Abstract
Providing high-quality, uncontaminated drinking water is an essential component of rodent husbandry. Acidification of drinking water is a common technique to control microbial growth but is not a benign treatment. In addition to its potential biologic effects, acidified water might interact with the water-delivery system, leading to the leaching of heavy metals into the drinking water. The goal of the current study was to evaluate the effects of water acidification and autoclaving on water-bottle assemblies. The individual components of the system (stainless-steel sipper tubes, rubber stoppers, neoprene stoppers, and polysulfone water bottles) were acid-digested and analyzed for cadmium, chromium, copper, iron, lead, magnesium, manganese, selenium, and zinc to quantify the metal composition of each material. In addition the amounts of these metals that leached into tap and acidified water with and without autoclaving were quantified after 1 wk of contact time. On a weight basis, sipper tubes contained the largest quantities of all metals except magnesium and zinc, which were greatest in the neoprene stoppers. Except for cadmium and selenium, all metals had leached into the water after 1 wk, especially under the acidified condition. The quantities of copper, lead, and zinc that leached into the drinking water were the most noteworthy, because the resulting concentrations had the potential to confound animal experiments. On the basis of these findings, we suggest that water-quality monitoring programs include heavy metal analysis at the level of water delivery to animals.
Abbreviation: ICP, inductively coupled plasma
Drinking water quality is an important factor in experimental outcomes and in the daily care and maintenance of laboratory animals. Animal Welfare Regulations1 and the Guide for the Care and Use of Laboratory Animals26 both stipulate that research animals must be provided clean, potable water, but typically water is only monitored for microbiologic contaminants in the academic environment.21,31,34 Good Laboratory Practice regulations require that animal drinking water be tested for potential confounding contaminants but do not identify specific agents.20 The literature supports the use of acidified water as a means to control bacterial contamination of rodent drinking water, but this practice has several biologic effects, and evidence suggests that it can affect the water-delivery system.28,33,46,49
Water acidification has been reported to have multiple biologic effects.48 When male CD1 mice ingested water acidified to pH 2.0, they had decreased water consumption, decreased weight gain, and decreased numbers of bacterial species in the terminal ileum.22 Rats provided water acidified to pH 2.0 for 24 wk had extensive corrosion of both the enamel and dentin of the first 2 mandibular molars.27,32,50 Mice provided water acidified to pH 2.0 for 120 d had reduced reticuloendothelial clearance rates, spleen weights, and spleen:body weight ratios, indicating a potential for alteration of the immune response.24 Although these effects were attributed to the acidification of the water, they may have been potentiated by the effects of acidified water on the water-delivery system and the subsequent ingestion of leached metals.
In addition, acidifying drinking water can cause metals to leach from water-bottle stoppers. In one study, acidified–deionized water leached more metals from polymer stoppers than did nonacidified–deionized water; the authors suggested that other types of stoppers would be more appropriate for specific nutritional and toxicological studies.28 Although 4 different types of polymer stoppers (rubber, neoprene, vinyl, and silicone) were evaluated, the study had several limitations that were never further examined.28 For example, metal analysis was performed on only 2 of the stopper types (rubber and neoprene), and the authors evaluated only 2 samples of each.28 Furthermore, the metal analysis of the stoppers was limited to 5 metals and the water analysis to 7 metals, with lead being the most notable metal that was absent from the analysis. Moreover, the study28 did not assess the effects of autoclaving on the water-delivery system, nor did the authors perform metal analysis of sipper tubes or the water bottle.
As part of our own facility water-quality assurance program, we recently extended our analysis to include point-of-delivery (water bottle) assessment in addition to source assessment for both heavy-metal and microbiologic contaminants. As a result, the goals of the current study were to identify potential heavy-metal contaminants in the water-bottle assemblies and to evaluate the leaching of those metals into the drinking water by using inductively coupled plasma (ICP) spectrophotometry. Specifically, we evaluated neoprene and rubber water-bottle stoppers, stainless-steel sipper tubes, and polysulfone water bottles for contaminant heavy metals after acid digestion. We then evaluated the effects of water acidification and autoclave treatment on the leaching of heavy metals from the water-bottle assemblies into the drinking water after 1 wk of inversion on an empty rodent cage.
Materials and Methods
Acid digestion of materials.
The individual components of a water bottle were digested in 1 N trace-metal HCl (Sigma, St Louis, MO) for 1 wk. Five samples of each constituent were digested and tested independently to identify variability within specific components. New double-seal rubber and neoprene water bottle stoppers (Ancare, Bellmore, NY) each were cut into 1 mm3 pieces by using a razor blade to maximize surface area and then digested in a 50-mL polypropylene tube (BD Labware, Franklin Lakes, NJ) containing 10 mL HCl per gram of sample. We poured 10 mL HCl into each of 5 polysulfone screw-top water bottles (Ancare), which subsequently sealed and stored on their sides to maximize the contact area. Stainless-steel open straight drinking tubes (Ancare) were placed individually in 50-mL polypropylene tubes containing 10 mL HCl. To control for potential leaching from the tubes, we placed 10 mL HCl in each of 5 empty tubes and let them sit for 1 wk. At the time of sample analysis, we also analyzed 5 aliquots of fresh HCl, as an additional control for contaminants in the HCl.
After 1 wk, each sample was diluted with deionized water to decrease the acid concentration to less than 5 weight–percent. Samples were passed over syringe filters (diameter, 25 mm; pore size, 0.45 μm; Aerodisc, PALL Life Science, Ann Arbor, MI) and analyzed for cadmium, chromium, copper, iron, lead, magnesium, manganese, selenium, and zinc by using a spectrophotometer (Vista-MPX ICP, Varian, Walnut Creek, CA). Before analysis, standard curves for each metal were established over the range of 0.02 to 100 ppm.
Leaching into drinking water.
Water-bottle components equivalent to those we used in the acid-digestion study were assembled to make complete water bottles that included either a rubber or neoprene stopper. The water conditions included: HCl-acidified (pH 2.2) water; autoclaved, HCl-acidified (pH 2.2) water; tap water; and autoclaved tap water. Samples were autoclaved in fully assembled water bottles. Water bottles remained inverted on an empty rodent cage for 1 wk to simulate the intended use. We assessed 5 replicates of stopper and water condition combination to accommodate intersample variation. At the time of analysis, fresh samples of tap and acidified water (n = 5 each) were included to serve as baseline controls for the leaching experiments.
Water samples (6 oz each) were submitted for analysis (Siemens Analytical Laboratory, Rockford, IL). Heavy-metal analysis was performed by using an ICP spectrophotometer (Ultima 2, Horiba Jobin Yvon, Edison, NJ) that was calibrated with a multielement standard prior to each sample run. The water samples were tested for cadmium, chromium, copper, iron, lead, magnesium, manganese, selenium, and zinc. In addition, all water samples were pH-analyzed by using an Accumet XL25 (Fisher Scientific, Waltham, MA) that was calibrated daily at pH 4 and 7.
Statistical analysis.
All statistical analysis was performed by using MATLAB (MathWorks, Natick, MA). The results of the acid digestion were analyzed by ANOVA and a multiple-comparisons procedure using a Bonferroni correction to identify the significance associated with metal concentrations in the individual water-bottle components. The leaching data were evaluated by using 3-way ANOVA to test for the interaction effects of stopper type, water acidification, and autoclaving on metal concentrations in the water. For all statistical tests, significance was defined as a P value of less than 0.05.
Results
Acid digestion.
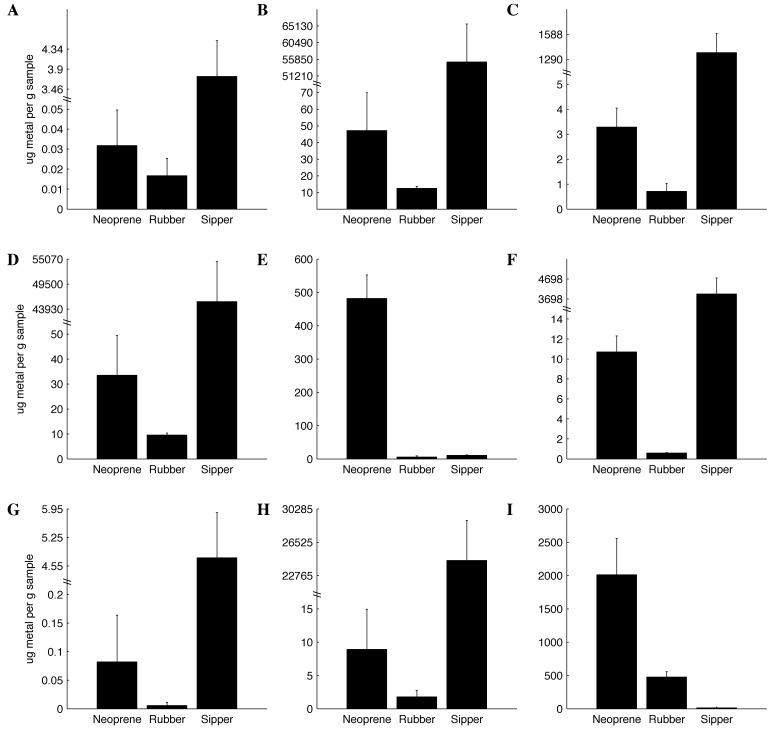
The quantities of heavy metals in the individual water-bottle components and controls are reported in Figure 1. New rubber and neoprene stoppers, old rubber stoppers, water bottles, and sipper tubes weighed 22.9 ± 0.1 g, 25.9 ± 0.3 g, 16.7 ± 0.4 g, 74.5 ± 3.4 g, and 8.0 ± 0.1 g each, respectively. The sipper tubes contained significantly (P < 0.05) more heavy metal (as micrograms metal per gram material) for all tested metals except magnesium and zinc (Figure 1). Compared with sipper tubes, neoprene stoppers contained more (P < 0.05) magnesium and zinc. Compared with neoprene stoppers, rubber stoppers contained less (P < 0.05) of all tested metals. The quantities of heavy metals in the water bottles and controls were at or below the limit of quantitation for all tested metals (data not shown).
Figure 1.
Heavy-metal composition (µg metal/g material; mean ± SEM; n = 5) of stoppers and sipper tubes as determined by ICP spectrometry. (A) Cadmium. (B) Chromium. (C) Copper. (D) Iron. (E) Magnesium. (F) Manganese. (G) Lead. (H) Selenium. (I) Zinc.
Leaching.
As determined by ICP spectroscopy, heavy metals leached into the water; the pH for each combination of stopper and water treatment (acid and autoclave) are reported in Table 1. There was no detectable leaching of either cadmium or selenium into the water from any water bottle combination. Acidification and stopper type had significant (P < 0.05) main effects on the leaching of copper, iron, zinc, and lead, as did the interaction between acidification and stopper type. Acidification was the only variable to have a significant (P < 0.05) effect on chromium. There were significant (P < 0.05) 3-way interactions between acidification, stopper type, and autoclaving on both manganese and magnesium, and each individual variable had significant (P < 0.05) main effects. Acidified water samples had a pH of 2.23 ± 0.03; tap water samples had a pH of 7.89 ± 0.02. The main effects of acidification, stopper type, and autoclaving; the interaction between acidification and autoclaving; and the interaction between acidification and stopper type all had significant (P < 0.05) effects on pH.
Table 1.
Heavy-metal levels in drinking water after 1 wk of contact time with water-bottle components
| Sample |
ppm (mean ± SE; n = 5) |
|||||||||||
| Stopper | Water | Autoclaved? | Cd | Cr | Cu | Fe | Pb | Mg | Mn | Se | Zn | pH |
| Rubber | Acid | No | <0.002 | 0.015 ± 0.01 | 0.022 ± 0.008 | 0.095 ± 0.035 | <0.02 | 12.10 ± 0.18 | 0.001 | <0.05 | 0.901 ± 0.056 | 2.15 ± 0.17 |
| Rubber | Acid | Yes | <0.002 | 0.009 ± 0.003 | 0.005a | 0.053 ± 0.013 | <0.02 | 13.26 ± 0.09 | <0.001 | <0.05 | 2.390 ± 0.225 | 2.29 ± 0.04 |
| Rubber | Tap | No | <0.002 | <0.002 | 0.030a | <0.002 | <0.02 | 11.76 ± 0.04 | <0.001 | <0.05 | 0.119 ± 0.063 | 7.01 ± 0.17 |
| Rubber | Tap | Yes | <0.002 | <0.002 | <0.002 | <0.002 | <0.02 | 12.88 ± 0.12 | <0.001 | <0.05 | 0.051 ± 0.012 | 8.12 ± 0.42 |
| Neoprene | Acid | No | <0.002 | 0.016 ± 0.011 | 3.250 ± 0.612 | 0.147 ± 0.050 | 0.196 ± 0.034 | 13.74 ± 0.19 | 0.003 ± 0.001 | <0.05 | 6.112 ± 1.823 | 2.21 ± 0.03 |
| Neoprene | Acid | Yes | <0.002 | 0.013 ± 0.001 | 3.110 ± 1.253 | 0.105 ± 0.016 | 0.240 ± 0.057 | 14.40 ± 0.10 | 0.004 ± 0.001 | <0.05 | 6.602 ± 2.474 | 2.22 ± 0.08 |
| Neoprene | Tap | No | <0.002 | <0.002 | 0.003a | <0.002 | <0.02 | 11.88 ± 0.04 | <0.001 | <0.05 | 0.180 ± 0.005 | 7.39 ± 0.23 |
| Neoprene | Tap | Yes | <0.002 | <0.002 | <0.002 | <0.002 | <0.02 | 13.48 ± 0.04 | <0.001 | <0.05 | 0.021 ± 0.003 | 8.14 ± 0.47 |
| None | Tap | No | <0.002 | <0.002 | <0.002 | <0.002 | <0.02 | 12.16 ± 0.09 | <0.001 | <0.05 | 0.003 ± 0.000 | 8.26 ± 0.12 |
| None | Acid | No | <0.002 | <0.002 | 0.004 ± 0.0002 | 0.007 ± 0.000 | <0.02 | 12.18 ± 0.02 | <0.001 | <0.05 | 0.415 ± 0.002 | 2.04 ± 0.13 |
| EPA maximal contaminant level 51 | 0.005 | 0.1 | 1.3 | 0.03b | 0.015 | — | 0.05b | 0.05 | 5.0b | 6.5–8.5b | ||
Only a single sample had a quantifiable concentration of copper.
This concentration is the national secondary drinking water regulation. Note that the Environmental Protection Agency (EPA) recommends these secondary standards but does not require compliance with these recommendations.
Discussion
This study is the first to report the metal analysis for all of components of a water-bottle assembly. Although we used a very similar analysis method (ICP spectroscopy) to that used previously (atomic absorption spectroscopy), the composition of the water-bottle stoppers differed slightly between studies.28 Rubber stoppers in the current study had less copper, iron, magnesium, manganese, and zinc per gram of polymer.28 Neoprene stoppers had less copper, iron, and magnesium but comparable quantities of manganese and zinc per gram of polymer to those evaluated previously.28 We also identified quantifiable amounts of lead, cadmium, chromium, and selenium in both the rubber and neoprene stoppers, which was not previously reported. We did not analyze any other stopper types, because they are not commercially available. The stainless-steel sipper tubes had large quantities of all tested metals, which were significantly greater than those in the stoppers for all metals except magnesium and zinc.
Similar to findings from the previous study,28 leaching in the current study was increased under the acidified condition. Of the metals tested, magnesium, zinc, and copper reached the highest concentrations in the water after 1 wk. Acidified water leached lead from the neoprene stoppers—a finding that has not previously been reported. In the current study, acidified water leached more chromium and magnesium but less copper, iron, and zinc and comparable amounts of manganese and selenium from rubber stoppers, compared with previous results.28 Similarly, acidified water leached more copper and magnesium but less iron and zinc and comparable amounts of chromium, manganese, and selenium from neoprene stoppers in the current study compared with the previous study.28 Slight differences between the studies may be partially responsible for some of these discrepancies. In particular, water bottles in the current study were left inverted for 7 d instead of 6 d; deionized water was not used; pH was 0.3 units lower; and the source of the stoppers was different.
The results of the current study and those obtained previously28 collectively suggest that water should be analyzed for contaminants at the level of delivery to animals. These studies further suggest that all materials used in water bottle assemblies should be analyzed for potential heavy-metal contaminants.28 The differences in the metal content of the stopper types between the 2 studies highlights the potential variability in stopper materials due to differences in sources and manufacturing processes. To address the variability in material contents, facilities should periodically test these materials for potential contaminants. This suggestion is further supported by the recent reports of lead and melamine contaminants in children's toys and pet foods from foreign suppliers.8,10,11,47,52
Heavy-metal leaching into drinking water may be problematic due to the individual biologic effects of these components after exposure. Lead has been documented to cause a variety of toxic effects and, as a result, the Environmental Protection Agency has set the ideal water concentration of lead at 0 ppm and the level of action at 0.015 ppm.51 In the current study, lead leached from neoprene stoppers into acidified drinking water. These lead levels reached 0.2 ppm, thus exceeding the human guidelines.51 The primary toxic effects of lead are immunologic and reproductive in nature, but lead also can affect other body systems in rodents when administered at high concentrations.7,9,13,14,23,39-42,45,53,54 Contaminant concentrations of dietary lead (0.4 μg/g wet weight of feed) have been associated with increased mortality rates in aged male CD1 mice.44 The amount of lead that these previous mice44 ingested over the course of their lives was comparable to the exposure (on a μg/g body weight basis) with that which theoretically would be ingested from acidified water in contact with neoprene stoppers. This finding suggests that animals on aging studies should not be provided acidified water from water bottles with neoprene stoppers, to mitigate potential premature death associated with chronic low-level lead toxicity.
Copper was detected in the acidified water at a maximal average concentration of 3.25 ppm for neoprene stoppers and 0.02 ppm for rubber stopper. The limit for copper in drinking water is 1.3 ppm due to the development of gastrointestinal distress acutely and liver or kidney damage chronically in humans.51 Although the amount of copper in acidified water exposed to a neoprene stopper exceeds this limit, the risk of rodents developing long-term liver or kidney damage as a result of long-term exposure under these watering conditions is minimal, given that they typically consume a larger quantity of copper from their diet (Table 2).
Table 2.
Amounts (µg) of zinc and copper potentially ingested daily by a 30-g mouse or 300-g rat according to average daily intakes for adult rodents
| Mouse |
Rat |
|||
| Zn | Cu | Zn | Cu | |
| Harlan Teklad 7912 | 315 | 115 | 945 | 345 |
| Teklad mineral–deficient diets | 7.5 | 1.6 | 22.5 | 4.8 |
| Acidified water, neoprene stopper | 42 | 21 | 190 | 95 |
| Acidified water, rubber stopper | 6 | 0.2 | 30 | 0.7 |
Zinc was detected in the largest concentrations in the neoprene stoppers and leached into the water that contacted these stoppers. When the water was acidified, the amount of zinc leached increased significantly to the maximal average of 6.6 ppm after 1 wk. Although acute zinc toxicity often is associated with the ingestion of nonfood items (for example, a penny or lead sinker), dietary zinc is relatively nontoxic.18 However, when supplemented at 10 to 20 times the recommended dietary allowance of 15 mg/d in humans, clinical signs of zinc toxicity do develop.18 The risk of rodents developing toxicity due to long-term exposure to zinc under these watering conditions is minimal because they normally consume a much larger quantity of zinc from their diet (Table 2).
Although the concentrations of copper and zinc that leached into acidified water in contact with neoprene stoppers is not expected to lead to acute toxicities, the potential to affect experimental outcomes remains. For example, an investigator studying the immune-modulating effects of copper and zinc might choose to feed their animals a diet low in copper and or zinc.4,12,15,16,19,29,30,37,38 In these cases, the potential quantity of zinc and copper ingested from acidified water on neoprene stoppers would exceed that from the diet by 5-fold and 20-fold, respectively (Table 2). This situation likely would negate the effects of feeding the metal-deficient diets and potentially confound the results of the animal experiment.
The Environmental Protection Agency currently does not have a threshold concentration for iron in drinking water, because iron toxicity is exceedingly less common than is iron deficiency in humans. Iron deficiency increases the absorption, potentially leading to toxicity, of other divalent metals, including lead, cadmium, aluminum, and manganese.55 The amount of iron in drinking water affects its palatability, which sharply declines at 0.3 ppm for humans.51 Iron leached into the water in the current study, especially when the water was acidified and a neoprene stopper was used, but the maximal iron concentration reached was only 0.1 ppm. This finding indicates that leaching of iron is unlikely to affect water palatability or cause toxicity in rodents.
Magnesium often is found in high concentrations in tap water, especially when the source is groundwater, and tap water is considered a major source of the required dietary magnesium in humans.55 As expected, magnesium was present in high concentrations in all water samples in the current study, including fresh tap water. Although additional magnesium leached from the neoprene stoppers exposed to acidified water, the potential quantity of magnesium ingested from drinking water remains far less that the amount consumed from the diet (2000 μg/g diet, Teklad 7912). These results suggest that the magnesium that leached from the neoprene stoppers is of minimal concern and not expected to result in toxicity.
Manganese and chromium were present in the sipper tubes and neoprene and rubber stoppers. Under all tested conditions, both metals leached at very low levels. Although manganese is a cofactor in several important enzymatic pathways and functions with vitamin K, leading to the formation of prothrombin, there are relatively few studies of toxicity, and deficiency is of a much larger clinical significance.25 When administered intraperitoneally to rats at high concentrations (10 mg/kg body weight), manganese causes hyperglycemia and hypoinsulinemina, indicating an effect on carbohydrate metabolism through alterations in insulin release and gluconeogenesis.25 Chromium is a required element in the diet and is important for normal growth and survival of rodents.35,44 Due to the potential for toxicity, the Environmental Protection Agency has set the limit for chromium at 0.1 ppm for people.51 The very small amounts of manganese and chromium that into the drinking water in the current study is not likely to pose as a significant source for toxicity.
Although neither cadmium nor selenium was detected in the water samples, both metals were present in the tested stoppers and sipper tubes, making ingestion a possibility. Excess dietary cadmium has immune-modulatory effects, is associated with an inability to thrive, and, over the long-term, can influence the absorption and distribution of essential elements and replace them in biologic enzymes.56 Rodent diets high in selenium (3.0 mg Se/kg diet) have been associated with gestational diabetes and insulin resistance, which can be passed onto the pups.36,43 Despite the potential for cadmium and selenium ingestion, toxicity is not anticipated.
Although few of the metal concentrations in the water reached individually toxic levels, quantifying the amounts of the metals in the materials and the amount leached into the water remains important. Rodents that are placed on diets deficient in specific micronutrients (for example, calcium, copper, iron) will be predisposed to toxicity from the nonessential metals ingested from the water due to the complex interaction between the metals and micronutrients.2,3,5,6,17,43,44 Furthermore, when otherwise healthy animals are fed a regular rodent chow but exposed to high concentrations of a given metal, the metabolism and activity of other metals can be affected, leading to relative deficiencies or toxicities.
In addition, rodents potentially can ingest the stopper material. Old rubber stoppers that had evidence of gnawing activity weighed an average of 6.2 g less than did new rubber stoppers. Although it is highly improbable that a single animal ingested the entire 6.2 g, rodents are likely to ingest small amounts of the stopper material. Exposure of the stopper material to the acidic environment of the stomach may lead to leaching of heavy metals, putting the animal at potential risk of toxicity. Further evaluation of this hypothesis is warranted to identify the actual risk associated with this route of heavy-metal exposure.
Although autoclaving had minimal effects on metal leaching, with the exception of manganese and magnesium, it did have degradative effects on the material properties of the stoppers. Specifically, the neoprene stoppers began to crack the first time they were autoclaved, and they subsequently crumbled after repeated cycles of autoclaving, requiring their removal from circulation after just 2 or 3 cycles. The rubber stoppers were not observed to degrade during this brief experimental period.
As illustrated in the current study, water acidification has unintended consequences that should not be ignored. The effects of water acidification on the water-bottle assembly can become a confounding variable, especially in rodent immunologic and aging studies. In light of these findings, we suggest that water-quality monitoring programs should include periodic analysis for heavy metals in addition to microbiologic contamination at the level of water delivery to the animal. We also suggest establishing a program to routinely perform metal-analysis testing of water bottle stoppers to identify potential sources of water contamination. Additional studies may be necessary to identify the biologic effects of long-term exposure to the metals leached from water bottle stoppers or gaskets in automatic water systems into acidified water.
Acknowledgments
We thank Saman Shafaie and Sarah Saslow (Northwestern University) for their useful discussions and assistance with the ICP spectroscopy and Mike Smith and Volta Perkins for support with water bottle assembly and autoclaving samples.
References
- 1.Animal Welfare Regulations. 2009. 9 CFR §3.2.
- 2.Abdel Rahim AG, Arthur JR, Mills CF. 1986. Effects of dietary copper, cadmium, iron, molybdenum, and manganese on selenium utilization by the rat. J Nutr 116:403–411 [DOI] [PubMed] [Google Scholar]
- 3.Ashraf MH, Fosmire GJ. 1985. Effects of marginal zinc deficiency on subclinical lead toxicity in the rat neonate. J Nutr 115:334–346 [DOI] [PubMed] [Google Scholar]
- 4.Beach RS, Gershwin ME, Hurley LS. 1982. Gestational zinc deprivation in mice: persistence of immunodeficiency for 3 generations. Science 218:469–471 [DOI] [PubMed] [Google Scholar]
- 5.Bremner I, Campbell JK. 1978. Effect of copper and zinc status on susceptibility to cadmium intoxication. Environ Health Perspect 25:125–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunn CR, Matrone G. 1966. In vivo interactions of cadmium, copper, zinc, and iron in the mouse and rat. J Nutr 90:395–399 [DOI] [PubMed] [Google Scholar]
- 7.Bunn TL, Parsons PJ, Kao E, Dietert RR. 2001. Gender-based profiles of developmental immunotoxicity to lead in the rat: assessment in juveniles and adults. J Toxicol Environ Health A 64:223–240 [DOI] [PubMed] [Google Scholar]
- 8.Chen KC, Liao CW, Cheng FP, Chou CC, Chang SC, Wu JH, Zen JM, Chen YT, Liao JW. 2009. Evaluation of subchronic toxicity of pet food contaminated with melamine and cyanuric acid in rats. Toxicol Pathol 37:959–968 [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Golemboski K, Piepenbrink M, Dietert R. 2004. Developmental immunotoxicity of lead in the rat: influence of maternal diet. J Toxicol Environ Health A 67:495–511 [DOI] [PubMed] [Google Scholar]
- 10.Cianciolo RE, Bischoff K, Ebel JG, Van Winkle TJ, Goldstein RE, Serfilippi LM. 2008. Clinicopathologic, histologic, and toxicologic findings in 70 cats inadvertently exposed to pet food contaminated with melamine and cyanuric acid. J Am Vet Med Assoc 233:729–737 [DOI] [PubMed] [Google Scholar]
- 11.Cocchi M, Vascellari M, Gallina A, Angeletti R, Agnoletti F, Mutinelli F, Cattai S. 2009. Renal failure in dogs in Italy associated with melamine-contaminated pet food. Vet Rec 164:407–408 [DOI] [PubMed] [Google Scholar]
- 12.Combs DK, Goodrich RD, Meiske JC. 1983. Influence of dietary zinc or cadmium on hair and tissue mineral concentrations in rats and goats. J Anim Sci 56:184–193 [DOI] [PubMed] [Google Scholar]
- 13.Cook JA, Di Luzio NR, Hoffmann EO. 1975. Factors modifying susceptibility to bacterial endotoxin: the effect of lead and cadmium. CRC Crit Rev Toxicol 3:201–229 [DOI] [PubMed] [Google Scholar]
- 14.Cook JA, Hoffmann EO, Luzio ND. 1975. Influence of lead and cadmium on the susceptibility of rats to bacterial challenge. Proc Soc Exp Biol Med 150:741–747 [DOI] [PubMed] [Google Scholar]
- 15.Davis CD, Feng Y. 1999. Dietary copper, manganese and iron affect the formation of aberrant crypts in colon of rats administered 3,2′-dimethyl-4-aminobiphenyl. J Nutr 129:1060–1067 [DOI] [PubMed] [Google Scholar]
- 16.DePasquale-Jardieu P, Fraker PJ. 1984. Interference in the development of a secondary immune response in mice by zinc deprivation: persistence of effects. J Nutr 114:1762–1769 [DOI] [PubMed] [Google Scholar]
- 17.Flanagan PR, Haist J, MacKenzie I, Valberg LS. 1984. Intestinal absorption of zinc: competitive interactions with iron, cobalt, and copper in mice with sex-linked anemia (SLA). Can J Physiol Pharmacol 62:1124–1128 [DOI] [PubMed] [Google Scholar]
- 18.Fosmire GJ. 1990. Zinc toxicity. Am J Clin Nutr 51:225–227 [DOI] [PubMed] [Google Scholar]
- 19.Fraker PJ, DePasquale-Jardieu P, Zwickl CM, Luecke RW. 1978. Regeneration of T-cell helper function in zinc-deficient adult mice. Proc Natl Acad Sci USA 75:5660–5664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Good Laboratory Practice for Nonclinical Laboratory Studies. 2011. 21 CFR §58.
- 21.Haist C, Cadillac J, Dysko R. 2004. Assessment of bacterial contamination of drinking water provided to mice. Contemp Top Lab Anim Sci 43:8–13 [PubMed] [Google Scholar]
- 22.Hall JE, White WJ, Lang CM. 1980. Acidification of drinking water: its effects on selected biologic phenomena in male mice. Lab Anim Sci 30:643–651 [PubMed] [Google Scholar]
- 23.Hemphill FE, Kaeberle ML, Buck WB. 1971. Lead suppression of mouse resistance to Salmonella typhimurium. Science 172:1031–1032 [DOI] [PubMed] [Google Scholar]
- 24.Hermann LM, White WJ, Lang CM. 1982. Prolonged exposure to acid, chlorine, or tetracycline in the drinking water: effects on delayed-type hypersensitivity, hemagglutination titers, and reticuloendothelial clearance rates in mice. Lab Anim Sci 32:603–608 [PubMed] [Google Scholar]
- 25.Hurley LS, Keen CL, Baly DL. 1984. Manganese deficiency and toxicity: effects on carbohydrate metabolism in the rat. Neurotoxicology 5:97–104 [PubMed] [Google Scholar]
- 26.Institute of Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 27.Karle EJ, Gehring F, Deerberg F. 1980. [Acidifying of drinking water and its effect on enamel lesions of rat teeth]. Z Versuchstierkd 22:80–88 [Article in German] [PubMed] [Google Scholar]
- 28.Kennedy BW, Beal TS. 1991. Minerals leached into drinking water from rubber stoppers. Lab Anim Sci 41:233–236 [PubMed] [Google Scholar]
- 29.Koller LD, Mulhern SA, Frankel NC, Steven MG, Williams JR. 1987. Immune dysfunction in rats fed a diet deficient in copper. Am J Clin Nutr 45:997–1006 [DOI] [PubMed] [Google Scholar]
- 30.Lim Y, Levy M, Bray TM. 2004. Dietary zinc alters early inflammatory responses during cutaneous wound healing in weanling CD1 mice. J Nutr 134:811–816 [DOI] [PubMed] [Google Scholar]
- 31.Lipman NS, Perkins SE. 2002. Factors that may influence animal research, p 1143–1184. In: Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed. New York (NY): Academic Press. [Google Scholar]
- 32.McClure FJ. 1943. The destructive action, in vivo, of dilute acids and acid drinks and beverages on the rat's molar teeth. J Nutr 26:251–259 [Google Scholar]
- 33.McPherson CW. 1963. Reduction of Pseudomonas aeruginosa and coliform bacteria in mouse drinking water following treatment with hydrochloric acid or chlorine. Lab Anim Care 13:737–744 [PubMed] [Google Scholar]
- 34.Meier TR, Maute CJ, Cadillac JM, Lee JY, Righter DJ, Hugunin KM, Deininger RA, Dysko RC. 2008. Quantification, distribution, and possible source of bacterial biofilm in mouse automated watering systems. J Am Assoc Lab Anim Sci 47:63–70 [PMC free article] [PubMed] [Google Scholar]
- 35.Padmavathi IJ, Rao KR, Venu L, Ganeshan M, Kumar KA, Rao Ch N, Harishankar N, Ismail A, Raghunath M. 2010. Chronic maternal dietary chromium restriction modulates visceral adiposity: probable underlying mechanisms. Diabetes 59:98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peraza MA, Ayala-Fierro F, Barber DS, Casarez E, Rael LT. 1998. Effects of micronutrients on metal toxicity. Environ Health Perspect 106 Suppl 1:203–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prohaska JR. 2011. Impact of copper limitation on expression and function of multicopper oxidase (ferroxidases). Adv Nutr 2:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prohaska JR, Lukasewycz OA. 1981. Copper deficiency suppresses the immune response of mice. Science 213:559–561 [DOI] [PubMed] [Google Scholar]
- 39.Ronis MJ, Badger TM, Shema SJ, Roberson PK, Shaikh F. 1996. Reproductive toxicity and growth effects in rats exposed to lead at different periods during development. Toxicol Appl Pharmacol 136:361–371 [DOI] [PubMed] [Google Scholar]
- 40.Ronis MJ, Badger TM, Shema SJ, Roberson PK, Shaikh F. 1998. Effects on pubertal growth and reproduction in rats exposed to lead perinatally or continuously throughout development. J Toxicol Environ Health A 53:327–341 [DOI] [PubMed] [Google Scholar]
- 41.Ronis MJ, Badger TM, Shema SJ, Roberson PK, Templer L, Ringer D, Thomas PE. 1998. Endocrine mechanisms underlying the growth effects of developmental lead exposure in the rat. J Toxicol Environ Health A 54:101–120 [DOI] [PubMed] [Google Scholar]
- 42.Ronis MJ, Gandy J, Badger T. 1998. Endocrine mechanisms underlying reproductive toxicity in the developing rat chronically exposed to dietary lead. J Toxicol Environ Health A 54:77–99 [DOI] [PubMed] [Google Scholar]
- 43.Schroeder HA, Nason AP. 1976. Interactions of trace metals in mouse and rat tissues; zinc, chromium, copper, and manganese with 13 other elements. J Nutr 106:198–203 [DOI] [PubMed] [Google Scholar]
- 44.Schroeder HA, Vinton WH, Jr, Balassa JJ. 1963. Effect of chromium, cadmium, and other trace metals on the growth and survival of mice. J Nutr 80:39–47 [DOI] [PubMed] [Google Scholar]
- 45.Selye H, Tuchweber B, Bertok L. 1966. Effect of lead acetate on the susceptibility of rats to bacterial endotoxins. J Bacteriol 91:884–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanner RS, James SA. 1992. Rapid bactericidal effect of low pH against Pseudomonas aeruginosa. J Ind Microbiol 10:229–232 [Google Scholar]
- 47.Thompson ME, Lewin-Smith MR, Kalasinsky VF, Pizzolato KM, Fleetwood ML, McElhaney MR, Johnson TO. 2008. Characterization of melamine-containing and calcium oxalate crystals in 3 dogs with suspected pet food-induced nephrotoxicosis. Vet Pathol 45:417–426 [DOI] [PubMed] [Google Scholar]
- 48.Thunert A, Heine W. 1975. [The water supply of SPF animal houses. III. Heating and acidification of drinking water]. Z Versuchstierkd 17:50–52 [Article in German] [PubMed] [Google Scholar]
- 49.Tober-Meyer BK, Bieniek HJ. 1981. Studies on the hygiene of drinking water for laboratory animals. 1. The effect of various treatments on bacterial contamination. Lab Anim 15:107–110 [DOI] [PubMed] [Google Scholar]
- 50.Tolo KJ, Erichsen S. 1969. [Acidified drinking water and dental enamel in rats]. Z Versuchstierkd 11:229–233 [Article in German] [PubMed] [Google Scholar]
- 51.United States Environmental Protection Agency. [Internet]. National primary drinking water standards. [Cited 11 April 2011 ]. Available at: http://water.epa.gov/drink/contaminants/index.cfm#Primary.
- 52.VanArsdale JL, Leiker RD, Kohn M, Merritt TA, Horowitz BZ. 2004. Lead poisoning from a toy necklace. Pediatrics 114:1096–1099 [DOI] [PubMed] [Google Scholar]
- 53.Wadi SA, Ahmad G. 1999. Effects of lead on the male reproductive system in mice. J Toxicol Environ Health A 56:513–521 [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Xun P, Zhao Y, Wang X, Qian L, Chen F. 2008. Effects of lead exposure on sperm concentrations and testes weight in male rats: a meta-regression analysis. J Toxicol Environ Health A 71:454–463 [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization. 2009. Calcium and magnesium in drinking water: public health significance. Geneva (Switzerland): World Health Organization. [Google Scholar]
- 56.Zeng MS, Li X, Liu Y, Zhao H, Zhou JC, Li K, Huang JQ, Sun LH, Tang JY, Xia XJ, Wang KN, Lei XG. 2012. A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic Biol Med 52:1335–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]