Abstract
Pulsatile drug delivery systems (PDDS) have attracted attraction because of their multiple benefits over conventional dosage forms. They deliver the drug at the right time, at the right site of action and in the right amount, which provides more benefit than conventional dosages and increased patient compliance. These systems are designed according to the circadian rhythm of the body, and the drug is released rapidly and completely as a pulse after a lag time. These products follow the sigmoid release profile characterized by a time period. These systems are beneficial for drugs with chronopharmacological behavior, where nocturnal dosing is required, and for drugs that show the first-pass effect. This review covers methods and marketed technologies that have been developed to achieve pulsatile delivery. Marketed technologies, such as PulsincapTM, Diffucaps®, CODAS®, OROS® and PULSYSTM, follow the above mechanism to render a sigmoidal drug release profile. Diseases wherein PDDS are promising include asthma, peptic ulcers, cardiovascular ailments, arthritis and attention deficit syndrome in children and hypercholesterolemia. Pulsatile drug delivery systems have the potential to bring new developments in the therapy of many diseases.
Keywords: capsular system, pulsatile drug delivery system, pulse, rupturable coating
Introduction
Oral drug delivery is the largest segment of the total drug delivery market. It is the most preferred route for drug administration. The oral controlled-release systems show a typical pattern of drug release in which the drug concentration is maintained in the therapeutic window for a prolonged period of time, thereby ensuring sustained therapeutic action. There are certain conditions for which such a release pattern is not suitable that demand release of a drug after a lag time. In other words, they require pulsatile drug delivery system (PDDS). The pulsatile system is gaining a lot of interest, as the drug is released completely after defined lag time (Fig. 1). Pulsatile drug delivery is time and site-specific drug delivery, thus providing spatial and temporal delivery and increasing patient compliance. Pulsatile drug delivery is defined as the rapid and transient release of certain amount of molecules within a short time period immediately after a predetermined off-released period, i.e., lag time, or these systems have a peculiar mechanism of delivering the drug rapidly and completely after a lag time, i.e., a period of no drug release. Such a release pattern is known as pulsatile release.1-4
Figure 1.
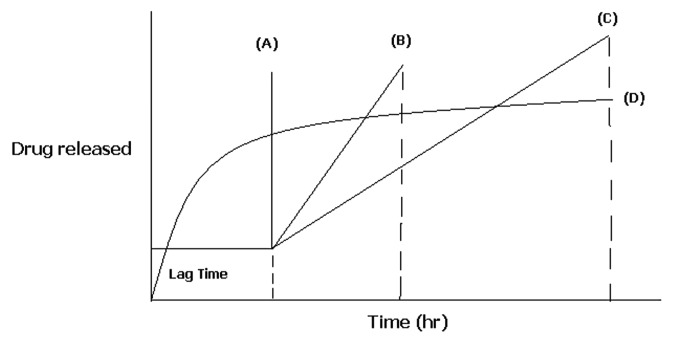
Schematic representation of different drug delivery systems where (A) sigmoidal release after lag time (B) delayed release after lag time (C) sustained release after lag time (D) extended release without lag time.
Humans exhibit endogenous circadian rhythms that are regulated by the master circadian clock of the body, the suprachiasmatic nucleus. Chronopharmacotherapy of diseases (bronchial asthma, myocardial infarction, angina pectoris, rheumatic disease, ulcer and hypertension) (Table 1) that show circadian rhythms in their pathophysiology and treatment of such diseases require pulsatile drug delivery systems, by which drug is released rapidly and completely as a pulse after a lag time.5-7 There are many other conditions that demand pulsatile release, like many body functions that follow circadian rhythms, such as secretion of hormones [including follicle stimulating hormone (FSH), luteinizing hormone (LH), luteinizing hormone releasing hormone (LHRH), estrogen and progesterone], acid secretion in the stomach, gastric emptying and gastrointestinal blood transfusion. Drugs that produce biological tolerance demand a system that will prevent their continuous presence at the biophase, as this tends to reduce their therapeutic effect. The lag time is essential for drugs that undergo degradation in gastric acidic medium (e.g., peptide drug) and irritate the gastric mucosa or induce nausea and vomiting. Targeting a drug to a distal organ of gastrointestinal tract (GIT), like the colon, requires that the release is prevented in the two-third portion of the GIT. Drugs (β-blockers or β-estradiol) that undergo first-pass metabolism, resulting in reduced bioavailability, altered steady-state levels of drug and metabolite and potential food drug interaction, require delayed released to the extent possible.8-10 All the above attributes can be taken into account in designing a delivery system that exhibits pulsatile release characteristics and releases the drug in a predetermined fashion at a particular site.
Table 1. Diseases that require pulsatile drug delivery.
| Disease | Chronological behavior | Drugs used |
|---|---|---|
| Peptic ulcer |
Acid secretion is high in the afternoon and at night. |
H2blockers |
| Cancer |
The blood flow to tumors is 3-fold greater during each daily activity phase of the circadian cycle than during the daily rest phase. |
Vinca alkaloids, Taxanes |
| Duodenal ulcer |
Gastric acid secretion is highest at night while gastric and small bowel motility and gastric emptying are all slower at night. |
Proton pump inhibitors |
| Neurological disorders |
The central pathophysiology of epilepsy and the behavioral classification of convulsive events. |
MAO-B inhibitor |
| Hypercholesterolemia |
Cholesterol synthesis is generally higher during night than day time. |
HMG CoA reductase Inhibitors |
| Diabetes mellitus |
Increase in the blood sugar level after meal. |
Sulfonylurea, Insulin |
| Arthritis |
Level of pain increases at night. |
NSAIDs, Glucocorticoids |
| Cardiovascular diseases |
BP is at its lowest during the sleep cycle and rises steeply during the early morning. |
Nitroglycerin, calcium channel blocker, ACE inhibitors |
| Asthma |
Precipitation of attacks during night or at early morning. |
Β2 agonist, Antihistamines |
| Attention deficit syndrome | Increase in DOPA level in afternoon. | Methylphenidate |
There are numerous advantages of the pulsatile drug delivery systems. Some of them are enlisted as below:
These systems can be used for extended day time or night time activity.
They reduce the dose frequency, dose size and cost, which ultimately reduces side effects, thereby improving patient compliance.
Drug adapts to suit circadian rhythms of body functions or diseases.
Drug targeting to a specific site, like the colon, can be achived.
They protect mucosa from irritating drugs.
Drug loss by extensive first pass metabolism is prevented.
They provide constant drug levels at the site of action and prevent the peak-valley fluctuations.11
Disadvantages:
Low drug loading capacity and incomplete release of drug.
Multiple manufacturing steps.
Classification of Pulsatile Drug Delivery Systems
Pulsatile drug delivery systems can be classified in to three categories:
Time-controlled pulsatile release systems
Delivery systems containing erodible coating layer
Bulk-eroding system
Bulk erosion means that the ingress of water is faster than the rate of degradation. In this case, degradation take places throughout the polymer sample and proceeds until a critical molecular weight is reached. At this point, degradation products become small enough to be solubilized, and the structure starts to become significantly more porous and hydrated. Hence, there is a time lag before the drug can be released corresponding to the time required for critical molecular weight to be reached. A number of research groups have investigated purposed formulation by using this system.12,13
Surface eroding system
In this type of system, the reservoir device is coated with a soluble or erodible layer, which dissolves with time and releases the drug after a specified lag period, as in case of a chronotropic system, where the drug is entrapped in the core layer with hydroxyl propyl methyl cellulose (HPMC) and with an additional layer of enteric-coated film outside it. The time clock system is another example of a surface eroding system and consists of solid dosage form coated with lipid barriers like carnauba wax and beeswax along with surfactants. When both these systems come in contact with aqueous medium, the coat emulsifies or erodes after the lag time. It is independent of the gastrointestinal motility, pH, enzyme and gastric residence. The lag time and onset of action are controlled by thickness and the viscosity grade of the polymer used.14-17
Delivery system with rupturable coating layer
These systems depend on the disintegration of the coating layer for the release of a drug. The pressure necessary for the rupture of the coating can be achieved by effervescent excipients, swelling agents or osmotic pressure.
An effervescent mixture of citric acid and sodium bicarbonate has been reported, wherein the mixture was incorporated in a tablet core coated with ethyl cellulose. The carbon dioxide developed after penetration of water into the core resulted in a pulsatile release of the drug after rupture of the coating. The release may depend on the mechanical properties of the coating layer. It is reported that the weak and non-flexible ethyl cellulose film ruptured adequately as compared with more flexible films. The lag time increases with increasing coating thickness and hardness of the core table.15,16 Highly swellable agents, called superdisintegrants, were used to design a capsule-based system comprising a drug, swelling agent and rupturable polymer layer.18-21
Bai et al. invented a pulsatile drug delivery system comprising a plurality of particles that were divided into several individual delivery units, each having its own distinct composition. The individual particles had the same composition as the internal core, but the thickness of the external coating layer varied. Drug delivery was controlled by the rupture of the membrane. The timing of release was controlled by the thickness of the coating and the amount of water-soluble polymer needed to achieve the pulsed release.22
Capsule-shaped system provided with release controlling plug
R.P. Scherer International Corporation, Michigan developed the pulsincap system, in which the lag time is continued by a plug that gets pushed away by swelling or erosion, releasing the drug as a pulse from the insoluble capsule body. The system is comprised of a water insoluble capsule enclosing the drug reservoir. A swellable hydrogel plug was used to seal the drug contents into the capsule body. When the capsule comes in contact with dissolution fluid, the plug gets swells, and after a lag time, the plug pushes itself outside the capsule and rapidly releases the drug. The length of the plug and its point of insertion into the capsule controlled the lag time.23,24
Stimuli-induced pulsatile release system
Stimuli-based drug delivery systems release the drug in response to stimuli that are induced by the biological environment. Release of the drug in response to those systems results from stimuli-induced changes in the gels or in the micelles, which may deswell, swell or erode in response to the respective stimuli. In these systems, the drug is released after stimulation by any biological factor, like temperature or any other chemical stimuli.25,26 The mechanisms of drug release include ejection of the drug from the gel as the fluid phase syneresis out, drug diffusion along a concentration gradient, electrophoresis of charged drugs toward an oppositely charged electrode and liberation of the entrapped drug as the gel or micelle complex erodes. There has been much interest in the development of a stimuli-sensitive delivery system that releases therapeutic agents in the presence of a specific enzyme or protein. These systems are considered excellent delivery candidates, since they can be modified according to the task to be achieved. They are further classified into:
Thermoresponsive pulsatile release
Hydrogels that undergo reversible volume changes in response to changes in temperature are known as thermosensitive gels. Thermosensitive hydrogels have been investigated as possible drug delivery carriers for stimuli-responsive drug delivery. Hydrogels are crosslinked networks of biological, synthetic or semi-synthetic polymers. These gels shrink at a transition temperature that is related to the lower critical solution temperature (LCST) of the linear polymer from which the gel is made. One of the common characteristics of temperature-sensitive polymers is the presence of hydrophobic groups, such as methyl, ethyl and propyl groups. From the many temperature-sensitive polymers, poly(N-isopropylacrylamide) (PINPAm) is probably the most extensively used. PINPA cross-linked gels have shown thermoresponsive, discontinuous swelling/deswelling phases, swelling, for example, at temperatures below 32°C and shrinking above this temperature. Krezanoski et al. describe the use of the reversed thermal gelation (RTG) system, consisting of a polyol polymer, such as Pluronic®. Gels of this type of polymer display low viscosity at ambient temperature, and exhibit a sharp increase in viscosity as the temperature rises. Yuk et al. developed temperature-sensitive drug delivery systems utilizing an admixture of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymer (F-68) and poly vinyl alcohol (PVA).28 The pulsatile release of acetaminophen occurred due to pulsatile change in temperature between 35°C and 40°C.29
Chemical stimuli-induced pulsatile release
The development of stimuli-sensitive delivery systems has been the latest topic of interest. These systems release therapeutic agents in the presence of any biological factor, like enzyme, pH or any other chemical stimuli. One prominent application of this technology has been the development of a system that can automatically release insulin in response to elevated blood glucose levels. Kazunori et al.30 developed a gel composed of PNIPAAm with phenylboronic acid moieties that showed a remarkable change in the swelling induced by glucose. This type of glyco-sensitive gel may have potential utilitiy in self-regulated drug releasing systems as well as in other applications, such as actuators, regulators and separation systems with glyco-sensitivity.
pH-dependent systems for glucose-stimulated drug delivery are based on the oxidation reaction of glucose to gluconic acid, catalyzed by glucose oxidase, which can lower pH to approximately 5.8 in a glucose-rich environment, such as the bloodstream after a meal. This reaction can be used to drive the swelling of a pH-dependent membrane. A dual membrane system was formed, with the first membrane referred to as the glucose-sensing membrane, in which glucose oxidase was immobilized on cross-linked polyacrylamide. The second membrane worked as an interface between the insulin reservoir and the sensing membrane. Composed of N, N-diethylaminoethyl methacrylate and 2-hydroxypropyl methacrylate (DEA-HPMA), it formed the barrier membrane.31,32
Externally regulated pulsatile release system
Electroresponsive pulsatile release
An electric field as an external stimulus has advantages, such as availability of equipment that allows precise control with regard to the magnitude of the current, duration of electric pulses, interval between pulses, etc. Electrically responsive delivery systems are prepared from polyelectrolytes and are thus pH responsive as well as electroresponsive. Under the influence of the electric field, electroresponsive hydrogels generally deswell, swell or erode. Poly(2-acrlamide-2-methylpropanesulfonic acid-co-butyl methacrylate) [P(AMPS-co-BMA)] hydrogels were used for electric stimuli-induced drug delivery systems.33,34 Kwon et al. exploited cross-linked poly(2-acrylamide-2-methylpropanesulfonic acid-co-butyl methacrylate) (P(AMPS-co-BMA)) hydrogels for electric stimuli-induced drug delivery.35 The mechanisms of drug release include expulsion of a drug from the gel as the fluid phase syneresis out, drug diffusion along a concentration gradient and electrophoresis of a charged drug toward an oppositely charged electrode and release of the entrapped drug as the gel complex erodes with regards to the magnitude of the current, duration of electric pulses, interval between pulses, etc. Electrically responsive delivery systems are prepared from polyelectrolytes and are thus pH responsive as well as electroresponsive. Under the influence of an electric field, electroresponsive hydrogels generally deswell, swell or erode. Poly(2-acrlamide-2-methylpropanesulfonic acid-co-butyl methacrylate) P(AMPS-co-BMA) hydrogels were used for electric stimuli-induced drug delivery system.36-41
Ultrasonically stimulated
Ultrasound is mostly used as an enhancer for the improvement of drug permeation through a biological barrier, such as skin, lungs, intestinal wall and blood vessels. There are several reports describing the effect of ultrasound on controlled drug delivery. Kost and coworkers depicted an ultrasound-enhanced polymer. Miyazaki et al. used ultrasound to achieve up to a 27-fold increase in the release of 5-fluorouracil from an ethylene and vinyl acetate (EVAc) matrix. Increasing the strength of the ultrasound resulted in a proportional increase in the amount of 5-fluorouracil released.42
Magnetically induced pulsatile release
Use of an oscillating magnetic to regulate the drug delivery from a polymer matrix was one of the first methodologies investigated to develop an externally controlled drug delivery system. Magnetic carriers receive a response to a magnetic field from incorporated materials, such as magnetite, iron, nickel, cobalt, etc. For biomedical applications, magnetic carriers must be water-based, biocompatible, non-toxic and non-immunogenic. Basically the mechanistic approach behind the strategy is based on slowing down the movement of oral drugs in the gastrointestinal system through magnetic attraction. This is possible by filling an additional magnetic component into capsules or tablets. The speed of travel through the stomach and intestines can then be slowed down at specific positions by an external magnet, thus changing the timing and/or extent of drug absorption into stomach or intestines.43-47
Marketed Technologies of Pulsatile Drug Delivery
Different marketed technologies has been developed for pulsatile drug delivery, such as PulsincapTM, Diffucap®, three-dimensional printing®, CODAS®, OROS®, IPDAS®, GEOCLOCK®, Ritalina®, Uniphyl®, Opana®ER.48-50 Some of them are discussed below:
PulsincapTM technology
Pulsincap was developed by R.R. Scherer International Corporation (Michigan). This device consists of a non-disintegrating half capsule body sealed at the open end with a hydrogel plug that is covered by a water-soluble cap. The whole unit is coated with an enteric polymer to avoid the problem of variable gastric emptying. When this capsule comes in contact with the dissolution fluid, it swells, and after a lag time, the plug pushs itself outside the capsule and rapidly releases the drug. Another formulation approach was in the form of a bead or granule with a four-layered spherical structure, which consists of a core, a drug, swelling agent (e.g., sodium starch glycolate or carboxy methyl cellulose sodium) and an outer membrane of water-insoluble polymer (e.g., ethyl cellulose, Eudragit® RL). The penetration of GI fluids through the outer membrane causes the expansion of the swelling agent. The resulting stress due to swelling force leads to the destruction of the membrane and subsequent rapid drug release. Polymers used for designing the hydrogel plug were various viscosity grades of hydroxyl propyl methyl cellulose, polymethyl methacrylates, polyvinyl acetate and poly ethylene oxide. Another new approach was enteric-coated, timed-release, press-coated tablets (ETP tablets). These tablets were developed by coating enteric polymer on timed-released, press-coated tablets composed of an outer shell of hydroxyl propyl cellulose and core tablets containing diltiazem hydrochloride as a model drug.51-53
Patel and Patel developed a modified Pulsincap device containing diclofenac sodium to target the drug in the colon. This is a site-specific and time-dependent formulation; i.e., by administering the formulation at bed time, symptoms that are experienced early in the morning are avoided. This therapeutic effect is prolonged by continuously releasing the medication over an extended period of time after administering a single dose. The objective of the study was to explore the time- and pH-dependent controlled drug delivery of Diclofenac Sodium using the pulsincap system.54
DIFFUCAPS® technology
Variations in pH throughout the GI tract affect the solubility and absorption of certain drugs. This pH dependency can cause a problem, particularly when developing a sustained or controlled release formula. Carvedilol and dipyridamole are drugs that are soluble in the acidic conditions of the stomach but are insoluble in the neutral/slightly alkaline conditions of the intestine, where absorption of active drug is ideal. Of particular concern are weak, basic drug compounds that are insoluble at a pH greater than five. Eurand’s Diffucaps® technology facilitates the development and commercialization of novel, controlled-release delivery systems for once- or twice-daily dosing of single drugs or drug combinations that exhibit extreme pH-dependent solubility profiles and/or are poorly soluble in physiological fluids. This proprietary technology has been developed specifically for weak, basic drugs and involves the incorporation of a pharmaceutically acceptable organic acid or a crystallization-inhibiting polymer onto inert cores and coating the drug-layered beads with proprietary functional polymers. Formulations using an acid core ensure that an acidic environment surrounds the drug at all times, thereby producing a soluble drug in an in vivo environment where it would otherwise be insoluble.55
Diffucaps® is a multiparticulate bead system comprised of multiple layers of drug, excipients and release-controlling polymers. The beads contain a layer of organic acid or alkaline buffer to control the solubility of a drug by creating an optimal pH microenvironment for drugs that exhibit poor solubility in intestinal pH, in environments with pH greater than 8.0 or in physiological fluids. Diffucaps® beads are < 1.5 mm in diameter and can be filled into capsules or compressed into orally disintegrating tablets. In addition, for patients who experience difficulty in swallowing tablets or capsules, Diffucaps® products are produced in capsules that allow the capsules to be opened and the contents used as a sprinkle on foods, providing a flexible dosage form. The flexibility of the Diffucaps® system allows for easy adjustment of the release profile and dosing strength to achieve targeted in vivo results. This flexibility simplifies dose-ranging studies for drug development partners involved in clinical testing, because the beads can be encapsulated separately to create separate study arms.56
Eurand’s Diffucaps® technology is used in several currently marketed products and in novel products in clinical development. Diffucaps® multiparticulate bead system enabled the development of a formulation with an initial release of the drug for quick onset of action to maintain consistent plasma levels throughout the day. AMRIX is available in two dose-proportional strengths, 15 and 30 mg capsules. This illustrates an advantage of the Diffucaps® technology—multiple product strengths can be readily developed by using differing amounts of drug-layered beads contained in the final capsule dosage form. Diffucaps® technology created a delayed release capsule that achieves peak plasma levels in the AMRIX, when patient risk is highest, and continued maintenance throughout the day.
Advantages of this multiparticulate system are its suitability for drugs exhibiting poor solubility in lower intestinal pH, in environments with a pH above 8.0 or in physiological fluids, where it can provide dosage strength flexibility and the required PK profile, give optimal release profiles for single drugs and drug combinations and can minimize food effect. The Diffucaps® drug release system can also be used in combination with other Eurand technologies to enhance drug solubility in the GI tract.57-59
Three-dimensional printing®
Three-dimensional printing (3DP) is a novel solid freeform fabrication technology that has been applied to the fabrication of complex pharmaceutical drug devices, or three-dimensional printing (3DP) is a rapid prototyping (RP) technology. Prototyping involves constructing specific layers that use powder processing and liquid binding materials. Reports in the literature have highlighted the many advantages of the 3DP system over other processes in enhancing pharmaceutical applications; these include new methods in design, development, manufacture and commercialization of various types of solid dosage forms. For example, 3DP technology is flexible in that it can be used in applications linked to linear drug delivery systems, colon-targeted delivery systems, oral fast disintegrating delivery systems, floating delivery systems, time-controlled and pulse release delivery systems as well as dosage forms with multiphase release properties and implantable DDS. In addition, 3DP can also provide solutions for resolving difficulties relating to the delivery of poorly water-soluble drugs, peptides and proteins, highly toxic and potent drugs and controlled release of multidrugs in a single dosage forms.
Due to its flexible and highly reproducible manufacturing process, 3DP has some advantages over conventional compressing and other RP technologies in fabricating solid DDS. This enables 3DP to be further developed for use in pharmaceutics applications. However, there are some problems that limit the further applications of the system, such as the selection of suitable excipients and the pharmacotechnical properties of 3DP products. Further developments are therefore needed to overcome these issues, so that 3DP systems can be successfully combined with conventional pharmaceutics.60
Limitations of the technology as relating to pharmaceuticals have been addressed, and prototype dosage forms have been fabricated. The resolution of the 3DP tablets was found to depend on particle size and liquid migration during printing and drying. The surface finish of 3DP tablets was enhanced by uniaxial pressing. Migration inhibiting additives were effective in limiting transport. Both aqueous and ethanol-based solutions showed a decrease in migration on the order of 20% when appropriate powder bed additives were introduced. Migration was also decreased by pre-printing barriers to confine secondary printed drug solutions. Low dosage forms were fabricated with as little as 2.3 nanograms. Lower dosages are expected upon dilution of the initial drug solution. Printing forms with high dosage is limited by powder void volume, filling efficiency and drug solubility limits.
Complex oral dosage forms were fabricated with 3DP to show lagged release, extended release, double release and zero-order release. Release properties, such as lag time and release rate, were manipulated by varying the printing parameters. Dual-release and zero-order-release forms were fabricated using a surface degradation/erosion system based on HPMC, lactose and Eudragid RL100. Erosion rate constants were used to model release from tablets with non-uniform drug distributions. Diclofenac and chlorpheniramine dual-release tablets were designed with three drug regions, and dissolution of the tablets followed the model closely, exhibiting two onsets.
Two types of zero-order tablets were invented and fabricated by 3DP. These contained drug concentration gradients designed to complement the volumetric non-uniformity of eroding shells. Three formulations showed constant release of diclofenac sodium over 1–7 h (9.6 mg/hr), 1–15 h (6.8 mg/hr) and 1–36 h (2.5 mg/ hr).61,62
CODAS® (chronotherapeutic oral drug absorption system)
In certain cases, immediate release of drug is undesirable. A delay of drug action may be required for a variety of reasons. Chronotherapy is an example of when drug release may be programmed to occur after a prolonged interval following administration. Elan Drug Technology developed CODAS® technology to achieve this prolonged interval. The many advantages of the CODAS® technology include a delivery profile designed to compliment circadian pattern, controlled onset, an extended release delivery system, rate of release essentially independent of pH, posture and food, “sprinkle” dosing by opening the capsule and sprinkling the contents on food, reduction in effective daily dose and drug exposure, gastrointestinal tract targeting for local effect and reduced systemic exposure to achieve a target profile.63,64
Verelan® PM uses the proprietary CODASTM technology, which is designed for bedtime dosing, incorporating a 4- to 5 h delay in drug delivery. The controlled-onset delivery system results in a maximum plasma concentration (Cmax) of verapamil in the morning hours. These pellet-filled capsules provide for extended release of the drug in the gastrointestinal tract. The Verelan® PM formulation has been designed to initiate the release of verapamil 4–5 h after ingestion. This delay is introduced by the level of non-enteric release-controlling polymer applied to drug-loaded beads. The release-controlling polymer is a combination of water soluble and water insoluble polymers. As water from the gastrointestinal tract comes into contact with the polymer-coated beads, the water soluble polymer slowly dissolves, and the drug diffuses through the resulting pores in the coating. The water insoluble polymer continues to act as a barrier, maintaining the controlled release of the drug. The rate of release is essentially independent of pH, posture and food. Multiparticulate systems, such as Verelan® PM, have been shown to be independent of gastrointestinal motility.65
OROS® technology
OROS delivery systems were adopted for poorly water soluble drugs. The push-pull system is comprised of a bilayer or trilayer tablet core consisting of one push layer and one or more drug layers. The drug layer contains the poorly soluble drugs, osmotic agents and a suspending agent. The push layer contains among other things, an osmotic agent and water swellable polymers. A semipermeable membrane surrounds the tablet core. A variety of OROS® systems (ALZA Corp.) have been developed: Procardia XL®, Ditropan XL® and Concerta® are notable examples. The recently developed L-OROS® SOFTCAPTM delivery system combines the features of a controlled-release and bioavailability-enhanced delivery system to enhance compliance and therapeutic effect.66,67
L-OROSTM technology was developed by Alza to over come the drug solubility issue. These formulations include self-emulsifying liquid carrier formulations (SEF) that allow a drug to be more readily absorbed through the gastrointestinal membrane and blood stream. The SEF in L-OROS systems consists of drugs in non-aqueous liquid carriers formulated to give either a solution or a nanosuspension. As a drug in solution is released in the GI tract, it forms very small droplets (< 100 nm), increasing the drugs solubility, thereby enhancing bioavailability. As the drug in a nanosuspension is released, the drug nano particles are dispersed, and aggregation is prevented.68-70
IPDAS®
The intestinal protective drug absorption system (IPDAS) is a new oral drug delivery approach that is applicable to gastrointestinal (GI) irritant drugs, including the nonsteroidal anti-inflammatory drug (NSAID) class. IPDAS® delivery system can also be employed to confer the advantages of multiparticulate technology in a tablet dosage form. The IPDAS® technology is composed of numerous high-density, controlled-release beads, which are compressed into a tablet form. Once an IPDAS® tablet is ingested, it disintegrates and disperses beads containing a drug in the stomach, which subsequently passes into the duodenum and along the gastrointestinal tract in a controlled and gradual manner, independent of the feeding state. Release of active ingredient is controlled by the polymer system used to coat the beads and/or the micromatrix of polymer/active ingredient formed in the extruded/spheronised multiparticulates. The intestinal protection of IPDAS® technology is inherent by virtue of the multiparticulate nature of the formulation, which ensures wide dispersion of irritant drug throughout the gastrointestinal tract. IPDAS® was initially designed as part of the development process for Elan Drug Technologies’ proprietary naproxen formulation, Naprelan®.71 Although naproxen, as the free acid or the sodium salt, has pharmacokinetic characteristics that are consistent with once-daily dosing, the GI irritant and ulcerogenic potential associated with a large bolus dose of naproxen precludes safe use of an immediate-release form. In addition, the desired pharmacodynamic activity of a once-daily dosage form of naproxen requires rapidly available naproxen for a prompt onset of analgesic activity as well as a prolonged phase of absorption to provide 24 h analgesic/anti-inflammatory activity. The objective was to develop a once-daily controlled release system with a fast onset of action and reduced gastric irritancy. The objective was achieved in Naprelan®; it has a proven onset of pain relief within 30 min that lasts up to 24 h and has been shown to be well-tolerated.59 The many advantages of the IPDAS® technology include high-density multiparticulate formulation, gastrointestinal protection for more locally irritant drugs (e.g., NSAIDs), advantages of multiparticulate in a tablet form and fast onset if required.72,73
Geoclock®
SkyePharma developed a new oral drug delivery technology, Geoclock®, in the form of chronotherapy-focused press-coated tablets. Geoclock® tablets have an active drug inside an outer tablet layer consisting of a mixture of hydrophobic wax and brittle material in order to obtain a pH-independent lag time prior to core drug delivery at a predetermined release rate. This dry coating approach is designed to allow the timed release of both slow release and immediate release active cores by releasing the inner tablet first, after which time, the surrounding outer shell gradually disintegrates.In addition to controlled release, the Geoclock® technology also has applications for the improved release of colonic drug delivery as well as for multiple pulse drug delivery to deliver doses of a drug at specific times throughout the day. Using SkyePharma’s proprietary GeoclockTM technology, LodotraTM took the form of a specially formulated tablet, which, once ingested, did not release the active ingredient, prednisone, until approximately four hours later. LodotraTM has been designed so that maximum plasma levels are reached six hours after intake. This enables a patient to swallow the tablet at 10 p.m. before going to sleep, with the dose of prednisone not being released until 2 a.m. and reaching maximum plasma levels at 4 a.m., which is regarded as the optimal timing to relieve the stiffness and pain on waking. This nighttime release formulation is especially suited to the treatment of early morning stiffness, which is associated with rheumatoid arthritis caused by the marked release of inflammatory cytokines, including interleukin-6 (IL-6) in the early hours of the morning.74
Uniphyl®
Uniphyl (theophylline, anhydrous) tablets in a controlled-release system allow a 24 h dosing interval for patients. Uniphyl administered in the fed state is completely absorbed after oral administration.
PULSYSTM
MiddleBrook Pharmaceuticals, Inc. has developed a delivery technology called PULSYS, which enables pulsatile delivery or delivery in rapid bursts of certain drugs. The technology provides the prolonged release and absorption of a drug. The company’s PULSYS product MOXATAG (amoxicillin extended-release) tablets, 775 mg are used for the treatment of pharyngitis/tonsillitis secondary to Streptococcus pyogenes, commonly known as strep throat, for adults and pediatric patients age 12 and older. MOXATAG’s once-daily extended-release tablet consists of three components: one immediate release and two delayed-release components. The three components are combined in a specific ratio using its PULSYS technology to prolong the release of amoxicillin from MOXATAG compared with immediate-release amoxicillin.75,76
Covera-HS
Covera-HS is the first once-daily formulation of an antihypertensive/anti-anginal agent that uses an advanced tablet coating and a novel drug delivery system to mimic the body’s typical 24 h circadian variations in blood pressure and heart rate. This unique delivery technology, called COER-24TM (Controlled-Onset-Extended-Release), was developed in conjunction with Alza Corp. Covera-HS is the only controlled-release verapamil formulation that is currently approved with an indication for the management of both hypertension (high blood pressure) and angina pectoris (chest pain). Available in both 180 mg and 240 mg tablets, Covera-HS is designed for oral dosing at bedtime. Peak concentration of Covera-HS is delivered in the early waking hours, when blood pressure and heart rate are rise at their highest rate. There is minimal drug delivery during sleep, when blood pressure and heart rate are at their physiologic lowest.66
TMDS (time multiple action delivery system)
This system controls release rates for multiple ingredients within a single tablet in a programmed manner. TMDS technology allows for the release of more than one active ingredient in a single tablet formulation to be released in multiple profiles over time.
GeomatrixTM
A new delivery device, in the form of a multi-layer tablet has recently been proposed for constant drug release based on Geomatrix® Technology. It consists of a hydrophilic matrix core, containing the active ingredient and one or two impermeable or semi-permeable polymeric coatings (films or compressed barriers) applied on one or both bases of the core. The GeomatrixTM technology is applied to achieve customized levels of controlled release of specific drugs and can achieve simultaneous release of two different drugs and different rates from a single tablet. The presence of the coatings modifies the hydration/swelling rate of the core and reduces the surface area available for drug release. These partial coatings provide a modulation of the drug dissolution profile; i.e., they reduce the release rate from the device and shift the typical time-dependent release rate toward constant drug release. To achieve controlled release, a multilayered tablet constructed using two basic components, hydrophilic polymers, such as hydroxypropyl methycellulose (HPMC), and surface-controlling barriers. Active loaded core surface that is available for drug release when exposed to the fluid is controlled by barrier layers. Using this novel technology, SkyePharma has developed LodotraTM, containing a rheumathoid arthritis drug. LodotraTM delivers the active pharmaceutical ingredient at the most suitable time of day to treat the disease. Advantages of the GeomatrixTM technology are their ability to be easily incorporated into the production line, to be manufactured by readily available equipment, reproducibility, efficacy, versatility of release control mechanisms, controlled release of poorly soluble drugs, timed release of drugs, bi-phasic release of drugs, release of two or more drugs at different rates, pulsed release of drugs and safety of use.59,74,78-81
OSDRC technology
The conventional dry-coated tablet (DC) method requires core tablet preparation before, and therefore, the complicated procedure of the conventional DC method increases the manufacturing cost and the chance of failure, which may lead to a rise in core tablet supply. To solve this problem OSDRC-technology (one-step dry-coated tablet system, OSDRC-system) was developed that employs a double-structure punch (center punch and outer punch) allowing for dry-coated tablets to be assembled in a single run. The manufacturing process consists of three steps: bottom layer (the 1st outer layer) compression, core compression and whole tablet compression, which includes the upper layer and side layer (the 2nd outer layer). Because the tablets are produced in a single step while the punches make one rotation on a turntable, there is no longer any need for a separate stage to deliver the core.81-84
Diffutab®
Diffutab technology enables customized release profiles and region-specific delivery. The Diffutab technology incorporates a blend of waxes and hydrophilic polymers that control drug release through diffusion and erosion of a matrix tablet. Diffutabs are particularly useful for high-dose products and drugs that require sustained release and/or once-a-day dosing. Eurand applied this technology to both soluble and insoluble products. Advantages of Diffutabs are high drug loading, supporting sustained-release and once-a-day dosing, as matrix tablets utilize a combination of water soluble particles and active drug.57,85
Orbexa®
Orbexa technology is a multiparticulate system that enables high drug loading and is suitable for products that require granulation. Eurand’s Orbexa technology produces beads of a controlled size and density using granulation spheronization and extrusion techniques. These beads provide higher drug concentration than other systems, can be coated with functional polymer membranes for additional release rate control, are flexible and are suitable for use with sensitive materials, such as enzymes. Eurand’s Orbexa technology can be used for gastric protection, delayed release, sustained release, site-specific delivery, pulsatile delivery, complex release pattern, separation of incompatibles and combination products. Orbexa beads can be filled into capsules or single-dose sachets.86,87
Minitabs®
Eurand’s Minitabs are tiny (2 mm x 2 mm) cylindrical tablets coated with a functional membrane to control the rate of drug release. Eurand Minitabs contain gel-forming excipients that control drug release rate. Additional membranes may be added to further control release rate. The tablets are filled into capsules, allowing a combination of multiple drugs and/or multiple release profiles in the same dosage form. The Eurand Minitabs can be formulated as matrix tablets prior to further coating. Eurand Minitabs can also be used as a sprinkle on food. Eurand Minitabs combine the simplicity of tablet formulation with the sophistication of multiparticulate systems, suitable for high drug loading, and can be used as a sprinkle for pediatric and geriatric patients who have difficulty swallowing tablets.87,88
Conclusion
Rapid advancement and newer developments in the field of drug delivery have led to the formulation of the pulsatile drug delivery system, which, on one hand, can be formulated with ease and, on the other hand, provides a significant amount of therapeutic benefits. These systems deliver the drug at right time, place and amount in the patient’s body. The circadian disorders generally require chronopharmacotherapy, which can be easily accomplished by pulsatile drug delivery system in a very organized manner. During the last two decades, pharmaceutical technology has grown leaps and bounds and, with the advent of pulsatile drug delivery, one can remain assured of accomplishment of goal for safe and effective therapy. There are a number of ailments that require that the drug/bioactive be delivered in a specific way. The same cannot be either achieved or the benefits are partial when it comes to conventional dosage forms. Significant modification and designing of the conventional delivery systems in the form of pulsatile delivery systems ensures the time-controlled pulsatile release of bioactive compounds, which is prerequisite in the treatment of such disorders. The etiology of the dreaded diseases can be linked to the release of the specific drugs through these systems, which would definitely result in the betterment of the therapy. Although several milestones have been reached in this respect, there are still some unexplored facets of pulsatile drug delivery that can open new vistas through better engineering of the same.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/17717
References
- 1.Kikuchi A, Okano T. Pulsatile drug release control using hydrogels. Adv Drug Deliv Rev. 2002;54:53–77. doi: 10.1016/S0169-409X(01)00243-5. [DOI] [PubMed] [Google Scholar]
- 2.Bussemer T, Otto I, Bodmeier R. Pulsatile drug-delivery systems. Crit Rev Ther Drug Carrier Syst. 2001;18:433–58. [PubMed] [Google Scholar]
- 3.Santini JJ, Jr., Richards AC, Scheidt R, Cima MJ, Langer R. Microchips as controlled drug-delivery devices. Angew Chem Int Ed Engl. 2000;39:2396–407. doi: 10.1002/1521-3773(20000717)39:14<2396::AID-ANIE2396>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 4.Prescott JH, Lipka S, Baldwin S, Sheppard NF, Jr., Maloney JM, Coppeta J, et al. Chronic, programmed polypeptide delivery from an implanted, multireservoir microchip device. Nat Biotechnol. 2006;24:437–8. doi: 10.1038/nbt1199. [DOI] [PubMed] [Google Scholar]
- 5.Shidhaye SS, Lotlikar VM, Ghule AM, Phutane PK, Kadam VJ. Pulsatile drug delivery systems: an approach for chronotherapeutic diseases. Sys Rev Pharma. 2010;1:55–61. doi: 10.4103/0975-8453.59513. [DOI] [Google Scholar]
- 6.Richards Grayson AC, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, et al. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat Mater. 2003;2:767–72. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 7.Santini JTJ, Jr., Cima MJ, Langer R. A controlled-release microchip. Nature. 1999;397:335–8. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 8.Ritschel WA, Forusz H. Chronopharmacology: a review of drugs studies, methods find. Exp Clin Pharmacol. 1994;16:57–75. [PubMed] [Google Scholar]
- 9.Reddy RK, Jyothsna MV, Mohamed TS. Saleem, Chetty CMS. Review on: pulsatile drug delivery systems. J Pharm Sci Res. 2009;1:109–15. [Google Scholar]
- 10.Yang SY, Yang JA, Kim ES, Jeon G, Oh EJ, Choi KY, et al. Single-file diffusion of protein drugs through cylindrical nanochannels. ACS Nano. 2010;4:3817–22. doi: 10.1021/nn100464u. [DOI] [PubMed] [Google Scholar]
- 11.Rathod S. Colon Targeted Pulsatile Drug Delivery: A Review. Pharmainfo net 2007; 5.
- 12.Kalantzi LE, Karavas E, Koutris EX, Bikiaris DN. Recent advances in oral pulsatile drug delivery. Recent Pat Drug Deliv Formul. 2009;3:49–63. doi: 10.2174/187221109787158337. [DOI] [PubMed] [Google Scholar]
- 13.Lalwani A, Santani DD. Pulsatile drug delivery systems. Indian J Pharm Sci. 2007;69:489–97. doi: 10.4103/0250-474X.36932. [DOI] [Google Scholar]
- 14.Belgamwar VS, Gaikwad MV, Patil GB, Surana S. Pulsatile drug delivery systems. Asian J Pharm 2008; 141-5. [Google Scholar]
- 15.Ross AC, MacRae RJ, Walther M, Stevens HN. Chronopharmaceutical drug delivery from a pulsatile capsule device based on programmable erosion. J Pharm Pharmacol. 2000;52:903–9. doi: 10.1211/0022357001774787. [DOI] [PubMed] [Google Scholar]
- 16.Arora S, Ali J, Ahuja A, Baboota S, Qureshi J. Pulsatile drug delivery systems: An Approach for controlled drug delivery. Indian J Pharm Sci. 2006;68:295–300. doi: 10.4103/0250-474X.26655. [DOI] [Google Scholar]
- 17.Nitin DG, Kadam VJ, Jadhav KR, Kyatanwar AU, Patel UJ. Pulsatile drug delivery system. Journal Pharm Res. 2010;3:120–1. [Google Scholar]
- 18.Shidhaye SS, Lotlikar VM, Ghule AM, Phutane PK, Kadam VJ. Pulsatile Delivery Systems: An approach for chronotherapeutic diseases. Sys Rev Pharm. 2010;1:55–61. doi: 10.4103/0975-8453.59513. [DOI] [Google Scholar]
- 19.Krögel I, Bodmeier R. Floating or pulsatile drug delivery systems based on coated effervescent cores. Int J Pharm. 1999;187:175–84. doi: 10.1016/S0378-5173(99)00189-1. [DOI] [PubMed] [Google Scholar]
- 20.Bussemer T, Bodmeier R. Pulsatile drug release from coated capsules. AAPS PharmSci. 1999;1:434. [Google Scholar]
- 21.Baker RW. Controlled release delivery system by an osmotic bursting mechanism. US Patent 1976; 3:952.
- 22.Bai J, inventor. 1997 May 15. Pulsatile drug delivery system. US 5840329.
- 23.Kumar M, Ali A, Kaldhone P, Shirode A, Kadam VJ. Platform technologies for colon targeted drug delivery system: A review article. J Pharm Res. 2010;3:543–7. [Google Scholar]
- 24.Hebden JM, Wilson CG, Spiller RC, Gilchrist PJ, Blackshaw E, Frier ME, et al. Regional differences in quinine absorption from the undisturbed human colon assessed using a timed release delivery system. Pharm Res. 1999;16:1087–92. doi: 10.1023/A:1018948102778. [DOI] [PubMed] [Google Scholar]
- 25.Dalvadia H, Pate JK. Chronpharmaceutics, pulsatile drug delivery system as current trend. Asian J Pharm Sci. 2010;5:204–30. [Google Scholar]
- 26.Lee DY, Chen CM, inventors. 2000 August 15. Delayed pulse release hydrogel matrix tablet. US 6103263.
- 27.Krezanoski JZ, inventor. 1980 Feb. 12. Clear, water-miscible, liquid pharmaceutical vehicles and compositions which gel at body temperature for drug delivery to mucous membranes. US 4188373.
- 28.Oh KS, Han SK, Choi YW, Lee JH, Lee JY, Yuk SH. Hydrogen-bonded polymer gel and its application as a temperature-sensitive drug delivery system. Biomaterials. 2004;25:2393–8. doi: 10.1016/j.biomaterials.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Ashwini KG, Bhat A, Lakshmi AP, Reddy K. An Overview of Stimuli-Induced Pulsatile Drug Delivery Systems. Int J Pharm Tech Res. 2010;2:2364–78. [Google Scholar]
- 30.Kataoka K, Miyazaki H, Bunya M. Totally synthetic polymer gels responding to external glucose concentration: their preparation and application to on-off egulation of insulin release. J Am Chem Soc. 1998;120:12694–5. doi: 10.1021/ja982975d. [DOI] [Google Scholar]
- 31.Ishihara K, Kobayashi M, Ishimura N, Shinohara I. Glucose Induced Permeation Control of Insulin through a Complex Membrane Consisting of Immobilized Glucose Oxidase and a Poly(amine) Polym J. 1984;16:625–31. doi: 10.1295/polymj.16.625. [DOI] [Google Scholar]
- 32.Sershen S, West J. Implantable, polymeric systems for modulated drug delivery. Adv Drug Deliv Rev. 2002;54:1225–35. doi: 10.1016/S0169-409X(02)00090-X. [DOI] [PubMed] [Google Scholar]
- 33.Burns JS, Stevens HNE, McEwen J, Pritchard G, Brewer FM, Clarke A, et al. Pulsatile drug delivery system. J Control Release. 1996;38:151. [Google Scholar]
- 34.Saeger H, Virley P. Pulsincap and Mac226: Pulsed-Release Dosage Form. Scherer DDS, Ltd. 2004. [Google Scholar]
- 35.Kwon IC, Bae YH, Okano T, Berner B, Kim SW. Electrically credible polymer gel for controlled release of drugs. Makromol Chem Makromol Symp 1990; 33:265-77. [Google Scholar]
- 36.Yoshida R, Kaneko Y, Sakai K, Okano T, Sakurai Y, Bae YH, et al. Positive thermosensitive pulsatile drug release using negative thermosensitive hydrogels. J Control Release. 1994;32:97–102. doi: 10.1016/0168-3659(94)90229-1. [DOI] [Google Scholar]
- 37.Kwon IC, Bae YH, Kim SW. Electrically erodible polymer gel for controlled release of drugs. Nature. 1991;354:291–3. doi: 10.1038/354291a0. [DOI] [PubMed] [Google Scholar]
- 38.Kwon IC, Bae YH, Okano T, Kim SW. Drug release from electric current sensitive polymers. J Control Release. 1991;17:149–56. doi: 10.1016/0168-3659(91)90054-H. [DOI] [Google Scholar]
- 39.Jeon G, Yang SY, Byun J, Kim JK. Electrically actuatable smart nanoporous membrane for pulsatile drug release. Nano Lett. 2011;11:1284–8. doi: 10.1021/nl104329y. [DOI] [PubMed] [Google Scholar]
- 40.Maloney JM, Uhland SA, Polito BF, Sheppard NF, Jr., Pelta CM, Santini JT., Jr. Electrothermally activated microchips for implantable drug delivery and biosensing. J Control Release. 2005;109:244–55. doi: 10.1016/j.jconrel.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 41.Wood KC, Zacharia NS, Schmidt DJ, Wrightman SN, Andaya BJ, Hammond PT. Electroactive controlled release thin films. Proc Natl Acad Sci U S A. 2008;105:2280–5. doi: 10.1073/pnas.0706994105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kost J. Ultrasound for controlled delivery of therapeutics. Clin Mater. 1993;13:155–61. doi: 10.1016/0267-6605(93)90103-E. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh DST, Langer RF, Folkman J. Magnetic modulation of release of macromolecules from polymers. Proc Natl Acad Sci U S A. 1981;78:1863–7. doi: 10.1073/pnas.78.3.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H, Langer R. Magnetically-responsive polymerized liposomes as potential oral delivery vehicles. Pharm Res. 1997;14:537–40. doi: 10.1023/A:1012124205524. [DOI] [PubMed] [Google Scholar]
- 45.Cai K, Luo Z, Hu Y, Chen X, Liao Y, Yang L, Linhong D. Magnetically triggered reversible controlled drug delivery from microfabricated polymeric multireservoir devices 2009; 21:4045-9.
- 46.Hoare T, Santamaria J, Goya GF, Irusta S, Lin D, Lau S, et al. A magnetically triggered composite membrane for on-demand drug delivery. Nano Lett. 2009;9:3651–7. doi: 10.1021/nl9018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoare T, Timko BP, Santamaria J, Goya GF, Irusta S, Lau S, et al. Magnetically triggered nanocomposite membranes: a versatile platform for triggered drug release. Nano Lett. 2011;11:1395–400. doi: 10.1021/nl200494t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Survase S, Kumar N. Pulsatile Drug Dlivery: Currrent scenario. CRIPS. 2007;8:23–7. [Google Scholar]
- 49.Endo Pharmaceuticals Inc. 2011 March. Opana.com. Available from: www.opana.com
- 50.Pfizer Inc. 2002–2009. Neurontin. Available from: www.pfizer.com/files/products/uspi_neurontin.pdf
- 51.MacNeil ME, Rashid A, Stevens HN. Dispensing device. World Patent 1990; 9009168.
- 52.Fukui E, Miyamura N, Uemura K, Kobayashi M. Preparation of enteric coated timed-release press-coated tablets and evaluation of their function by in vitro and in vivo tests for colon targeting. Int J Pharm. 2000;204:7–15. doi: 10.1016/S0378-5173(00)00454-3. [DOI] [PubMed] [Google Scholar]
- 53.Jose S, Dhanya K, Cinu TA, Litty J, Chacko AJ. Colon targeted drug delivery: Different approaches. J Young Pharmacist. 2009;1:13–9. doi: 10.4103/0975-1483.51869. [DOI] [Google Scholar]
- 54.Patel GC, Patel MM. Developing a modified pulsincap system. Pharm Tech Europe 2009; 21. [Google Scholar]
- 55.Steve E. Bioenhancement technologies improve absorption of insoluble drugs in oral dosage forms. Drug Deliv Syst. 2007;2:4. [Google Scholar]
- 56.Percel P, Vishnupad KS, Venkatesh GM, inventors. 2002 Dec. 31Timed sustained release systems for Propranolol. US 6500454.
- 57.Aptalis. 2011. Aptalis homepage. Available from: http://www.aptalispharma.com/
- 58.Roy P. Aliasgar Shahiwala, Multiparticulate formulation approach to pulsatile Drug delivery: Current perspectives. J Contr Rele. 2009;134:74–80. doi: 10.1016/j.jconrel.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Verma RK, Garg S. Current status of drug delivery technologies and future directions. Pharmaceutical Technology. 2001;25:1–14. [Google Scholar]
- 60.Yu DG, Zhu LM, Branford-White CJ, Yang XL, Xiang LY. Three-dimensional printing in pharmaceutics: promises and problems. J Pharm Sci. 2008;97:3666–90. doi: 10.1002/jps.21284. [DOI] [PubMed] [Google Scholar]
- 61.Rowe CW, Katstra WE, Palazzolo RD, Giritlioglu B, Teung P, Cima MJ. Multimechanism oral dosage forms fabricated by three dimensional printing. J Control Release. 2000;66:11–7. doi: 10.1016/S0168-3659(99)00224-2. [DOI] [PubMed] [Google Scholar]
- 62.Katstra WE. Fabrication of complex oral drug delivery forms by Three Dimensional Printing, Massachusetts Institute of Technology 2001; 237-41. [Google Scholar]
- 63.Elan Drug Technologies. 2010. Oral Controlled Release. Available from: www.elandrugtechnologies.com/oral_controlled_release
- 64.Panoz DE. Geoghegan, Edward J, inventors. 1989 Sept. 15/ Controlled absorption pharmaceutical composition, US Patent 4863742.
- 65.Troy MH. Timing drug availability with therapeutic need. Speciality Pharma. 2006;2:1. [Google Scholar]
- 66.Alza. Available from: www.alza.com
- 67.Rappar D. Oral extended release: Snapshots and benefits. Drug Delivery Technol. 2007;7:42. [Google Scholar]
- 68.Devesh AB, Pethe AM. Lipid technology—a promising drug delivery system for poorly water soluble drugs. Int J Pharm Res Devel. 2010;2:1–11. [Google Scholar]
- 69.Shram M, Romach M, Sellers E, Thipphawong J. Assessing the abuse potential of an oral osmotic-controlled extended release (OROS) hydromorphone compared to immediate release hydromorphone. J Pain. 2009;10:45–54. doi: 10.1016/j.jpain.2009.01.188. [DOI] [Google Scholar]
- 70.Zentner GM, Rork GS, Himmelstein KJ. The controlled porosity osmotic pump. J Control Release. 1985;1:269–82. doi: 10.1016/0168-3659(85)90003-3. [DOI] [Google Scholar]
- 71.Elan Drug Technologies. 2010. IPDAS® Controlled Release Technology. Available from: www.elandrugtechnologies.com/nav/30/
- 72.Elan Corporation. 1997 Nov 4. Intestinal protective drug absorption system. US Patent 75111480.
- 73.Shaji J, Chadawar V, Talwalkar P. Multiparticulate Drug Delivery System. The Indian Pharmacist. 2007;6:21–8. [Google Scholar]
- 74.SkyePharma. 2010. SkyePharma - Drug Delivery Specialists. Available from: www.skypharma.com
- 75.MiddleBrook Pharmaceuticals. Available from: www.victorypharma.com/
- 76.Steven AG. Biotherapeutics—from drug discovery to drug delivery, control release society Newsletter 2004; 21:3.
- 77.Conte U, Maggi L, Colombo P, La MA. Multi-layered hydrophilic matrices as constant release devices (Geomatrix® systems) J Contr Rel. 1993;26:39–47. doi: 10.1016/0168-3659(93)90207-L. [DOI] [Google Scholar]
- 78.Conte U, Colombo P, Maggi L, La MA. Compressed barrier layers for constant drug release in swellable matrix tablets. STP Pharma Sci. 1994;4:107–13. [Google Scholar]
- 79.Conte U, Maggi L. Modulation of the dissolution profiles from Geomatrix multi-layer matrix tablets containing drugs of different solubility. Biomaterials. 1996;17:889–96. doi: 10.1016/0142-9612(96)83284-4. [DOI] [PubMed] [Google Scholar]
- 80.Conte U, La Manna A, Colombo P. 1992. Tablets with controlled-rate release of active substances. US Patent Application 07/926,380.
- 81.OSDrC. 2007. OSDrC Technologies. Available from: www.osdrc.com
- 82.Ozeki Y, Danjo K. Development of one-step dry-coated tablet system (OSDRC-System) and the comparison of its compression characteristics with those of conventional dry-coated tablets. J Pharm Sci Technol. 2004;64:59–66. [Google Scholar]
- 83.Yuichi O, Masaki A, Yukinao W, Kazumi D. Evaluation of novel one-step dry-coated tablets as a platform for delayed-release tablets. J Cont Rele. 2004;95:51–60. doi: 10.1016/j.jconrel.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 84.Ozeki Y, Watanabe Y, Okamoto H, Danjo K. Development of dividable one-step dry-coated tablets (dividable-OSDRC) and their evaluation as a new platform for controlled drug release. Pharm Res. 2004;21:1177–83. doi: 10.1023/B:PHAM.0000033004.88953.bc. [DOI] [PubMed] [Google Scholar]
- 85.Bussemer T, Otto I, Bodmeier R. Pulsatile drug-delivery systems. Crit Rev Ther Drug Carrier Syst. 2001;18:433–58. [PubMed] [Google Scholar]
- 86.Shaji J, Chadawar V, Talwalkar P. Multiparticulate Drug Delivery System. The Indian Pharmacist. 2007;6:21–8. [Google Scholar]
- 87.Dey NS, Majumdar S, Rao MEB. Multiparticulate drug delivery systems for controlled release. Trop J Pharm Res. 2008;7:1067–75. doi: 10.4314/tjpr.v7i3.14692. [DOI] [Google Scholar]
- 88.Eurand S.P.A. Corporation. 2003. Minitabs in multiparticulate drug delivery. 75843477.


