Abstract
Primordial follicles are formed prenatally in mammalian ovaries, and at birth they are fated to be activated to primary follicles, to be dormant, or to die. During the early stage of folliclulogenesis, the oocyte undergoes dynamic alterations in expression of numerous genes, which are regulated by transcription factors. Several germ-cell specific transcriptional regulators are critical for formation and maintenance of follicles. These transcriptional regulators include: Figla, Lhx8, Nobox, Sohlh1, and Sohlh2. A subset of these transcriptional regulators is mutated in women with ovarian insufficiency and infertility. Establishment of this oocyte pool is essential for fertility. This review focuses on these transcriptional regulators of female primordial follicles.
Keywords: Primordial follicles, Transcription factors, Figla, Lhx8, Nobox, Sohlh1, Sohlh2
Introduction
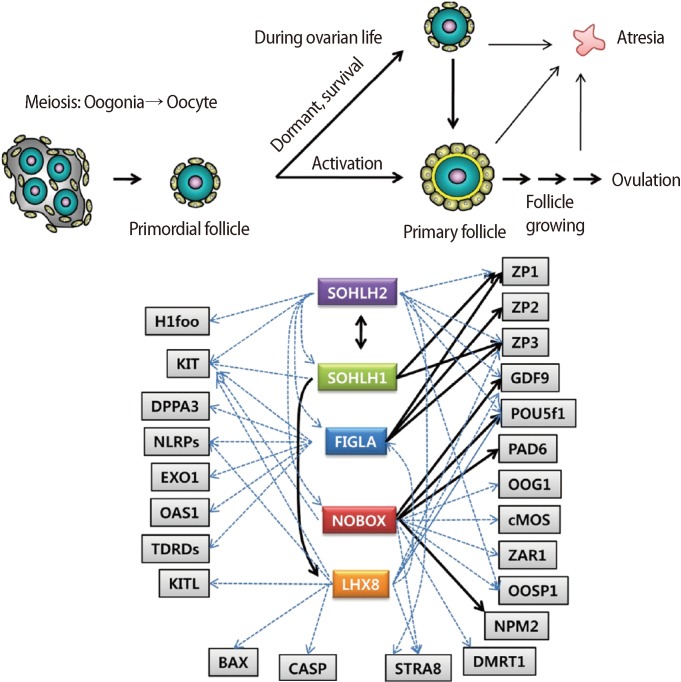
Oogenesis, a type of gametogenesis, is the production of ova or egg cells in females. Oogenesis occurs in all sexually reproductive species, and it consists of different stages of ovum production. There are several stages in ovum maturation in mammals, namely, the oogonium, primary oocyte, secondary oocyte, ootid, and ovum. In the first stage of oogenesis, the oogonium undergoes oocytogenesis, generating a primary oocyte through meiosis. Like the oogonium, the diploid primary oocyte contains two complete sets of chromosomes (4n). During meiosis, the primary oocyte produces a haploid secondary oocyte (2n). This process is halted halfway through until ovulation, when ootidogenesis continues to produce a released egg. In the final stage of oogenesis, the egg develops into the ovum, which is a mature egg cell. In humans and other mammals, the secondary oocyte becomes an ovum soon after fertilization with a sperm (Figure 1).
Figure 1.
Regulatory networks of transcriptional regulators, SOHLH1, SOHLH2, NOBOX, LHX8 and FIGLA (Modified from Choi and Rajkovic [5]). Black line, direct regulation; Blue line, putative regulation; ZP, zona pellucida; GDF9, growth differentiation factor 9; POU5f1, POU class 5 homeobox 1; PAD6, peptidyl arginine deiminase type VI; OOG1, oogenesin 1; cMOS, moloney sarcoma oncogene; ZAR1, zygote arrest 1; OOSP1, oocyte secreted protein 1; NPM2, nucleoplasmin 2; DMRT1, doublesex and mab-3 related transcription factor 1; STRA8, stimulated by retinoic acid gene 8; CASP, caspase; BAX, BCL2-associated X protein; KITL, kit ligand; TDRD, tudor domain containing; OAS1, 2'-5' oligoadenylate synthetase 1; EXO1, exonuclease 1; NLRP, NLR family, pyrin domain containing; DPPA3, developmental pluripotency-associated 3; H1foo, H1 histone family; member O, oocyte-specific.
Oogenesis occurs with folliculogenesis in mammals. Folliculogenesis is a complex process that depends on numerous factors including both extra-ovarian and intra-ovarian factors. Early folliculogenesis proceeds during embryonic development. Primordial germ cells (PGCs) are the origin of oocytes. PGCs appear in the endoderm near the yolk sac at the third to fourth weeks of gestation in the human and then migrate to the primitive gonad along the hindgut and dorsal mesentery by the 7th week of gestation [1]. After the arrival of PGCs in the gonad, the PGCs proliferate rapidly and then their numbers reach their maximum of up to 6 to 7 million at the 20th week of gestation. The PGCs enter meiosis and become primary oocytes from the 15th week of gestation [2]. Meiosis in primary oocytes is arrested at prophase I around birth. During meiosis, the primary oocyte becomes a primordial follicle by individual lapping with surrounding pregranulosa cells. Once primordial follicles are formed, they have three possible fates. Some of them are recruited for growth and differentiation to be ovulated, which is accompanied by the synthesis of a unique set of proteins in both germ cells and surrounding somatic cells. Most of them remain dormant during the ovarian cycle. Among them, some undergo the apoptotic pathway during follicle growth. Development of high quality oocytes during folliculogenesis is highly critical for proper fertilization and development because it affects early embryonic survival, the establishment and maintenance of pregnancy, and fetal development [3,4]. Primordial follicles are the smallest follicles in the mammalian ovary. During follicular development, the primary oocyte in the primordial follicle undergoes numerous germ-cell specific transcriptions which are crucial for the follicle's maintenance and survival until the end of the ovarian cycle. However, the mechanism(s) of maintenance and survival of primordial follicles remains unknown. Recent studies have described several germ cell-specific transcription factors in the ovary including factor in the germline alpha (FIGLA), newborn ovary homeobox protein (NOBOX), LIM-homeobox protein 8 (LHX8), spermatogenesis and oogenesis-specific basic helix-loop-helix transcription factor 1 (SOHLH1), and SOHLH2 [5]. The knockout mouse models show premature ovarian failure (POF). This review is focused on these germ-cell specific transcriptional factors, which might be crucial for understanding of the regulatory mechanism of maintenance and survival of primary oocytes until ovulation.
Factor in the germline alpha (FLGLA)
FIGLA is one of the well-known germ cell-specific transcriptional factors. FIGLA expresses in the oocyte as early as embryonic day 14.5 (E14.5) [6]. FIGLA contains a basic helix-loop-helix (bHLH) domain which binds to a specific DNA binding element, E-box (CANNTG). FIGLA is involved in regulation of numerous germ-cell specific genes including zona pellucida protein 1 (Zp1), Zp2, and Zp3 through an E-box motif (CANNTG) [6]. The E-boxes on the promoter of Zp1, Zp2, and Zp3 are conserved among species [7]. The physiological function of FIGLA was revealed by using a gene knockout mouse model. Figla deficient mice are sterile due to a defect of the primordial follicles in the ovary. Figla deficient mice lose all of the primordial follicles right after birth [8]. A recent study that performed whole gene profile analysis of the Figla deficient ovary revealed more downstream target genes including Nlrp family members (Nlrp4a, Nlrp4b, Nlrp4f, Nlrp5 [Mater], Nlrp14), Pou5f1, Kit, Exo1, Zbed3, Dppa3, Oas1h, and Padi6 [9]. Interestingly, Figla deficiency shows a sexually dimorphic phenotype because Figla null males are fertile with normal testicular morphology [8]. The ectopic expression of Figla represses testis specific genes, such as a Tdrd family member 6 (Tdrd6), Mvh, and Mili [10]. These findings suggest that Figla activates numerous oocyte-specific genes and represses testis-specific genes at the same time [9,10] and that the dimorphic regulation of FIGLA is crucial for the formation and maintenance of primordial follicles. A recent study showed that a mutation of FIGLA, the deletion c.419-421 delACA (p.140 delN), was found in patients with POF [11]. The deletion c.419-421 delACA disrupted the binding affinity of FIGLA to TCF3 [11]. TCF3 is known to be one of the FIGLA interacting factors [6,12].
Newborn ovary homeobox gene (NOBOX)
Nobox encodes a transcription factor containing a homeobox domain as a DNA binding site [13]. NOBOX belongs to a group of tissue-specific homeobox genes and may play an important role in oogenesis and ovarian development. Nobox is preferentially expressed during mammalian folliculogenesis. Nobox can be first detected in the oogonia as early as E15.5 and then remains abundant in oocytes throughout folliculogenesis [13,14]. A deficiency in Nobox shows a sexually dimorphic phenotype like that of Figla null mice. The gene knockout males are fertile and normal, but null females are infertile. Nobox null ovaries appear morphologically normal, containing primordial follicles [14]. However, Nobox null ovaries lost follicles rapidly after birth and impaired the transition of primordial follicles to primary follicles [14]. In microarray analysis, numerous germ cell-specific genes are regulated in the newborn ovary from Nobox null mice including Gdf9, Bmp15, Pou5f1, Zar1, c-Mos, Oog1, Pad6, Oosp1, Oas1d, Oas1d, Oas1e, and Oas1h. These are regulated either directly or indirectly. NOBOX transregulates its target genes through NOBOX binding elements (NBE; TAA/GTTG/A) [15]. NOBOX appears to directly bind to Gdf9, Pou5f1, and Pad6 through the NBE(s) present on their promoters [15,16]. In addition, one of the maternal genes, Npm2, contains one NBE which is required for basal transcriptional activity in oocytes [17]. Interestingly, one of the male-determining genes, Dmrt1, is overexpressed in the null ovaries. This suggests that NOBOX regulates sexually dimorphically during gametogenesis like FIGLA. A missense mutation, p.Arg355His, was found in the NOBOX homeobox domain in a POF patient [18]. The mutation disrupted the NOBOX affinity to targeting DNA binding elements and had a dominant negative effect on the binding of wild-type NOBOX to DNA.
LIM-homeobox protein 8 (LHX8)
LHX8 is a member of the LIM-homeobox transcription factor family. LHX8 contains two LIM domains and one homeobox domain that are highly conserved with 93% identity between mice and humans. LHX8 plays an important role in tissue patterning and differentiation including that of neural tissues [19-21]. In the ovary, Lhx8 transcripts localize to oocytes in germ cell clusters and primordial, primary, and antral follicles in the mouse ovary [22]. Lhx8 is detectable as early as E13.5 [22]. A deficiency of Lhx8 leads to female infertility [7,22]. Lhx8 null mice are infertile due to the absence of oocytes and impairment of the transition of primordial follicles into primary follicles [22]. At birth, histological examination shows that Lhx8-deficient ovaries are grossly similar to newborn wild-type ovaries. However, Lhx8 null ovaries fail to maintain the primordial follicles after birth. This phenotype is similar to that in Nobox deficient mice. The oocytes in primordial follicles of the Lhx8 null ovary have a problem with the transition from primordial to growing follicles and survival of the follicles. They exist until postnatal day 7. This is shorter than the survival in Nobox deficient mice. An Lhx8 deficiency causes the misexpression of many genes, such as Gdf9, Pou5f1, Nobox, Kit, and Kitl [22]. The comparison of genes regulated in Lhx8 null and Nobox null newborn ovaries revealed a common set of genes including the Nlrp family members, such as Nlrp14, Nlrp4c, and Nlrp4f [14,22,23]. These factors are also down-regulated in Figla null mice [9]. The function of Nlrp family members in the oocyte remains unclear. In addition, Stra8 is drastically up-regulated in both Lhx8 and Nobox null newborn ovaries. Stra8 is detectable in the ovary between E12.5 and E16.5 during embryo development and then its expression is not detectable in the ovary [24]. Stra8 is crucial for the initiation of meiosis with premeiotic DNA replication, meiotic chromosome condensation, cohesion, synapsis, and recombination [25]. This suggests that the repression of the meiotic factor or the activation of other factors including Pou5f1, Gdf9, and Nlrp family members by either NOBOX or LHX8 may be important for the maintenance and survival of primordial follicles after birth.
Spermatogenesis and oogenesis specific basic helix-loop-helix protein 1 (SOHLH1) and SOHLH2
SOHLH1 and SOHLH2 are bHLH germ cell-specific transcription factors in the development of both the ovary and testis. The expression patterns of SOHLH1 and SOHLH2 are different from that of FIGLA, NOBOX, and LHX8. In the ovary, the expression of Sohlh1 and Sohlh2 are confined to the germ cell, primordial follicles, and primary follicles, but not in secondary and growing follicles [7,26]. This indicates that SOHLH1 and SOHLH2 may play important and unique roles in primordial follicle formation, activation, and survival.
Ovaries of Sohlh1 deficient mice are completely devoid of follicles by 3 weeks after birth [7]. During early development, most oocytes in the 7-day-old Sohlh1 deficient ovaries are still enveloped by flat somatic cells similar to primordial follicles but now also contain multiple empty follicles in the ovary [7]. The defect in primordial follicle maintenance and survival may result from the surrounding pre-granulosa cells. The disruption of Sohlh1 misregulates many germ-cell specific genes. Among those, Lhx8 is regulated directly by SOHLH1 [7]. The phenotype of Sohlh2 deficiency has been shown to be very similar to that of Sohlh1 knockout [26,27]. Sohlh2 null ovaries form primordial follicles, but the primordial follicles limit growth and do not differentiate from surrounding granulosa cells into cuboidal and multilayered structures [26,27]. Sohlh2 deficient ovaries rapidly lose the follicles after birth, with few remaining by 14 days of postnatal life. The knockout mice ovaries misexpress numerous germ cell- and oocyte-specific genes, including Sohlh1, Nobox, Figla, Gdf9, Pou5f1, Zp1, Zp3, Kit, Oosp1, Nlrp14, H1foo, and Stra8. SOHLH2 may work together with SOHLH1. SOHLH1 and SOHLH2 can form heterodimer [27]. In addition, they coordinate germ cell development by the regulation of Kit expression [28-30].
Conclusion
The molecular mechanisms of maintenance and survival of primordial follicles during ovarian cycles remain unclear. New insights from recent studies of a series of germ-cell specific transcriptional regulators indicate that complex regulatory networks are involved in follicle formation and maintenance in the ovary. A knockout mouse model shows that they do not share redundancy among factors, but a unique pathway. Analyzing their downstream target genes might elucidate more mechanisms and pathways for developing a better understanding of the mechanism of activation and survival of ovarian follicles. The regulatory networks of primordial follicles will give us understanding ovary-specific pathways and clues to regulate and treat female fertility like premature ovarian failure.
Footnotes
This work was supported by the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0093821), and by the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084923).
No potential conflict of interest relevant to this article was reported.
References
- 1.McKAY DG, Hertig AT, Adams EC, Danziger S. Histochemical observations on the germ cells of human embryos. Anat Rec. 1953;117:201–219. doi: 10.1002/ar.1091170206. [DOI] [PubMed] [Google Scholar]
- 2.Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci. 1963;158:417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- 3.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 4.Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod. 2010;25:2944–2954. doi: 10.1093/humrep/deq275. [DOI] [PubMed] [Google Scholar]
- 5.Choi Y, Rajkovic A. Genetics of early mammalian folliculogenesis. Cell Mol Life Sci. 2006;63:579–590. doi: 10.1007/s00018-005-5394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang L, Soyal SM, Dean J. FIGalpha, a germ cell specific transcription factor involved in the coordinate expression of the zona pellucida genes. Development. 1997;124:4939–4947. doi: 10.1242/dev.124.24.4939. [DOI] [PubMed] [Google Scholar]
- 7.Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, et al. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci U S A. 2006;103:8090–8095. doi: 10.1073/pnas.0601083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soyal SM, Amleh A, Dean J. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127:4645–4654. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- 9.Joshi S, Davies H, Sims LP, Levy SE, Dean J. Ovarian gene expression in the absence of FIGLA, an oocyte-specific transcription factor. BMC Dev Biol. 2007;7:67. doi: 10.1186/1471-213X-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu W, Gauthier L, Baibakov B, Jimenez-Movilla M, Dean J. FIGLA, a basic helix-loop-helix transcription factor, balances sexually dimorphic gene expression in postnatal oocytes. Mol Cell Biol. 2010;30:3661–3671. doi: 10.1128/MCB.00201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao H, Chen ZJ, Qin Y, Shi Y, Wang S, Choi Y, et al. Transcription factor FIGLA is mutated in patients with premature ovarian failure. Am J Hum Genet. 2008;82:1342–1348. doi: 10.1016/j.ajhg.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayne RA, Martins da Silva SJ, Anderson RA. Increased expression of the FIGLA transcription factor is associated with primordial follicle formation in the human fetal ovary. Mol Hum Reprod. 2004;10:373–381. doi: 10.1093/molehr/gah056. [DOI] [PubMed] [Google Scholar]
- 13.Suzumori N, Yan C, Matzuk MM, Rajkovic A. Nobox is a homeobox-encoding gene preferentially expressed in primordial and growing oocytes. Mech Dev. 2002;111:137–141. doi: 10.1016/s0925-4773(01)00620-7. [DOI] [PubMed] [Google Scholar]
- 14.Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- 15.Choi Y, Rajkovic A. Characterization of NOBOX DNA binding specificity and its regulation of Gdf9 and Pou5f1 promoters. J Biol Chem. 2006;281:35747–35756. doi: 10.1074/jbc.M604008200. [DOI] [PubMed] [Google Scholar]
- 16.Choi M, Lee OH, Jeon S, Park M, Lee DR, Ko JJ, et al. The oocyte-specific transcription factor, Nobox, regulates the expression of Pad6, a peptidylarginine deiminase in the oocyte. FEBS Lett. 2010;584:3629–3634. doi: 10.1016/j.febslet.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 17.Tsunemoto K, Anzai M, Matsuoka T, Tokoro M, Shin SW, Amano T, et al. Cis-acting elements (E-box and NBE) in the promoter region of three maternal genes (Histone H1oo, Nucleoplasmin 2, and Zygote Arrest 1) are required for oocyte-specific gene expression in the mouse. Mol Reprod Dev. 2008;75:1104–1108. doi: 10.1002/mrd.20863. [DOI] [PubMed] [Google Scholar]
- 18.Qin Y, Choi Y, Zhao H, Simpson JL, Chen ZJ, Rajkovic A. NOBOX homeobox mutation causes premature ovarian failure. Am J Hum Genet. 2007;81:576–581. doi: 10.1086/519496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori T, Yuxing Z, Takaki H, Takeuchi M, Iseki K, Hagino S, et al. The LIM homeobox gene, L3/Lhx8, is necessary for proper development of basal forebrain cholinergic neurons. Eur J Neurosci. 2004;19:3129–3141. doi: 10.1111/j.0953-816X.2004.03415.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Guo YJ, Tomac AC, Taylor NR, Grinberg A, Lee EJ, et al. Isolated cleft palate in mice with a targeted mutation of the LIM homeobox gene lhx8. Proc Natl Acad Sci U S A. 1999;96:15002–15006. doi: 10.1073/pnas.96.26.15002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Marín O, Hermesz E, Powell A, Flames N, Palkovits M, et al. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc Natl Acad Sci U S A. 2003;100:9005–9010. doi: 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi Y, Ballow DJ, Xin Y, Rajkovic A. Lim homeobox gene, lhx8, is essential for mouse oocyte differentiation and survival. Biol Reprod. 2008;79:442–449. doi: 10.1095/biolreprod.108.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi Y, Qin Y, Berger MF, Ballow DJ, Bulyk ML, Rajkovic A. Microarray analyses of newborn mouse ovaries lacking Nobox. Biol Reprod. 2007;77:312–319. doi: 10.1095/biolreprod.107.060459. [DOI] [PubMed] [Google Scholar]
- 24.Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- 25.Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, et al. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–1434. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- 26.Choi Y, Yuan D, Rajkovic A. Germ cell-specific transcriptional regulator sohlh2 is essential for early mouse folliculogenesis and oocyte-specific gene expression. Biol Reprod. 2008;79:1176–1182. doi: 10.1095/biolreprod.108.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyoda S, Miyazaki T, Miyazaki S, Yoshimura T, Yamamoto M, Tashiro F, et al. Sohlh2 affects differentiation of KIT positive oocytes and spermatogonia. Dev Biol. 2009;325:238–248. doi: 10.1016/j.ydbio.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Barrios F, Filipponi D, Campolo F, Gori M, Bramucci F, Pellegrini M, et al. SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development. J Cell Sci. 2012;125:1455–1464. doi: 10.1242/jcs.092593. [DOI] [PubMed] [Google Scholar]
- 29.Ding X, Zhang X, Mu Y, Li Y, Hao J. Effects of BMP4/SMAD signaling pathway on mouse primordial follicle growth and survival via up-regulation of Sohlh2 and c-kit. Mol Reprod Dev. 2012 Dec 04; doi: 10.1002/mrd.22138. [Epub]. http://dx.doi.org/10.1002/mrd.22138. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Ahn HW, Chu T, Bowden W, Gassei K, Orwig K, et al. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Dev Biol. 2012;361:301–312. doi: 10.1016/j.ydbio.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]