given in the clinical setting beyond the short neuroprotective window. We are able to stimulate recovery of neurological function without altering the lesion volume, which has also been demonstrated in our experimental studies of stroke,19,42,43 and is in essence, enhancement of neurorecovery.19 The extended 24-hour window for treatment which improves neurological recovery, without altering CCI cortical volume, is a major benefit of the neurorestorative therapy. Recently, we evaluated the efficacy of delayed Tβ4 treatment on spatial learning and sensorimotor functional recovery in rats after TBI induced by CCI.34 Briefly, TBI rats received Tβ4 at a dose of 6 mg/kg or a vehicle (saline) administered i.p. starting at 24 hours after injury and then every third day for 2 weeks. The dose of Tβ4 was selected based on our previous studies in animal models of stroke and EAE.25,27 Tβ4 did not alter lesion volume (14.2 ± 3.9% for saline treatment vs. 15.7 ± 3.6% for Tβ4 treatment). TBI caused neuronal cell loss in the ipsilateral CA3 and DG examined 35 days after injury compared to sham controls. Tβ4 treatment initiated 24 hours post injury significantly reduced cell loss in these two regions compared to saline controls. Tβ4-treated TBI rats showed significant improvement in spatial learning (MWM test) and sensorimotor (mNSS test) functional recovery compared to the saline-treated TBI rats.34
Delayed (24 hours post injury) Tβ4 treatment promotes neurogenesis after TBI in rats
Evidence accumulated over the past decades has overturned the traditional dogma that the adult mammalian brain cannot generate new neurons. Adult neurogenesis has been identified in all vertebrate species examined thus far including humans.44-49 Newly generated neuronal cells originate from neural stem cells in the adult brain. Neural stem cells are the self-renewing, multipotent cells that generate the neuronal and glial cells of the nervous system.50 The major function of neurogenesis in adult brain seems to replace the neurons that die regularly in certain brain areas. Granule neurons in the DG continuously die and the progenitors in the subgranular zone of the DG may proliferate at the same rate as mature neuronal death to maintain a constant DG cell number.51 Similarly, the newly proliferated cells from the subventricular zone migrate and replenish the dead olfactory bulb neurons.52 Here, we focus on DG neurogenesis which is important for spatial learning and memory. In normal adult rats, newborn neural cells migrate from the subgranular zone of the DG of the hippocampus into the granule cell layer and eventually become mature granule neurons.53 These new granule neurons extend axonal processes to their postsynaptic targets54-57 and receive synaptic input.58 TBI stimulates widespread cellular proliferation in rats and results in focal neurogenesis in the DG of the hippocampus.59,60 Some of the newly generated granule neurons integrate into the hippocampus. The integration of the injury-induced neurogenic population into the existing hippocampal circuitry coincides with the time point when cognitive recovery is observed in injured animals.44
Bromodeoxyuridine (BrdU), a thymidine analogue, can be incorporated into the DNA of dividing cells and is widely used to label new cells.61-63 To label proliferating cells, BrdU (100 mg/kg) was injected i.p. daily starting at day 1 post TBI for 10 days. The number of BrdU-positive cells found in the ipsilateral cortex, DG, and CA3 areas was significantly increased 35 days after TBI compared with sham controls.18,34,64,65 Tβ4 treatment further increased the number of BrdU-positive cells compared to saline controls.34 The increased number of BrdU-positive cells may result from effects of Tβ4 on either increasing cell proliferation or reducing cell death of newborn cells. Our recent data show Tβ4 increases oligodendrocyte precursor cell proliferation and differentiation in animal models of stroke25 and experimental autoimmune encephalomyelitis.27 Tβ4 may not directly affect cell proliferation but inhibit cell death, for example, in corneal and conjunctival epithelial cells treated with benzalkonium chloride in vitro66 and endothelial precursor cells under serum deprivation.67 Our data further show that neurogenesis increases in TBI rats treated with Tβ4, suggesting that Tβ4 promotes newborn cells to differentiate into neurons. This is consistent with the effect of Tβ4 on promoting epicardium-derived progenitor cell differentiation into endothelial and smooth muscle cells to form the coronary vasculature.22 Whether the increased number of BrdU-positive cells in the brain of TBI rats treated with Tβ4 is tissue specific remains unknown. Tβ4 may not directly affect cell proliferation. Increased cell proliferation and neurogenesis are also possibly secondary to that Tβ4-mediated angiogenesis, as described later.
To identify newborn neurons, double immunofluorescent staining for BrdU/NeuN (mature neuronal marker) was performed (Fig.1). TBI alone significantly increased the number of newborn neurons (NeuN/BrdU-colabeled cells) in the DG of injured hemisphere. Tβ4 treatment significantly further increased the number of newborn neurons compared to saline controls. These data suggest that Tβ4 administration initiated 24 hours after TBI promotes neurogenesis in rats.
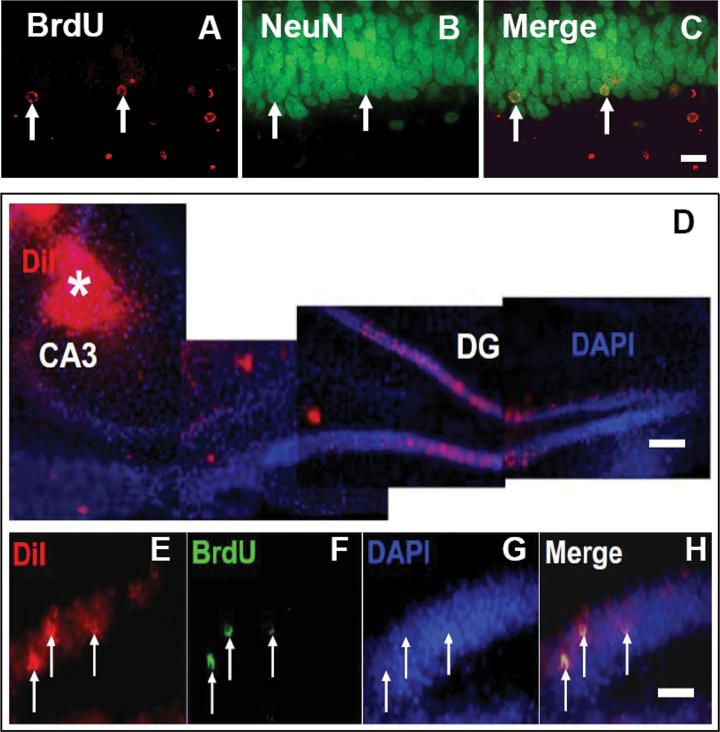
Fig. 1.
Double immunofluorescent staining for BrdU (red, A) and NeuN (green, B) to identify newborn neurons (yellow after merge, C) in the dentate gyrus of hippocampus from rats examined 35 days after TBI. Micrographs (D) show location of DiI injection in the CA3 region (indicated by white asterisk). In the CA3 region, axons projected from granule neurons in the dentate gyrus will take up injected DiI to their cell bodies. Co-localization (merge, H) of BrdU-positive nuclei (green, F) within retrogradely DiI labeled (red, E) granule cells were examined at 35 days after TBI. Scale bar = 25 μm (C, H). Scale bar = 50 μm (D).
To investigate whether the newborn neurons generated in the DG are capable of projecting their axons into the CA3 region of the hippocampus after TBI, we stereotactically injected a fluorescent tracer, 1,1″-dioleyl-3,3,3″,3″-tetramethylindocarbocyanine methanesulfonate (Dil, Delta 9-DiI; AnaSpec, San Jose, CA) into the ipsilateral CA3 region (stereotaxic coordinates AP, -3.6 mm bregma, ML, 3.6 mm, DV, 3.0 mm, Paxinos and Watson, 1994) at day 28 after TBI. BrdU (100mg/kg, ip) was injected i.p. daily starting at day 1 after TBI for 10 days to label newly generated cells. One week after DiI injection (i.e., 35 days after TBI), the animals were anesthetized and sacrificed. Their brains were fixed in 4% paraformaldehyde. The brain was cut into seven equally spaced 2-mm coronal blocks using a rat brain matrix. The brain blocks containing the hippocampus were processed for vibratome sections (100 μm) followed by BrdU staining. BrdU and DiI labeling in the hippocampus on brain sections was analyzed with a Bio-Rad MRC 1024 (argon and krypton) laser-scanning confocal imaging system mounted onto a Zeiss microscope (Bio-Rad, Cambridge, MA). Co-localization of BrdU-positive nuclei within retrogradely DiI-labeled granule cells was found, indicating that newborn granule neurons extend axons into the CA3 region that are capable of retrogradely transporting DiI from the CA3 to their cell bodies within the DG after TBI (Fig.2). This finding suggests that newborn granule neurons may be incorporated into functional hippocampal circuitry after TBI.
Fig. 2.
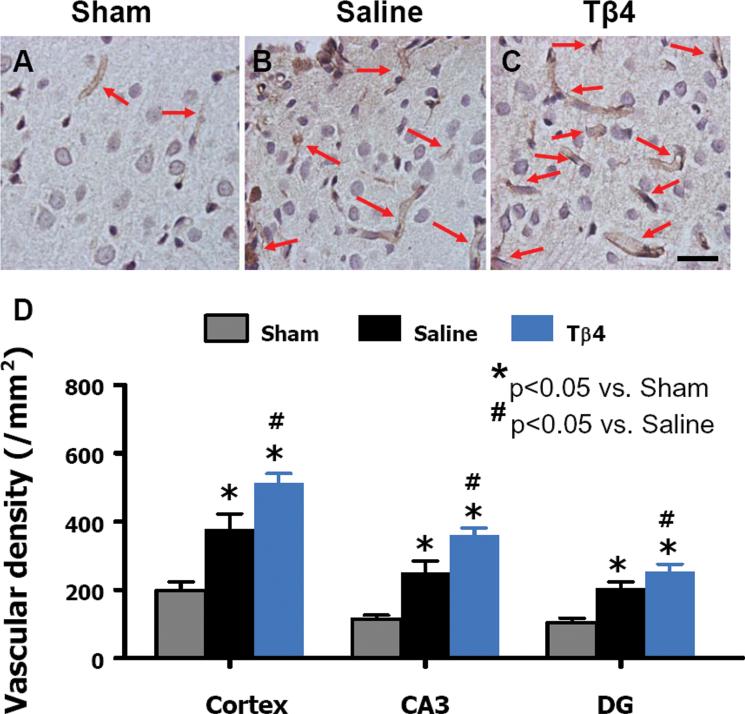
Delayed Tβ4 treatment increases vascular density in the injured cortex, ipsilateral dentate gyrus, and CA3 region 35 days after TBI. Arrows show vWF-stained vascular structure. TBI alone (B) significantly increases the vascular density in the injured cortex compared to sham controls (A, P < 0.05). Tβ4 treatment (C) further enhances angiogenesis after TBI compared to the saline-treated groups (P < 0.05). The density of vWF-stained vasculature in different regions is shown in (D). Scale bar = 25 μm (C). Data represent mean + SD. *P < 0.05 vs Sham group. #P < 0.05 vs Saline group. N (rats/group) = 6 (Sham); 9 (Saline); and 10 (Tβ4).
Delayed (24 hours post injury) Tβ4 treatment promotes angiogenesis after TBI in rats
The vascular system in the normal adult brain is stable, but is activated in response to certain pathological conditions including injuries.68 Von Willebrand factor (vWF) staining has been used to identify vascular structure in the brain after TBI.69 TBI alone significantly increased vascular density in the injured cortex, CA3, and DG of the ipsilateral hemisphere when examined at day 35 after TBI compared to sham controls.18,34,64,65 Tβ4 treatment significantly increased the vascular density in these regions compared to saline treatment.34 This is in agreement with in vitro and in vivo pro-angiogenic effect of Tβ4.70,71
Coupling of neurogenesis and angiogenesis
Neurovascular units within the central nervous system consist of endothelial cells, pericytes, neurons and glial cells, as well as growth factors and extracellular matrix proteins that are close to the endothelium.72,73 Neurovascular units provide niches for neural stem/progenitor cells in the adult brain and, within these units, newly-generated immature neurons are closely associated with the remodeling vasculature. The generation of new vasculature facilitates several coupled neurorestorative processes including neurogenesis and synaptogenesis, which improve functional recovery.74-76 The vascular production of stromal-derived factor 1 and angiopoietin 1 is involved in neurogenesis and promotes behavioral recovery after stroke.77 The disruption of this neurovascular coordination has been observed in a variety of brain conditions including infection, stroke and trauma.78 The injured brain promotes angiogenesis and neurogenesis,13,32,69,79-84 that may contribute to spontaneous functional recovery from injuries such as stroke and TBI. Neurorestorative agents that increase angiogenesis and neurogenesis have been shown to improve functional outcome following brain injury.19,33 Vascular endothelial cells within the neurovascular niche affect neurogenesis directly via contact with neural progenitor cells, while soluble factors from the vascular system that are released into the CNS enhance neurogenesis via paracrine signaling.85 Here, we demonstrate that Tβ4 treatment promotes both angiogenesis and neurogenesis in rats after TBI, suggesting that the neurovascular remodeling at least partially contributes to Tβ4-mediated improvement in functional recovery. A better understanding of molecular mechanisms in the neurovascular niches will be important for developing novel angiogenic and neurogenic therapies for brain injuries.
Conclusion
These studies demonstrate that in the animal model of TBI, early (6 hours post injury) treatment with Tβ4 i.p. at doses of 6 and 30 mg/kg reduces cortical lesion volume and hippocampal cell loss and improves functional recovery, suggesting its potential as a neuroprotective therapy for TBI. More importantly, delayed (24 hours post injury) treatment with Tβ4 administered i.p. at a dose of 6 mg/kg does not reduce lesion volume but significantly improves functional outcome in rats.34 Tβ4-induced angiogenesis, neurogenesis and oligodendrogenesis may contribute to functional recovery.34 Therefore, our data suggest that promoting endogenous neurorestorative processes using Tβ4 provides a novel therapeutic option for TBI. It should be noted that systemic administration of Tβ4 is safe and well-tolerated by animals and humans.26 Further investigation of the molecular mechanisms underlying Tβ4-mediated neuroprotection and neurorestoration is warranted.
Acknowledgement
The authors would like to thank Susan MacPhee-Gray for editorial assistance. Tβ4 was provided by RegeneRx Biopharmaceuticals, Inc. (Rockville, MD) under Materials Transfer Agreement. This work was supported by NIH grants RO1 NS62002 (Ye Xiong), PO1 NS42345 (Asim Mahmood, Michael Chopp), and PO1 NS023393 (Michael Chopp).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Janowitz T, Menon DK. Exploring new routes for neuroprotective drug development in traumatic brain injury. Sci. Transl. Med. 2010;2:27rv21. doi: 10.1126/scitranslmed.3000330. [DOI] [PubMed] [Google Scholar]
- 3.Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein DG, Wright DW. Progesterone in the clinical treatment of acute traumatic brain injury. Expert Opin. Investig. Drugs. 2010;19:847–857. doi: 10.1517/13543784.2010.489549. [DOI] [PubMed] [Google Scholar]
- 5.Aarabi B, Simard JM. Traumatic brain injury. Curr. Opin. Crit. Care. 2009;15:548–553. doi: 10.1097/MCC.0b013e32833190da. [DOI] [PubMed] [Google Scholar]
- 6.Xiong Y, Mahmood A, Chopp M. Emerging treatments for traumatic brain injury. Expert Opin. Emerg. Drugs. 2009;14:67–84. doi: 10.1517/14728210902769601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beauchamp K, Mutlak H, Smith WR, et al. Pharmacology of traumatic brain injury: where is the “golden bullet”? Mol. Med. 2008;14:731–740. doi: 10.2119/2008-00050.Beauchamp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolias CM, Bullock MR. Critical appraisal of neuroprotection trials in head injury: what have we learned? NeuroRx. 2004;1:71–79. doi: 10.1602/neurorx.1.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayan RK, Michel ME, Ansell B, et al. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada K, Chatzipanteli K, Busto R, et al. Role of nitric oxide in traumatic brain injury in the rat. J. Neurosurg. 1998;89:807–818. doi: 10.3171/jns.1998.89.5.0807. [DOI] [PubMed] [Google Scholar]
- 11.Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br. J. Pharmacol. 2011;164:1207–1229. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menon DK. Unique challenges in clinical trials in traumatic brain injury. Crit. Care Med. 2009;37:S129–135. doi: 10.1097/CCM.0b013e3181921225. [DOI] [PubMed] [Google Scholar]
- 13.Lu D, Mahmood A, Qu C, et al. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J. Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 14.Lu D, Goussev A, Chen J, et al. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J. Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Xiong Y, Mahmood A, et al. Sprouting of corticospinal tract axons from the contralateral hemisphere into the denervated side of the spinal cord is associated with functional recovery in adult rat after traumatic brain injury and erythropoietin treatment. Brain Res. 2010;1353:249–257. doi: 10.1016/j.brainres.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshima T, Lee S, Sato A, et al. TNF-alpha contributes to axonal sprouting and functional recovery following traumatic brain injury. Brain Res. 2009;1290:102–110. doi: 10.1016/j.brainres.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Richardson RM, Sun D, Bullock MR. Neurogenesis after traumatic brain injury. Neurosurg. Clin. N. Am. 2007;18:169–181. xi. doi: 10.1016/j.nec.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Xiong Y, Mahmood A, Meng Y, et al. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J. Neurosurg. 2010;113:598–608. doi: 10.3171/2009.9.JNS09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong Y, Mahmood A, Chopp M. Neurorestorative treatments for traumatic brain injury. Discov. Med. 2010;10:434–442. [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein AL, Hannappel E, Kleinman HK. Thymosin beta4: actinsequestering protein moonlights to repair injured tissues. Trends Mol. Med. 2005;11:421–429. doi: 10.1016/j.molmed.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Smart N, Risebro CA, Melville AA, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 23.Philp D, Huff T, Gho YS, et al. The actin binding site on thymosin beta4 promotes angiogenesis. FASEB. J. 2003;17:2103–2105. doi: 10.1096/fj.03-0121fje. [DOI] [PubMed] [Google Scholar]
- 24.Sun W, Kim H. Neurotrophic roles of the beta-thymosins in the development and regeneration of the nervous system. Ann. N. Y. Acad. Sci. 2007;1112:210–218. doi: 10.1196/annals.1415.013. [DOI] [PubMed] [Google Scholar]
- 25.Morris DC, Chopp M, Zhang L, et al. Thymosin beta4 improves functional neurological outcome in a rat model of embolic stroke. Neuroscience. 2010;169:674–682. doi: 10.1016/j.neuroscience.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crockford D, Turjman N, Allan C, et al. Thymosin beta4: structure, function, and biological properties supporting current and future clinical applications. Ann. N. Y. Acad. Sci. 2010;1194:179–189. doi: 10.1111/j.1749-6632.2010.05492.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Zhang ZG, Morris D, et al. Neurological functional recovery after thymosin beta4 treatment in mice with experimental auto encephalomyelitis. Neuroscience. 2009;164:1887–1893. doi: 10.1016/j.neuroscience.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox GB, Fan L, Levasseur RA, et al. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J. Neurotrauma. 1998;15:599–614. doi: 10.1089/neu.1998.15.599. [DOI] [PubMed] [Google Scholar]
- 29.Fujimoto ST, Longhi L, Saatman KE, et al. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Kline AE, Massucci JL, Marion DW, et al. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- 31.Colicos MA, Dixon CE, Dash PK. Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res. 1996;739:111–119. doi: 10.1016/s0006-8993(96)00819-0. [DOI] [PubMed] [Google Scholar]
- 32.Lu D, Qu C, Goussev A, et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J. Neurotrauma. 2007;24:1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Xiong Y, Mahmood A, et al. Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit. Brain Res. 2009;1294:153–164. doi: 10.1016/j.brainres.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong Y, Mahmood A, Meng Y, et al. Treatment of traumatic brain injury with thymosin beta in rats. J. Neurosurg. 2011;114:102–115. doi: 10.3171/2010.4.JNS10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Day LB, Schallert T. Anticholinergic effects on acquisition of place learning in the Morris water task: spatial mapping deficit or inability to inhibit nonplace strategies? Behav. Neurosci. 1996;110:998–1005. doi: 10.1037//0735-7044.110.5.998. [DOI] [PubMed] [Google Scholar]
- 37.Choi SH, Woodlee MT, Hong JJ, et al. A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J. Neurosci. Methods. 2006;156:182–193. doi: 10.1016/j.jneumeth.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Xiong Y, Zhang Y, Mahmood A, et al. Neuroprotective and neurorestorative effects of thymosin beta4 treatment initiated 6 hours after traumatic brain injury in rats. J. Neurosurg. 2012 doi: 10.3171/2012.1.JNS111729. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cherian L, Goodman JC, Robertson C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J. Pharmacol. Exp. Ther. 2007;322:789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- 40.Dietrich WD, Alonso O, Busto R, et al. Posttreatment with intravenous basic fibroblast growth factor reduces histopathological damage following fluid-percussion brain injury in rats. J. Neurotrauma. 1996;13:309–316. doi: 10.1089/neu.1996.13.309. [DOI] [PubMed] [Google Scholar]
- 41.Menon DK, Zahed C. Prediction of outcome in severe traumatic brain injury. Curr. Opin. Crit. Care. 2009;15:437–441. doi: 10.1097/MCC.0b013e3283307a26. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Chopp M. Neurorestorative treatment of stroke: cell and pharmacological approaches. NeuroRx. 2006;3:466–473. doi: 10.1016/j.nurx.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 44.Sun D, McGinn MJ, Zhou Z, et al. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Kempermann G, Kronenberg G. Depressed new neurons--adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol. Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- 46.Kempermann G, Gage FH. Genetic influence on phenotypic differentiation in adult hippocampal neurogenesis. Brain Res. Dev. Brain Res. 2002;134:1–12. doi: 10.1016/s0165-3806(01)00224-3. [DOI] [PubMed] [Google Scholar]
- 47.van Praag H, Schinder AF, Christie BR, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Richardson RM, Singh A, Sun D, et al. Stem cell biology in traumatic brain injury: effects of injury and strategies for repair. J. Neurosurg. 2010;112:1125–1138. doi: 10.3171/2009.4.JNS081087. [DOI] [PubMed] [Google Scholar]
- 50.Taupin P. The therapeutic potential of adult neural stem cells. Curr. Opin. Mol. Ther. 2006;8:225–231. [PubMed] [Google Scholar]
- 51.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 52.Yamashima T, Tonchev AB, Yukie M. Adult hippocampal neurogenesis in rodents and primates: endogenous, enhanced, and engrafted. Rev. Neurosci. 2007;18:67–82. doi: 10.1515/revneuro.2007.18.1.67. [DOI] [PubMed] [Google Scholar]
- 53.Cameron HA, Woolley CS, McEwen BS, et al. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 54.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J. Comp. Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- 55.Stanfield BB, Trice JE. Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp. Brain Res. 1988;72:399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- 56.Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J. Comp. Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 57.Hastings NB, Seth MI, Tanapat P, et al. Granule neurons generated during development extend divergent axon collaterals to hippocampal area CA3. J. Comp. Neurol. 2002;452:324–333. doi: 10.1002/cne.10386. [DOI] [PubMed] [Google Scholar]
- 58.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 59.Urrea C, Castellanos DA, Sagen J, et al. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor. Neurol. Neurosci. 2007;25:65–76. [PubMed] [Google Scholar]
- 60.Kernie SG, Erwin TM, Parada LF. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J. Neurosci. Res. 2001;66:317–326. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- 61.Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 62.Taupin P. Protocols for studying adult neurogenesis: insights and recent developments. Regen. Med. 2007;2:51–62. doi: 10.2217/17460751.2.1.51. [DOI] [PubMed] [Google Scholar]
- 63.Kuhn HG, Cooper-Kuhn CM. Bromodeoxyuridine and the detection of neurogenesis. Curr. Pharm. Biotechnol. 2007;8:127–131. doi: 10.2174/138920107780906531. [DOI] [PubMed] [Google Scholar]
- 64.Meng Y, Xiong Y, Mahmood A, et al. Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats. J. Neurosurg. 2011;115:550–560. doi: 10.3171/2011.3.JNS101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ning R, Xiong Y, Mahmood A, et al. Erythropoietin promotes neurovascular remodeling and long-term functional recovery in rats following traumatic brain injury. Brain Res. 2011;1384:140–150. doi: 10.1016/j.brainres.2011.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sosne G, Albeiruti AR, Hollis B, et al. Thymosin beta4 inhibits benzalkonium chloride-mediated apoptosis in corneal and conjunctival epithelial cells in vitro. Exp. Eye Res. 2006;83:502–507. doi: 10.1016/j.exer.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Y, Qiu F, Xu S, et al. Thymosin beta4 activates integrin-linked kinase and decreases endothelial progenitor cells apoptosis under serum deprivation. J. Cell Physiol. 2011;226:2798–2806. doi: 10.1002/jcp.22624. [DOI] [PubMed] [Google Scholar]
- 68.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 69.Lu D, Mahmood A, Zhang R, et al. Upregulation of neurogenesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats. J. Neurosurg. 2003;99:351–361. doi: 10.3171/jns.2003.99.2.0351. [DOI] [PubMed] [Google Scholar]
- 70.Smart N, Rossdeutsch A, Riley PR. Thymosin beta4 and angiogenesis: modes of action and therapeutic potential. Angiogenesis. 2007;10:229–241. doi: 10.1007/s10456-007-9077-x. [DOI] [PubMed] [Google Scholar]
- 71.Dettin M, Ghezzo F, Conconi MT, et al. In vitro and in vivo proangiogenic effects of thymosin-beta4-derived peptides. Cell Immunol. 2011;271:299–307. doi: 10.1016/j.cellimm.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 72.Lok J, Gupta P, Guo S, et al. Cell-cell signaling in the neurovascular unit. Neurochem. Res. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 73.Guo S, Lo EH. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke. 2009;40:S4–7. doi: 10.1161/STROKEAHA.108.534388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta. Neuropathol. 2009;117:481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 75.Chopp M, Li Y. Treatment of stroke and intracerebral hemorrhage with cellular and pharmacological restorative therapies. Acta. Neurochir. Suppl. (Wien) 2008;105:79–83. doi: 10.1007/978-3-211-09469-3_16. [DOI] [PubMed] [Google Scholar]
- 76.Li Y, Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci. Lett. 2009;456:120–123. doi: 10.1016/j.neulet.2008.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohab JJ, Fleming S, Blesch A, et al. A neurovascular niche for neurogenesis after stroke. J. Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han HS, Suk K. The function and integrity of the neurovascular unit rests upon the integration of the vascular and inflammatory cell systems. Curr. Neurovasc. Res. 2005;2:409–423. doi: 10.2174/156720205774962647. [DOI] [PubMed] [Google Scholar]
- 79.Chopp M, Li Y, Zhang ZG. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke. 2009;40:S143–145. doi: 10.1161/STROKEAHA.108.533141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- 81.Chopp M, Li Y, Zhang J. Plasticity and remodeling of brain. J. Neurol. Sci. 2008;265:97–101. doi: 10.1016/j.jns.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 82.Wang L, Zhang Z, Wang Y, et al. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 83.Xiong Y, Mahmood A, Lu D, et al. Histological and functional outcomes after traumatic brain injury in mice null for the erythropoietin receptor in the central nervous system. Brain Res. 2008;1230:247–257. doi: 10.1016/j.brainres.2008.06.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiong Y, Lu D, Qu C, et al. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J. Neurosurg. 2008;109:510–521. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang XT, Bi YY, Feng DF. From the vascular microenvironment to neurogenesis. Brain Res. Bull. 2011;84:1–7. doi: 10.1016/j.brainresbull.2010.09.008. [DOI] [PubMed] [Google Scholar]