Background: The mechanism of EGF signaling in the regulation of prostate cancer (PCa) metastasis remains unclear.
Results: EGF promotes epithelial-mesenchymal transition (EMT) and induces degradation of epithelial protein lost in neoplasm (EPLIN), a putative suppressor of PCa metastasis.
Conclusion: EGF activates ERK1/2-dependent phosphorylation, ubiquitination, and protein turnover of EPLIN.
Significance: This study suggested that blockade of EGF signaling could retard EMT and inhibit invasiveness of PCa cells.
Keywords: Epidermal Growth Factor (EGF), Epithelial to Mesenchymal Transition, ERK, Invasion, Metastasis, Prostate Cancer, Protein Degradation, Protein Phosphorylation, Protein Turnover, EPLIN
Abstract
Aberrant expression of EGF receptors has been associated with hormone-refractory and metastatic prostate cancer (PCa). However, the molecular mechanism for EGF signaling in promoting PCa metastasis remains elusive. Using experimental models of PCa metastasis, we demonstrated that EGF could induce robust epithelial-mesenchymal transition (EMT) and increase invasiveness. Interestingly, EGF was found to be capable of promoting protein turnover of epithelial protein lost in neoplasm (EPLIN), a putative suppressor of EMT and tumor metastasis. Mechanistic study revealed that EGF could activate the phosphorylation, ubiquitination, and degradation of EPLIN through an extracellular signal-regulated kinase 1/2 (ERK1/2)-dependent signaling cascade. Pharmacological inhibition of the ERK1/2 pathway effectively antagonized EGF-induced EPLIN degradation. Two serine residues, i.e. serine 362 and serine 604, were identified as putative ERK1/2 phosphorylation sites in human EPLIN, whose point mutation rendered resistance to EGF-induced protein turnover. This study elucidated a novel molecular mechanism for EGF regulation of EMT and invasiveness in PCa cells, indicating that blockade of EGF signaling could be beneficial in preventing and retarding PCa metastasis at early stages.
Introduction
Aberrant overexpression of EGF receptors (EGFRs) has been associated with hormone-refractory and metastatic prostate cancer (PCa)2 (1–6). Activation of EGFR signaling cascade could promote the proliferation, survival, and invasion of PCa cells (7). As the predominant ligand to EGFR/ErbB1, EGF has been shown to induce epithelial-mesenchymal transition (EMT), a crucial mechanism for the acquisition of metastatic capabilities. Several mechanisms, such as mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK)-dependent up-regulation of TWIST (8), Snail, or Slug (9) may mediate the effects of EGF on EMT and acquired invasiveness.
Epithelial protein lost in neoplasm (EPLIN) is an actin-binding protein that functions as a key molecule linking the cadherin-catenin complex to the actin cytoskeleton (10, 11). Two EPLIN isoforms, the 600-residue EPLIN-α and 759-residue EPLIN-β, are expressed in epithelial and endothelial cells in a context-dependent manner (11–13, 15). EPLIN was initially thought to be a potential tumor suppressor that is preferentially expressed in human epithelia but frequently lost in cancerous cells (11, 16). Recently, we reported a novel role of EPLIN in the regulation of EMT and invasiveness (17). EPLIN depletion in epithelial-like PCa cells promoted the disassembly of adherens junction, structurally distinct actin remodeling, and activation of β-catenin signaling. We further identified a subset of putative EPLIN target genes associated with EMT, invasion, and metastasis. Significantly, EPLIN down-regulation was associated with clinical lymph node metastases of human solid tumors, including PCa, breast cancer, colorectal cancer, and squamous cell carcinoma of the head and neck (SCCHN). These data were further supported by recent reports that EPLIN may be a negative regulator of tumor aggressiveness in PCa, breast cancer, and esophageal cancer (18–20).
Using experimental models of PCa progression, we observed that EGF could induce EMT and promote invasiveness in epithelial-like PCa cells. Intriguingly, the morphological and behavioral changes were associated with a significant decrease in EPLIN expression at protein level. We further elucidated that EGF could activate the phosphorylation, polyubiquitination, and degradation of EPLIN via an extracellular signal-regulated kinase 1/2 (ERK1/2)-dependent signaling cascade. Mutation of certain serine residues of EPLIN rendered resistance to EGF-induced protein degradation. These studies revealed a novel mechanism of EPLIN regulation that could contribute to PCa metastasis.
EXPERIMENTAL PROCEDURES
Cell Culture
Human PCa cell lines ARCaPE, ARCaPM, PC3, and C4-2 were routinely maintained in T-medium (Invitrogen) with 5% fetal bovine serum (FBS). Human SCCHN cell lines 686LN, M4e, 37A, and 37B (21) were maintained in Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 10% fetal bovine serum. Where specified, cells were starved overnight with serum-free RPMI 1640 and treated with recombinant human EGF (R&D Systems, Minneapolis, MN). For blocking ERK1/2 signaling, U0126 (1 μm; Calbiochem, San Diego, CA) or PD98059 (10 μm; Calbiochem) were preincubated with PCa cells for 1 h prior to EGF treatment. Proteasome inhibitors PS341 (1 μm; LC Laboratories, Woburn, MA) or MG132 (10 μm; LC Laboratories) were preincubated with PCa cells for 2 h prior to EGF treatment for the analysis of protein ubiquitination.
Western Blot Analysis
Total cell lysates were prepared using radioimmune precipitation assay buffer (Santa Cruz Biotechnology). Nuclear proteins were extracted using a Novagen kit (EMD Biosciences, San Diego, CA). Immunoblotting analysis followed standard procedure (22). Information for the antibodies used in this study is described in Table 1.
TABLE 1.
Antibodies used in Western blot analyses, immunoprecipitation, confocal microscopy, and immunohistochemistry
| Antibody | Sources | Catalog no. |
|---|---|---|
| EPLIN (immunoblotting) | Novus Biologicals | NB100-2305 |
| EPLIN (immunoprecipitation) | Novus Biologicals | NB100-2310 |
| EPLIN (immunoblotting/immunofluorescence) | BD Transduction Laboratories | 612115 |
| EPLIN (immunohistochemistry) | Santa Cruz Biotechnology | Sc-133553 |
| E-cadherin (immunoblotting/immunofluorescence) | BD Transduction Laboratories | 610181 |
| E-cadherin (immunohistochemistry) | Santa Cruz Biotechnology | Sc-7870 |
| β-catenin (immunoblotting/immunofluorescence) | Santa Cruz Biotechnology | sc-7199 |
| β-catenin (immunohistochemistry) | BD Transduction Laboratories | 610154 |
| TBP | Santa Cruz Biotechnology | 96818 |
| β-actin | Sigma-Aldrich | A5316 |
| EGFR | Cell Signaling Technology | 4267 |
| p-EGFR (Tyr-1068) | Cell Signaling Technology | 2236 |
| p-EGFR (Tyr-1173) | Santa Cruz Biotechnology | sc-12351 |
| neu | Abcam | ab2428 |
| p-neu (Tyr-1221/1222) | Cell Signaling Technology | 2243 |
| GFP | Santa Cruz Biotechnology | sc-9996 |
| Ubiquitin | Enzo Life Sciences | BML-PW8810 |
| p-serine | Upstate | 05-1000 |
| ERK1 | Santa Cruz Biotechnology | sc-94 |
| p-ERK1/2 | Santa Cruz Biotechnology | sc-16982 |
Immunofluorescence and Confocal Imaging
Immunofluorescence was performed as described previously (17). Mouse anti-EPLIN antibody, rabbit anti-E-cadherin antibody, or rabbit anti-β-catenin antibody were used (Table 1). Actin filaments were stained by phalloindin (Invitrogen) at a dilution of 1:500. Cells were imaged on a Zeiss LSM 510 META. In all cases, either a 63× or 100× Zeiss Plan-Apo oil objective was used (numerical aperture of 1.3 and 1.4, respectively). All images had contrast expansion performed in Adobe Photoshop.
Immunoprecipitation
The Immunoprecipitation Starter Pack (GE Healthcare) was used according to the manufacturer's instructions. Total lysates (1 mg) were immunoprecipitated with 5 μg of rabbit anti-EPLIN antibody, rabbit anti-green fluorescence protein (GFP) antibody, or normal rabbit IgG (R&D Systems, AB-105-C) (Table 1). Protein A/G-Sepharose 4 Fast Flow beads were added to precipitate proteins and then washed and eluted. The samples were further processed for Western blot analysis.
Plasmids and Constructs
GFP-tagged expression plasmid for EPLIN-β (pCMV-EPLIN-AC-GFP) and control plasmid pCMV-AC-GFP were purchased from Origene (Rockville, MD). Human EPLIN-β reporter was obtained from SwitchGear Genomics (Menlo Park, CA). pTK-RL plasmid was purchased from Promega (Madison, WI).
Site-directed Mutagenesis
Single-point mutations at Ser-362 or Ser-604 and a double-point mutation at Ser-362 and Ser-604 were performed using a QuikChange Lightning site-directed mutagenesis kit (Stratagene) following the manufacturer's instructions. The primer sequences are described in Table 2.
TABLE 2.
Primer sequences for generating EPLIN-GFP mutants
| Construct | Primer |
|---|---|
| EPLIN (S362A)-GFP | 5′-ATCCCAAGCCACTAGCTCCAGATTCCAGAGC-3′ |
| EPLIN (S604A)-GFP | 5′-GCACCTCTGTCAAGGCCCCAAAAACTGTGTCC-3′ |
Transfection and Infection
Transient expression of cDNA plasmids was mediated by transfection with Lipofectamine 2000, and transient expression of siRNA was performed using Oligofectamine reagent (Invitrogen), according to the manufacturer's protocols.
EPLIN Reporter Assay
Subconfluent PCa cells were plated and grown overnight on 12-well plates. 1.25-μg human EPLIN-β reporter plasmids were transfected with 0.25-μg pRL-TK (internal control) as described previously (17). Cells were serum-starved overnight and further incubated in the absence or presence of EGF at varying concentrations. Luciferase activities were measured 48 h later using a Dual-Luciferase reporter assay system (Promega). Relative luciferase units were expressed as firefly luciferase intensity normalized to Renilla luciferase activity.
Quantitative Reverse Transcription PCR and RT-PCR
Total RNA was prepared with Qiagen RNeasy kit (Valencia, CA). The first-strand cDNA was synthesized using SuperScript® III First-Strand synthesis system (Invitrogen). Quantitative PCR was performed by the LightCycler 480 system (Roche Applied Science) using a Brilliant® SYBR® Green QPCR Master Mix (Stratagene) according to the manufacturer's instructions. For end point RT-PCR, the SuperScript® III One-Step RT-PCR kit (Invitrogen) was used following the manufacturer's protocol. The specific primer pairs are described in Table 3. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was amplified with a pair of primers described previously (22) and used to normalize RNA inputs.
TABLE 3.
Primer sequences for PCR analysis
| Genes | Primers |
|---|---|
| EPLIN-α | |
| Forward | 5′-AAGCAAAAATGAAAACGAAG-3′ |
| Reverse | 5′-ACTGAACCTGACCGTACAGACACCCACCTTAGCAATAG-3′ |
| EPLIN-β | |
| Forward | 5′-AGAGGAAAGGCGGCTTTTAG-3′ |
| Reverse | 5′-CAGTGCTGCTGTTCCGTAGA-3′ |
Determination of Protein Half-life (T½)
ARCaPE or C4-2 cells or PC3 cells transiently transfected (48 h) with individual wild-type and mutated EPLIN-GFP cDNAs, were incubated with cycloheximide (10 μg/ml, Calbiochem) to inhibit further protein synthesis. Following incubation for 0, 1, 2, 4, and 6 h, cells were harvested and lysed, and Western blotting was performed as described above. A rabbit anti-EPLIN (Novus Biologicals, Inc) was used for the detection of endogenous EPLIN protein in ARCaPE or C4-2 cells, or an anti-GFP antibody was used to recognize the ectopically expressed EPLIN-GFP proteins in PC3 cells (Table 1). Desired protein bands from the Western blots were quantitated and normalized by the intensity of corresponding β-actin controls using the ImageJ program (National Institutes of Health), and the data were graphed using the SigmaPlot program (Systat Software Inc., San Jose, CA). Protein degradation rate is expressed as half-life (T½), the time for degradation of 50% of the protein, which was determined by exponential decay fitting algorithm.
In Vitro Invasion Assay
A modified Boyden chamber with individual 8-μm polyester membrane inserts of a 24-well plate was coated with Matrigel. 1 × 105 cells in 200 μl of defined medium were seeded into the upper chamber, and 300 μl of serum-free RPMI 1640 medium supplemented with recombinant EGF (50 ng/ml) were added to the lower chamber. Eighteen h later, invasive cells to the lower surface of the membrane were fixed with methanol, stained with 5% Giemsa solution, and counted with a light microscope.
Animal Study
Six-week-old athymic nude mice (BALB/c; National Cancer Institute) were used. Animal protocols were approved by Emory University Institutional Animal Care and Use Committee. 2 × 106 ARCaPE cells per 100 μl per site were injected subcutaneously using a previously established procedure (23). After confirmation of tumor growth to a size of ∼1 mm3 (2–4 weeks after tumor inoculation), mice were randomly divided and treated with either PBS or recombinant EGF once a week at a dose of 5 mg/kg body weight via the intraperitoneal route. Tumor size was measured with a digital caliper every week. Animals were sacrificed 6 weeks later, and tumor specimens were collected.
Immunohistochemistry Analysis
ARCaPE xenograft tumor tissues were analyzed by immunohistochemistry staining using standard procedures. Briefly, tissues were deparaffinized, rehydrated, and subjected to 5-min pressure-cooking antigen retrieval, 10-min double endogenous enzyme block, and overnight primary antibody incubation, and subjected to prediluted biotinylated pan-specific universal secondary antibody for 10 min. Signals were detected by adding 3,3′-diaminobenzidine substrate hydrogen peroxide and counterstained by hematoxylin QS. All immunohistochemistry reagents were obtained from Vector Laboratories (Burlingame, CA). Antibodies against E-cadherin, EPLIN, and phosphorylated EGFR (Tyr-1173) were purchased from Santa Cruz Biotechnology, and β-catenin antibody was purchased from BD Transduction Laboratories (Table 1). Positive expression was defined as >15% positive staining in cell population.
Statistical Analysis
Significance levels for comparisons of protein expression in tumor tissue specimens were calculated by using the two-sample t test. Treatment effects were evaluated using a two-sided Student's t test. All data represent three or more experiments. Errors are S.E. values of averaged results, and values of p ≤ 0.05 were taken as a significant difference between means.
RESULTS
EGF Induces EMT in PCa Cells
ARCaP (androgen refractory cancer of the prostate) cells are a novel human PCa model that resembles the classical descriptions of EMT and closely mimics the clinical pathophysiology of PCa metastasis (24, 25). Upon exposure to certain soluble factors or host bone microenvironment, low-invasive and epithelial-like ARCaPE cells undergo EMT and acquire invasive properties (26, 27). Western blot analysis showed that both EGFR and neu (HER2/ErbB2) were highly expressed and phosphorylated in ARCaPE cells and their highly metastatic, mesenchymal-like counterparts ARCaPM cells (Fig. 1A, left panel). Treatment with recombinant human EGF at low doses (20–50 ng/ml) induced rapid phosphorylation of EGFR and neu in ARCaPE cells (Fig. 1A, right panel), suggesting an active EGFR signaling. Significantly, EGF promoted EMT within 72 h (Fig. 1B). The appearance of mesenchymal morphology upon EGF treatment was associated with decreased E-cadherin and increased nuclear β-catenin (Fig. 1, C and D). Confocal microscopy further revealed the disassembly of actin stress fibers with a radial structure to short, disordered actin cores, formation of peripheral and dorsal ruffles, and a lower degree of co-localization of EPLIN and actin filaments (Fig. 1E). In a modified Boyden chamber assay, invasion of ARCaPE cells through Matrigel was markedly increased (by 5-fold) in the presence of EGF (Fig. 1F). Consistently, EGF treatment promoted the appearance of a mesenchymal-like morphology in 686LN SCCHN cells (21) (Fig. 1G). These data indicated that EGF could induce EMT in certain human cancer cells, which may involve active remodeling of the actin cytoskeleton and the disruption of epithelial adhesions.
FIGURE 1.
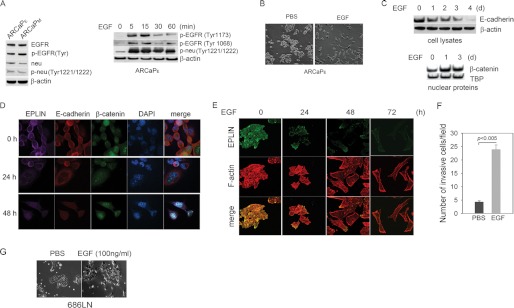
EGF induces EMT in PCa and SCCHN cells. A, a functional EGFR signaling system in ARCaP cells. Left panel, expression and phosphorylation of EGFR and neu(ErbB2) in ARCaP cells. A pan-p-EGFR antibody that detects phosphorylation at tyrosine residues 992, 1045, 1068, 1148, and 1173 was used. Right panel, EGF treatment (50 ng/ml) rapidly activates the phosphorylation of both EGFR and ErbB2 in ARCaPE cells. B, EGF (50 ng/ml, 72 h) induces EMT in ARCaPE cells. C, Western blot analyses of E-cadherin expression and nuclear translocation of β-catenin in ARCaPE cells in response to EGF treatment (50 ng/ml). D, confocal microscopy showed that EGF (50 ng/ml) induces down-regulation and disassociation of EPLIN and E-cadherin, and nuclear translocation of β-catenin in ARCaPE cells. E, EGF treatment (50 ng/ml) induces re-organization of the actin cytoskeleton in ARCaPE cells. F, EGF (50 ng/ml, 18 h) treatment increases invasion of ARCaPE cells through Matrigel in modified Boyden chamber. Error bars denote S.E. (n = 3). G, EGF treatment (100 ng/ml, 72 h) induces EMT-like morphological changes in 686LN cells.
EGF Promotes EPLIN Protein Turnover
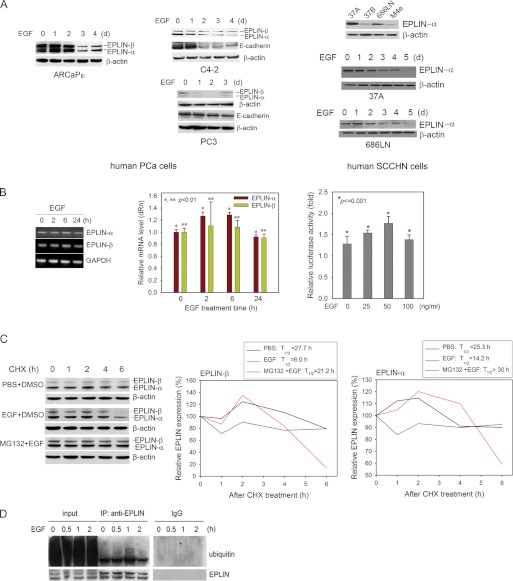
Following EGF treatment, a progressive reduction of EPLIN proteins was observed in ARCaPE, C4-2, and PC3 cells, which was accompanied by a decrease in E-cadherin expression (Fig. 2A, left and middle panels). EGF treatment also resulted in a reduction of EPLIN protein in epithelial-like SCCHN cells (Fig. 2A, right panel). On the contrary, EGF did not significantly affect mRNA expression of either EPLIN isoforms in PCa cells, as demonstrated by RT-PCR, quantitative RT-PCR, and EPLIN-β promoter activity assay (Fig. 2B). These results suggested that EGF could promote EPLIN down-regulation primarily at post-transcriptional levels.
FIGURE 2.
EGF induces EPLIN protein degradation in PCa and SCCHN cells. A, left panel: EGF treatment (50 ng/ml) induces progressive decrease in protein expression of both EPLIN isoforms in ARCaPE cells. The total lysates were the same as used in Fig. 1C. Middle panel: EGF treatment (50 ng/ml) reduces protein expression of EPLIN and E-cadherin in C4-2 and PC3 cells. Right panel: EPLIN-α is the predominant EPLIN isoform in several human SCCHN cell lines, which is reduced in the more aggressive cell lines (37B, M4e) when compared with that in the low invasive cell lines (37A, 686LN) (top). EPLIN-α expression is reduced upon EGF treatment in 37A (50 ng/ml; middle) and 686LN (100 ng/ml; bottom) cells. B, the effects of EGF on the mRNA expression of EPLIN isoforms in ARCaPE cells were assessed by regular (left panel) and real-time quantitative (middle panel) PCR and EPLIN-β reporter assay (right panel). Expression of GAPDH was used as control. Bars denote S.E. (n = 3). C, EGF (50 ng/ml) promotes protein turnover of both EPLIN isoforms, whereas treatment with a proteasome inhibitor MG132 partially rescues EPLIN in ARCaPE cells in ARCaPE cells. D, EGF induces polyubiquitination of EPLIN in a time-dependent manner. ARCaPE cells were treated with 50 ng/ml EGF. Immunoprecipitation was performed at the indicated times with an anti-EPLIN antibody, and Western blot analyses were performed with an anti-polyubiquitin antibody. Input, total cell lysates; DMSO, dimethyl sulfoxide; CHX, cycloheximide.
We investigated whether EGF induced EPLIN down-regulation by promoting protein turnover. The half-life (T½) of EPLIN protein in ARCaPE cells was determined in the presence of cycloheximide, a de novo protein synthesis inhibitor (Fig. 2C). In the absence of EGF, EPLIN-α and -β isoforms have a half-life of ∼25.3 and 27.7 h, respectively. EGF treatment significantly reduced their T½ to 14.2 and 6.0 h, respectively. Addition of a proteasome inhibitor MG132 effectively antagonized EGF-induced EPLIN degradation in ARCaPE cells (T½ > 30.0 h for EPLIN-α, and 21.2 h for EPLIN-β). These results indicated that EGF may facilitate protein turnover of both EPLIN isoforms through a proteasome-dependent mechanism.
Polyubiquitination serves as a triggering signal leading to protein degradation in the proteasome (28). To examine the mechanism responsible for EGF-induced EPLIN turnover, ARCaPE cells were firstly treated with a proteasome inhibitor PS341 and analyzed for the ubiquitination of EPLIN using an immunoprecipitation procedure. Fig. 2D showed that EGF induced a time-dependent polyubiquitination of EPLIN in ARCaPE cells, with the peak appearing at 60 min. The data suggested that EGF may promote EPLIN degradation through a ubiquitin proteasome-dependent mechanism.
EGF Induces Serine Phosphorylation and EPLIN Turnover through ERK1/2 Pathway
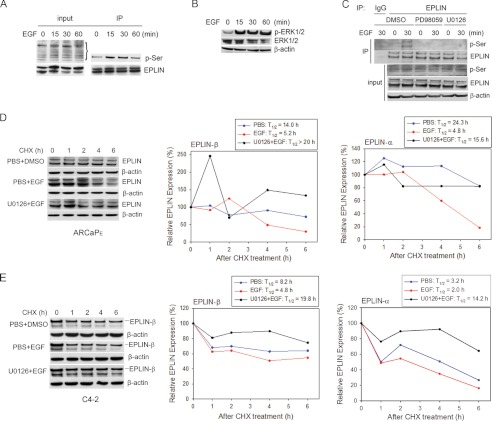
Phosphorylation in response to extracellular signals or stress is a common mechanism “priming” certain proteins for subsequent recruitment of degradation machinery (29, 30). We investigated whether EGF could induce phosphorylation of EPLIN in ARCaPE cells, which may be a prerequisite for ubiquitination and degradation of EPLIN. Because current antibodies against phosphorylated mouse EPLIN (31) were not suitable for detecting phosphorylation of human EPLIN, an immunoprecipitation protocol was used to precipitate EPLIN and determine its phosphorylation status using a pan-phosphoserine (p-Ser) antibody in an immunoblotting assay. Serine phosphorylation of EPLIN was found to be rapidly increased, with a peak at 15–30 min following EGF stimulation (Fig. 3A).
FIGURE 3.
EGF induces serine phosphorylation and degradation of EPLIN through the ERK1/2 pathway. A, EGF (50 ng/ml) induces EPLIN phosphorylation at serine residues in ARCaPE cells. B, EGF (50 ng/ml) activates ERK1/2 signaling in ARCaPE cells. C, blockade of ERK1/2 signaling with PD98059 or U0126 inhibits EGF-induced EPLIN phosphorylation in ARCaPE cells. Input: total cell lysates. D, blockade of ERK1/2 signaling inhibits EGF-induced EPLIN degradation in ARCaPE cells. E, blockade of ERK1/2 signaling with U0126 inhibits EGF-induced EPLIN degradation in C4-2 cells. CHX, cycloheximide; DMSO, dimethyl sulfoxide.
Several EGFR downstream pathways were tested for their potential role in mediating EGF-induced EPLIN phosphorylation. Among them, ERK1/2 were found to be significantly activated by EGF treatment (Fig. 3B). Blockade of the ERK1/2 pathway with specific MEK inhibitors U0126 or PD98059 abrogated EGF-induced EPLIN phosphorylation at serine sites (Fig. 3C). Importantly, inhibition of ERK1/2 activity effectively antagonized EGF-induced EPLIN turnover. As shown in Fig. 3D, EGF treatment significantly accelerated EPLIN degradation in the presence of cycloheximide (T½ = 4.8 h for EPLIN-α and 5.2 h for EPLIN-β, respectively), whereas preincubation with U0126 resulted in longer T½ (>15.0 h) for both EPLIN isoforms. Similarly, EGF reduced the T½ of EPLIN proteins in C4-2 cells, an effect that was attenuated by the pretreatment with U0126 (Fig. 3E). These data suggested a pivotal role of ERK1/2 pathway in mediating EGF regulation of EPLIN phosphorylation and degradation in PCa cells.
Ser-362 and Ser-604 Are Required for EGF-induced Phosphorylation and Degradation of EPLIN
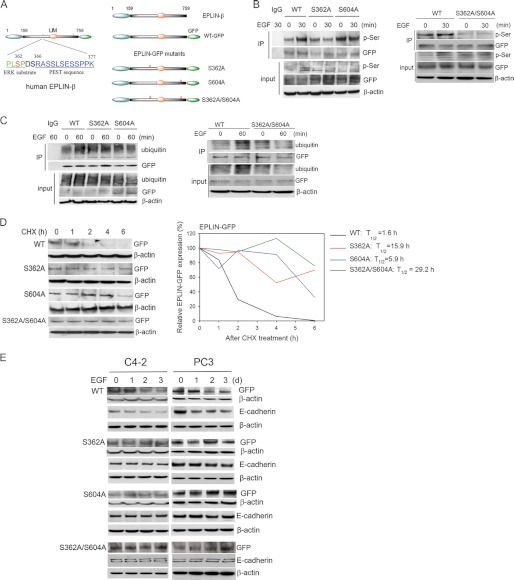
Two web-based programs, PhosphoSitePlusTM and the Eukaryotic Linear Motif (ELM) server, were used to predict putative phosphorylation sites within EPLIN-β. Ten serine-containing sequences were identified as potential ERK1/2 substrates, including these spanning amino acids 359–365 and 601–607. A recent study searching for candidate substrates for ERK1/2 by a proteomic approach found that ERK1/2 phosphorylates mouse EPLIN at several serine residues including Ser-360 and Ser-602 (31). Their counterpart residues in human EPLIN protein are Ser-362 and Ser-604, respectively. Of particular interest, Ser-362 appears to be a consensus ERK phosphorylation site (Pro-Leu-Ser-Pro) (Fig. 4A, left panel) (32). To investigate whether these serine residues are required for EGF-induced phosphorylation of EPLIN, we characterized two single-point mutants (S362A, S604A) and a double-point mutant (S362A/S604A) using GFP-tagged wild-type EPLIN-β cDNA as the template (Fig. 4A, right panel). Wild-type (WT) and mutated EPLIN-GFP cDNAs were transiently expressed in the PC3 cell line for its high transfection efficiency and relatively low endogenous EPLIN expression (17). Immunoprecipitation with anti-GFP antibody and subsequent blotting with p-Ser antibody were performed on cells treated with EGF. Consistent with our previous observation (Fig. 3A), EGF treatment significantly induced serine phosphorylation of wild-type EPLIN-GFP within 30 min, whereas point mutations at Ser-362 or Ser-604 inhibited EPLIN phosphorylation by EGF (Fig. 4B, left panel). The double-point mutation appeared to be more effective in antagonizing EGF-induced EPLIN phosphorylation at serine residues (Fig. 4B, right panel). Furthermore, immunoblotting with anti-ubiquitin antibody showed that mutations of S362A, S604A, and S362A/S604A attenuated polyuniquitination of EPLIN in response to EGF treatment (Fig. 4C). The data indicated that these serine residues are required for EGF-mediated EPLIN phosphorylation and ubiquitination.
FIGURE 4.
Ser-362 and Ser-604 are critical to EGF-induced phosphorylation and degradation of EPLIN. A, left panel: Ser-362 is an ERK substrate and adjacent to a putative PEST sequence in human EPLIN protein. Right panel: schematic diagram of the EPLIN-GFP construct and its point mutants at Ser-362, Ser-604, or Ser-362/Ser-604. B, mutation at Ser-362, Ser-604, or Ser-362/Ser-604 abrogates EGF-induced EPLIN phosphorylation in PC3 cells. Immunoprecipitation with anti-GFP antibody and subsequent blotting with p-Ser antibody were performed. Input: total cell lysates. C, mutation at Ser-362, Ser-604, or Ser-362/Ser-604 inhibits EGF-induced ubiquitination of EPLIN in PC3 cells. Immunoprecipitation with anti-GFP antibody and subsequent blotting with polyubiquitin antibody were performed. Input: total cell lysates. D, mutation at Ser-362, Ser-604, or Ser-362/Ser-604 inhibits EGF-induced EPLIN degradation in PC3 cells. Protein T½ assays were performed in PC3 cells transiently expressing wild type (WT) EPLIN or the point mutants. An anti-GFP antibody was used to detect the presence of EPLIN-GFP proteins. E, mutation at Ser-362, Ser-604, or Ser-362/Ser-604 inhibits EGF-induced down-regulation of EPLIN and E-cadherin in C4-2 and PC3 cells.
To examine whether Ser-362 and Ser-604 residues are involved in EGF-induced EPLIN degradation, the stability of wild-type and mutated EPLIN-GFP proteins in the presence of EGF were compared in PC3 cells (Fig. 4D). Following addition of cycloheximide, expression of wild-type EPLIN-GFP was rapidly reduced with a half-life of ∼1.6 h. S362A and S604A mutants exhibited longer half-lives of ∼15.9 and 5.9 h, respectively, and the double mutation at Ser-362 and Ser-604 significantly increased the stability of EPLIN-GFP protein in PC3 cells, with a predicted T½ of 29.2 h. These results indicated that the presence of these two serine residues is required for EGF-mediated EPLIN turnover in PCa cells.
Mutations at Ser-362 and Ser-604 Inhibit EGF-induced Down-regulation of E-cadherin in PCa Cells
Using E-cadherin down-regulation as a molecular indicator of EMT, we examined whether a stabilized EPLIN could inhibit EGF-induced EMT process in PCa cells. Consistent with Fig. 2A, EGF induced a decrease in protein expression of both EPLIN and E-cadherin in PCa cells transfected with wild-type EPLIN-GFP. On the contrary, transient expression of mutated EPLIN-GFP (S362A, S604A, and S362A/S604A) effectively blocked EGF-induced E-cadherin down-regulation (Fig. 4E). These data indicated that these “degradation-resistant” EPLIN proteins could render PCa cells less sensitive to EGF-induced EMT.
EGF Induces in Vivo Down-regulation of EPLIN and E-cadherin in ARCaPE Xenograft Tumors
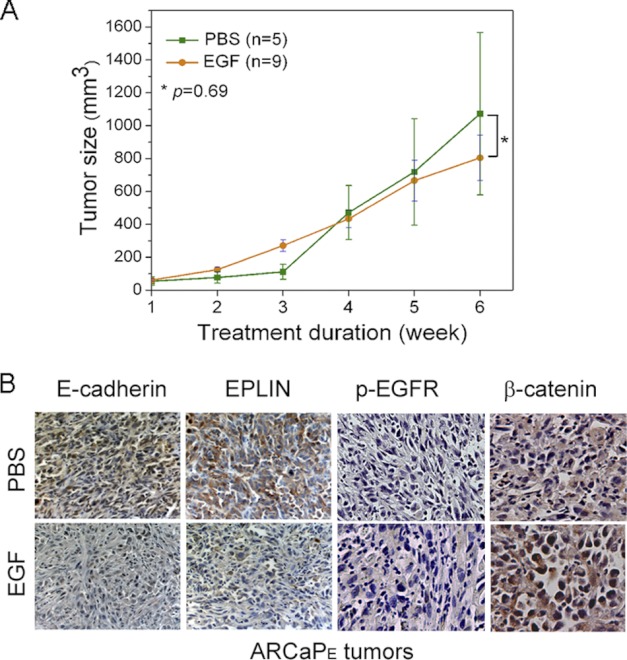
To investigate the physiological significance of EGF-EPLIN signaling in PCa progression, we determined whether activation of EGFR signaling is associated with EPLIN down-regulation and EMT in an ARCaPE xenograft model. As shown in Fig. 5A, a 6-week treatment with recombinant EGF did not significantly affected ARCaPE tumor growth (1.07 ± 0.49 cm3 versus 0.80 ± 0.14 cm3 of control group). However, immunohistochemistry analyses showed that tissue expression of EPLIN and E-cadherin was markedly reduced following EGF treatment. Increased p-EGFR in EGF-treated tissues suggested an activation of the EGFR signaling, which was associated with increased β-catenin at the tissue level (Fig. 5B). The data indicated that activation of EGFR signaling could promote in vivo EMT and EPLIN down-regulation in PCa.
FIGURE 5.
EGF induces in vivo down-regulation of EPLIN and E-cadherin in ARCaPE xenograft tumors. A, intraperitoneal administration of recombinant EGF does not significantly affect the subcutaneous growth of ARCaPE tumor in athymic nude mice. (B) EGF treatment markedly reduces tissue expression of EPLIN and E-cadherin, whereas increases the expression of p-EGFR and β-catenin in subcutaneous ARCaPE tumors.
DISCUSSION
Our recent studies demonstrated that EPLIN could function as a suppressor of tumor metastasis by negatively regulating EMT in PCa cells (17). Down-regulation of EPLIN, therefore, could significantly contribute to the progression of epithelial cancer toward metastatic status. Yet, no previous studies have attempted to elucidate the regulatory mechanism of EPLIN in cancer cells. In this study, we provide biochemical evidence supporting a mechanism by which EGF negatively regulates EPLIN expression through ERK1/2-dependent protein turnover. Gene mutation experiments identified two amino acid residues that are required for the phosphorylation, polyubiquitination, and protein degradation of EPLIN. Animal studies further supported a physiological function of EGF in promoting EPLIN down-regulation and in vivo EMT in an experimental model of PCa. Taken together, these results elucidated an important mechanism for EPLIN down-regulation in epithelial cancer cells.
EGF-induced EMT has been demonstrated as a crucial mechanism for the acquisition of invasiveness in a variety of solid tumors, including breast, ovarian, and head and neck cancer (8, 33). Mechanistically, EGF could activate distinct signaling pathways, including ERK1/2, Akt, Wnt-β-catenin, and signal transducer and activator of transcription 3, to suppress the expression of epithelial proteins (e.g. E-cadherin) and to increase the expression of mesenchymal proteins (e.g. vimentin). Although activation of EGF-EGFR pathway has been associated with aggressiveness and progression-free interval in PCa patients, it remains largely unknown on the mechanisms by which EGF-EGFR signaling promotes metastasis (1–6, 34–36). Earlier studies in DU145 PCa cells found that EGF induced the disruption of epithelial cell adhesion to the extracellular membrane through dephosphorylation and inactivation of the focal adhesion kinase signaling, resulting in enhanced motility and invasion (37). EGF also caused the disruption of cell-cell adherens junctions by caveolin-1-mediated E-cadherin endocytosis, followed by nuclear translocation of β-catenin and activation of T cell-specific factor-dependent transcription (38). Recently, Gan et al. (39) demonstrated that EGF treatment led to up-regulation of Snail and down-regulation of E-cadherin through an Akt-dependent mechanism, which eventually promoted EMT and enhanced invasiveness in DU145 and PC3 cells. In the present study, we elucidated a novel signaling mechanism by which PCa cells undergo EMT in response to EGF stimulation. We demonstrated that EPLIN, a key component of the cell-cell adhesion complex, is a downstream target of EGF-EGFR signaling in PCa cells. Down-regulation of EPLIN via accelerated protein turnover could result in the disruption of the adherens junctions and active reorganization of the actin cytoskeleton, both characteristics of EMT and indicators of invasive cancer cells. In addition to these structural alterations, EPLIN down-regulation may also affect the expression of multiple target genes, as we described previously (17). We acknowledged that in parallel to EPLIN degradation, other signaling pathways (such as E-cadherin down-regulation and Wnt/β-catenin activation) could simultaneously be activated by EGF treatment, which together contribute to EGF-induced EMT in PCa cells.
So far, information on the regulation of EPLIN expression is very limited (12). Although the two EPLIN isoforms appear to exert similar functions in the maintenance of epithelial structures (10, 40), their varied expression in different cancer types suggested that EPLIN may be delicately regulated in a context-dependent manner (11, 12, 17, 18). One can envision that extracellular signals may play a major role in the regulation of EPLIN expression. Indeed, mRNA level of EPLIN-α, but not of EPLIN-β, could be readily induced in NIH3T3 fibroblasts upon serum stimulation. A transcription complex composed of serum response factor and megakaryoblastic acute leukemia was formed and subsequently bound a region containing a conserved CArG consensus site within EPLIN-α promoter (41). In PCa cells, however, it appeared that EGF did not have a significant effect on mRNA expression of either EPLIN isoforms, which led us to postulate that EGF-induced EPLIN down-regulation may primarily occur at protein level by affecting protein stability. In fact, as a major EGFR downstream signaling pathway, ERK1/2 has been shown to be capable of directly binding and phosphorylating certain serine residues on mouse EPLIN (31). Intrigued by these observations, we investigated whether phosphorylation of EPLIN could be a prerequisite of EGF-induced protein turnover. In the present study, we identified at least two serine residues (Ser-362 and Ser-604) of human EPLIN protein are involved in the regulation of EPLIN degradation. It is interesting that the double-point mutation of Ser-362 and Ser-604 appears to be more effective in attenuating EGF-induced phosphorylation and polyubiquitination of EPLIN-GFP, suggesting a compensatory and synergistic effect between Ser-362 and Ser-604 residues. Nonetheless, it is potentially possible that other residues/sequences may also be involved in the regulation of EPLIN stability. For example, it has been demonstrated that the presence of one or more PEST motifs, i.e. the primary sequence rich in Pro, Glu, Ser, and Thr flanked by positively charged amino acids, may serve as a signal to direct ubiquitination for proteolytic degradation. Using a web-based algorithm PESTfind (42), we identified a PEST sequence (RASSLSESSPPK) with a PEST score of +5.89 (PEST scores greater than +5 are considered significant) (Fig. 4A, left panel). Interestingly, this PEST sequence is adjacent to one of the putative ERK1/2 phosphorylation site Ser-362 and contains five serine residues and one lysine residue that may be phosphorylation and ubiquitination sites. It is also worth of noting that the two putative ERK1/2 phosphorylation sites are present in both EPLIN isoforms, which may explain the observed effect of EGF on EPLIN protein expression regardless of which isoform is more prevalent in various cancer cells (Fig. 2A).
Interaction between epithelial cancer cells and adjacent tumor-associated “reactive” stroma is essential to the acquisition of invasive phenotypes (43, 44). Clinical data have indicated that reactive stroma could be a predictor of biochemical-free recurrence in PCa patients independent of Gleason score and other pathologic variables (45, 46). A recent microarray study found that EGF transcripts increased significantly in laser-captured reactive prostatic stroma compared with that in matched normal stroma (47). It is possible that EGF abundantly expressed by reactive prostatic stroma could provide a niche to promote EMT and convert certain PCa cells into a highly invasive form (48). Supporting this concept, our animal studies showed that short-term administration of recombinant EGF could significantly reduce EPLIN expression in ARCaPE xenograft tumors, which was associated with increased p-EGFR and β-catenin at tissue level. Importantly, the expression of E-cadherin in ARCaPE tumors was markedly reduced following EGF treatment, indicating the occurrence of in vivo EMT. It is equally important to note that the in vivo administration of recombinant EGF did not significantly affect tumor growth in athymic nude mice. Consistently, EGF did not exhibit any mitogenic effect in PCa cells (data not shown).
These findings could have important clinical implications. In fact, although EGFR overexpression has been observed in ∼30% of PCa patients (49), it remains controversial on the contribution of EGFR signaling to PCa progression. Most of the speculations were based on the failure of a clinical trial with gefitinib (Iressa), a small molecule EGFR inhibitor, in showing significant benefits in PCa patients (1, 50). Our present study highlighted the importance of EGF-EPLIN signaling in EMT, suggesting a primary role of EGFR signaling in the regulation of PCa invasiveness, but not of PCa growth. Therefore, these observations could provide a molecular explanation for the ineffectiveness of gefitinib in suppressing the growth of primary PCa in clinical setting. On the other hand, the blockade of EGFR signaling could be more effective in preventing and retarding PCa progression toward metastasis. Supporting this notion, gefitinib treatment significantly reduced bone metastatic incidence in an experimental model of PCa (14).
Acknowledgments
We thank Dr. Anthea Hammond at Winship Cancer Institute for editorial assistance, and Dr. Oskar Laur at Emory University Custom Cloning Core Facility for technical assistance.
This work was supported, in whole or in part, by NCI, National Institutes of Health Grants 1R21CA164612-01A1 (to D. W), 1R43CA141870 (to Y. A. W), P01 CA098912 and R01 CA122602 (to L. W. K. C), and Georgia Cancer Coalition Distinguished Scholar Grant (to O. K). This work was also supported by American Cancer Society Grant RSG-10-140-01 (to D. W.).
- PCa
- prostate cancer
- EPLIN
- Epithelial Protein Lost in Neoplasm
- EGFR
- EGF receptor
- EMT
- epithelial-mesenchymal transition
- SCCHN
- squamous cell carcinoma of the head and neck.
REFERENCES
- 1. DeHaan A. M., Wolters N. M., Keller E. T., Ignatoski K. M. (2009) EGFR ligand switch in late stage prostate cancer contributes to changes in cell signaling and bone remodeling. Prostate 69, 528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah R. B., Ghosh D., Elder J. T. (2006) Epidermal growth factor receptor (ErbB1) expression in prostate cancer progression: correlation with androgen independence. Prostate 66, 1437–1444 [DOI] [PubMed] [Google Scholar]
- 3. Mimeault M., Pommery N., Hénichart J. P. (2003) New advances on prostate carcinogenesis and therapies: involvement of EGF-EGFR transduction system. Growth Factors 21, 1–14 [DOI] [PubMed] [Google Scholar]
- 4. Hernes E., Fosså S. D., Berner A., Otnes B., Nesland J. M. (2004) Expression of the epidermal growth factor receptor family in prostate carcinoma before and during androgen-independence. Br. J. Cancer 90, 449–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Lorenzo G., Tortora G., D'Armiento F. P., De Rosa G., Staibano S., Autorino R., D'Armiento M., De Laurentiis M., De Placido S., Catalano G., Bianco A. R., Ciardiello F. (2002) Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin. Cancer Res. 8, 3438–3444 [PubMed] [Google Scholar]
- 6. Zellweger T., Ninck C., Bloch M., Mirlacher M., Koivisto P. A., Helin H. J., Mihatsch M. J., Gasser T. C., Bubendorf L. (2005) Expression patterns of potential therapeutic targets in prostate cancer. Int. J. Cancer 113, 619–628 [DOI] [PubMed] [Google Scholar]
- 7. Mimeault M., Batra S. K. (2006) Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis 27, 1–22 [DOI] [PubMed] [Google Scholar]
- 8. Lo H. W., Hsu S. C., Xia W., Cao X., Shih J. Y., Wei Y., Abbruzzese J. L., Hortobagyi G. N., Hung M. C. (2007) Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 67, 9066–9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu Z., Jiang Y., Steed H., Davidge S., Fu Y. (2010) TGFβ and EGF synergistically induce a more invasive phenotype of epithelial ovarian cancer cells. Biochem. Biophys. Res. Commun. 401, 376–381 [DOI] [PubMed] [Google Scholar]
- 10. Abe K., Takeichi M. (2008) EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc. Natl. Acad. Sci. U.S.A. 105, 13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maul R. S., Chang D. D. (1999) EPLIN, epithelial protein lost in neoplasm. Oncogene 18, 7838–7841 [DOI] [PubMed] [Google Scholar]
- 12. Chen S., Maul R. S., Kim H. R., Chang D. D. (2000) Characterization of the human EPLIN (Epithelial Protein Lost in Neoplasm) gene reveals distinct promoters for the two EPLIN isoforms. Gene. 248, 69–76 [DOI] [PubMed] [Google Scholar]
- 13. Chervin-Pétinot A., Courçon M., Almagro S., Nicolas A., Grichine A., Grunwald D., Prandini M. H., Huber P., Gulino-Debrac D. (2012) Epithelial protein lost in neoplasm (EPLIN) interacts with α-catenin and actin filaments in endothelial cells and stabilizes vascular capillary network in vitro. J. Biol. Chem. 287, 7556–7572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Angelucci A., Gravina G. L., Rucci N., Millimaggi D., Festuccia C., Muzi P., Teti A., Vicentini C., Bologna M. (2006) Suppression of EGF-R signaling reduces the incidence of prostate cancer metastasis in nude mice. Endocr. Relat. Cancer 13, 197–210 [DOI] [PubMed] [Google Scholar]
- 15. Sanders A. J., Ye L., Mason M. D., Jiang W. G. (2010) The impact of EPLINα (Epithelial protein lost in neoplasm) on endothelial cells, angiogenesis and tumorigenesis. Angiogenesis 13, 317–326 [DOI] [PubMed] [Google Scholar]
- 16. Song Y., Maul R. S., Gerbin C. S., Chang D. D. (2002) Inhibition of anchorage-independent growth of transformed NIH3T3 cells by epithelial protein lost in neoplasm (EPLIN) requires localization of EPLIN to actin cytoskeleton. Mol. Biol. Cell. 13, 1408–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang S., Wang X., Osunkoya A. O., Iqbal S., Wang Y., Chen Z., Müller S., Chen Z., Josson S., Coleman I. M., Nelson P. S., Wang Y. A., Wang R., Shin D. M., Marshall F. F., Kucuk O., Chung L. W., Zhau H. E., Wu D. (2011) EPLIN downregulation promotes epithelial-mesenchymal transition in prostate cancer cells and correlates with clinical lymph node metastasis. Oncogene 30, 4941–4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang W. G., Martin T. A., Lewis-Russell J. M., Douglas-Jones A., Ye L., Mansel R. E. (2008) Eplin-α expression in human breast cancer, the impact on cellular migration and clinical outcome. Mol. Cancer 7, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanders A. J., Martin T. A., Ye L., Mason M. D., Jiang W. G. (2011) EPLIN is a negative regulator of prostate cancer growth and invasion. J. Urol. 186, 295–301 [DOI] [PubMed] [Google Scholar]
- 20. Liu Y., Sanders A. J., Zhang L., Jiang W. G. (2012) EPLIN-α expression in human oesophageal cancer and its impact on cellular aggressiveness and clinical outcome. Anticancer Res. 32, 1283–1289 [PubMed] [Google Scholar]
- 21. Zhang X., Su L., Pirani A. A., Wu H., Zhang H., Shin D. M., Gernert K. M., Chen Z. G. (2006) Understanding metastatic SCCHN cells from unique genotypes to phenotypes with the aid of an animal model and DNA microarray analysis. Clin. Exp. Metastasis. 23, 209–222 [DOI] [PubMed] [Google Scholar]
- 22. Wu D., Zhau H. E., Huang W. C., Iqbal S., Habib F. K., Sartor O., Cvitanovic L., Marshall F. F., Xu Z., Chung L. W. (2007) cAMP-responsive element-binding protein regulates vascular endothelial growth factor expression: implication in human prostate cancer bone metastasis. Oncogene 26, 5070–5077 [DOI] [PubMed] [Google Scholar]
- 23. Gleave M., Hsieh J. T., Gao C. A., von Eschenbach A. C., Chung L. W. (1991) Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 51, 3753–3761 [PubMed] [Google Scholar]
- 24. Xu J., Wang R., Xie Z. H., Odero-Marah V., Pathak S., Multani A., Chung L. W., Zhau H. E. (2006) Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate 66, 1664–1673 [DOI] [PubMed] [Google Scholar]
- 25. Zhau H. Y., Chang S. M., Chen B. Q., Wang Y., Zhang H., Kao C., Sang Q. A., Pathak S. J., Chung L. W. (1996) Androgen-repressed phenotype in human prostate cancer. Proc. Natl. Acad. Sci. U. S. A. 93, 15152–15157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhau H. E., Odero-Marah V., Lue H. W., Nomura T., Wang R., Chu G., Liu Z. R., Zhou B. P., Huang W. C., Chung L. W. (2008) Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin. Exp. Metastasis. 25, 601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Josson S., Nomura T., Lin J. T., Huang W. C., Wu D., Zhau H. E., Zayzafoon M., Weizmann M. N., Gururajan M., Chung L. W. (2011) β2-microglobulin induces epithelial to mesenchymal transition and confers cancer lethality and bone metastasis in human cancer cells. Cancer Res. 71, 2600–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schrader E. K., Harstad K. G., Matouschek A. (2009) Targeting proteins for degradation. Nat. Chem. Biol. 5, 815–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou B. P., Liao Y., Xia W., Zou Y., Spohn B., Hung M. C. (2001) HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell. Biol. 3, 973–982 [DOI] [PubMed] [Google Scholar]
- 30. Tang X., Jang S. W., Wang X., Liu Z., Bahr S. M., Sun S. Y., Brat D., Gutmann D. H., Ye K. (2007) Akt phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nat. Cell. Biol. 9, 1199–1207 [DOI] [PubMed] [Google Scholar]
- 31. Han M. Y., Kosako H., Watanabe T., Hattori S. (2007) Extracellular signal-regulated kinase/mitogen-activated protein kinase regulates actin organization and cell motility by phosphorylating the actin cross-linking protein EPLIN. Mol. Cell. Biol. 27, 8190–8204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gonzalez F. A., Raden D. L., Davis R. J. (1991) Identification of substrate recognition determinants for human ERK1 and ERK2 protein kinases. J. Biol. Chem. 266, 22159–22163 [PubMed] [Google Scholar]
- 33. Hardy K. M., Booth B. W., Hendrix M. J., Salomon D. S., Strizzi L. (2010) ErbB/EGF signaling and EMT in mammary development and breast cancer. J. Mammary Gland Biol. Neoplasia 15, 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Teixeira A. L., Ribeiro R., Cardoso D., Pinto D., Lobo F., Fraga A., Pina F., Calais-da-Silva F., Medeiros R. (2008) Genetic polymorphism in EGF is associated with prostate cancer aggressiveness and progression-free interval in androgen blockade-treated patients. Clin. Cancer Res. 14, 3367–3371 [DOI] [PubMed] [Google Scholar]
- 35. Bell D. W., Lynch T. J., Haserlat S. M., Harris P. L., Okimoto R. A., Brannigan B. W., Sgroi D. C., Muir B., Riemenschneider M. J., Iacona R. B., Krebs A. D., Johnson D. H., Giaccone G., Herbst R. S., Manegold C., Fukuoka M., Kris M. G., Baselga J., Ochs J. S., Haber D. A. (2005) Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J. Clin. Oncol. 23, 8081–8092 [DOI] [PubMed] [Google Scholar]
- 36. She Q. B., Solit D. B., Ye Q., O'Reilly K. E., Lobo J., Rosen N. (2005) The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 8, 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu Z., Jiang G., Blume-Jensen P., Hunter T. (2001) Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol. Cell. Biol. 21, 4016–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu Z., Ghosh S., Wang Z., Hunter T. (2003) Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer Cell. 4, 499–515 [DOI] [PubMed] [Google Scholar]
- 39. Gan Y., Shi C., Inge L., Hibner M., Balducci J., Huang Y. (2010) Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 29, 4947–4958 [DOI] [PubMed] [Google Scholar]
- 40. Maul R. S., Song Y., Amann K. J., Gerbin S. C., Pollard T. D., Chang D. D. (2003) EPLIN regulates actin dynamics by cross-linking and stabilizing filaments. J. Cell. Biol. 160, 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leitner L., Shaposhnikov D., Descot A., Hoffmann R., Posern G. (2010) Epithelial Protein Lost in Neoplasm α (Eplin-α) is transcriptionally regulated by G-actin and MAL/MRTF coactivators. Mol. Cancer 9, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rogers S., Wells R., Rechsteiner M. (1986) Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 234, 364–368 [DOI] [PubMed] [Google Scholar]
- 43. Chung L. W. (1991) Fibroblasts are critical determinants in prostatic cancer growth and dissemination. Cancer Metastasis Rev. 10, 263–274 [DOI] [PubMed] [Google Scholar]
- 44. Mundy G. R. (1997) Mechanisms of bone metastasis. Cancer 80, 1546–1556 [DOI] [PubMed] [Google Scholar]
- 45. Ayala G., Tuxhorn J. A., Wheeler T. M., Frolov A., Scardino P. T., Ohori M., Wheeler M., Spitler J., Rowley D. R. (2003) Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin. Cancer Res. 9, 4792–4801 [PubMed] [Google Scholar]
- 46. Yanagisawa N., Li R., Rowley D., Liu H., Kadmon D., Miles B. J., Wheeler T. M., Ayala G. E. (2008) Reprint of: Stromogenic prostatic carcinoma pattern (carcinomas with reactive stromal grade 3) in needle biopsies predicts biochemical recurrence-free survival in patients after radical prostatectomy. Hum. Pathol. 39, 282–291 [DOI] [PubMed] [Google Scholar]
- 47. Dakhova O., Ozen M., Creighton C. J., Li R., Ayala G., Rowley D., Ittmann M. (2009) Global gene expression analysis of reactive stroma in prostate cancer. Clin. Cancer Res. 15, 3979–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bailey J. M., Singh P. K., Hollingsworth M. A. (2007) Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J. Cell. Biochem. 102, 829–839 [DOI] [PubMed] [Google Scholar]
- 49. Peraldo-Neia C., Migliardi G., Mello-Grand M., Montemurro F., Segir R., Pignochino Y., Cavalloni G., Torchio B., Mosso L., Chiorino G., Aglietta M. (2011) Epidermal Growth Factor Receptor (EGFR) mutation analysis, gene expression profiling and EGFR protein expression in primary prostate cancer. BMC Cancer 11, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Small E. J., Fontana J., Tannir N., DiPaola R. S., Wilding G., Rubin M., Iacona R. B., Kabbinavar F. F. (2007) A phase II trial of gefitinib in patients with non-metastatic hormone-refractory prostate cancer. BJU Int. 100, 765–769 [DOI] [PubMed] [Google Scholar]