Abstract
Protein kinase M ζ (PKMζ) is a constitutively active form of atypical PKC that is exclusively expressed in the brain and implicated in the maintenance of long-term memory1–9. Most studies that support a role for PKMζ in memory maintenance have used pharmacological PKMζ inhibitors such as the myristoylated zeta inhibitory peptide (ZIP) or chelerythrine. Here, we used a genetic approach and targeted exon 9 of the Prkcz gene to generate mice that lack both protein kinase C ζ (PKCζ) and PKMζ (Prkcz−/− mice). Prkcz−/− mice showed normal behavior in a cage environment and in baseline tests of motor function and sensory perception, but displayed reduced anxiety-like behavior. Surprisingly, they did not show deficits in learning or memory in tests of cued fear conditioning, novel object recognition, object location recognition, conditioned place preference (CPP) for cocaine, or motor learning, when compared with wild-type littermates. ZIP injection into the nucleus accumbens (NAc) reduced expression of cocaine CPP in Prkcz−/− mice. In vitro, ZIP and scrambled ZIP inhibited PKMζ, PKCι and PKCζ with similar Ki values. Chelerythrine was a weak inhibitor of PKMζ (Ki = 76 µM). Our findings show that absence of PKMζ does not impair learning and memory in mice, and that ZIP can erase reward memory even when PKMζ is not present.
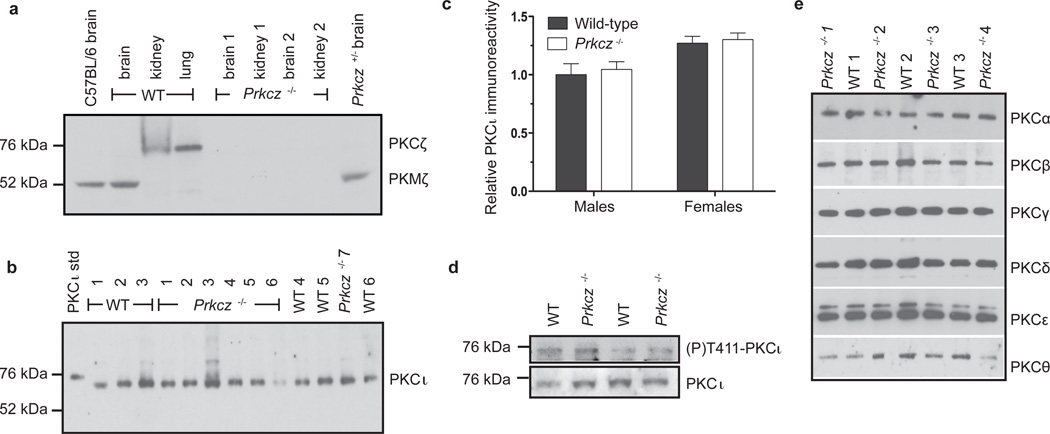
PKMζ is a constitutively active atypical kinase that is transcribed from an internal promoter in the Prkcz gene10. PKMζ and PKCζ show complementary patterns of expression, with PKMζ mainly expressed in the brain and PKCζ primarily expressed outside of the nervous system10. We used homologous recombination to target exon 9 of the Prkcz gene, which encodes the purine-binding site in the catalytic domain of PKCζ and PKMζ, to generate mice that lack both kinases. We confirmed the effect of Prkcz gene deletion on protein expression of PKCζ and PKMζ by western blot analysis. We detected an immunoreactive band at 52 kDa corresponding to PKMζ in brain samples from C57BL/6, wild-type and heterozygous Prkcz+/− mice, but not Prkcz−/− mice (Fig. 1a). A 70 kDa protein band, corresponding to PKCζ, was present in kidney and lung samples from wild-type mice but not from Prkcz−/− mice (Fig. 1a). These data confirm that both PKCζ and PKMζ are absent in Prkcz−/− mice.
Figure 1. Absent PKCζ and PKMζ immunoreactivity in Prkcz −/− mouse tissues.
a, PKCζ (~72 kDa) and PKMζ (~52 kDa) were detected in lung, kidney and brain samples from C57BL/6, wild-type (WT) and heterozygous Prkcz +/− mice but not from Prkcz −/− mice. b, PKCι could be detected in wild-type and Prkcz−/− samples at ~72 kDa. His-tagged human PKCι (PKCι std) was run as a positive control. c, Females showed more brain PKCι immunoreactivity than males without a difference between genotypes (n=8 wild-type females, n=6 wild-type males, n=5 Prkcz −/− females, n=7 Prkcz −/− males. Data are shown as mean + s.e.m.). d, The phospho-PKCι/PKCι ratio was similar between Prkcz −/− (n=7) and wild-type mice brain samples (n=7, P=0.47). e, All PKCs, except for PKCη, were detectable by western blot analysis in wild-type and Prkcz −/− mouse brain samples, and were of similar abundance in both genotypes.
PKCι is the third member of the atypical PKC subfamily that includes PKCζ and PKMζ. Although it is expressed in the brain, nothing is known about its role in regulating behavior. Although female mice had higher levels of brain PKCι immunoreactivity than males, levels were similar in Prkcz−/− and wild-type mice [Fsex (1,22)=13.56, P=0.001; Fgenotype (1,22)=0.292, P=0.59; Fsex×genotype (1,22)=0.009, P=0.9224; Fig. 1b,c]. Since all PKC isoforms require phosphorylation at the activation loop by phosphoinositide-dependent kinase-1 (PDK-1) for catalytic activity11, 12, we investigated if there was increased phosphorylation of PKCι at this site (T411) in Prkcz−/− mice. The ratio of phospho-T411-PKCι/totalPKCι immunoreactivity (Fig. 1d) did not differ by sex or genotype [Fsex(1,10)=0.096, P=0.76; Fgenotype (1,10)=0.567, P=0.47; Fsex×genotype (1,10)=1.01, P=0.34], and there was also no genotype difference when we combined male and female data (t=0.744, P=0.47). These results indicate that loss of PKMζ and PKCζ does not result in a compensatory increase in the abundance of PKCι or in PDK-1-mediated phosphorylation of PKCι. We were able to detect all other PKC isozymes in brain samples except PKCη, and found that their abundance was similar in Prkcz−/− and wild-type mice (Fig. 1e and Supplementary Fig. 1.
Prkcz−/− mice did not show morphological abnormalities or unusual behaviors compared with wild-type mice on a standardized behavioral screen13. Prkcz−/− (n=11) and wild-type mice displayed intact visual perception (n=22) on the visual cliff test with both genotypes avoiding the perceived cliff more than 50% of the time and to a similar extent (U=116.5, P=0.88). There was no genotype difference in the tail-flick test for thermal nociception (n=13 per genotype; t=0.163, P=0.87), or in total distance traveled in an open field (n=32 wild-type, n=30 Prkcz−/− mice; t=0.748,P=0.46).
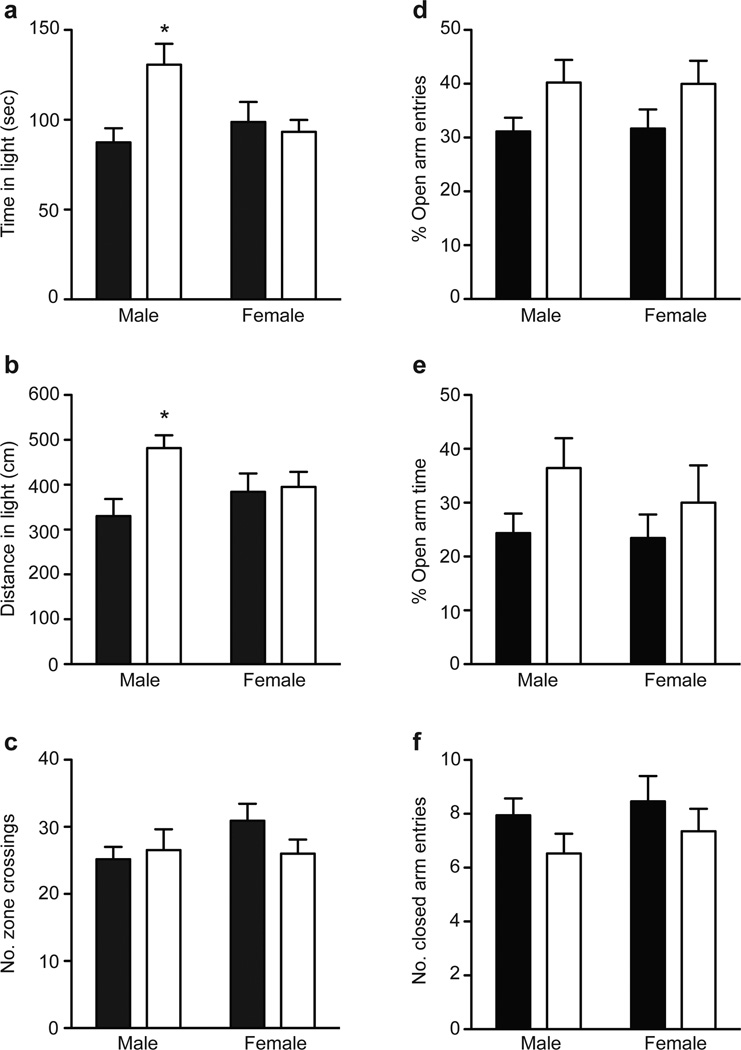
We analyzed anxiety-like behavior using the light-dark box and the elevated plus maze, which exploit the conflict between the desire to explore a novel environment and aversion to brightly lit, open spaces. In the light-dark box test, male Prkcz−/− mice spent 51% more time [Fgenotype×sex (1,52)=6.567, P=0.01] and traveled 36% farther in the lit compartment [Fgenotype×sex (1,51)=5.803, P=0.02] than wild-type mice (Fig. 2a,b). The number of zone crossings was not different between genotypes or sexes [Fgenotype (1,52)=0.560, P=0.46; Fsex (1,52)=1.184, P=0.28; Fgenotype×sex (1,52)=1.725, P=0.19] (Fig. 2c). On the elevated plus maze, Prkcz−/− mice of both sexes (n=31) made more entries into the open arms [Fgenotype (1,60)=5.615, P=0.02; Fsex (1,60)=0.002, P=0.97; Finteraction (1,60)=0.013, P=0.91] and tended to spend more time in the open arms [Fgenotype (1,60)=3.302, P=0.07; Fsex (1,60)=0.514, P=0.48; Finteraction (1,60)=0.285, P=0.60] than wild-type mice (n=33) (Fig. 2d,e). The number of closed arm entries was not different between genotypes or sexes [Fgenotype (1,60)=2.629, P=0.11; Fsex (1,60)=0.752, P=0.39; Finteraction (1,60)=0.039, P=0.85] (Fig. 2f). These findings indicate that Prkcz modulates anxiety-like behavior, particularly in male mice.
Figure 2. Reduced anxiety-like behavior in Prkcz −/− mice.
a,b, Male Prkcz −/− mice (n=16) spent more time and traveled farther in the lit compartment compared with male wild-type mice (n=13). There was no genotype difference in female mice (n=14 wild-type mice, n=13 Prkcz −/− mice). c, The total number of zone crossings, a measure of locomotor activity, was similar between Prkcz −/− and wild-type mice of both sexes. d,e, Prkcz −/− mice (n=31) made more open arm entries and trended towards spending more time in the open arms than wild-type mice (n=33). f, The number of closed arm entries was similar in both genotypes. Black bars represent wild-type mice, white bars represent Prkcz −/− mice, data are shown as mean + s.e.m.
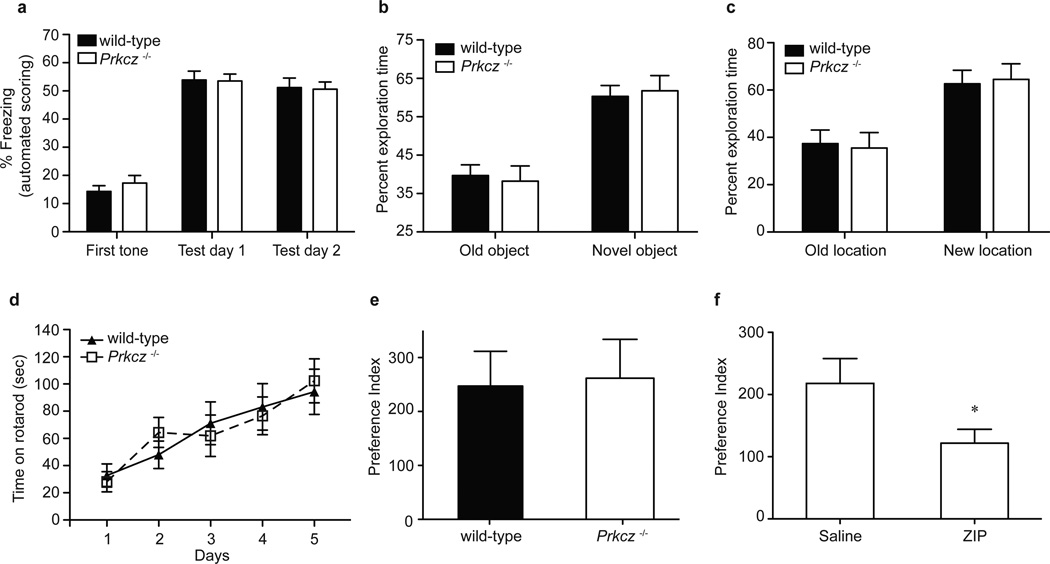
Since targeting the Prkcz gene reduced anxiety-like behavior, we also examined fear responses using a cued fear conditioning procedure, by which animals learn to associate a tone with a foot shock. In this paradigm, subsequent presentations of the tone alone evoke defensive freezing behavior. Performance in this task is impaired in rats after ZIP administration into the amygdala7. We first compared the response to different shock intensities and found no genotype difference [Fgenotype (1,108)=0.848, P=0.37; Fshock (6,108)=152.3, P<0.0001; Fgenotype×shock (6,108)=1.298, P=0.26]. Automated scoring correlated well with hand scoring for the first tone presentation on the training day (r2=0.863, P<0.0001; Supplementary Fig. 2a), the average of five tone presentations on test day 1 (r2=0.894; P<0.0001; Supplementary Fig. 2b) and the average of three tone presentations on test day 2 (r2=0.786; P<0.0003; Supplementary Fig. 2c). There was a low level of freezing during the first tone presentation on the training day when the mice had not yet been exposed to the shock (Fig. 3a). On test day 1, freezing to the tone was significantly greater indicating that mice had learned to associate the tone with the foot shock. The mice exhibited a similar level of freezing on test day 2. We found no genotype difference in freezing during any of the sessions, indicating that Prkcz−/− and wild-type mice learned the association equally well [Fgenotype (1,90)=0.070, P=0.79; Fsession (2,90)=134.6, P<0.0001; Fgenotype×session (2,90)=0.30, P=0.745].
Figure 3. Intact learning and memory in Prkcz −/− mice.
a, In cued fear conditioning, wild-type (n=20) and Prkcz −/− mice (n=27) showed similar levels of freezing during all three sessions. b, During the novel object task, Prkcz −/− (n=17) and wild-type mice (n=16) spent more time exploring the new object compared with the old object. There was no genotype difference in exploration of old or novel object. c, In the spatial memory task, Prkcz −/− (n=13) and wild-type mice (n=12) spent more time exploring the new location compared with the old location and there was no genotype difference in exploration of either location. d, Prkcz −/− (n=11) and wild-type mice (n=12) remained on the accelerating rotarod for similar amounts of time and showed similar improvement in this task over successive trials. e, In the conditioned place preference test, Prkcz −/− (n=7) and wild-type mice (n=8) showed similar preference for the cocaine-paired chamber when tested one day after the last conditioning session. f, Compared with injection of saline (n=9), injection of ZIP (n=13) into the NAc significantly reduced expression of cocaine CPP in Prkcz −/− mice. All data is shown as mean ± s.e.m.
Given that fear memory was unimpaired in Prkcz−/− mice, we investigated other tests of learning and memory. We first used a novel object recognition task to examine hippocampal-dependent learning and memory14. Wild-type (t=3.69, P=0.002) and Prkcz−/− mice (t=2.98, P=0.01) showed greater exploration of the novel object compared with chance, and there was no genotype difference in time exploring the novel object (t=0.31, P=0.76) (Fig. 3b). We tested object location memory using a procedure in which performance is impaired in rats administered ZIP bilaterally into the hippocampus1. Mice of both genotypes spent more time exploring the new location [Fgenotype (1,23)=−6.9, P=1.00; Flocation (1,23)=9.59, P=0.005; Fgenotype×location (1,23)=0.04, P=0.83] (Fig. 3c). We assessed motor learning by measuring improvement in ability to remain on an accelerating rotarod over successive trials (Fig. 3d). There was no genotype difference in improvement of performance over time [Fgenotype (1,84)=0.002, P=0.96; Fsession (4,84)=33.29, P<0.0001; Fgenotype×session (4,84)=1.53, P=0.20], indicating that Prkcz−/− and wild-type mice learned this task equally well. We also assessed drug reward memory in male mice by measuring cocaine CPP15. Wild-type (t=3.838, P=0.006) and Prkcz−/− mice (t=3.645, P=0.01) spent significantly more time in the cocaine-paired chamber after conditioning and there was no genotype difference in the cocaine CPP index (t=0.153, P=0.88) (Fig. 3e). Since ZIP injection into the NAc can erase cocaine reward memory in rats3, we tested if ZIP reduces cocaine reward memory in male and female Prkcz−/− mice using the same cocaine treatment protocol3. We found that compared with saline, ZIP impaired cocaine CPP in Prkcz−/− mice (t=2.258, P=0.04) (Fig. 3f).
The finding that ZIP inhibits memory in Prkcz−/− mice suggests that its effect on memory maintenance1–9 occurs through PKMζ-independent mechanisms. Recently, the specificity of ZIP and chelerythrine for inhibiting PKMζ has been called into question16–19. Part of this concern arises because the PKC zeta and iota pseudosubstrate peptide sequences are identical (SIYRRGARRWRKL), and PKCι is widely expressed in the nervous system20. To determine the specificity of ZIP, scrambled ZIP and chelerythrine for PKMζ, we tested these compounds in an in vitro kinase assay using purified PKMζ, PKCζ and PKCι (Table 1, Supplementary Fig. 3). We found that both ZIP and scrambled ZIP inhibited PKMζ in the micromolar range, which is less potent than recently reported21. There was only a 7.3-fold difference in Ki values between ZIP and scrambled ZIP, and this modest difference in Ki values suggests that scrambled ZIP is not an ideal control peptide for ZIP inhibition of PKMζ. Interestingly, ZIP and scrambled ZIP were equally potent inhibitors of PKCι and PKCζ compared with PKMζ (Table 1). Chelerythrine was a weak inhibitor of PKMζ when assayed without dithiothreitol (DTT), and lost all inhibitory activity when 1 mM DTT was included (Table 1, Supplementary Fig. 3d). These results question the use of ZIP and chelerythrine as specific inhibitors of PKMζ.
Table 1.
In vitro inhibitory activity of compounds against purified atypical PKC isozymes (Ki, 95% CI).
| Compound | PKCζ (µM) | PKMζ (µM) | PKCι (µM) |
|---|---|---|---|
| ZIP | 1.70 (1.14–2.54) |
2.11 (1.91–2.33) |
1.43 (1.21–1.68) |
| Scrambled ZIP | 5.51 (3.12–9.71) |
15.4 (14.7–16.2) |
4.92 (3.48–6.96) |
| Chelerythrine | NA | 75.97 (68.44–84.25) |
NA |
NA = Not assessed.
In summary, our in vitro studies suggest that the current pharmacological reagents commonly used to inhibit PKMζ are not specific for this kinase. More importantly, our in vivo studies with Prkcz−/− mice indicate that PKMζ is not required for long-term memory and that ZIP can impair memory through mechanisms that do not involve PKMζ. These findings cast doubt on the importance of PKMζ in the maintenance of long-term memory.
Methods Summary
Generation and testing of Prkcz−/− mice
A targeting construct containing a 1.1 kb floxed region of exon 9 was used to generate chimeric mice that were crossed to produce wild-type and Prkcz−/− littermates. To detect PKCζ and PKMζ, an anti-PKCζ antibody (T. Sacktor, SUNY Downstate, NY, USA) was used. Anti-phospho-PKC (pan) (ζThr410), which detects PKCι phosphorylated at T411, was purchased from Cell Signaling Technology, as were antibodies to detect PKCα, PKCδ, PKCι and PKCθ. Anti-PKCβ, PKCγ and PKCη antibodies were purchased from BD Transduction Laboratories. Anti-PKCε antibody was previously generated (SN134)22.
Behavior
The behavioral screen was based on methods described by J. Crawley13. Methods for other behavioral tests are available in the online version of the manuscript. Data were examined for normality using a D’Agostino and Pearson omnibus normality test. Light-dark box and elevated plus maze results were analyzed by two-factor ANOVA with a Bonferroni post-hoc test. The relationship between machine and hand scoring of fear conditioning was analyzed by calculating a Pearson product-moment correlation coefficient. In all tests of learning and memory we did not detect a sex difference; to increase power and the possibility of detecting a genotype difference, we combined results from male and female mice and analyzed these data by ANOVA, t-, or Mann-Whitney tests.
Full Methods and any associated references are available in the online version of the paper.
Methods
Generation of Prkcz−/− mice
A 14.5 kb targeting construct containing exon 9 flanked by loxP sites was used to generate ES cells by homologous recombination. Targeted ES cells (W4 line, Taconic) were injected into C57BL/6J blastocysts to generate chimeric mice that were mated with Flpase C57BL/6J mice (Jackson Laboratories) to remove the neomycin selection cassette in the targeting vector. F1 generation progeny were crossed with C57BL/6J CMV-Cre mice to delete exon 9. Hybrid C57BL/6JX129S6 wild-type and Prkcz−/− littermates were genotyped using the forward primer (GGTATAGTAGGCAGCTATTGCG) located in the long arm of the construct and a reverse primer (TCCTGCCTCAGCCAGAAAACAAACCACACGG) located outside of the construct. All mice were 8–12 weeks old and housed under a 12-h light:12-h dark cycle, with lights on at 6 AM and off at 6 PM. Food and water were freely available. All procedures were conducted in accordance with guidelines of the NIH and the Gallo Center Institutional Animal Care and Use Committee.
Western Blotting
Tissue samples were homogenized in RIPA buffer with EGTA, protease and phosphatase inhibitors (G Biosciences). Anti-PKCζ (from T. Sacktor, SUNY Downstate, NY, USA) or anti-PKCε (SN13422) was used at 1:1000. Anti-PKCζ(phospho-Thr410, cat#2060), PKCα, PKCδ, PKCι and PKCθ antibodies (Cell Signaling Technology) were used at 1:500-1:1000. Mouse monoclonal anti-PKCβ, PKCγ and PKCη antibodies were from BD Transduction Laboratories. All antibodies were incubated in 5% nonfat dry milk, except anti-PKC ζ(phospho-Thr410) which was incubated in 5% BSA. HRP-conjugated donkey anti-rabbit or donkey anti-mouse secondary antibodies were used (Jackson Immuno Research Labs, Inc.). Immunoreactive bands were quantified using ImageJ (http://rsbweb.nih.gov/ij/). Phospho-PKC and PKC samples were normalized to proteins (38–102 kDa) detected on a Coomassie Blue stained gel run in parallel. Data were expressed relative to the mean immunoreactivity determined in wild-type samples.
Behavioral testing
We examined mice for morphological defects, body weight, and startle to a sudden loud noise13. Vision was assessed using a visual cliff assay13, and the % entries onto the normal perspective surface out of 10 trials was calculated. Thermal sensation was tested using a tail-flick apparatus (Columbus Instruments). Locomotor activity was recorded as the distance traveled in an open field chamber23, 24 after 60 min. Anxiety-like behavior was measured using a light-dark box (Med Associates Inc.) and an elevated plus maze as in previous work23, 24. Novel object recognition14 and object location memory1 were measured using published methods. Motor learning was assessed using a rotarod (Accuscan Instruments) that accelerated from 0 to 40 rpm in 5 min. Mice were placed on the rotarod at 4 rpm, and had to stay on the rotarod for at least 15 seconds for a trial to be considered successful. The latency to fall in three successful trials was recorded for 5 consecutive days.
The response to footshock was determined by administering 0.5-sec shocks every 3 min in 0.1 mA increments from 0.1 to 0.7 mA. Responses were scored as: 0-no reaction, 1-flinch, 2-small hop, 3-dash, 4-small jump, 5-large jump, with an extra 0.25 added for any vocalization. Fear conditioning was tested by subjecting naïve mice to an 11-min session with five pairings of a 30-sec, 85-dB tone that co-terminated with a 1-sec, 0.3-mA footshock. The chambers (San Diego Instruments) had transparent walls with a metal rod floor on the training day. On test days the chambers had a solid floor and wallpaper. On test day 1, 24 h after the training day, each mouse was returned to the chamber with the new context and exposed to five 30-sec tones over 12 minutes. On test day 2, the mouse was returned to the chamber and exposed to three 30-sec tones over 7 minutes. Beam breaks were measured every second. If there was no new beam break during a 1-sec interval, the mouse was considered to be freezing during that interval. The chamber was also equipped with a video camera mounted in the corner for subsequent hand-scored freezing, which was measured as the time during which the mouse exhibited no movement except for breathing. The amount of time spent freezing was expressed as a percentage of total session time.
Cocaine conditioned place preference was measured in non-cannulated Prkcz−/− and wild-type mice based on the protocol in Li, et al.3, but using a two-chamber apparatus (Med Associates Inc.) and 20-min conditioning sessions. Injections of saline or 10 mg/kg cocaine i.p. (Sigma-Aldrich) were counterbalanced across groups. Cocaine preference index was calculated as the time (seconds) spent in the cocaine-paired chamber on test day minus the time spent in the same chamber before conditioning. Three mice in the non-cannulated group (1 wild-type and 2 Prkcz−/− mice) and three Prkcz−/− mice (2 saline-treated and 1 ZIP-treated) in the cannulated group did not develop CPP and were therefore excluded from analysis. One outlier in the cannulated group that was identified by a Grubb’s test was also removed from the analysis.
Surgery and microinjection
Mice were anaesthetized with ketamine (100 mg/kg i.p.) and xylazine (7 mg/kg i.p.) and placed in a digital stereotaxic alignment system (model 1900, David Kopf Instruments). Bilateral guide cannulae (C235GS-5-2.0, 26 gauge, Plastics One) were aimed at the NAc (1.40 mm anterior to bregma, ± 1.0 mm mediolateral, −3.8 mm ventral from skull surface) and secured with dental cement (DenMat). Mice recovered from surgery for one week prior to the start of experiments. The amount of ZIP peptide (Tocris Bioscience, R&D Systems) in a 1 mg vial was assessed using a guanidine hydrochloride-based Bradford assay (Sigma-Aldrich). The reported peptide purity by Tocris Bioscience closely matched the measured peptide purity. ZIP was dissolved in 0.9% physiological saline, adjusting for peptide purity, to a 10 mM concentration (10 nmol/µl). Mice were injected with 1 µl of ZIP or saline per side at 0.25 µl/minute using injectors that extended 0.7 mm beyond the guide cannulae. The injectors were left in place for 1 minute to allow for diffusion, after which they were removed and the obstructers replaced. The mice were returned to their home cage after injection.
Histological verification of cannulae placements
After completion of the experiment, mice were sacrificed and the brain was removed and placed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were transferred to a 20% sucrose solution in 4% paraformaldehyde for 2 days. Brains were frozen, cut into sections and Nissl stained to verify cannulae placement. Mice with injection sites outside of the NAc were excluded from the analysis.
Kinase assay
Kinase activity was measured using the LANCE PKC Assay Kit (PerkinElmer Life Sciences Inc.). Flag-affinity purified rat PKCζ (1nM), PKMζ (0.5nM) or PKCι (2.5nM) was added to the buffer with 50 nM ULight-PKC peptide substrate, CRFARKGSLRQKNV, (#TRF0108-D, PerkinElmer Life Sciences Inc.) and increasing concentrations of test compound. The reaction was initiated by adding 2.5 µM ATP and terminated after 60 min by adding 2X Stop Solution/Detection Mix containing 20 mM EDTA and 4 nM Eu-anti-phospho-PKC (Ala25Ser) (#TRF0207-D, PerkinElmer Life Sciences Inc.). Increasing concentrations of the PKC peptide substrate (2.5–50 nM) were used to determine Km values for each atypical PKC. ZIP and scrambled ZIP were obtained from Tocris Bioscience and were dissolved in 0.9% physiological saline, after adjusting for reported peptide purity. Chelerythrine was obtained from Sigma-Aldrich, dissolved in DMSO and assayed using PKMζ prepared in the absence of DTT since chelerythrine changed color and lost all inhibitory activity when 1 mM DTT was present. Phosphorylation was detected using a FlexStation III Microplate Reader in LANCE TR-FRET mode (excitation=340 nm, emission=665 nm) and was expressed as relative fluorescence units (RFU). The percentage of inhibition by each test compound was calculated as: (signal without test compound - signal with test compound) / (signal without test compound - signal in the absence of ATP)×100. Data were analyzed by nonlinear regression and Ki values were calculated by the Cheng-Prusoff equation using Prism 5.0c (GraphPad Software, San Diego, CA, USA).
Supplementary Material
Acknowledgments
We thank V.N. Kharazia and A. J. Lean for assistance. This work was supported by National Institutes of Health grant AA017072 (R.O.M.), a Canadian Institute of Health Research Post-doctoral Fellowship (A.M.L.), and funds provided by the State of California for medical research on alcohol and drug abuse to UCSF.
Footnotes
Supplementary information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions
A.M.L. designed experiments, collected and analyzed data, and wrote the manuscript. B.R.K., J.P.L., M.E.Z., C.Q., T.M., S.C.F-W. collected and analyzed data. D.W. collected and analyzed the data from the in vitro kinase assays. J.D. produced the constructs for the kinase assays and for generation of the mutant mice, and genotyped the mice. R.O.M. designed experiments, analyzed data, and coauthored the manuscript.
The authors declare no competing financial interest.
REFERENCES
- 1.Hardt O, Migues PV, Hastings M, Wong J, Nader K. PKMzeta maintains 1-day- and 6-day-old long-term object location but not object identity memory in dorsal hippocampus. Hippocampus. 2010;20:691–695. doi: 10.1002/hipo.20708. [DOI] [PubMed] [Google Scholar]
- 2.He YY, et al. PKMzeta maintains drug reward and aversion memory in the basolateral amygdala and extinction memory in the infralimbic cortex. Neuropsychopharmacology. 2011;36:1972–1981. doi: 10.1038/npp.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li YQ, et al. Inhibition of PKMzeta in nucleus accumbens core abolishes long-term drug reward memory. J Neurosci. 2011;31:5436–5446. doi: 10.1523/JNEUROSCI.5884-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migues PV, et al. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13:630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- 5.Parsons RG, Davis M. Temporary disruption of fear-potentiated startle following PKMzeta inhibition in the amygdala. Nat Neurosci. 2011 doi: 10.1038/nn.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastalkova E, et al. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 7.Serrano P, et al. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shabashov D, Shohami E, Yaka R. Inactivation of PKMzeta in the NAc Shell Abolished Cocaine-Conditioned Reward. J Mol Neurosci. 2011 doi: 10.1007/s12031-011-9671-7. [DOI] [PubMed] [Google Scholar]
- 9.Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez AI, et al. Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J Biol Chem. 2003;278:40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- 11.Chou MM, et al. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 12.Le Good JA, et al. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 13.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-Garcia JM. Plastic modifications induced by object recognition memory processing. Proc Natl Acad Sci U S A. 2010;107:2652–2657. doi: 10.1073/pnas.0915059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brabant C, Quertemont E, Tirelli E. Influence of the dose and the number of drug-context pairings on the magnitude and the long-lasting retention of cocaine-induced conditioned place preference in C57BL/6J mice. Psychopharmacology (Berl) 2005;180:33–40. doi: 10.1007/s00213-004-2138-6. [DOI] [PubMed] [Google Scholar]
- 16.Lisman J. Memory erasure by very high concentrations of ZIP may not be due to PKM-zeta. Hippocampus. 2011 doi: 10.1002/hipo.20980. [DOI] [PubMed] [Google Scholar]
- 17.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu-Zhang AX, Schramm CL, Nabavi S, Malinow R, Newton AC. Cellular pharmacology of protein kinase Mzeta (PKMzeta) contrasts with its in vitro profile: implications for PKMzeta as a mediator of memory. J Biol Chem. 2012;287:12879–12885. doi: 10.1074/jbc.M112.357244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- 20.Naik MU, et al. Distribution of protein kinase Mzeta and the complete protein kinase C isoform family in rat brain. J Comp Neurol. 2000;426:243–258. doi: 10.1002/1096-9861(20001016)426:2<243::aid-cne6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Yao Y, et al. Matching biochemical and functional efficacies confirm ZIP as a potent competitive inhibitor of PKMzeta in neurons. Neuropharmacology. 2013;64:37–44. doi: 10.1016/j.neuropharm.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi DS, Wang D, Dadgar J, Chang WS, Messing RO. Conditional rescue of protein kinase C epsilon regulates ethanol preference and hypnotic sensitivity in adult mice. J Neurosci. 2002;22:9905–9911. doi: 10.1523/JNEUROSCI.22-22-09905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, et al. The type 1 equilibrative nucleoside transporter regulates anxiety-like behavior in mice. Genes Brain Behav. 2007;6:776–783. doi: 10.1111/j.1601-183X.2007.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodge CW, et al. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase Cepsilon. J Clin Invest. 2002;110:1003–1010. doi: 10.1172/JCI15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.