Abstract
Cancer vaccines are typically formulated for bolus injection and often produce short-lived immunostimulation resulting in poor temporal control over immune cell activation and weak oncolytic activity. One means of overcoming these limitations utilizes immunologically active biomaterial constructs. We previously reported that antigen-laden, macroporous PLG scaffolds induce potent dendritic cell (DC) and cytotoxic T-lymphocyte (CTL) responses via the controlled signaling of inflammatory cytokines, antigen and toll-like receptor agonists. In this study, we describe the kinetics of these responses and illustrate their fundamental relationship to potent tumor rejection when implanted subcutaneously in a mouse B16 model of melanoma. By explanting scaffolds from mice at times ranging from 1–7 d, a seamless relationship was observed between the production of controlled CTL responses, tumor growth and long-term survival in both prophylactic and therapeutic models. Scaffolds must be implanted for > 7 d to augment CTL responses via the prolonged presentation of tumor antigen, and the benefits included a notable regression of established tumors. Host DC infiltration into the porous material persisted for 12 days (peaking at day 5 ~1.4 x 106 cells), and a sharp attenuation in DC numbers coincided with peak CD8+ CTL infiltration at day 12 (~8 x 105 cells). Importantly, these PLG systems enhanced DC numbers in the draining lymph node, resulting in increased CD8(+) CTL subsets at days 10–16 of vaccination. These results indicate that material systems can finely control innate and adaptive immune cell responses to kill typically untreatable melanoma tumors and provide critical kinetic data for the design of vaccine carriers.
Keywords: biomaterials, cancer, dendritic cells, drug delivery, host response, immunity, immunotherapy, vaccine
Introduction
As components of the innate immune response, dendritic cells (DCs) are involved in the initial reactions to infection as they detect foreign antigens and are activated by stimuli, such as pathogen associated molecular patterns (PAMPs) unique to invading pathogens.1-3 PAMPs, including lipopolysacharides and cytosine-guanosine (CpG) sequences in bacterial DNA, trigger a particular toll-like receptor (TLR) that allows DCs to classify the pathogen and induce their expression of T-cell activating molecules.4 Activated DCs then migrate to draining lymph nodes (LNs) where they prime the appropriate T-cell response, via the presentation of antigen, costimulatory molecules and the appropriate cytokines.1 While innate immunity encompasses many rapid reactions to infection, adaptive immunity, in contrast, is learned or acquired more slowly via cellular messengers (for example, DCs), over time frames of days to weeks.5-9 For example, transplantation of infected tissue into mice results in DC infiltration and innate activation, within 3 d, followed by peak T-cell expansion after 9 d.10
Current vaccine methods produce short-lived bioactivity, and cancer vaccines have failed to reproducibly cause the regression of solid tumors.11,12 Current delivery methods for experimental vaccines often include bolus injections/effusions of antigen and adjuvants with short half-lives, or activated cell products that lose significant viability upon in vivo transplantation.1-3,13 Biomaterials may be utilized to extend the duration of immunostimulation, and have been studied extensively in vaccine formulations as adjuvants and to enable controlled presentation of antigens to the immune system.14,15 More recently, material-based particulate systems have been developed to control antigen localization in tissue or to target host DCs specifically via antibody or ligand conjugation.14,16,17 Although current biomaterial-based vaccines can enhance antigen loading to DCs, leading to antigen specific T-cell activation, they lack concurrent regulation of effector T-cell activity. Therapeutic vaccination against an established disease, including solid tumors, requires methods that stimulate not only innate immunity and DCs, but also persistent cytotoxic T lymphocyte (CTL) responses, which kill tumor cells, until the disease has cleared. Therefore, the natural kinetics of innate and adaptive immune responses and the coordination of these responses by vaccine vectors should impact vaccine design and application.
We recently described the development of 3-dimensional, macroporous poly(lactide-co-glycolide; PLG) matrices that regulate the trafficking and activation of dendritic cells (DCs) in situ.5,6 These implantable, matrices spatially and temporally control the in vivo presentation of cytokines, tumor antigens and danger signals.18,19 Fabricated with GM-CSF, a CpG oligonucleotide (ODN), and tumor lysate these PLG vaccines release GM-CSF into the surrounding tissue, forming GM-CSF gradients that recruit host DCs18-21 following subcutaneous implantation. CpG-rich ODNs, which act as danger signals, and antigen (tumor lysate incorporated within the PLG) are embedded in the matrix or slowly released to matrix resident DCs which programs DC development and maturation. The coordination of DC activation induced by these biomaterial-based vaccines, promotes potent, prolonged, and specific cytotoxic, T-cell mediated immunity that has been shown to eradicate large established peripheral tumors in mice.18,19 In side-by-side comparisons, this vaccine system outperformed clinically tested cell-based vaccines and formulations of equivalent doses delivered in bolus, demonstrating the importance of the material backbone.19 Importantly, in controlled experiments of vaccine efficacy it was demonstrated that the presentation of all 3 bioactive components (GM-CSF, CpG-ODN, and tumor lysate) of the vaccine system were required to induce immune mediated destruction of tumors.18,19
In this report, we study the relationship between vaccine duration and efficacy in melanoma models and its correlation to local kinetics of DC and T-cell responses to the aforementioned PLG vaccine system. The duration of vaccination was varied to determine the impact on vaccine efficacy. The report also describes the kinetics of innate (DCs) and adaptive cellular responses (cytotoxic T cells) to these vaccines, and highlights the inflection point when innate responses transition to effective anti-tumor immune responses after vaccination. Because the cytokines interleukin (IL)-12 and interferon (IFN)-γ are important mediators and indicators of T-helper (Th)-1 and cytotoxic T-cell responses to viruses and tumors,5,22,23 we also measured their concentrations at the PLG vaccine site over time. Additionally, the vaccine’s induction of systemic cellular responses were monitored at the inguinal LN draining the site of vaccination, as these are points where naïve T cells interact with antigen presenting cells (APCs; DCs and macrophages) and are important locations where CD8+ CTL cells are primed.9
Results
The cross section of the macroporous PLG vaccine is shown in an SEM micrograph in (Fig. 1A). As previously reported the matrices were largely porous, isoreticulated scaffolds with an overall porosity of approximately 85%. The melanoma vaccines contained 680 +/− 26 μg of protein (n = 4) derived from melanoma tumor lysate, 2.5 +/− 0.3 μg of GM-CSF (n = 8), and 150 +/− 2.0 μg of CpG-ODN (n = 8).
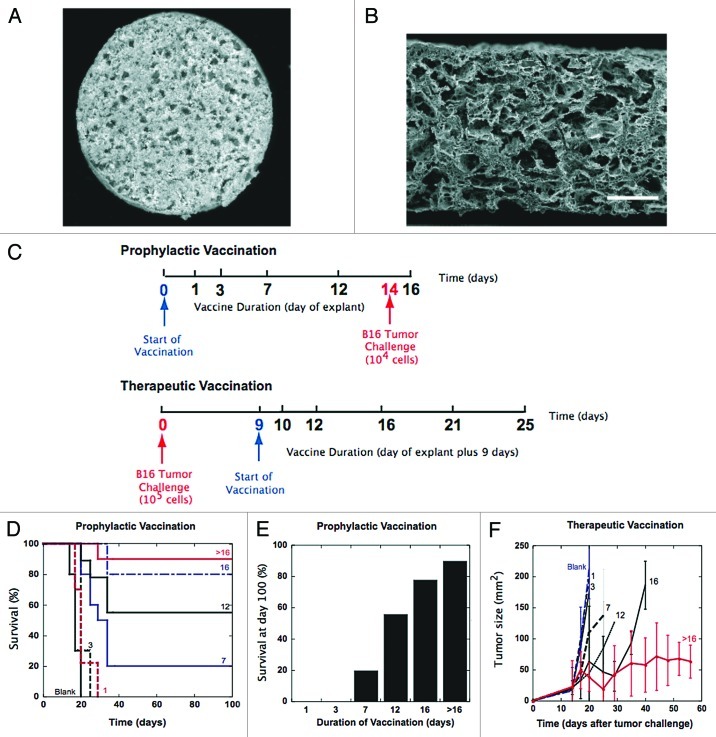
Figure 1.
Effect of PLG vaccine duration on efficacy in B16 melanoma tumor models. (A) Photomicrograph of surface of macroporous, PLG-based vaccine loaded with GM-CSF, tumor lysates and CpG-ODN. (B) SEM micrograph of cross section of macroporous, PLG vaccine. Scale bar – 200 μm. (C) Schematic of PLG vaccine regimen for both prophylactic and therapeutic B16 models in mice. [Prophylactic] Vaccine duration is varied from 0, 1, 3, 7, 12, and 16 d. (D) A comparison of the survival time and (E) the survival percentage at day 100 in mice treated with blank PLG scaffolds, and PLG vaccines for a duration of 1, 3, 7, 12, 16 d or indefinitely (> 16). Mice were challenged with 104 B16-F10 melanoma tumor cells at day 14 after the start of vaccination. (F) Effect of vaccine duration on therapeutic efficacy [Therapeutic]. Tumor growth curves for mice vaccinated at day 9 after tumor challenge (105 B16 melanoma cells), with PLG vaccines for a duration of 1, 3, 7, 12, 16 d or indefinitely (> 16). The total GM-CSF dose was 3000ng, and CpG-ODN dose was 100μg. Values in E represent mean and standard error.
Effect of vaccine duration on vaccine efficacy
As described previously, macroporous PLG scaffolds were fabricated to control the presentation of GM-CSF, tumor lysate (B16 melanoma), and CpG-ODN18,19 to serve as vaccines (PLG vaccines) (Fig. 1A and B). The duration of vaccination was controlled by explanting the material system at various timepoints, and we tested the relationship between vaccine duration and efficacy in prophylactic and therapeutic B16-F10 melanoma models. PLG vaccines were implanted subcutaneously into the backs of C57BL/6J mice and removed at days 1, 3, 7, 12 and 16 after implantation and these mice were challenged at day 14 with an otherwise lethal dose of B16-F10 cells (Fig. 1C). Prophylactic vaccination times of less than 7 d resulted in little survival benefit (Fig. 1D). However, vaccination times of 7, 12 and 16 d resulted in significant long-term survival, as 60, and 80 percent of animals survived tumor challenge (Fig. 1D and 1E). Continuous vaccination (matrix not removed) conferred a small increase in immune protection (90% survival) over vaccination times of 16 d (80% survival). These results clearly indicate that longer-term antigen/immune cell stimulation was required to induce significant protection.
The relationship between vaccine duration and tumor rejection was also determined by varying vaccination times in therapeutic treatment of established B16 melanomas. Mice were inoculated with 105 B16 tumor cells and melanomas were allowed to develop for 9 d, at which point animals received PLG vaccines. Vaccination was then arrested by the removal of the implant at days 1, 3, 7, 12 and 16 after implantation (Fig. 1C). Therapeutic vaccination times of less than 7 d had no impact on tumor progression (Fig. 1D). Vaccination times of greater than 12 d significantly impacted tumor progression and extended the lifespan of mice (Fig. 1F). Importantly, continuous vaccination (> 16 d) prolonged anti-tumor attack, the suppression of tumor growth, and conferred a 3-fold increase in median survival (Fig. 1F).
Kinetics of DC and T-cell responses at PLG vaccine site
To further delineate the relation between the duration of vaccination and the associated immunologic response, the kinetics of cell recruitment by the PLG vaccines was quantified. The engineered PLG matrices were designed to release a pulse of GM-CSF to recruit host DCs.18,20,21 Histological analysis at day 14 post-implantation of PLG matrices loaded with 3000ng of GM-CSF revealed enhanced cellular infiltration and penetration into the void volume of the material, relative to cell infiltration at 3 d after implantation (Fig. 2A). As described previously, these PLG vaccines are embedded with B16-melanoma tumor lysates and CpG-ODN molecules, to serve as tumor antigen and danger signals to infiltrating immune cells.18 Incorporation of approximately 680 μg of protein derived from B16-F10 melanoma tumor lysate resulted in sustained release of tumor-associated proteins for at least 40 d, in vitro, with approximately 80 μg being released between days 5 and 40 (Fig. S1). At day 40 the percent of tumor protein retained within the matrix remained high (77%) indicating that the vaccine serves as a site of sustained antigen presentation that may program immune cells for extended periods.
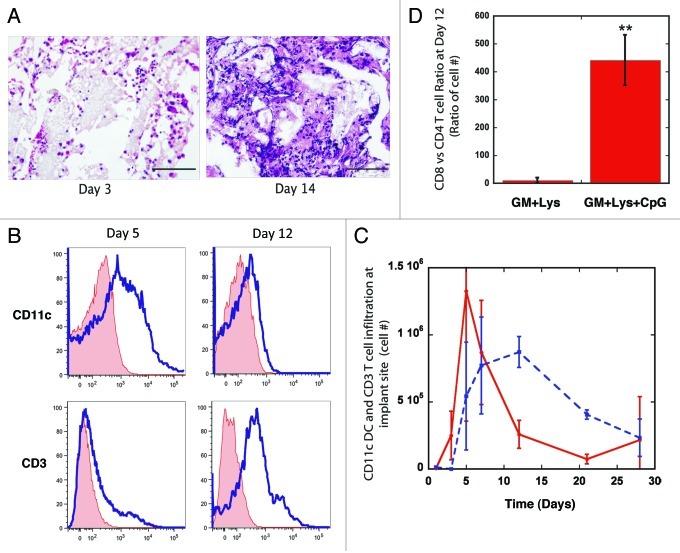
Figure 2.
Kinetics of DC and T cell infiltration into PLG vaccine site. (A) Hematoxylin and Eosin staining of sectioned PLG vaccines explanted from subcutaneous pockets in the backs of C57BL/6J mice after 3 and 14 d. (B) FACS histograms of CD11c(+) DCs and CD3(+) T cells infiltrating PLG matrices loaded with 3000ng GM-CSF, 100μg CpG-ODN and tumor antigens at days 5 and 12. Histograms of isotype control (tinted line) are also included. (C) The total number of CD11c(+) DCs and CD3(+) T cells isolated from PLG vaccines as a function of time post implantation. (D) The ratio of CD8 Tcells vs. CD4 Tcells residing within PLG vaccines at day 12 post-implantation. Values in B and C represent mean and standard deviation (n = 4 or 5). ** p < 0.01 as compared with controls.
To assess the kinetics of DC and T-cell responses to PLG vaccines, matrices were explanted at various times and total cell infiltrates were isolated and analyzed using FACS analysis to determine CD11c(+) DC and the CD3(+) T-cell subpopulations. DC numbers were detectable at day 3 post-implantation, peaked at days 5 and 7 (Fig. 2 B and C), and dropped sharply at Day 12 post-implantation. The T-cell response to PLG vaccines is predominantly comprised of CD8(+) cytotoxic T cells (Fig. 2D). Local cytotoxic T-cell responses persisted at significant levels between days 7 and 28 after implantation. These data indicate that the vaccine site transitions from primarily activating innate immune responses and DCs to a T-cell effector site between 7 to 12 d after implantation, and this CD8(+) T-cell responses is maintained for at least 28 d.
Kinetics of IL-12 and IFN-γ production at vaccine site
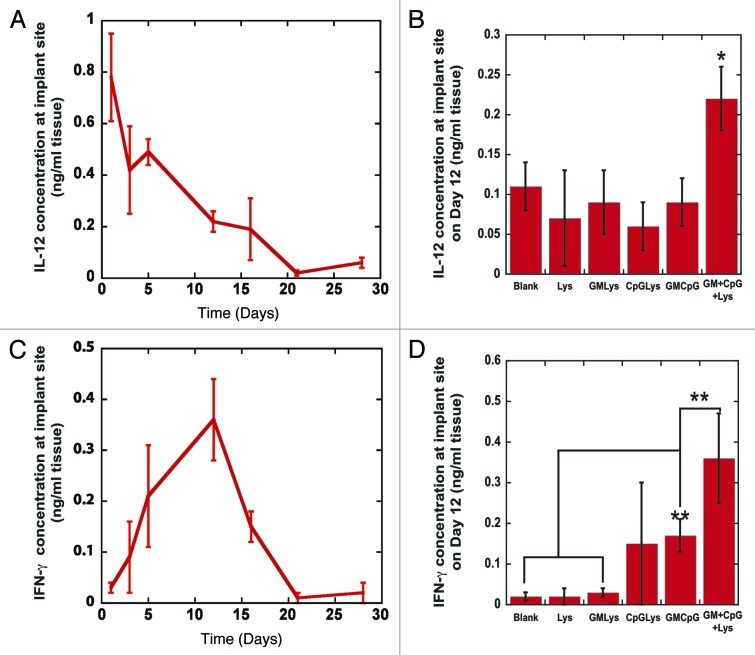
IL-12, which is a T-cell growth and stimulating factor and an activating factor for DCs, is produced by DCs and macrophages in response to intercellular pathogens and tumor cells. Local IL-12 concentrations peaked at 800 ρg/ml after one day of vaccination, and then subsided to approximately 300 ρg/ml between days 5–16 of vaccination (Fig. 3A). Peak levels of IL-12 correlated with the infiltration of CD14(+) monocytes and CD11b(+) macrophages and these cells were likely the primary producers of IL-12 from days 1–3 after vaccination (Fig. 3A and S2). All 3 components of the vaccine, GM-CSF, CpG-ODN and tumor lysates were required to promote and maintain high IL-12 concentrations, as blank controls and all other combinations of the vaccine’s bioactive factors produced significantly lower IL-12 levels (Fig. 3B). Interestingly, the IL-12 concentration subsided to undetectable levels after day 21 of vaccination, and the time over which IL-12 was detected coincided with the time course of macrophage, monocyte and DC infiltration (Fig. 2B,2C and S2) at the vaccine site. IFN-γ levels at the vaccine sites were first detected at day 3 after vaccination, peaked at day 12, and subsided at days 16–21; these kinetics mirror the time-course of T-cell infiltration (Fig. 3C and 2B). Altogether, this data indicates DCs are exposed to high IL-12 concentrations while the CpG-ODN danger signals and tumor lysates are presented from the vaccine. Provision of CpG-ODN signaling into the vaccine dramatically increased IFN-γ production in situ (Fig. 3D), likely due to their role in promoting DC activation (including ligation of TLR-9),24 and this cytokine is also hallmark of cytotoxic T-cell activity and Th1 responses.22-24 Importantly, PLG vaccines sustained the induction of IL-12 and IFN-γ from infiltrating immune cells for 16 d, demonstrating Th1 polarization in the immune response and prolonged CD8(+) CTL activity5,19,22,23 to the tumor antigens embedded within the vaccine’s matrix.18,19
Figure 3.
Kinetics of IL-12 and IFN-γ production at vaccine site. (A) The concentrations of IL-12 at the implant site of PLG vaccines as a function of time post-implantation. (B) The in vivo concentration of IL-12 at the implant site of blank matrices (Blanks), PLG vaccines (GM+CpG+LYS) or matrices loaded with either Lysate alone (Lys) or Lysate with 3000ng of GM-CSF (GM+Lys) or Lysate with100 μg PEI-CpG-ODN (CpG+Lys) or the combination of GM-CSF and PEI-CpG-ODN (GM+CpG) at Day 12 after implantation into the backs of C57BL/6J mice. (C) The concentration of IFN-γ at the implant site of PLG vaccines as a function of time post-implantation. (D) The in vivo concentration of IFN-γ at the implant site of blank matrices (Blanks), PLG vaccines (GM+CpG+LYS) or matrices loaded with either Lysate alone (Lys) or Lysate with 3000ng of GM-CSF (GM+Lys) or Lysate with100 μg PEI-CpG-ODN (CpG+Lys) or the combination of GM-CSF and PEI-CpG-ODN (GM+CpG) at Day 12 after implantation into the backs of C57BL/6J mice. Values in A, B, C and D represent mean and standard deviation (n = 4 or 5). *p < 0.05** p < 0.01 as compared with controls.
Effects of PLG vaccination on cell populations in draining LN
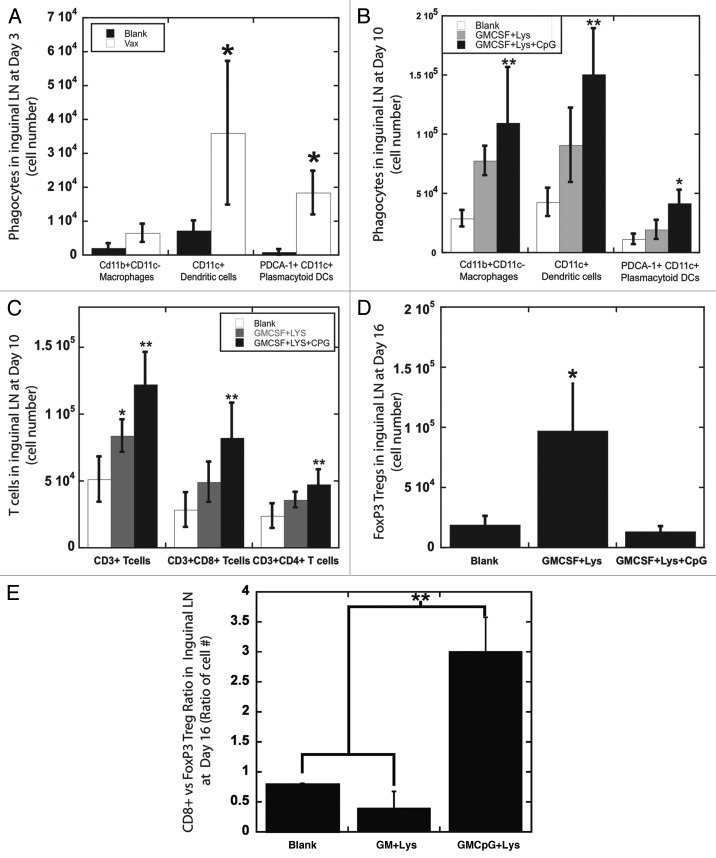
To determine the systemic effects of PLG vaccines, cellular populations in the draining LNs were monitored over time. Blank matrices and PLG matrices containing GM-CSF, lysate and CpG-ODN (PLG vaccines) were implanted subcutaneously into the backs of mice at a distance of approximately 9 mm from the inguinal LN. PLG vaccines resulted in a rapid enhancement in the numbers of phagocytic cells in the proximal inguinal LN, as 4 fold increases in resident DCs and plasmacytoid DCs numbers were observed at day 3 (Fig. 4A). Vaccination induced persistent APC activity in LNs, and at day 10 PLG vaccines maintained approximately 3-fold increases in macrophage, DC and plasmacytoid DC numbers in LNs (in comparison to blank PLG matrices) (Fig. 4B). Consequently, significant T-cell expansion was observed at the draining inguinal LNs at day 10 of vaccination, as a 2.5-fold increase in CD3(+) T-cell numbers (~1.3x106 T cells) was generated in comparison to blank matrices (5X105 T cells). Importantly, the T-cell expansion induced by PLG vaccines was mostly due to the expansion of the CD8(+) CTL subset, as their numbers in LNs increased similarly in scale from 2.8x105 CTLs (blank matrices) to 8.2x105 CTLs (PLG vaccines) at day 10 of vaccination (Fig. 4C). Provision of persistent CpG-ODN signaling alongside antigenic signals (tumor lysate), was required of the vaccines ability to induce polarization of LN T cells toward CD8(+) CTL expansion (Fig. 4C), and to downregulate FoxP3(+) T regulatory cell activity in LNs (Fig. 4D and E). The ratio of CD8(+), cytotoxic T cells to Treg cells to T reg cells was approximately 3-fold higher in animals treated with scaffold formulations that included danger signaling in addition to GM-CSF and tumor lysate delivery (Fig. 4E). FoxP3 T cells may suppress the cytotoxicity of CD8(+) T cells and extinguish vaccine activity, and these results presented in this study confirm previous observations that persistent or unopposed GM-CSF signaling can cause the generation of Treg cells and immunosuppressive pathways and TLR antagonism by danger signaling may override these effects and enhance anti-tumor responses.19,25-28
Figure 4.
Effects of PLG vaccination on cells in draining lymph nodes (A and B) The number of phagocytic cells: CD11b(+)CD11c(-) macrophages, CD11c(+) DCs, and PDCA-1(+)CD11c(+) plasmacytoid DCs, in the draining inguinal lymph nodes of mice at (A) day 3 and at (B) day 10 after implantation of blank matrices (Blank) or PLG matrices containing GM-CSF and Lysate (GMCSF+LYS) only or PLG vaccines (GMCSF+Lys+CpG). (C) The number of T cells: CD3(+) T cells, CD3(+)CD8(+) T cells, and CD3(+)CD4(+) T cells, in the draining inguinal lymph nodes of mice at day 10 after implantation of blank matrices (Blank) or PLG matrices containing GM-CSF and Lysate (GMCSF+LYS) only or with CpG-ODN (GMCSF+Lys+CpG). (D) The number of CD3(+)FoxP3(+) Tregs in the draining inguinal lymph nodes of mice at day 16 after implantation of blank matrices (Blank) or PLG matrices containing GM-CSF and Lysate (GMCSF+LYS) only or with CpG-ODN (GMCSF+Lys+CpG). (E) The ratio of CD8 T cells vs. CD3(+)FoxP3(+) Tregs in the draining inguinal lymph nodes of mice at day 16 after implantation of PLG matrices containing GM-CSF and Lysate (GMCSF+LYS) only or with CpG-ODN (GMCSF+Lys+CpG). Values represent mean and standard deviation (n = 4 or 5). *p < 0.05** p < 0.01 as compared with controls.
Discussion
Many infections provide antigenic and stimulatory signals to the immune system that activate DCs, which then process and present instructive signals to prime effector T-cell responses. The kinetics of these responses, to infection have been described extensively - including the rapid recognition and activation of DC populations and the time lag associated with T-cell priming and expansion.5-10 Optimally, these effector T cells mediate the killing of infectious agents and persist until antigens and pathogens are cleared, but tumors have evolved mechanisms to evade and subdue these destructive responses.1-3,11,12 Based on these considerations it is intuitively appealing to hypothesize that vaccine-based therapy for established tumors may require not only the stimulation of innate DC responses, but also the maintenance of adaptive T-cell responses. This implies that vaccine formulations must be able to present antigen in an immunostimulatory environment for extended periods to maintain T-cell responses until all tumor cells have been killed by effectors.
In this study, we determined the long-term vaccine kinetics of both innate DC responses and effector T-cell responses to a biomaterial based vaccine, and its relationship to vaccine efficacy. Previously, the PLG vaccine system demonstrated significant CD8(+), specific CTL induction, both locally at the vaccine site and systemically in spleens and via the eradication of B16 melanoma tumors.18,19 The immune responses to PLG vaccines as outlined here, propagate with kinetics similar to viral and bacterial infections, as described by other researchers.6-10 These results also indicate that the vaccine’s ability to coordinate persistent DC activation and T-cell responses in situ was manifested both locally and systemically, resulting in enhanced APC generation in LNs followed by T-cell expansion with similar kinetics (Figs. 2 and 5). A unique feature of these PLG vaccines is their prolonged maintenance of local T-cell responses (detectable at 28 d) by sustained antigen presentation after the conclusion of DC recruitment and activation, which likely underlies the therapeutic efficacy of the vaccine.
Vaccination utilizing melanoma vaccines fabricated into PLG matrices resulted in 2 distinct phases of immune responses in situ. First, innate responses included macrophages/monocyte infiltration detected at 24 h (Fig. S2) and DC activation began at day 3, peaked at day 5 and subsided at day 12 post-vaccination (Fig. 2A and B). The second phase consisted of adaptive T-cell responses consisting mostly of CD8(+) cytotoxic T-lymphocytes (CTLs) (Fig. 2C), which began at day 5, peaked at day 12 and subsided at day 28 (Fig. 2A and B). Indeed, CTL responses to the PLG matrix manifested potent effector function, with vaccination resulting in a prototypical activation phase that gradually plateaus, and was followed by a contraction phase as the antigen was cleared. The time lag associated with the onset of the effector response (detected at Day 5 after implantation) is likely the time required to induce sufficient DC recruitment and priming.
The vaccine also induced persistent IL-12 and IFN-γ production consistent with DC and T-cell infiltration into the vaccine site; this is a hallmark of Th1 and cytotoxic T-cell responses against tumors. Previously, it was shown that these PLG vaccines can induce CTLs expressing TCRs specific for the melanoma antigen, Trp-2,18,19 and the kinetics of cytotoxic T-cell homing to the vaccine site is likely in response to prolonged antigen presentation (tumor lysates) by the PLG matrix. The timing of the peak levels of T-cell infiltration and IFN-γ production at the vaccine site coincided with a significant drop in local DC numbers at days 7–12 and was likely due to CD8(+)T-cell cytotoxicity against these antigen-presenting cells18,19 - as these cells continue to process and present the tumor antigens that killer T cells are being primed against. Importantly, similar kinetics of APC recruitment and CTL expansion were observed systemically in the draining, inguinal LNs. These results indicate that vaccination with this system requires stimulation for at least 3 d to induce the onset of innate immunity (i.e., DC responses) and that effector CTL responses can be maintained until antigen is depleted from the vaccine site.
The relationship between vaccine duration and vaccine efficacy suggests the importance of prolonged maintenance of effector responses against established tumors. By explanting the PLG vaccines between 1 and 7 d – before the onset of significant effector CTL responses in situ – little to no benefit was observed in regards to tumor progression or survival. When the scaffolds were implanted for greater than 7 d, the engineered matrices efficiently maintained potent T-cell responses by providing a secondary site of tumor antigen presentation, after the primary induction of innate DC activation had occurred. Vaccination times that included the maintenance of T-cell responses greater than 7 d resulted in enhanced prophylactic protection and the significant regression of solid tumors. In tumor bearing animals, prolonged durations of vaccination (> 16 d) significantly augmented persistent CTL responses locally and slowed tumor progression, resulting in an almost 3-fold increase in mouse survival.
Current vaccination methods include protein fusions, antibody conjugation to antigen and viral gene therapy to enhance the half-life, targeting and immunogenicity of antigen. Biomaterials have also been utilized to sustain or target antigen delivery to immune cells in animal models,13,14 predominantly as particulate systems. Although, current biomaterials are capable of extending the in vivo half-life of antigen delivery from hours to days, allowing for prolonged DC stimulation, this lifespan is shortened by biodegradation, lymphatic drainage, and renal clearance.13,14,29-31 Thus, the ability of current methods to effectively coordinate adaptive responses (for example, T cells) is likely inadequate, as exemplified by their inability to stimulate reproducible tumor regression against solid and invasive tumors in mice and man, and this may be attributed to a rapid loss of vaccine activity (days to a week) in vivo.
In summary, this study highlights the benefit of augmenting immune cell responses in therapeutic vaccine formulations and suggests specific temporal requirements for the bioactivity of vaccine systems. Our data combined with past studies of immune responses to infection2-10 indicate that approximately 7 d of immunostimulation by vaccines is required to stimulate effector T-cell responses. Importantly, long-term antigen stimulation (> 12 d; after DC activation) provided a distinct benefit in the maintenance of CTL responses and the efficacy of therapeutic vaccines to established tumors. This duration of stimulation is predominantly absent in current vaccine approaches.
Materials and Methods
GM-CSF and CpG-ODN incorporation and bioactivity
To determine the incorporation of GM-CSF and CpG-ODN into PLG scaffolds, 125I-labeled hr-GM-CSF (Perkin Elmer) and P33-CpG-ODN 1826 (oligofactory; radiolabeled at Perkin Elmer) were utilized as a tracer. The amount of 125I-hr-GM-CSF and P33-CpG-ODN in PLG vaccines was determined at each time point by counting the radioactivity in a gamma counter or a scintillation counter.
Total protein content
To measure the total protein incorporated (tumor lysate) into the PLG vaccines, 1.0N NaOH was utilized to dissolve the PLG scaffold and solubilize the protein incorporated into the device. Protein was quantified using the Micro BCA Protein Assay Kit. A set of 5 devices were used for these analyses.
Matrix Fabrication
An 85:15, 120 kD copolymer of D,L-lactide and glycolide (PLG) (Lakeshore Biomaterials, Birmingham, Al) was utilized in a gas-foaming process to form porous PLG matrices.32 In brief, PLG microspheres encapsulating GM-CSF were first made using a standard double emulsion process.33 PLG microspheres were then mixed with 150 mg of the porogen, sucrose (sieved to a particle size between 250 μm and 425 μm), and compression molded. The resulting disc was allowed to equilibrate within a high-pressure CO2 environment, and a rapid reduction in pressure causes the polymer particles to expand and fuse into an interconnected structure.20 The sucrose was leached from the scaffolds by immersion in water yielding scaffolds that were 90% porous. To incorporate tumor lysates into PLG scaffolds, biopsies of B16-F10 tumors that had grown subcutaneously in the backs of C57BL/6J mice (Jackson Laboratory, Bar Harbor Maine), were digested in collagenase (250 U/ml) (Worthington, Lakewood, NJ) and suspended at a concentration equivalent to 107 cells per ml after filtration through 40 μm cell strainers. The tumor cell suspension was subjected to 4 cycles of rapid freeze in liquid nitrogen and thaw (37°C) and then centrifuged at 400 rpm for 10 min. The supernatant (1ml) containing tumor lysates was collected, incubated with the PLG microspheres and lyophilized and the resulting mixture was used to make PLG scaffold-based cancer vaccines. To incorporate CpG-ODNs into PLG scaffolds, CpG-ODN 1826, 5′-tcc atg acg ttc ctg acg tt-3′, (Oligofactory, Holliston, MA) was first condensed with poly(ethylenimine) (PEI, Mn ~60,000, Sigma Aldrich) molecules by dropping ODN-1826 solutions into PEI solution, while vortexing the mixture.20,23,34 The charge ratio between PEI and CpG-ODN (NH3+:PO4–) was kept constant at 7 during condensation. PEI-CpG-ODN condensate solutions were then vortexed with 60 μl of 50% (wt/vol) sucrose solution, lyophilized and mixed with dry sucrose to a final weight of 150 mg. The sucrose containing PEI-CpG-ODN condensate was then mixed with blank, GM-CSF and/or tumor lysate loaded PLG microspheres to make PLG cancer vaccines.
In situ identification of DC and T-cell infiltration
Blank PLG matrices and matrices containing 3000ng GM-CSF alone or in combination with either 1, 10, 50, or 100 μg CpG-ODN (studies were also performed with tumor lysates co-presented with either 3000ng GM-CSF or 100 μg CpG-ODN alone or in combination) were implanted into subcutaneous pockets on the back of 7–9 week old male C57BL/6J mice. For histological examination scaffolds were excised and fixed in Z-fix solution, embedded in paraffin, and stained with hematoxylin and eosin. For SEM analysis, matrices were sputter coated with gold prior to visualization.
To analyze DC and T-cell recruitment, scaffolds were excised at various time-points and the ingrown tissue was digested into single cell suspensions using a collagenase solution (Worthingtion, 250 U/ml) that was agitated at 37°C for 45 min. The cell suspensions were then poured through a 40μm cell strainer to isolate cells from scaffold particles and the cells were pelleted and washed with cold PBS and counted using a Z2 coulter counter (Beckman Coulter). To assess DC infiltration, subsets of the total cell population isolated from PLG matrices were then stained with primary antibodies (BD PharMingen, San Diego, CA) conjugated to fluorescent markers to allow for analysis by flow cytometry. APC-conjugated CD11c (dendritic cell marker) stains were conducted for DC recruitment analysis. To assess T-cell infiltration, PE-Cy7 conjugated CD3 stains were performed in conjunction with APC-conjugated CD8a (CD8 T cells), FITC-conjugated CD4 (CD4 T cells) and analyzed with flow cytometry. Cells were gated according to positive FITC, APC and PE using isotype controls, and the percentage of cells staining positive for each surface antigen was recorded.
Tumor growth assays, and in situ cytokine concentrations
PLG-based vaccines were developed utilizing matrices incorporating melanoma tumor lysates, 3,000ng GM-CSF and 100 μg PEI-CpG-ODN condensates and were implanted subcutaneously into the lower left flank of C57BL/6J mice. For prophylactic vaccinations, animals were challenged 14 d later with a subcutaneous injection of 105 B16-F10 melanoma cells (ATCC, Manassas, NJ) in the back of the neck. To assess the effect of the duration of vaccination, PLG vaccines were explanted at days, 1, 3, 7, 12, and 16 d after implantation. Animals were monitored for the onset of tumor growth (approximately 1mm3) and sacrificed for humane reasons when tumors grew to 20 - 25 mm (longest diameter).
To determine in vivo GM-CSF concentrations in adjacent tissue and IL-12p70, and IFN-γ, concentrations at the matrix implant site excised tissue was digested with tissue protein extraction reagent (Pierce). After centrifugation, the concentration of GM-CSF, IL-12p70, and IFN-γ in the supernatant was then analyzed with ELISA (R&D systems), according to the manufacturers instructions.
Statistical analysis
All values in the present study were expressed as mean ± SD. The significant differences between the groups were analyzed by a Student’s t test and a P value of less than 0.05 was considered significant
Supplementary Material
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/16277
References
- 1.Steinman RM,, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Gilboa E. Dendritic cell based vaccines. J Clin Invest. 2007;117:1195–203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–47. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct TH responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–9. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–7. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 6.Pope C, Kim A, Marzo D, Masopust K, Williams J, Jiang H, et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–9. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 7.Sano G, Hafalla JC, Morrot A, Abe R, Lafaille JJ, Zavala F. Swift development of protective effector functions in naive CD8 T cells against malaria liver stages. J Exp Med. 2001;194:173–80. doi: 10.1084/jem.194.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8 T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–42. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- 9.Padilla AM, Simpson LJ, Tarleton RL. Insufficient TLR Activation Contributes to the Slow Development of CD8+ T Cell Responses in Trypanosoma cruzi Infection. J Immunol. 2009;183:1245–52. doi: 10.4049/jimmunol.0901178. [DOI] [PubMed] [Google Scholar]
- 10.Wakim LM, Waithman J, Rooijen NV, Heath WR, Carbone FR. Dendritic Cell-Induced Memory T Cell Activation in Nonlymphoid Tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–24. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali OA, Mooney DJ. “Converging Cell Therapy with Biomaterials.” Cell Transplantation from Laboratory to Clinic. Elsevier Inc. Burlington, MA. 591-609 (2006). [Google Scholar]
- 14.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–60. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 15.Uchida M, Natsume H, Kishino T, Seki T, Ogihara M, Juni K, et al. Immunization by particle bombardment of antigen-loaded poly-(DL-lactide-co-glycolide) microspheres in mice. Vaccine. 2006;24:2120–30. doi: 10.1016/j.vaccine.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–64. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 17.Kwon YJ, James E, Shastri N, Fréchet JM. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proc Natl Acad Sci USA. 2005;102:18264–8. doi: 10.1073/pnas.0509541102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–8. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali OA, Emerich D, Dranoff G, Mooney DJ. In Situ Regulation of DC Subsets and T Cells Mediates Tumor Regression in Mice Sci Transl Med. 1, 8-19. (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pulendran B, Banchereau J, Burkeholder S, Kraus E, Guinet E, Chalouni C, et al. Flt3-Ligand and Granulocyte Colony-Stimulating Factor Mobilize Distinct Human Dendritic Cell Subsets In Vivo. J Immunol. 2000;165:566–72. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 22.O'Shea JJ, Visconti R. Type 1 IFNs and regulation of TH1 responses: enigmas both resolved and emerge. Nat Immunol. 2000;1:17–9. doi: 10.1038/76872. [DOI] [PubMed] [Google Scholar]
- 23.Magram J, Sfarra J, Connaughton S, Faherty D, Warrier R, Carvajal D, et al. IL-12-deficient mice are defective but not devoid of type 1 cytokine responses. Ann N Y Acad Sci. 1996;795:60–70. doi: 10.1111/j.1749-6632.1996.tb52655.x. [DOI] [PubMed] [Google Scholar]
- 24.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–94. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. G. Dranoff Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jinushi M, Hodi SF, Dranoff G. Enhancing the clinical activity of granulocyte-macrophage colony-stimulating factor-secreting tumor cell vaccines. Immunol Rev. 2008;222:287–98. doi: 10.1111/j.1600-065X.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 28.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–37. [PMC free article] [PubMed] [Google Scholar]
- 29.Pooyan S, Qiu B, Chan MM, Fong D, Sinko PJ, Leibowitz MJ, et al. Conjugates bearing multiple formyl-methionyl peptides display enhanced binding to but not activation of phagocytic cells. Bioconjug Chem. 2002;13:216–23. doi: 10.1021/bc0100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan L, Pooyan S, Hu P, Leibowitz MJ, Stein S, Sinko PJ. Peritoneal macrophage uptake, pharmacokinetics and biodistribution of macrophage-targeted PEG-fMLF (N-formyl-methionyl-leucyl-phenylalanine) nanocarriers for improving HIV drug delivery. Pharm Res. 2007;24:2110–9. doi: 10.1007/s11095-007-9402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachelder EM, Beaudette TT, Broaders KE, Paramonov SE, Dashe J, Frochet JM. Acid-Degradable Polyurethane Particles for Protein-Based Vaccines: Biological Evaluation and in Vitro Analysis of Particle Degradation Products. Mol Pharm. 2008;5:876–84. doi: 10.1021/mp800068x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris LD, Kim BS, Mooney DJ. Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Res. 1998;42:396–402. doi: 10.1002/(SICI)1097-4636(19981205)42:3<396::AID-JBM7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm Res. 1991;8:713–20. doi: 10.1023/A:1015841715384. [DOI] [PubMed] [Google Scholar]
- 34.Ali OA, Mooney DJ. Sustained GM-CSF and PEI condensed pDNA presentation increases the level and duration of gene expression in dendritic cells. J Control Release. 2008;132:273–8. doi: 10.1016/j.jconrel.2008.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.