Abstract
The soy-derived phytoestrogen genistein has received attention for its potential to improve vascular function, but its mechanism remains unclear. Here, we report that genistein at physiologically relevant concentrations (0.1–10 μM) significantly inhibited thrombin-induced increase in endothelial monolayer permeability. Genistein also reduced the formation of stress fibers by thrombin and suppressed thrombin-induced phosphorylation of myosin light chain (MLC) on Ser19/Thr18 in endothelial cells (ECs). Genistein had no effect on resting intracellular [Ca2+] or thrombin-induced increase in Ca2+ mobilization. Addition of the inhibitors of endothelial nitric oxide synthase or estrogen receptor did not alter the protective effect of genistein. RhoA is a small GTPase that plays an important role in actin-myosin contraction and endothelial barrier dysfunction. RhoA inhibitor blocked the protective effect of genistein on endothelial permeability and also ablated thrombin-induced MLC-phosphorylation in ECs. Inhibition of PKA significantly attenuated the effect of genistein on thrombin-induced EC permeability, MLC phosphorylation, and RhoA membrane translocation in ECs. Furthermore, thrombin diminished cAMP production in ECs, which were prevented by treatment with genistein. These findings demonstrated that genistein improves thrombin-induced endothelial barrier dysfunction in ECs through PKA-mediated suppression of RhoA signaling.
Cardiovascular diseases, especially atherosclerosis, are the major cause of morbidity and mortality in the industrialized world and claim the lives of over 40% of the nearly 2.4 million Americans who die each year (1). Vascular inflammation and endothelial barrier dysfunction-induced vascular permeabilities play a fundamental role in the initiation and progression of atherosclerosis. Indeed, the development of atherosclerosis is associated with increased permeability of vascular endothelial monolayers for macromolecules such as low-density lipoprotein (2). Thrombin is a multifunctional serine protease generated at the site of vascular injury, which plays a significant role in inflammatory diseases and endothelial barrier dysfunction. Thrombin elevates intracellular Ca2+ concentration and thereby activates Ca2+/calmodulin-dependent myosin light chain (MLC) kinase, leading to the phosphorylation of MLC and cell contraction (3, 4). Previous studies show that thrombin-induced endothelia cell (EC) permeability is mediated through RhoA, a GTPase. RhoA and its kinase inhibit dephosphorylation of MLC, thereby leading to MLC phosphorylation and cell contraction, resulting in increased vascular permeability (5). As such, RhoA signaling may be a potential target for preventing thrombin-induced endothelial barrier dysfunction.
Genistein, an important soy-derived phytoestrogen, has received wide attention because of its potential beneficial effects on various human degenerative diseases, such as cardiovascular diseases. Genistein has a weak estrogenic effect (6) by binding to estrogen receptors (ERs) (7) and by inhibiting protein tyrosine kinase (PTK) (8). Recent human intervention studies suggest a beneficial effect of genistein on atherosclerosis (9), markers of cardiovascular risk (10, 11), vascular motor tone (12, 13), vascular endothelial function (14), and systemic arterial compliance (15). However, the effect of genistein on plasma lipid profiles, such as low-density lipoprotein and triglycerides, has been found to be essentially neutral (13, 15, 16), suggesting that the antiatherogenic effect of genistein is not due to a change in plasma lipids. Data from animal and in vitro studies also suggest a protective role of genistein in the vasculature (17, 18). Studies demonstrate that genistein has antiatherogenic effects by inhibiting proliferation of vascular endothelial (19) and smooth muscle cells (20). While these data are of great interest, most of the results in these studies reflected a pharmacological effect of genistein (>30 μM) that are well above the achievable plasma genistein concentrations (≤5 μM) in both rodents and humans following the consumption of genistein (21, 22). Recently, we have reported that genistein at physiological relevant concentrations (≤5 μM) reduce hyperglycemia-induced vascular inflammation in ECs (23). While this study supports a vascular-protective effect of genistein, the effect and molecular mechanisms of genistein on thrombin-induced endothelial permeability remains unknown.
Cyclic AMP is a central signaling molecule in a variety of cellular systems and plays an important role in maintaining normal vascular function (24–27). We have recently demonstrated that genistein at physiologically achievable doses directly acts on vascular ECs, leading to accumulation of intracellular cAMP and subsequent activation of protein kinase A (PKA) (28). The activation of the cAMP-signaling system is not related to any known action of genistein, such as inhibition of PTK or binding to ERs (28, 29), suggesting a novel effect of genistein on vasculature. Cyclic AMP/PKA signaling has been suggested to play a very important role in maintaining normal vascular function by inhibiting vascular endothelial from proinflammatory cytokine-induced damage (24), depressing leukocyte adhesion to ECs (25), and maintaining normal endothelial barrier function (26, 27). However, the molecular action of genistein on endothelial barrier dysfunction is unknown. In this study, we investigated the hypothesis that genistein protects thrombin-induced endothelial barrier dysfunction through cAMP/PKA-mediated suppression of RhoA signaling.
Materials and Methods
Materials
Bovine aortic endothelial cells (BAECs) and vascular endothelial growth factors were from Lonza Walkersville, Inc. (Walkersville, MD); M199 media, fetal bovine serum (FBS), L-glutamine, penicillin-streptomycin, Fura-2AM, and phalloidin Alexa-488 were purchased from Invitrogen (Carlsbad, CA); RhoA, phosphor-specific MLC and MLC antibodies were from Cell Signaling Inc. (Danvers, MA); nitrocellulose membranes were from Schleicher & Schuell (Keene, NH); superSignal chemiluminescence detection system, stripping buffer, and avidin-conjugated fluorescein (avidin-FITC) were purchased from Pierce (Rockville, IL); fibronectin-coated Transwell plates were obtained from BD Bioscience Labware (Boston, MA); cAMP enzyme immunoassay (EIA) kit was from Assay Design Inc. (Ann Arbor, MI); ICI 182,780 (ICI) was purchased from Tocris Cookson (Balwin, MO); genistein (from soybean, ≥98%, HPLC), H89, α-thrombin, C3-transferase (C3), NG-nitro-L-arginine methyl ester (L-NAME), protease inhibitor cocktail, phosphatase inhibitor cocktail I, 8-Bromo-cAMP, and general chemicals were from Sigma-Aldrich (St. Louis, MO).
Cell culture
BAECs were grown in M199 medium supplemented with 20% FBS, 50 U/ml penicillin, and 0.05 mg/ml streptomycin and incubated at 37°C in a 5% CO2, 95% air environment. Medium was changed every second day until confluence. BAECs were serially passaged after 0.05% trypsin treatment and passages 4-8 were used in all experiments. Before experiments, cells were cultured for 24 h in M199 medium containing 10% FBS and subsequently, BAECs were serum-starved in M199 medium containing 1% FBS and 0.5% bovine serum albumin (BSA) for 12 h. For endothelial barrier permeability study, BAECs were cultured on fibronectin-coated polycarbonate filters (0.6 cm, 3 μm pore size) of the Transwell plate in M199 medium with 20% FBS for 3-4 d until a tight monolayer formed. Before experiment, cells were kept in M199 medium containing 10% FBS for 12 h and then were washed and incubated in medium containing 1% FBS for 2 h.
Evaluation of endothelial barrier function in ECs
To evaluate the protective effect of genistein against thrombin-induced endothelial barrier dysfunction, we measured avidin-FITC (∼64 kD) passage cross the endothelial monolayer, an established in vitro model for study of endothelial permeability. Passage of avidin-FITC through BAEC monolayers, as an index of barrier function, was assessed as described previously (30, 31). BAECs were cultured on fibronectin-coated polycarbonate porous filters and treated with genistein, or vehicle (dimethylsulfoxide) in medium containing 1% serum for 30 minutes at 37°C. In some experiments, cells were preincubated with or without 0.3 mM endothelial nitric oxide synthase (eNOS) inhibitor L-NAME, 1 μM estrogen receptor antagonist ICI for 30 minutes, 5 μg/ml RhoA inhibitor C3 for 24 h, or 10 μM PKA-specific inhibitor H89 for 30 minutes prior to addition of 5 μM genistein for 30 minutes. Thrombin (2U/ml) and avidin-FITC (1 μM) were then added to the upper compartment of the Transwells in the continued presence of genistein or vehicle for 1 hour. Samples were taken from the bottom compartment and the concentration of avidin-FITC in each sample was determined using a fluorometer plate reader (BMG Labtech, Inc, Durham, NC). Passage rates were expressed as nanogram per square centimeter per hour (31).
Assessment of cytoskeleton changes in ECs
BAECs were incubated in 4-well glass chamber slides in M199 medium near confluence, and serum-starved for 24 hours. Cells were then treated with genistein or vehicle in phenol-red free medium for 10 minutes at 37°C. Afterward, thrombin (2U/ml) was added to the medium in the continued presence of genistein or vehicle for 5 minutes. Cells were then fixed with paraformaldehyde for 10 minutes at room temperature. After three washes in PBS, fixed cells were permeabilized with 0.1% Triton X-100 for 1 minute at room temperature. After a new series of three washes in PBS, cells were incubated with phalloidin Alexa-488 (dilution: 1:100 in PBS) for 1 hour and were then observed with a computer-operated Nikon Fluorescence microscope (Nikon, Japan). Images were acquired with a high-resolution color camera (Nikon, Japan).
Intracellular calcium concentration ([Ca2+]) measurement
The concentration of cytosolic free [Ca2+] was measured using the intracellular fluorescent Ca2+ probe of Fura-2 AM as previously described (32, 33). Briefly, confluent BAECs were loaded with 1 μg/mL of Fura-2 AM at 37°C in culture medium for 30 minutes and then washed and placed in PBS containing 20 mM HEPES. The cells were preincubated with various concentrations of genistein or vehicle for 30 minutes at 37°C and were then treated with or without 2 U/ml thrombin in the continued presence of genistein for 10 minutes. Fura-2 AM fluorescence data were obtained at an emission wavelength of 505 nm, with alternating excitation wavelengths of 340 and 380 nm, using a dual- excitation monochromator.
Intracellular cAMP assay
The accumulation of cAMP in BAECs was determined by a specific EIA assay kit. Serum-starved BAECs were preincubated with Hank's balanced salt solution buffer and then treated with 5 μM genistein or vehicle at 37°C; 30 minutes later, thrombin (2 U/ml) was added to cell culture in the continued presence or genistein or vehicle for 5 minutes. After treatment period, the supernatant was rapidly aspirated and intracellular cAMP content was measured as we previously described (28).
Analysis of MLC phosphorylation and RhoA activation in ECs
Following experimental treatment, BAECs were harvested by scraping into lysis buffer (20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM Na4P2O7, 1 mM β-glycerolphosphate, 1 mM Na3VO4), supplemented with protease inhibitor cocktail (1:500) and phosphatase inhibitor cocktail I (1:100). The extracts were sonicated and centrifuged at 10,000 × g for 5 minutes. Protein levels were measured using a Bio-Rad assay kit. Detergent-extracted proteins were mixed with Laemmli sample buffer, heated for 5 minutes at 95°C, and equal amounts of cell lysate proteins (50 μg) were resolved on 10% SDS-PAGE gels. The gels were blotted onto nitrocellulose membranes, probed with rabbit anti-phospho-MLC (Thr18/Ser19), mouse anti-MLC, or rabbit anti-RhoA primary antibodies overnight at 4°C, and incubated with secondary antibody conjugated to horseradish peroxidase for 1 hour at room temperature. The immunoreactive proteins were detected by superSignal chemiluminescence. The protein bands were digitally imaged for densitometric quantitation with a software program (Silk Scientific, Inc., Orem, UT). The amount of phosphorylated MLC was normalized with the total MLC contents from the same sample.
Statistical analysis
All data were subjected to one-way ANOVA analysis using Sigmaplot® software, and treatment differences were subjected to Tukey's multiple-comparison tests, where P < .05 was considered significantly different. Values are expressed as mean ± standard error (SE).
Results
Genistein prevents thrombin-induced endothelial barrier dysfunction
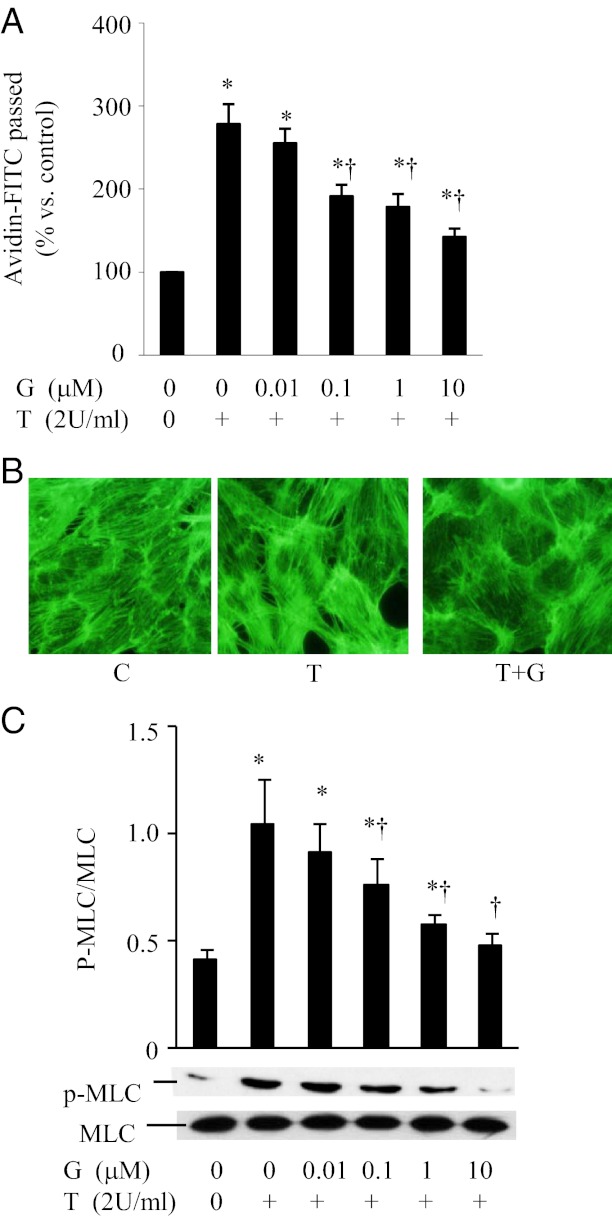
Treatment of thrombin significantly induced endothelial barrier dysfunction in ECs as shown by an increase in avidin-FITC passage (Figure 1A). Genistein pretreatment at 0.1 μM, 1 μM, and 10 μM concentrations reduced thrombin-induced increase in avidin-FITC passage by 31%, 37%, and 49%, respectively (P < .05) (Figure 1A). The actin cytoskeleton of ECs is important in maintaining the structural integrity of ECs, and thrombin-induced EC contraction is associated with increased formation of F-actin stress fiber (34). We further evaluated thrombin-induced cytoskeletal reorganization using fluorescence microscopy. Thrombin induced a dramatic increase in the formation of stress fibers in ECs (Figure 1B). Treatment with genistein prevented the formation of stress fibers, suggesting that genistein can prevent the thrombin-induced changes in the EC cytoskeleton (Figure 1B). Phosphorylation of MLC is a key mechanism by which thrombin signals are converted into the mechanochemical force for cell contraction (3). We then tested whether genistein suppresses thrombin-induced MLC phosphorylation in ECs. Exposure of ECs to thrombin leads to the phosphorylation of MLC on Ser19/Thr18. However, genistein treatment blocked the thrombin-induced MLC phosphorylation (Figure 1C). To determine whether this genistein effect is only restricted to thrombin, we further examined the effect of genistein on tumor necrosis factor (TNF)–α induced endothelial monolayer permeability in ECs. Genistein similarly suppressed TNF-α-stimulated monolayer permeability (data is not shown), further conforming the protective effect of genistein on acute inflammatory mediator-induced endothelial barrier dysfunction.
Figure 1.
Genistein protects thrombin-induced EC barrier dysfunction. A, BAEC monolayers were preincubated with various concentrations of genistein (G) or vehicle (C) for 30 minutes. Cells were then treated with or without 2U/ml thrombin (T) in the continued presence of genistein for 1 hour and avidin-FITC passage was measured. B, BAECs were preincubated with genistein (G, 5 μM) or vehicle (C) for 30 minutes, followed by stimulation with thrombin (T; 2 U/ml) for 5 minutes. Cells were then fixed with paraformaldehyde, permeabilized with Triton X-100, and stained with Alexa-phalloidin, and viewed with a digital fluorescence microscope. C, BAECs were preincubated with indicated concentrations of genistein for 30 minutes, followed by stimulation with thrombin (T; 2 U/ml) for 5 minutes. Phosphorylation of MLC (upper panel) and total MLC (lower panel) were detected by Western blot. Data are expressed as mean ± SE of four different experiments, each performed in duplicate. *P < .05 vs control; †P < .05 vs thrombin-alone-treated cells.
Genistein did not affect thrombin-induced intracellular calcium release
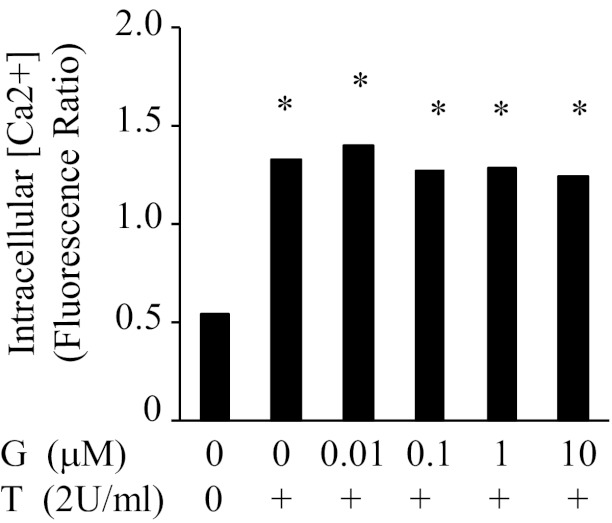
It is well established that thrombin elevates intracellular Ca2+ concentration and thereby activates Ca2+/calmodulin-dependent MLC kinase, leading to the phosphorylation of MLC and cell contraction (3, 4). We tested whether genistein protective action against thrombin-induced endothelial permeability is associated with the inhibition of thrombin-induced increased in intracellular [Ca2+]. Genistein (10 nM to 10 μM) did not alter either resting [Ca2+] or thrombin-induced increase in Ca2+ mobilization (Figure 2), suggesting that the protective effect of genistein against thrombin-induced endothelial barrier dysfunction is not mediated via reducing intracellular [Ca2+].
Figure 2.
Genistein does not affect thrombin-induced intracellular calcium release in BAECs. BAECs were loaded with 1 μg/mL of fura-2 AM (30 min at 37°C in culture medium). The cells were preincubated with various concentrations of genistein (G) or vehicle (C) for 30 minutes at 37°C and were then treated with or without 2 U/ml thrombin (T) for 5 minutes. [Ca2+] was measured in fura-2 loaded BAECs at an emission wavelength of 505 nm with alternating excitation wavelengths of 340 and 380 nm. Data are expressed as mean ± SE from three independent experiments, each assayed in duplicate. *P < .05 vs vehicle-treated control.
Effect of genistein on thrombin-induced endothelial permeability is not mediated through nitric oxide (NO)
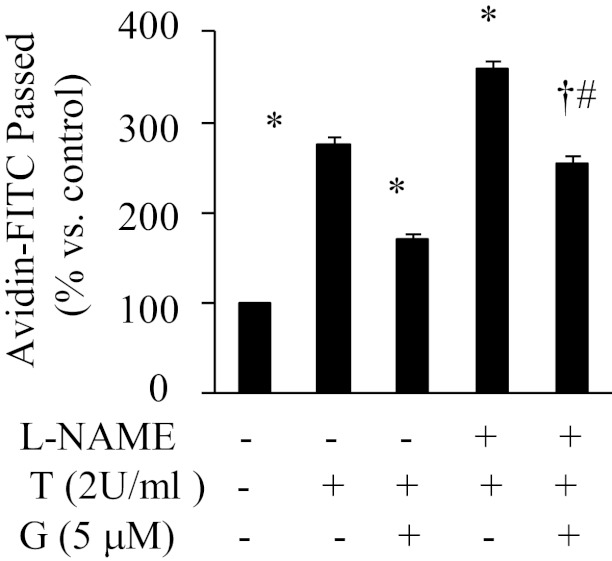
Previous studies have shown that NO can attenuate thrombin-induced endothelial permeability through cGMP-mediated inhibition of phosphodiesterase III or intracellular Ca2+ accumulation (35). We assessed whether genistein activity on endothelial permeability is mediated via NO. Inhibition of NO production by L-NAME enhanced the thrombin-induced increase in endothelial permeability (Figure 3). In the presence of L-NAME, the protective action of genistein against thrombin-induced endothelial permeability was attenuated by about 20% (Figure 3), suggesting the existence of an alternative mechanism, which may more importantly mediate genistein activity that counteracts the barrier disruptive effects of thrombin.
Figure 3.
Inhibition of NO did not block the effect of genistein on thrombin-induced endothelial permeability. BAEC monolayers were preincubated with or without 0.3 mM Nϖ-nitro-L-arginine methyl ester (L-NAME) for 30 minutes, then with the presence of 5 μM genistein (G) for 30 minutes, followed by addition of 2 U/ml thrombin (T) for 1 hour. Avidin-FITC passage was measured, and data are expressed as mean ± SE of three different experiments, each performed in duplicate. *P < .05 vs thrombin alone-treated cells; †P < .05 vs T+G-treated cells; #P < .05 vs L-NAME + T-treated cells.
Effect of genistein on thrombin-induced endothelial permeability is independent of ERs or PTK
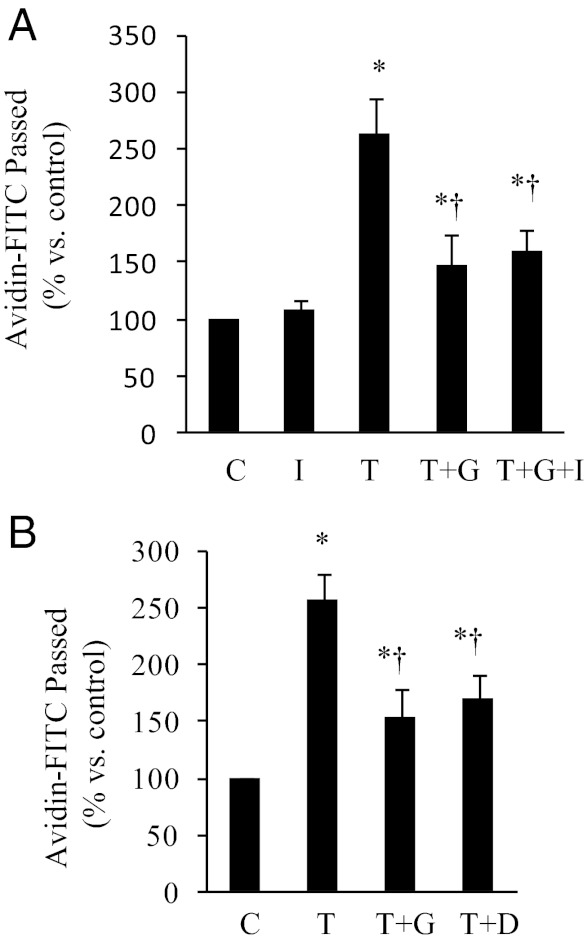
Because genistein has weak estrogenic effects in some tissues by binding to ERα and ERβ (6), we examined whether this genistein effect is mediated via ERs. The ER antagonist ICI 182,780, which successfully inhibited the effect of 17β-estradiol in ECs in our previous study (29), did not inhibit the protective effect of genistein on thrombin-induced endothelial permeability (Figure 4A), suggesting that genistein activity is not mediated through ERs. Genistein is an inhibitor of PTK (36). However, our previous studies have shown that genistein at low concentrations (10 nM to 10 μM) had no effect on the basal or endothelial growth factor–induced PTK activity (29). Genistein only inhibits PTK at 100-μM concentration (29, 37). We further compared the effect of genistein with that of daidzein, an analog of genistein that is inactive for PTK inhibition, on thrombin-induced endothelial permeability. As shown in Figure 4B, daidzein also ameliorated thrombin-induced endothelial barrier dysfunction, although to a slightly lesser degree than did genistein. These results suggest that genistein action against thrombin-induced endothelial barrier dysfunction is not mediated through inhibition of PTK.
Figure 4.
The effect of genistein on thrombin-induced endothelial permeability is independent of ERs or PTK. A, BAEC monolayers were preincubated with or without (C) ICI 182,780 (I) for 30 minutes, then with the presence of 5 μM genistein (G) for 30 minutes, followed by addition of 2 U/ml thrombin (T) for 1 hour. B, BAEC monolayers were pretreated with 5 μM genistein (G) or daidzein (D) for 30 minutes prior to addition of 2 U/ml thrombin (T) for 1 hour. Avidin-FITC passage was measured, and data are expressed as mean ± SE of three different experiments, each performed in duplicate. *P < .05 vs control; †P < .05 vs thrombin-alone-treated cells.
Genistein suppression of thrombin-induced EC permeability is mediated via RhoA in ECs
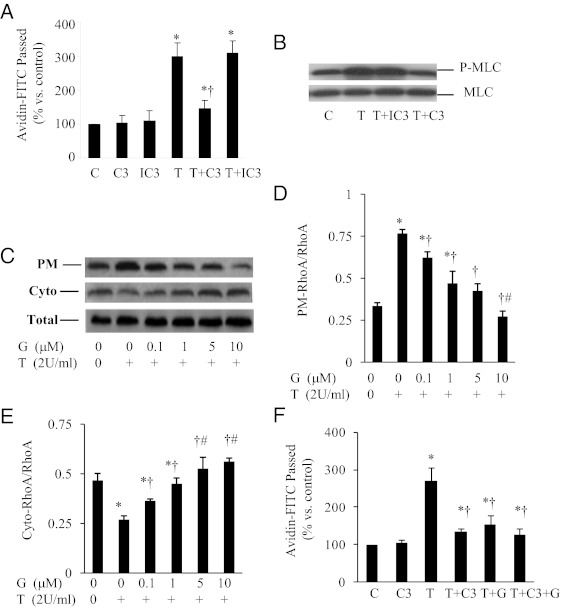
To analyze whether RhoA is involved in EC contraction, we measured thrombin-stimulated increase in vascular permeability in the absence or presence of the specific RhoA inhibitor C3-transferase (C3). Preincubation of the cells with C3 inhibited thrombin-induced endothelial permeability, whereas heat-inactivated C3 had no such an effect (Figure 5A). Consistently, active C3 but not the heat-inactivated C3 ablated thrombin-induced MLC phosphorylation (Figure 5B), suggesting that RhoA plays an important role in mediating thrombin-induced endothelial barrier dysfunction. The activity of RhoA is regulated by GDP/GTP cycling. Once activated, RhoA releases bound GDP in exchange for GTP and translocates to plasma membranes from cytosolic fractions (31, 38, 39). To further confirm the role of RhoA in mediating the inflammatory action of thrombin, we measured RhoA membrane translocation in the presence or absence of genistein. Incubation of ECs with thrombin greatly induced RhoA membrane translocation, which was diminished by genistein treatment. Dose-response studies showed that genistein as low as 0.1 μM attenuated RhoA activation, with a maximal effect at 10 μM genistein, which was also significantly more potent than ≤5 μM genistein (Figure 5, C-E). Consistently, the addition of genistein did not produce further inhibitory effect of C3 on thrombin-induced endothelial permeability (Figure 5F). Together, these results indicate that genistein may protect vascular barrier function through inhibition of RhoA signaling-mediated MLC phosphorylation.
Figure 5.
The effect of genistein on thrombin-induced endothelial permeability is mediated by inhibition of RhoA[b]. A, BAEC monolayers were incubated for 24 h with 5 μg/ml C3 transferase (C3), heat-inactivated C3 (IC3), or vehicle (C), followed by addition of 2 U/ml thrombin (T) for 1 hour to stimulate and measure avidin-FITC passage. B, BAECs were preincubated with 5 μg/ml C3 transferase (C3), inactivated C3 (IC3), or vehicle (C) for 24 hours. Cells were then stimulated with thrombin (T; 2 U/ml) for 5 minutes. Phosphorylation of MLC (P-MLC) and total MLC were detected by Western blot. Representative images from three independent experiments with similar results are shown. C–E, BAECs were preincubated with 5 μg/ml C3 transferase (C3), inactivated C3 (IC3), or vehicle (C) for 24 hours. The cells were then incubated with genistein (G, 0.1–10 μM) for 30 minutes prior to addition of thrombin (T; 2 U/ml) for 5 minutes. The plasma membranes (PM) and cytoplasm (Cyto) of the cells were isolated, and RhoA in the PM, cytosol, and whole cell extracts (Total) were detected by Western blots. F, BAEC monolayers were incubated with 5 μg/ml C3 transferase (C3) or vehicle (C) for 24 hours, followed by the addition of 5 μM genistein (G) for 30 minutes before stimulation with 2 U/ml thrombin (T) for 1 hour. Avidin-FITC passage was measured. All data are expressed as mean ± SE of four independent experiments, each performed in duplicate.*P < .05 vs control; †P < .05 vs thrombin-alone-treated cells; #P < .05 vs ≤5 μM genistein-treated cells (D) or ≤ 1 μM genistein-treated cells (E).
The inhibitory effect of genistein on RhoA and endothelial barrier dysfunction are mediated by PKA
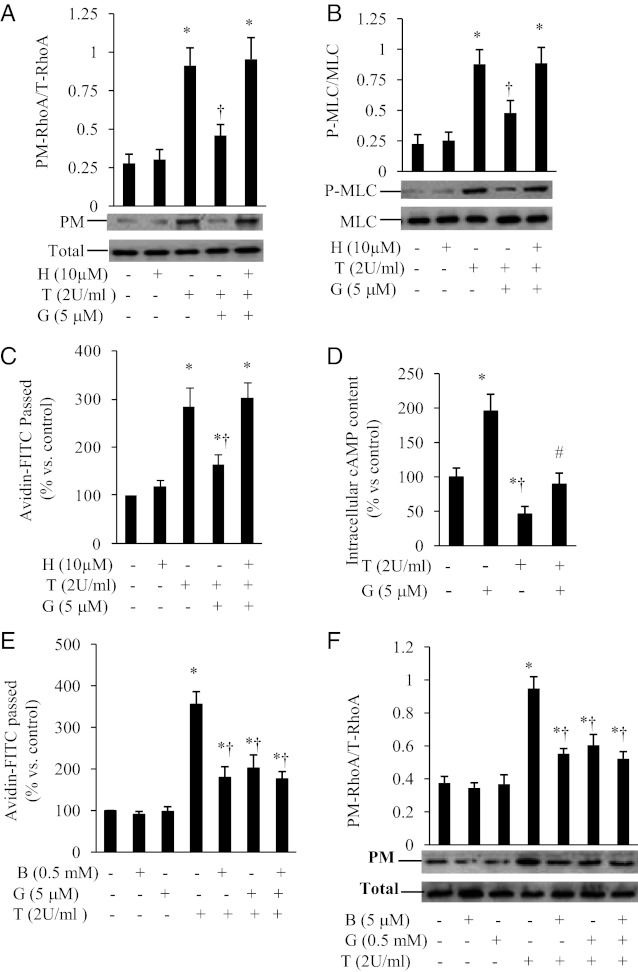
The cAMP/PKA signaling pathway was shown to decrease isometric tension development, intercellular gap formation, and vascular permeability in multiple experimental preparations (27, 40–43). Our recent study showed that genistein activates the cAMP signaling system in ECs (28). We investigated whether the cAMP/PKA signaling pathway is involved in the protective action of genistein against thrombin-induced-RhoA membrane translocation, MLC phosphorylation, and EC permeability. Inhibition of PKA by H89, a selective PKA inhibitor, abolished the inhibitory effects of genistein on thrombin-induced RhoA membrane translocation (Figure 6A) and MLC phosphorylation (Figure 6B) in ECs. The addition of PKA inhibitor significantly attenuated the protective effect of genistein against thrombin-induced EC permeability (Figure 6C). Furthermore, genistein stimulated intracellular cAMP accumulation in ECs (Figure 6D), which is consistent with our previous finding (28). Interestingly, thrombin greatly reduced intracellular cAMP levels in ECs (Figure 6D). However, coincubation with genistein largely prevented this detrimental effect of thrombin (Figure 6D). To confirm the role of cAMP in regulating endothelial barrier function and RhoA activity, we further examined the effect of 8-bromo-cAMP, a cell-permeable cAMP analog that is widely used in studies related to cAMP-mediated cellular events (44), on thrombin-induced endothelial permeability and RhoA activation. 8-Bromo-cAMP significantly reduced the thrombin-induced increase in avidin-FITC passage (Figure 6E) and diminished the activity of RhoA membrane translocation in ECs (Figure 6F), the effects that slightly more potent than those of genistein, suggesting that cAMP signaling plays an important role in maintaining endothelial barrier function and regulating RhoA activity in ECs. These results suggest that genistein inhibition of RhoA and endothelial barrier dysfunction is largely mediated via the cAMP/PKA pathway.
Figure 6.
Genistein inhibition of RhoA and endothelial barrier dysfunction is mediated via PKA. BAECs were preincubated for 30 minutes with 10 μM H89 (H) or vehicle, followed by addition of 5 μM genistein (G) for 30 minutes prior to stimulation with 2 U/ml thrombin (T) for 5 minutes. Cells were then used for measuring RhoA in the plasma membranes (PM) and whole cell extracts (panel A) or for detecting the phosphorylated MLC (P-MLC) and total MLC (panel B). Representative images and quantitative data from four independent experiments are shown. Panel C, BAEC monolayers were preincubated with 10 μM H89 (H) or vehicle for 30 minutes, followed by the addition of 5 μM genistein (G) for 30 minutes prior to stimulation with 2 U/ml thrombin (T) for 1 hour. Avidin-FITC passage was measured. Panel D, BAECs were preincubated with 5 μM genistein (G) or vehicle for 30 minutes followed by the addition of 2 U/ml thrombin (T) for 5 minutes. Intracellular cAMP was measured by EIA. Panel E, BAEC monolayers were preincubated with 0.5 mM 8-bromo-cAMP (B) or 5 μM genistein (G) for 30 minutes. The thrombin-stimulated monolayer permeability was measured. Panel F, BAECs were preincubated with 0.5 mM 8-bromo-cAMP (B) or 5 μM genistein (G) for 30 minutes prior to the addition of thrombin (T; 2 U/ml) for 5 minutes. RhoA in the plasma membranes (PM) and whole cell extracts (Total) were detected by Western blots. Data are expressed as mean ± SE of 3-4 independent experiments, each performed in duplicate. *P < .05 vs control; †P < .05 vs thrombin-alone-treated cells.
Discussion
Vascular inflammation and endothelial barrier dysfunction-induced vascular permeability play a fundamental role in the initiation and progression of atherosclerosis. Thrombin produced by the injured endothelium induces endothelial contraction and simultaneous cytoskeletal rearrangements, which promote intercellular gap formation that leads to increases in vascular permeability (3, 34). In the present study, we showed that genistein suppresses thrombin-induced increase in endothelial monolayer permeability via inhibition of MLC phosphorylation and RhoA membrane translocation. This action of genistein is independent of [Ca2+], PTK, and ERs but dependent on PKA. Unlike many previously published in vitro studies in which high doses of genistein were used with the results therefore reflecting a pharmacological rather than physiological actions of this phytoestrogen, the effective dose of genistein observed in the present study is likely physiologically relevant. Human studies reported that serum genistein levels can reach 4.6 and 4.1 μM, following consumption of three meals per day containing soy milk and a single soy meal, respectively (21, 22). The serum concentrations of genistein were reported to be 0.16–0.89 μM (45) in Japanese men consuming soy products. In the present study, genistein at a very low concentration (0.1 μM) ameliorated thrombin-induced increase in endothelial monolayer permeability. Therefore, the findings in the present study demonstrate for the first time that targeting PKA-mediated suppression of RhoA activity by genistein may be an important mechanism for its previously reported protective action against inflammation-related vascular dysfunction in vivo such as atherosclerosis (9–15, 17–20).
Endothelial permeability is associated with a dramatic change in the structural integrity of the endothelial monolayer as well as actomyosin-based cell contractility regulated by the phosphorylation state of MLC (5). Indeed, phosphorylation of regulatory MLC is a key mechanism of EC contraction and thrombin-induced barrier dysfunction (5). In the present study, genistein suppressed the thrombin-induced phosphorylation of MLC in ECs, providing further evidence showing the protective action of genistein against endothelial barrier dysfunction.
As genistein reportedly has both estrogenic and antiestrogenic actions in some tissues, which may largely be due to its binding activity to the ERs, which are present in ECs, we considered the possibility that the effect of genistein on endothelial permeability is mediated through the ER-mediated mechanism. However, blockage of ERs with ICI 182,780, a pure antagonist for both ERα− and ERβ by inhibiting receptor dimerization and inducing their degradation (33–35), did not inhibit the effect of genistein on vascular permeability. It is unlikely that the inability of this agent to block the effect of genistein on thrombin-induced barrier dysfunction is due to a lack of efficacy, because we recently reported that, at the same concentration used, ICI completely abolished the 17β-estradiol-elicited various biological events in ECs (23, 46). Indeed, a number of previous studies have shown that activation of these classical ERs by 17β-estradiol can disrupt cytoskeletal architecture and lead to increased EC monolayer permeability and migration (47–49), an effect that may involve the activation of RhoA signaling (48, 50), which was also inhibited by genistein in the present study. These results suggest that the effect of genistein on EC monolayer permeability provoked by thrombin is independent of the ER-mediated signaling mechanisms.
Genistein at high concentrations can inhibit PTK (36), and studies showed that activation of PTK may facilitate cell contraction and barrier dysfunction (51, 52). However, we do not believe that the activity of genistein on thrombin-induced endothelial barrier dysfunction is mediated through inhibition of PTK. First, genistein concentrations (0.1 to 10 μM) used in present studies that had protective effect of genistein against thrombin-induced endothelial barrier dysfunction had no effect on basal or agonist-stimulated PTK activity in ECs as we previously examined (29). Genistein inhibited PTK only in ECs at 100-μM concentration (29), which is as high as 10- to 1000-fold of the effective doses of genistein used in the present study. Second, daidzein, an analog of genistein that does not inhibit PTK, also inhibit the effect of genistein on vascular permeability.
Endothelial-derived NO regulates a variety of vascular functions including anti-inflammation (53, 54). It is well established that the thrombin-induced increase in intracellular [Ca2+] leads to the activation of Ca2+/calmodulin-dependent MLC kinase (MLCK), which subsequently phosphorylates Thr-18 and Ser-19 of MLC, a key mechanism of thrombin-induced EC barrier dysfunction. Genistein did not alter either resting [Ca2+] or thrombin-induced increase in Ca2+ mobilization. These data suggest that the activity of genistein on thrombin-induced endothelial barrier dysfunction is not significantly mediated through modulation of intracellular [Ca2+]. Previous studies have shown that NO can reduce thrombin-induced endothelial permeability (35). We recently demonstrated that genistein stimulates rapid NO production via nongenomic activation of eNOS in ECs (29). In the present study, genistein action on thrombin-induced EC permeability was only moderately attenuated by eNOS inhibitor L-NAME, although this dose of L-NAME completely blocked genistein-induced eNOS activity in ECs in our previous study (29). However, inhibition of NO production by L-NAME also enhanced the thrombin-induced increase in permeability. Interestingly, it was reported that thrombin can also increase rapid NO production by stimulating eNOS activity in ECs (55, 56). These findings suggest that the observed attenuating effect of L-NAME on barrier protective action of genistein is at least partially attributable to the inhibition of thrombin-induced NO production. The data from the present study also provide evidence that the elevated endogenous NO production by thrombin or genistein is insufficient to reverse thrombin-stimulated endothelial barrier permeability, and that there should be an alternative mechanism that is believed to play a more important role in mediating the protective effect of genistein in ECs.
RhoA has been suggested to substantially mediate the thrombin-induced hyperpermeability of endothelium by various mechanisms (5, 39, 57–59). Once activated, RhoA activates its downstream target Rho kinase, which increases MLC phosphorylation by inactivation of myosin-specific phosphatase (60, 61). Rho kinase also directly increases MLC phosphorylation and cytoskeletal reorganization (62). Actin-myosin contraction and subsequent EC barrier dysfunction are primarily mediated through MLC phosphorylation (60, 61). In the present study, genistein ablated the activity of RhoA membrane translocation and MLC phosphorylation stimulated by thrombin, suggesting that genistein acts on the Rho/MLC signaling pathway to exert this cytoprotective action in ECs, a novel function of genistein that has not been previously recognized.
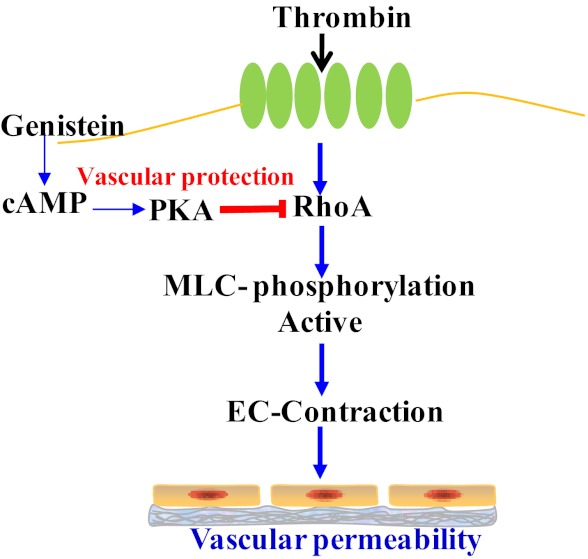
It has been consistently shown that activation of the cAMP/PKA signaling pathway protects against endothelial barrier dysfunction promoted by various inflammatory mediators, including thrombin (39, 41–43, 63, 64). Indeed, we showed in the present study that elevation of intracellular cAMP concentration profoundly inhibited endothelial permeability, further confirming a critical role for cAMP in counteracting endothelial barrier dysfunction. Previous studies found that thrombin-induced intercellular gap formation is always paralleled by a rapid decrease in cAMP production in ECs, which is necessary for disrupting barrier function by this inflammatory mediator (65, 66). Thrombin may decrease cAMP levels by Ca2+ entry-mediated inhibition of type 6 adenylate cyclase (AC) (65). In the present study, thrombin greatly reduced intracellular cAMP levels in ECs, which was negated by treatment with genistein. This genistein effect is not due to the suppression of thrombin-stimulated intracellular [Ca2+] rise but rather a direct stimulation of cAMP production, because genistein alone elevated intracellular cAMP levels and our recent study shows that genistein can directly activate AC, leading to cAMP/PKA signaling in primary ECs (29). Accordingly, inhibition of PKA abolished the inhibitory effects of genistein on thrombin-induced EC permeability, MLC phosphorylation, and RhoA membrane translocation, and these PKA-mediated actions of genistein are mimicked by application of a cAMP analog. Taken all together, these results indicate that genistein ameliorates thrombin-induced endothelial barrier dysfunction by activation of the AC/cAMP/PKA cascade, leading to inhibition of RhoA signaling in ECs (Figure 7). However, how AC is activated by genistein is unclear. The GTP-binding protein Gαs is often required for cell membrane receptor-mediated activation of AC (67, 68). Interestingly, it was shown that genistein can bind to an orphan plasma membrane receptor GPR30 in cancer cells (69). While the physiological role of GPR30 is still unclear, it was shown that GPR30 is coupled to Gαs to stimulate AC in cancer cells (67). Because GPR30 is also expressed in vascular ECs (70), there is a possibility that genistein activates AC via GPR30-mediated mechanisms. This aspect is currently under investigation in our laboratory.
Figure 7.
Schematic illustration of proposed mechanisms by which genistein protects against thrombin-induced endothelial barrier dysfunction. EC, endothelial cell; MLC, myosin light chain, PKA, protein kinase A.
In summary, we showed that genistein inhibits thrombin-induced increase in endothelial monolayer permeability. This protective activity of genistein in EC-formed barrier is novel because it is not mediated through its known actions or inhibition of thrombin-induced increase in intracellular [Ca2+], but it depends on PKA-mediated suppression of RhoA activity. Given that EC inflammation and dysfunction play a critical role in the pathogenesis of various vascular diseases and that genistein exerts this protective effect on ECs at readily achievable concentrations by dietary intake of this compound, our findings may provide a novel cellular mechanism that may underlie some of the reported beneficial effects of genistein in modulation of vascular function.
Acknowledgments
This work was supported by grants from the American Heart Association Mid-Atlantic Affiliate Beginning Grant-in-Aid (D. Liu), National Center for Complementary and Alternative Medicine in the National Institutes of Health (R21AT004694, 3R21AT004694-02S1, and 1R01AT007077- 01 to D. Liu and 1R15AT005372 and 7R15AT005372 to Z. Jia). Current address for P.V.A.B.: Division of Nutrition, University of Utah, Salt Lake City, UT 84112.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AC
- adenylate cyclase
- avidin-FITC
- avidin conjugated fluorescein
- BAECs
- bovine endothelial cells
- C3
- C3-transferase
- eNOS
- endothelial NO synthase
- ER
- estrogen receptor
- FBS
- fetal bovine serum
- ICI
- ICI 182,780
- L-NAME
- NG-nitro-L-arginine methyl ester
- MLC
- myosin light chain
- MLCK
- MLC kinase
- NO
- nitric oxide
- PKA
- protein kinase A
- PTK
- protein tyrosine kinase.
References
- 1. Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171 [DOI] [PubMed] [Google Scholar]
- 2. Hennig B, Chung BH, Watkins BA, Alvarado A. Disruption of endothelial barrier function by lipolytic remnants of triglyceride-rich lipoproteins. Atherosclerosis. 1992;95:235-247 [DOI] [PubMed] [Google Scholar]
- 3. Bogatcheva NV, Garcia JG, Verin AD. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochemistry (Moscow). 2002;67:75-84 [DOI] [PubMed] [Google Scholar]
- 4. Sandoval R, Malik AB, Naqvi T, Mehta D, Tiruppathi C. Requirement for Ca2+ signaling in the mechanism of thrombin-induced increase in endothelial permeability. Am J Physiol. 2001;280:L239–L247 [DOI] [PubMed] [Google Scholar]
- 5. Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273:21867-21874 [DOI] [PubMed] [Google Scholar]
- 6. Kim H, Peterson TG, Barnes S. Mechanisms of action of the soy isoflavone genistein: emerging role for its effects via transforming growth factor beta signaling pathways. Am J Clin Nutr. 1998;68:1418S–1425S [DOI] [PubMed] [Google Scholar]
- 7. Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863-870 [DOI] [PubMed] [Google Scholar]
- 8. Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592-5595 [PubMed] [Google Scholar]
- 9. Anthony MS, Clarkson TB, Williams JK. Effects of soy isoflavones on atherosclerosis: potential mechanisms. Am J Clin Nutr. 1998;68:1390S–1393S [DOI] [PubMed] [Google Scholar]
- 10. van der Schouw YT, de Kleijn MJ, Peeters PH, Grobbee DE. Phyto-oestrogens and cardiovascular disease risk. Nutr Metab Cardiovasc Dis. 2000;10:154-167 [PubMed] [Google Scholar]
- 11. Wangen KE, Duncan AM, Xu X, Kurzer MS. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am J Clin Nutr. 2001;73:225-231 [DOI] [PubMed] [Google Scholar]
- 12. Walker HA, Dean TS, Sanders TA, Jackson G, Ritter JM, Chowienczyk PJ. The phytoestrogen genistein produces acute nitric oxide–dependent dilation of human forearm vasculature with similar potency to 17ss-estradiol. Circulation. 2001;103:258-262 [DOI] [PubMed] [Google Scholar]
- 13. Squadrito F, Altavilla D, Morabito N, et al. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium-dependent vasodilation in postmenopausal women. Atherosclerosis. 2002;163:339-347 [DOI] [PubMed] [Google Scholar]
- 14. Squadrito F, Altavilla D, Crisafulli A, et al. Effect of genistein on endothelial function in postmenopausal women: a randomized, double-blind, controlled study. Am J Med. 2003;114:470-476 [DOI] [PubMed] [Google Scholar]
- 15. Nestel PJ, Yamashita T, Sasahara T, et al. Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arterioscler Thromb Vasc Biol. 1997;17:3392-3398 [DOI] [PubMed] [Google Scholar]
- 16. Hodgson JM, Puddey IB, Beilin LJ, Mori TA, Croft KD. Supplementation with isoflavonoid phytoestrogens does not alter serum lipid concentrations: a randomized controlled trial in humans. J Nutr. 1998;128:728-732 [DOI] [PubMed] [Google Scholar]
- 17. Valsecchi AE, Franchi S, Panerai AE, Rossi A, Sacerdote P, Colleoni M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur J Pharmacol. 650:694-702 [DOI] [PubMed] [Google Scholar]
- 18. Walker HA, Dean TS, Sanders TA, Jackson G, Ritter JM, Chowienczyk PJ. The phytoestrogen genistein produces acute nitric oxide–dependent dilation of human forearm vasculature with similar potency to 17beta-estradiol. Circulation. 2001;103:258-262 [DOI] [PubMed] [Google Scholar]
- 19. Fotsis T, Pepper M, Adlercreutz H, Hase T, Montesano R, Schweigerer L. Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis. J Nutr. 1995;125:790S–797S [DOI] [PubMed] [Google Scholar]
- 20. Dubey RK, Gillespie DG, Imthurn B, Rosselli M, Jackson EK, Keller PJ. Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension. 1999;33:177-182 [DOI] [PubMed] [Google Scholar]
- 21. Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S. Bioavailability of soybean isoflavones depends upon gut microflora in women. Journal of Nutrition. 1995;125:2307-2315 [DOI] [PubMed] [Google Scholar]
- 22. King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr. 1998;67:867-872 [DOI] [PubMed] [Google Scholar]
- 23. Babu PV, Si H, Fu Z, Zhen W, Liu D. Genistein prevents hyperglycemia-induced monocyte adhesion to human aortic endothelial cells through preservation of the cAMP signaling pathway and ameliorates vascular inflammation in obese diabetic mice. J Nutr. 2012;142:724-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D'Angelo G, Lee H, Weiner RI. cAMP-dependent protein kinase inhibits the mitogenic action of vascular endothelial growth factor and fibroblast growth factor in capillary endothelial cells by blocking Raf activation. J Cell Biochem. 1997;67:353-366 [PubMed] [Google Scholar]
- 25. Morandini R, Ghanem G, Portier-Lemarie A, Robaye B, Renaud A, Boeynaems JM. Action of cAMP on expression and release of adhesion molecules in human endothelial cells. Am J Physiol. 1996;270:H807–H816 [DOI] [PubMed] [Google Scholar]
- 26. Moy AB, Bodmer JE, Blackwell K, Shasby S, Shasby DM. cAMP protects endothelial barrier function independent of inhibiting MLC20-dependent tension development. Am J Physiol. 1998;274:L1024–L1029 [DOI] [PubMed] [Google Scholar]
- 27. Lum H, Jaffe HA, Schulz IT, Masood A, RayChaudhury A, Green RD. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am J Physiol. 1999;277:C580–C588 [DOI] [PubMed] [Google Scholar]
- 28. Liu D, Jiang H, Grange RW. Genistein activates the 3′,5′-cyclic adenosine monophosphate signaling pathway in vascular endothelial cells and protects endothelial barrier function. Endocrinology. 2005;146:1312-1320 [DOI] [PubMed] [Google Scholar]
- 29. Liu D, Homan LL, Dillon JS. Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5′-monophosphate-dependent mechanism. Endocrinology. 2004;145:5532-5539 [DOI] [PubMed] [Google Scholar]
- 30. Langeler EG, van Hinsbergh VW. Norepinephrine and iloprost improve barrier function of human endothelial cell monolayers: role of cAMP. Am J Physiol. 1991;260:C1052–C1059 [DOI] [PubMed] [Google Scholar]
- 31. van Nieuw Amerongen GP, Vermeer MA, Negre-Aminou P, Lankelma J, Emeis JJ, van Hinsbergh VW. Simvastatin improves disturbed endothelial barrier function. Circulation. 2000;102:2803-2809 [DOI] [PubMed] [Google Scholar]
- 32. Nakano T, Watanabe H, Ozeki M, et al. Endoplasmic reticulum Ca2+ depletion induces endothelial cell apoptosis independently of caspase-12. Cardiovasc Res. 2006;69:908-915 [DOI] [PubMed] [Google Scholar]
- 33. Fu Z, Liu D. Long-term exposure to genistein improves insulin secretory function of pancreatic beta-cells. Eur J Pharmacol. 2009;616:321-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273:21867-21874 [DOI] [PubMed] [Google Scholar]
- 35. Draijer R, Atsma DE, van der Laarse A, van Hinsbergh VW. cGMP and nitric oxide modulate thrombin-induced endothelial permeability. Regulation via different pathways in human aortic and umbilical vein endothelial cells. Circ Res. 1995;76:199-208 [DOI] [PubMed] [Google Scholar]
- 36. Nakashima S, Koike T, Nozawa Y. Genistein, a protein tyrosine kinase inhibitor, inhibits thromboxane A2-mediated human platelet responses. Mol Pharmacol. 1991;39:475-480 [PubMed] [Google Scholar]
- 37. Peterson G, Barnes S. Genistein inhibits both estrogen and growth factor-stimulated proliferation of human breast cancer cells. Cell Growth Differ. 1996;7:1345-1351 [PubMed] [Google Scholar]
- 38. Seasholtz TM, Zhang T, Morissette MR, Howes AL, Yang AH, Brown JH. Increased expression and activity of RhoA are associated with increased DNA synthesis and reduced p27(Kip1) expression in the vasculature of hypertensive rats. Circ Res. 2001;89:488-495 [DOI] [PubMed] [Google Scholar]
- 39. Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol. 2003;284:L972–L980 [DOI] [PubMed] [Google Scholar]
- 40. Stevens T, Creighton J, Thompson WJ. Control of cAMP in lung endothelial cell phenotypes. Implications for control of barrier function. Am J Physiol. 1999;277:L119–L126 [DOI] [PubMed] [Google Scholar]
- 41. Westendorp RG, Draijer R, Meinders AE, van Hinsbergh VW. Cyclic-GMP-mediated decrease in permeability of human umbilical and pulmonary artery endothelial cell monolayers. J Vasc Res. 1994;31:42-51 [DOI] [PubMed] [Google Scholar]
- 42. Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol. 1995;163:510-522 [DOI] [PubMed] [Google Scholar]
- 43. Patterson CE, Lum H, Schaphorst KL, Verin AD, Garcia JG. Regulation of endothelial barrier function by the cAMP-dependent protein kinase. Endothelium. 2000;7:287-308 [DOI] [PubMed] [Google Scholar]
- 44. Han X, Kobzik L, Balligand JL, Kelly RA, Smith TW. Nitric oxide synthase (NOS3)–mediated cholinergic modulation of Ca2+ current in adult rabbit atrioventricular nodal cells. Circ Res. 1996;78:998-1008 [DOI] [PubMed] [Google Scholar]
- 45. Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209-1210 [DOI] [PubMed] [Google Scholar]
- 46. Liu D, Si H, Reynolds KA, Zhen W, Jia Z, Dillon JS. Dehydroepiandrosterone protects vascular endothelial cells against apoptosis through a Galphai protein-dependent activation of phosphatidylinositol 3-kinase/Akt and regulation of antiapoptotic Bcl-2 expression. Endocrinology. 2007;148:3068-3076 [DOI] [PubMed] [Google Scholar]
- 47. Groten T, Pierce AA, Huen AC, Schnaper HW. 17 beta-estradiol transiently disrupts adherens junctions in endothelial cells. Faseb J. 2005;19:1368-1370 [DOI] [PubMed] [Google Scholar]
- 48. Simoncini T, Scorticati C, Mannella P, et al. Estrogen receptor alpha interacts with Galpha13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol. 2006;20:1756-1771 [DOI] [PubMed] [Google Scholar]
- 49. Aberdeen GW, Wiegand SJ, Bonagura TW, Jr., Pepe GJ, Albrecht ED. Vascular endothelial growth factor mediates the estrogen-induced breakdown of tight junctions between and increase in proliferation of microvessel endothelial cells in the baboon endometrium. Endocrinology. 2008;149:6076-6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oviedo PJ, Sobrino A, Laguna-Fernandez A, et al. Estradiol induces endothelial cell migration and proliferation through estrogen receptor-enhanced RhoA/ROCK pathway. Mol Cell Endocrinol. 2011;335:96-103 [DOI] [PubMed] [Google Scholar]
- 51. Shi S, Verin AD, Schaphorst KL, et al. Role of tyrosine phosphorylation in thrombin-induced endothelial cell contraction and barrier function. Endothelium. 1998;6:153-171 [DOI] [PubMed] [Google Scholar]
- 52. Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vasc Pharmacol. 2002;39:213-223 [DOI] [PubMed] [Google Scholar]
- 53. Yu J, Rudic RD, Sessa WC. Nitric oxide-releasing aspirin decreases vascular injury by reducing inflammation and promoting apoptosis. Lab Invest. 2002;82:825-832 [DOI] [PubMed] [Google Scholar]
- 54. Kataoka C, Egashira K, Inoue S, et al. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39:245-250 [DOI] [PubMed] [Google Scholar]
- 55. Thors B, Halldorsson H, Thorgeirsson G. eNOS activation mediated by AMPK after stimulation of endothelial cells with histamine or thrombin is dependent on LKB1. Biochim Biophys Acta. 2011;1813:322-331 [DOI] [PubMed] [Google Scholar]
- 56. Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26:5933-5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Nieuw Amerongen GP, Draijer R, Vermeer MA, van Hinsbergh VW. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circ Res. 1998;83:1115-1123 [DOI] [PubMed] [Google Scholar]
- 58. Hirase T, Kawashima S, Wong EY, et al. Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J Biol Chem. 2001;276:10423-10431 [DOI] [PubMed] [Google Scholar]
- 59. Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vasc Pharmacol. 2002;39:187-199 [DOI] [PubMed] [Google Scholar]
- 60. Garcia JG, Verin AD, Schaphorst K, et al. Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60(src). Am J Physiol. 1999;276:L989–L998 [DOI] [PubMed] [Google Scholar]
- 61. Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett. 2002;527:101-104 [DOI] [PubMed] [Google Scholar]
- 62. Birukov KG, Jacobson JR, Flores AA, et al. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch [comment]. Am J Physiol. 2003;285:L785–L797 [DOI] [PubMed] [Google Scholar]
- 63. Ogawa S, Koga S, Kuwabara K, et al. Hypoxia-induced increased permeability of endothelial monolayers occurs through lowering of cellular cAMP levels. Am J Physiol. 1992;262:C546–C554 [DOI] [PubMed] [Google Scholar]
- 64. Koga S, Morris S, Ogawa S, et al. TNF modulates endothelial properties by decreasing cAMP. Am J Physiol. 1995;268:C1104–C1113 [DOI] [PubMed] [Google Scholar]
- 65. Cioffi DL, Moore TM, Schaack J, Creighton JR, Cooper DM, Stevens T. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J Cell Biol. 2002;157:1267-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Baumer Y, Spindler V, Werthmann RC, Bunemann M, Waschke J. Role of Rac 1 and cAMP in endothelial barrier stabilization and thrombin-induced barrier breakdown. J Cell Physiol. 2009;220:716-726 [DOI] [PubMed] [Google Scholar]
- 67. Filardo EJ, Quinn JA, Frackelton AR, Jr., Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70-84 [DOI] [PubMed] [Google Scholar]
- 68. Ghahremani MH, Cheng P, Lembo PM, Albert PR. Distinct roles for Galphai2, Galphai3, and Gbeta gamma in modulation offorskolin- or Gs-mediated cAMP accumulation and calcium mobilization by dopamine D2S receptors. J Biol Chem. 1999;274:9238-9245 [DOI] [PubMed] [Google Scholar]
- 69. Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175-179 [DOI] [PubMed] [Google Scholar]
- 70. Broughton BR, Miller AA, Sobey CG. Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol. 298:H1055–H1061 [DOI] [PubMed] [Google Scholar]