Abstract
Objective To assess the efficacy of vitamin and antioxidant supplements in the prevention of cardiovascular diseases.
Design Meta-analysis of randomised controlled trials.
Data sources and study selection PubMed, EMBASE, the Cochrane Library, Scopus, CINAHL, and ClinicalTrials.gov searched in June and November 2012. Two authors independently reviewed and selected eligible randomised controlled trials, based on predetermined selection criteria.
Results Out of 2240 articles retrieved from databases and relevant bibliographies, 50 randomised controlled trials with 294 478 participants (156 663 in intervention groups and 137 815 in control groups) were included in the final analyses. In a fixed effect meta-analysis of the 50 trials, supplementation with vitamins and antioxidants was not associated with reductions in the risk of major cardiovascular events (relative risk 1.00, 95% confidence interval 0.98 to 1.02; I2=42%). Overall, there was no beneficial effect of these supplements in the subgroup meta-analyses by type of prevention, type of vitamins and antioxidants, type of cardiovascular outcomes, study design, methodological quality, duration of treatment, funding source, provider of supplements, type of control, number of participants in each trial, and supplements given singly or in combination with other supplements. Among the subgroup meta-analyses by type of cardiovascular outcomes, vitamin and antioxidant supplementation was associated with a marginally increased risk of angina pectoris, while low dose vitamin B6 supplementation was associated with a slightly decreased risk of major cardiovascular events. Those beneficial or harmful effects disappeared in subgroup meta-analysis of high quality randomised controlled trials within each category. Also, even though supplementation with vitamin B6 was associated with a decreased risk of cardiovascular death in high quality trials, and vitamin E supplementation with a decreased risk of myocardial infarction, those beneficial effects were seen only in randomised controlled trials in which the supplements were supplied by the pharmaceutical industry.
Conclusion There is no evidence to support the use of vitamin and antioxidant supplements for prevention of cardiovascular diseases.
Introduction
Cardiovascular diseases are the leading causes of deaths and disability worldwide.1 Over the past few decades, observational epidemiological studies have reported that intake of fruit and vegetables rich in various vitamins and antioxidants reduce the risk.2 It has been estimated that if an individual increases fruit and vegetable intake up to 600 g daily, the worldwide burden of disease could be reduced by 31% for ischaemic heart disease and 19 % for ischaemic stroke.3 Unlike the evidence for fruit and vegetables, however, many randomised controlled trials have reported inconsistent findings regarding the efficacy of vitamin and antioxidant supplementation on cardiovascular diseases.4
Several meta-analyses have reported conflicting evidence from randomised controlled trials. In 2003, a meta-analysis of 12 trials indicated that vitamin E supplements did not provide benefit in cardiovascular death or cerebrovascular events.5 Instead, it showed that β carotene supplementation led to a small increase in all cause mortality and cardiovascular death. In 2006, Flores-Mateo et al reported that the use of supplements containing selenium did not reduce the risk of coronary heart disease in the meta-analysis of six trials.6 More recently, however, Lee et al found that folic acid supplementation with B vitamins had potential small benefits in the prevention of stroke,7 and Qin et al indicated that folic acid treatment decreased the risk of cardiovascular disease by 15% in patients with end stage renal disease or advanced chronic kidney disease.8 Even though several meta-analyses of randomised controlled trials have been published regarding the efficacy of vitamins and antioxidant supplements on cardiovascular diseases, they involved individual vitamins or antioxidants, and there was no published comprehensive meta-analysis that reviewed this topic all together in one report. To the best of our knowledge, no meta-analyses have performed subgroup analyses by important factors such as methodological quality or funding source.
We investigated the efficacy of vitamin and antioxidant supplements on cardiovascular diseases through a comprehensive meta-analysis of randomised controlled trials by various factors such as by type of prevention (primary v secondary), type of vitamins and antioxidants, dose of supplement, type of cardiovascular outcomes, study design, methodological quality (high v low), duration of treatment (<5 years v ≥5 years), funding source (independent organisation v pharmaceutical industry), provider of supplements (pharmaceutical industry v not pharmaceutical industry), type of control (placebo v no placebo), number of participants in each trial (<10 000 v ≥10 000), and supplements given singly or in combination with other vitamin or antioxidant supplements.
Methods
Literature search
We searched PubMed, Embase, the Cochrane Library, Scopus, CINAHL, and ClinicalTrials.gov in June and November 2012 using common keywords related to vitamin or antioxidant supplements and cardiovascular diseases. The keywords were as follows: “vitamin supplement,” “antioxidant supplement,” “vitamin A supplement,” “vitamin B6 supplement,” “vitamin B12 supplement,” “folic acid supplement,” “vitamin C supplement,” “vitamin D supplement,” “vitamin E supplement,” “selenium supplement,” “beta-carotene supplement,” “lycopene supplement,” or “isoflavone supplement,”; and “cardiovascular disease,” “angina,” “acute myocardial infarction,” “transient ischemic attack,” or “stroke.” We also reviewed the bibliographies of relevant articles to locate additional publications. The language of publication was not restricted.
Selection criteria
To be included randomised controlled trials had to report the efficacy of vitamin or antioxidant supplements for the prevention of cardiovascular diseases and follow participants for at least six months. If data were duplicated or shared in more than one study, we included the first published or more comprehensive study in the analysis.
Selection of relevant studies
Based on the predetermined selection criteria, two of the authors (S-KM, WJ) independently selected all trials retrieved from the databases and bibliographies. Disagreements were resolved by discussion or in consultation with a third author (S-WO).
Assessment of methodological quality
We assessed the methodological quality of included trials with the Jadad scale.9 Its score ranges from 0 (very poor) to 5 (rigorous). The five point quality scale consists of points for randomisation (described as randomised, 1 point; table of random numbers or computer generated randomisation, additional 1 point), double blind (described as double blind, 1 point; use masking such as identical placebo, additional 1 point), and follow-up (state the numbers and reasons for withdrawal in each group; 1 point) in the report of each trial. All trials were classified into two groups, those with a score of ≤4 or versus 5 because the mean score for the 47 trials assessed in the current study (the full text for three trials was not available) was 4.3, and then subgroup meta-analyses were performed.
Main and subgroup analyses
We investigated the association between vitamin or antioxidants supplementation and major cardiovascular events. Major cardiovascular events included cardiovascular death, fatal or non-fatal myocardial infarction, angina, sudden cardiac death, fatal or non-fatal stroke, and transient ischaemic attack. We also performed subgroup meta-analyses by type of prevention (primary v secondary: in this study, trials involving healthy populations or patients with any specific disease except for cardiovascular disease were classified as primary prevention trials, and trials involving patients with cardiovascular disease were classified as secondary prevention trials), type of supplement by quality and dose (each supplement, vitamins only, antioxidants only, or antioxidants excluding vitamins), type of outcome (cardiovascular death, angina, fatal or non-fatal myocardial infarction, stroke, or transient ischaemic attack), type of outcome in each supplement, type of study design (randomised, double blind, placebo controlled trial v open label, randomised controlled trial), methodological quality (high v low), duration of treatment (<5 years v ≥5 years), funding source (pharmaceutical industry v independent organisation), provider of supplements (pharmaceutical industry v not pharmaceutical industry), type of control (placebo v no placebo), number of participants (≥10 000 v <10 000), and supplements given singly or in combination with other vitamin or antioxidant supplements by quality.
Statistical analysis
We calculated the relative risks with 95% confidence intervals by using crude 2×2 tables on the basis of intention to treat analysis, whenever possible, from the original publications. For the test of heterogeneity, we used Higgins I2, which measures the percentage of total variation across trials.10 I2 was calculated as follows:
I2 = 100% × (Q – df)/Q,
where Q is Cochran’s heterogeneity statistic and df indicates the degrees of freedom. Negative values of I2 are set to zero. I2 ranges from 0% (no observed heterogeneity) to 100% (maximal heterogeneity).
To calculate pooled relative risks with 95% confidence intervals, we used both the fixed effects and random effects models. An I2 value >50% was considered as substantial heterogeneity. When there was no substantial heterogeneity, we reported the pooled estimate calculated from the fixed effects model. When there was substantial heterogeneity, we reported the pooled estimate calculated from the random effects model.
We assessed publication bias with Begg’s funnel plot and Egger’s test. If publication bias exists, the Begg’s funnel plot is asymmetric or the Egger’s test P value is <0.05. We used Stata SE version 10.0 software (StataCorp, College Station, TX) for all the statistical analyses.
Results
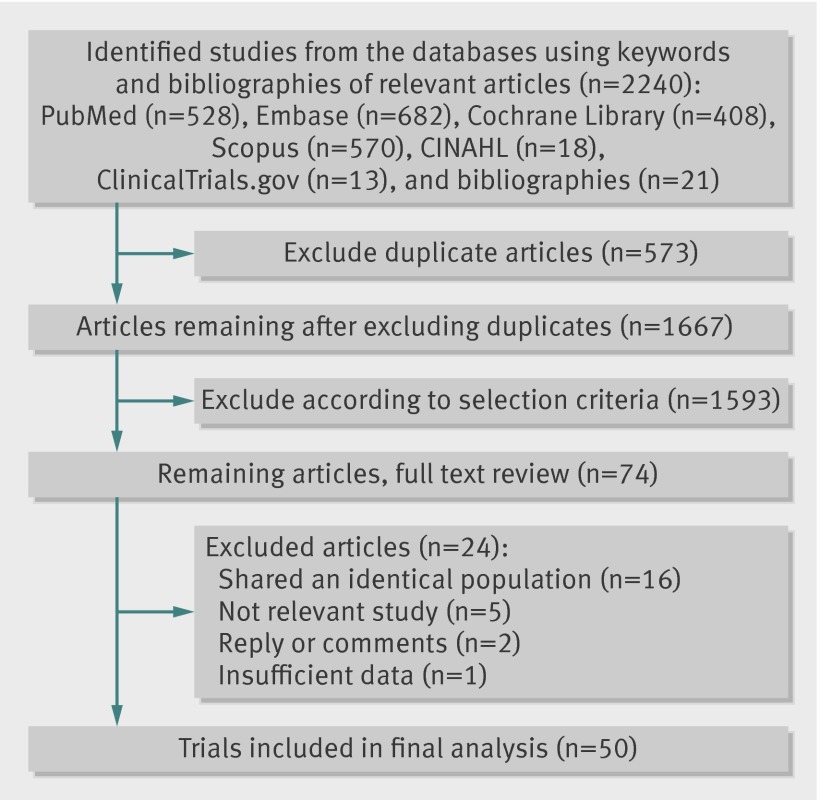
By searching databases (PubMed, Embase, the Cochrane Library, Scopus, CINAHL, and ClinicalTrials.gov) and hand searching relevant bibliographies, we identified 2240 articles (fig 1). After excluding 573 duplicated articles, two of authors independently reviewed and excluded 1593 articles that did not satisfy the predetermined selection criteria based on each article’s title and abstract. We reviewed the full texts of the 74 remaining articles and excluded 24 articles (16 were identical trials within the same population; five were not related to the subject of this study; two were replies or comments; and one reported insufficient data). A total of 5011 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 trials were included in the final analysis. The full details of all included trials are in appendix 1. The 50 trials included 294 478 participants with 156 663 in intervention groups and 137 815 in control groups. In the trials reporting age, the mean age of the participants ranged from 49 to 82. The year of publication of the included trials ranged between 1989 and 2012, spanning 23 years. Twelve trials were conducted in the United State, four in the United Kingdom, three in Finland, three in France, three in Italy, two in Canada, two in Israel, two in Australia, two in China, two in Germany, two in Norway, one in Sweden, one in Switzerland, one in the Netherlands, one in US/Canada, one in US/Canada/Scotland), one in Germany/the Netherlands, one in Canada/US, one in 13 countries, and one in 20 countries. The range of supplementation and follow-up periods was 6 months to 12 years. The number of participants ranged from 61 to 39 876.
Fig 1 Flow diagram for identification of relevant clinical trials examining effect of vitamins and supplements in prevention of cardiovascular disease
Among the 50 trials, 30 were primary prevention trials (general populations, smokers and workers exposed to asbestos, patients with oesophageal dysplasia, male physicians, patients with non-melanoma skin cancer, postmenopausal women, patients undergoing chronic haemodialysis, patients with end stage renal disease, ambulatory elderly women with vitamin D insufficiency, patients with chronic renal failure, older people with femoral neck fractures, patients with diabetes mellitus, elderly women with a low serum 25-hydroxyvitamin D concentration, health professionals, people with a high fasting plasma total homocysteine concentration, or kidney transplant recipients), and 20 were secondary prevention trials (patients with cardiovascular disease, coronary heart disease, acute myocardial infarction, unstable angina, transient ischaemic attack, stroke, angiographically proved coronary atherosclerosis, vascular disease, or aortic valve stenosis).
Forty five trials were randomised, double blind, placebo controlled trials, and five were open label, randomised controlled trials. All vitamin or antioxidant supplements and placebos were administered orally either singly or in combination with other vitamin or antioxidant supplements.
The dose regimens used in each trial were as follows: vitamin A (10 000 or 25 000 IU daily), vitamin B6 (3, 6, 10, 12.5, 25, 40, 48, 50, or 100 mg daily; 20 mg three times weekly), vitamin B12 (0.4, 0.5, 1, or 2 mg daily; 6, 18, 20, 60, or 400 µg daily; 50 µg three times weekly), vitamin C (60, 120, 180, 250, 500, or 1000 mg daily), vitamin D (800 or 1000 IU daily; 200 IU twice daily; 400 IU twice daily; 300 IU daily and 100 IU daily; 100 000 IU every four months), vitamin E (60, 200, 400, 600, 800 IU daily; 400 or 600 IU alternate day; 400 IU twice daily; 30, 50, 300, 600 mg daily), β carotene (6, 15, 20, 25, 30, or 50 mg daily; 50 mg alternate day), folic acid (560 or 800 µg daily; 0.5, 0.8, 1, 1.2, 2, 2.5, 5, 15, or 40 mg daily; 5 mg three times weekly), and selenium (50, 75, 100, 122, or 122 µg daily).
Thirty nine trials used vitamin supplements only, and 22 trials used antioxidant supplements only. The additional nutritional supplements or drugs used in each trial were aspirin (325 mg daily); coenzyme Q10 (100 mg daily); calcium (93 mg daily) with or without hormone replacement therapy; application of a sun protection factor 15+ sunscreen; ramipril (angiotensin converting enzyme inhibitor; with or without aspirin (100 mg daily); zinc (20 mg daily); multivitamins and minerals; calcium carbonate (500 mg twice daily); calcium (1000 mg daily); calcium citrate (1000 mg daily); omega 3 fatty acids (eicosapentaenoic acid and docosahexaenoic acid at a ratio of 2:1). The main outcomes used in each trial were fatal or non-fatal acute myocardial infarction, unstable angina, coronary heart disease, ischaemic heart disease, major coronary events, cardiovascular death, sudden death, transient ischaemic stroke, stroke, and cardiovascular disease.
Out of 47 trials reporting funding source, five were funded by pharmaceutical companies and 42 were funded mainly by public or governmental organisations or independent scientific foundations. Also, in 29 trials, vitamin or antioxidant supplements were provided at no cost from the pharmaceutical industry, while 18 trials paid for them or did not mention whether the pharmaceutical industry charged for them.
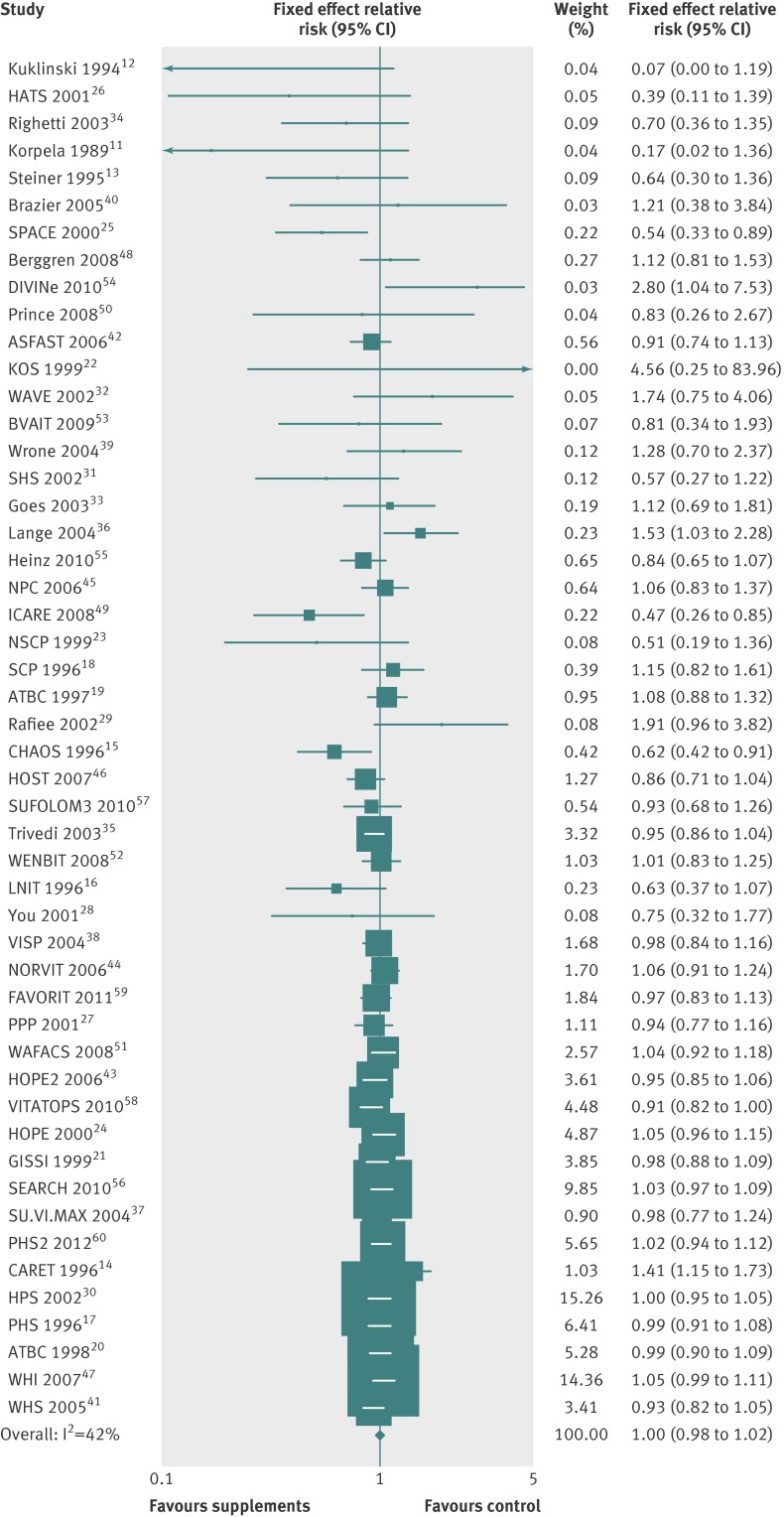
In the fixed effects meta-analysis of all 50 trials, use of vitamin or antioxidant supplements was not associated with reduced risks of major cardiovascular events (relative risk 1.00; 95% confidence interval 0.98 to 1.02; I2=42%) (fig 2). Overall, the effect sizes of the smaller randomised controlled trials tend to be less than 1.0, while the effect sizes of the larger ones tend to be null. In the 48 selected trials, the Begg’s funnel plot was symmetrical, and P for bias was 0.11 in the Egger’s test (two12 22 of the 50 trials were not included because of “zero” cells in the 2×2 table) (the Begg’s funnel plot is not shown in the figure).
Fig 2 Efficacy of vitamin and antioxidant supplements in prevention of major cardiovascular events in meta-analysis of 50 randomised controlled trials sorted in ascending order of number of participants
Based on the Jadad scales, the mean score for the 47 trials assessed was 4.3, ranging from 2 to 5 (table 1).
Table 1.
Methodological quality, based on Jadad scale, of 47* included trials on efficacy of vitamin and antioxidant supplements in prevention of cardiovascular diseases
| Randomisation | Description of randomisation methods | Double blind | Used identical placebo | Follow-up reporting | Total score | |
|---|---|---|---|---|---|---|
| Steiner, 199513 | 1 | 0 | 1 | 1 | 1 | 4 |
| Omenn, 1996 (CARET)14 | 1 | 1 | 1 | 1 | 1 | 5 |
| Stephens, 1996 (CHAOS)15 | 1 | 1 | 1 | 1 | 1 | 5 |
| Mark, 1996 (LNIT)16 | 1 | 1 | 1 | 1 | 0 | 4 |
| Hennekens, 1996 (PHS)17 | 1 | 0 | 1 | 1 | 1 | 4 |
| Greenberg, 1996 (SCP)18 | 1 | 1 | 1 | 1 | 1 | 5 |
| Rapola, 1997 (ATBC)19 | 1 | 1 | 1 | 1 | 1 | 5 |
| Virtamo, 1998 (ATBC)20 | 1 | 1 | 1 | 1 | 1 | 5 |
| GISSI, 199921 | 1 | 1 | 0 | 0 | 1 | 3 |
| Komulainen, 1999 (KOS)22 | 1 | 0 | 1 | 1 | 1 | 4 |
| Green, 1999 (NSCP)23 | 1 | 0 | 0 | 1 | 1 | 3 |
| Yusuf, 2000 (HOPE)24 | 1 | 0 | 1 | 1 | 0 | 3 |
| Boaz, 2000 (SPACE)25 | 1 | 1 | 1 | 1 | 0 | 4 |
| Brown, 2001 (HATS)26 | 1 | 0 | 1 | 1 | 1 | 4 |
| De Gaetano, 2001 (PPP)27 | 1 | 1 | 0 | 0 | 1 | 3 |
| You, 200128 | 1 | 0 | 1 | 1 | 1 | 4 |
| HPS, 200230 | 1 | 1 | 0 | 1 | 1 | 4 |
| Schnyder, 2002 (SHS)31 | 1 | 0 | 1 | 1 | 1 | 4 |
| Waters, 2002 (WAVE)32 | 1 | 1 | 1 | 1 | 1 | 5 |
| Liem, 2003 (Goes)33 | 1 | 1 | 0 | 0 | 1 | 3 |
| Righetti, 200334 | 1 | 1 | 0 | 0 | 1 | 3 |
| Trivedi, 200335 | 1 | 0 | 1 | 1 | 1 | 4 |
| Lange, 200436 | 1 | 0 | 1 | 1 | 1 | 4 |
| Hercberg, 2004 (SU.VI.MAX)37 | 1 | 1 | 1 | 1 | 1 | 5 |
| Toole, 2004 (VISP)38 | 1 | 1 | 1 | 1 | 1 | 5 |
| Wrone, 200439 | 1 | 1 | 1 | 1 | 1 | 5 |
| Brazier, 200540 | 1 | 1 | 1 | 1 | 1 | 5 |
| Lee, 2005 (WHS)41 | 1 | 1 | 1 | 1 | 1 | 5 |
| Zoungas, 2006 (ASFAST)42 | 1 | 0 | 1 | 1 | 1 | 4 |
| Lonn, 2006 (HOPE-2)43 | 1 | 1 | 1 | 1 | 1 | 5 |
| Bønaa, 2006 (NORVIT)44 | 1 | 1 | 1 | 1 | 1 | 5 |
| Stranges, 2006 (NPC)45 | 1 | 1 | 1 | 1 | 0 | 4 |
| Jamison, 2007 (HOST)46 | 1 | 1 | 1 | 1 | 1 | 5 |
| Hsia, 2007 (WHI)47 | 1 | 0 | 1 | 1 | 0 | 3 |
| Berggren, 200848 | 1 | 0 | 0 | 0 | 1 | 2 |
| Milman, 2008 (ICARE)49 | 1 | 1 | 1 | 1 | 1 | 5 |
| Prince, 200850 | 1 | 1 | 1 | 1 | 1 | 5 |
| Albert, 2008 (WAFACS)51 | 1 | 0 | 1 | 1 | 1 | 4 |
| Ebbing, 2008 (WENBIT)52 | 1 | 1 | 1 | 1 | 1 | 5 |
| Hodis, 2009 (BVAIT)53 | 1 | 1 | 1 | 1 | 1 | 5 |
| House, 2010 (DIVINe)54 | 1 | 1 | 1 | 1 | 1 | 5 |
| Heinz, 201055 | 1 | 0 | 1 | 1 | 1 | 4 |
| Armitage, 2010 (SEARCH)56 | 1 | 1 | 1 | 1 | 1 | 5 |
| Galan, 2010 (SU.FOL.OM3)57 | 1 | 1 | 1 | 1 | 1 | 5 |
| VITATOPS 201058 | 1 | 1 | 1 | 1 | 1 | 5 |
| Bostom, 2011 (FAVORIT)59 | 1 | 1 | 1 | 1 | 1 | 5 |
| Sesso, 2012 (PHS2)60 | 1 | 1 | 1 | 1 | 1 | 5 |
*We were unable to retrieve full text from three trials: Korpela et al, 1989,11 Kuklinski et al, 1994,12 and Rafiee et al, 2002.29
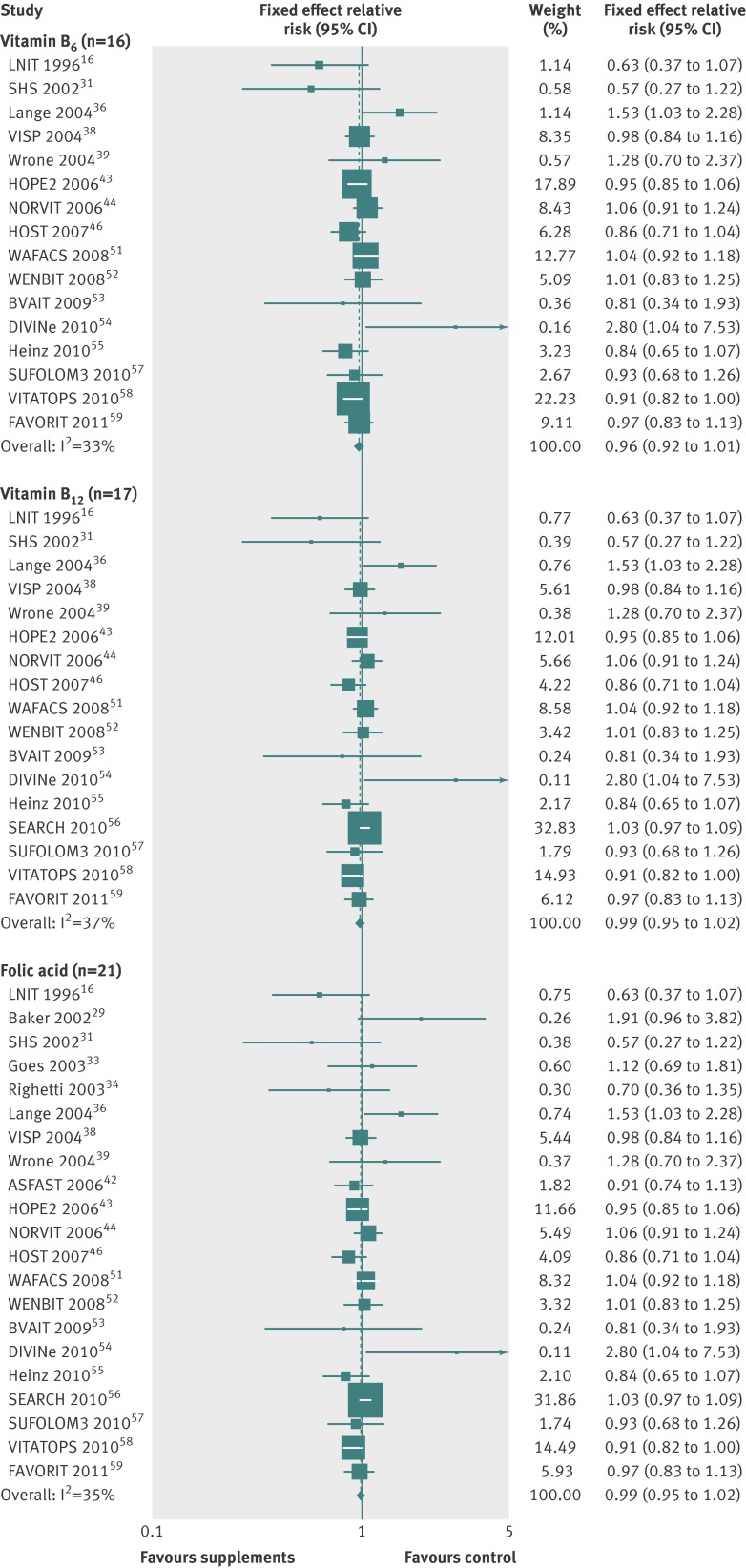
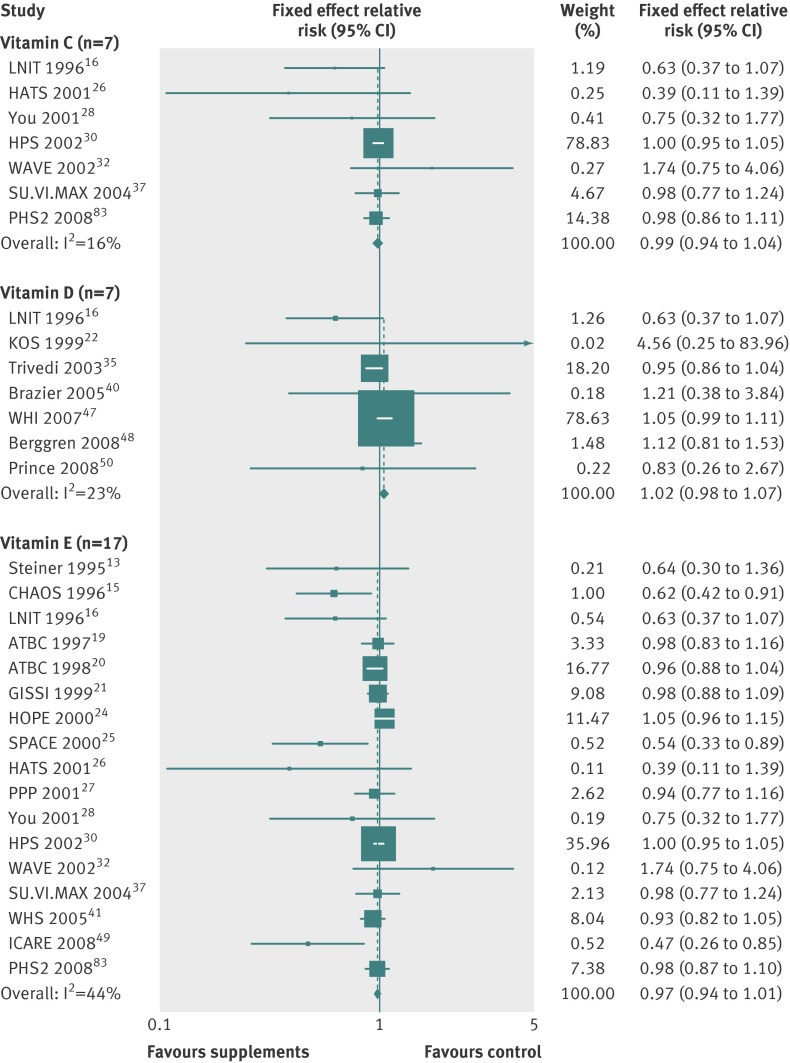
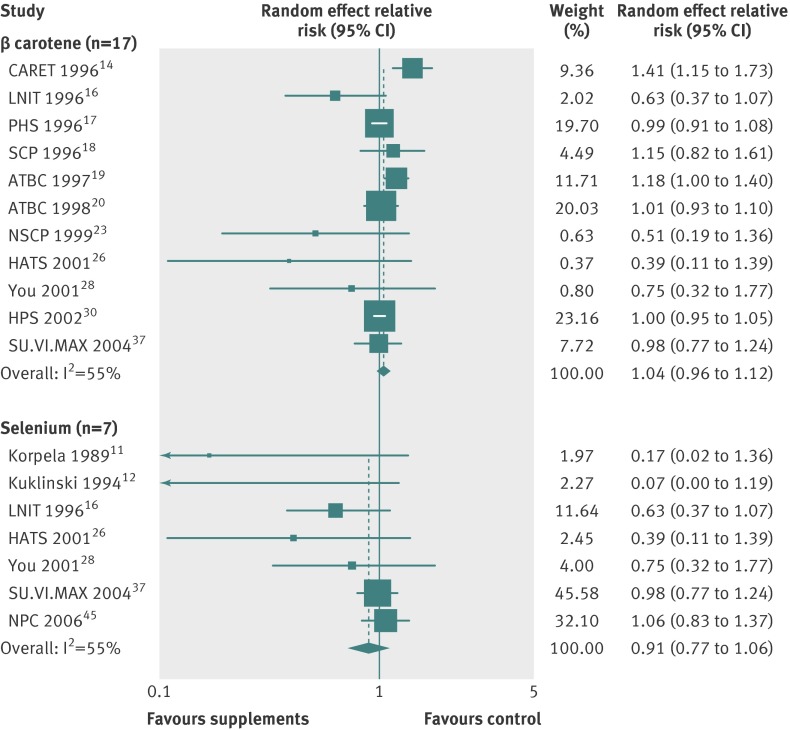
Tables 2-4 show the efficacy of vitamin or antioxidant supplements in the prevention of the major cardiovascular events in subgroup meta-analysis by various factors. Overall, subgroup meta-analyses by type of supplement (table 2) showed that there was no significant association between vitamin or antioxidant supplements and the risk of the major cardiovascular events (figs 3-6, and table 2), while low dose vitamin B6 supplementation slightly decreased the risk of major cardiovascular events (relative risk 0.92, 95% confidence interval 0.85 to 0.99; I2=35%; fig 7) in the fixed effects meta-analysis. Similarly, we found no significant association in the overall subgroup meta-analysis by type of outcome (cardiovascular death, fatal or nonfatal myocardial infarction, stroke, or transient ischaemic attack; table 3), type of prevention (primary v secondary; table 2), type of study design, methodological quality (high v low by score of 5), duration of treatment (<5 years v ≥5 years), funding source (independent organisation v pharmaceutical industry) (table 4), and provider of the supplements (pharmaceutical industry v at cost or no mention), while vitamin B6 and vitamin E supplements were associated with a reduced risk of cardiovascular death (relative risk 0.91, 95% confidence interval 0.83 to 0.99; I2=0%) and myocardial infarction (0.77, 0.65 to 0.91; I2=76%), respectively, and vitamin and antioxidant supplementation marginally increased the risk of angina pectoris (1.04, 1.00 to 1.08; I2=36%) (table 3).
Table 2.
Efficacy of vitamin and antioxidant supplements in the prevention of the major cardiovascular events in subgroup meta-analysis by type of prevention and type of supplement
| Factor | No of trials | Relative risk (95% CI) | Heterogeneity I2 (%) | Model |
|---|---|---|---|---|
| All | 50 | 1.00 (0.98 to 1.02) | 42 | Fixed effects |
| Prevention: | ||||
| Primary | 30 | 1.01 (0.98 to 1.03) | 42 | Fixed effects |
| Secondary | 20 | 1.00 (0.97 to 1.03) | 44 | Fixed effects |
| Type of supplement: | ||||
| Vitamins only | 39 | 0.99 (0.97 to 1.01) | 44 | Fixed effects |
| Low quality trials | 17 | 0.99 (0.96 to 1.02) | 32 | Fixed effects |
| High quality trials | 22 | 0.99 (0.94 to 1.05) | 53 | Random effects |
| Antioxidants only | 22 | 0.98 (0.96 to 1.02) | 41 | Fixed effects |
| Low quality trials | 11 | 0.99 (0.96 to 1.03) | 25 | Fixed effects |
| High quality trial | 9 | 0.97 (0.91 to 1.02) | 49 | Fixed effects |
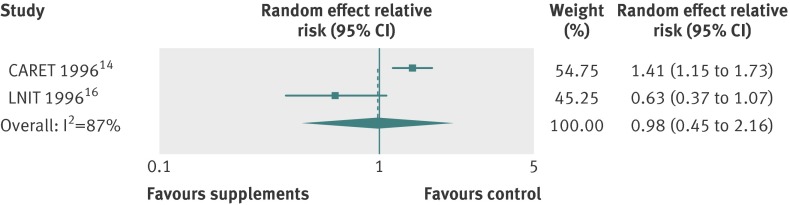
| Vitamin A | 2 | 0.98 (0.45 to 2.16) | 87 | Random effects |
| Low dose (10 000 IU/day) | 1 | 0.63 (0.37 to 1.07) | NA | NA |
| High dose (25 000 IU/day) | 1 | 1.41 (1.15 to 1.73)* | NA | NA |
| Vitamin B6 | 16 | 0.96 (0.92 to 1.01) | 33 | Fixed effects |
| Low dose (3-25 mg/day) | 8 | 0.92 (0.85 to 0.99)* | 35 | Fixed effects |
| Low quality trials | 3 | 0.76 (0.61 to 0.94)* | 0 | Fixed effects |
| High quality trials | 5 | 0.94 (0.87 to 1.02) | 39 | Fixed effects |
| High dose (40-100 mg/day) | 8 | 0.99 (0.94 to 1.05) | 22 | Fixed effects |
| Vitamin B12 | 17 | 0.99 (0.95 to 1.02) | 37 | Fixed effects |
| Low dose (6 µg-0.5 mg/day) | 11 | 0.96 (0.90 to 1.02) | 34 | Fixed effects |
| High dose (1-2 mg/day) | 6 | 1.00 (0.96 to 1.05) | 43 | Fixed effects |
| Folic acid | 21 | 0.99 (0.95 to 1.02) | 35 | Fixed effects |
| Low dose (500 µg-5 mg/day) | 17 | 0.99 (0.96 to 1.03) | 39 | Fixed effects |
| High dose (10-40 mg/day) | 4 | 0.89 (0.78 to 1.03) | 0 | Fixed effects |
| Vitamin C† | 7 | 0.99 (0.94 to 1.04) | 16 | Fixed effects |
| Low dose (120-250 mg/day) | 3 | 0.99 (0.94 to 1.04) | 31 | Fixed effects |
| High dose (500-1000 mg/day) | 4 | 0.98 (0.94 to 1.12) | 28 | Fixed effects |
| Vitamin D | 7 | 1.02 (0.98 to 1.07) | 23 | Fixed effects |
| Low dose (120-250 mg/day) | 2 | 1.05 (0.99 to 1.12) | 0 | Fixed effects |
| High dose (500-1000 mg/day) | 5 | 0.94 (0.86 to 1.03) | 0 | Fixed effects |
| Vitamin E† | 17 | 0.97 (0.94 to 1.01) | 44 | Fixed effects |
| Low dose (60 IU-250 mg/day) | 13 | 0.96 (0.92 to 1.01) | 35 | Fixed effects |
| High dose (500-600 mg/day) | 4 | 0.84 (0.52 to 1.35) | 69 | Random effects |
| β carotene | 11 | 1.04 (0.96 to 1.12) | 55 | Random effects |
| Low dose (6-25 mg/day) | 6 | 0.99 (0.95 to 1.03) | 7 | Fixed effects |
| High dose (30-50 mg/day) | 5 | 1.14 (0.96 to 1.35) | 69 | Random effects |
| Selenium | 7 | 0.91 (0.77 to 1.06) | 47 | Fixed effects |
| Low dose (50-100 µg/day) | 5 | 0.85 (0.70 to 1.04) | 44 | Fixed effects |
| High dose (122-200 µg /day) | 2 | 0.57 (0.10 to 3.16) | 67 | Fixed effects |
NA=not applicable.
*P≤0.05.
†For subgroup meta-analysis of vitamin C and vitamin E, we used data from 2008 PHS2 article83 because data were not available in 2012 PHS article.
Table 3.
Efficacy of vitamin and antioxidant supplements in prevention of major cardiovascular events in subgroup meta-analysis by outcome
| No of trials | Relative risk (95% CI) | Heterogeneity I2 (%) | Model | |
|---|---|---|---|---|
| Cardiovascular death | 32 | 1.01 (0.97 to 1.05) | 41 | Fixed effects |
| Vitamin A | 2 | 0.98 (0.45 to 2.16) | 87 | Random effects |
| Vitamin B6 | 8 | 0.91 (0.83 to 0.99)* | 0 | Fixed effects |
| Low quality trials | 4 | 0.93 (0.75 to 1.14) | 12 | Fixed effects |
| High quality trials | 4 | 0.90 (0.82 to 0.99)* | 0 | Fixed effects |
| Not supplied by pharmaceutical industry | 2 | 0.96 (0.84 to 1.10) | 0 | Fixed effects |
| Supplied by pharmaceutical industry | 2 | 0.85 (0.75 to 0.97)* | 0 | Fixed effects |
| Not supplied by pharmaceutical industry | 3 | 0.96 (0.83 to 1.10) | 0 | Fixed effects |
| Supplied by pharmaceutical industry | 5 | 0.88 (0.79 to 0.98)* | 0 | Fixed effects |
| Vitamin B12 | 9 | 0.96 (0.90 to 1.03) | 27 | Fixed effects |
| Folic acid | 11 | 0.96 (0.89 to 1.03) | 11 | Fixed effects |
| Vitamin C† | 6 | 1.03 (0.95 to 1.12) | 27 | Fixed effects |
| Vitamin D | 3 | 0.90 (0.76 to 1.07) | 27 | Fixed effects |
| Vitamin E† | 15 | 0.98 (0.92 to 1.04) | 37 | Fixed effects |
| β carotene | 10 | 1.10 (0.96 to 1.27) | 61 | Random effects |
| Selenium | 15 | 0.98 (0.92 to 1.04) | 37 | Fixed effects |
| Vitamin | 2 | 0.98 (0.45 to 2.16) | 87 | Random effects |
| Angina | 10 | 1.04 (1.00 to 1.08)* | 36 | Fixed effects |
| Low quality trials | 6 | 1.05 (1.00 to 1.09)* | 25 | Fixed effects |
| High quality trials | 4 | 1.01 (0.86 to 1.18) | 57 | Random effects |
| Vitamin B6 | 4 | 0.93 (0.72 to 1.20) | 77 | Random effects |
| Vitamin B12 | 4 | 0.93 (0.72 to 1.20) | 77 | Random effects |
| Folic acid | 4 | 0.93 (0.72 to 1.20) | 77 | Random effects |
| Vitamin C† | 2 | 0.94 (0.85 to 1.03) | 0 | Fixed effects |
| Vitamin D | 1 | 1.07 (0.93 to 1.23) | NA | NA |
| Vitamin E† | 3 | 1.15 (0.99 to 1.33) | 0 | Fixed effects |
| Myocardial infarction | 34 | 1.00 (0.96 to 1.03) | 0 | Fixed effects |
| Vitamin B6 | 13 | 0.99 (0.91 to 1.07) | 11 | Fixed effects |
| Vitamin B12 | 14 | 0.99 (0.93 to 1.06) | 4 | Fixed effects |
| Folic acid | 15 | 0.99 (0.93 to 1.06) | 0 | Fixed effects |
| Vitamin C† | 4 | 0.96 (0.87 to 1.07) | 0 | Fixed effects |
| Vitamin D | 2 | 1.06 (0.92 to 1.21) | 0 | Fixed effects |
| Vitamin E† | 12 | 0.77 (0.65 to 0.91)* | 76 | Random effects |
| Low quality trials | 5 | 0.76 (0.57 to 1.01) | 81 | Random effects |
| High quality trials | 7 | 0.75 (0.58 to 0.97)* | 75 | Random effects |
| Not supplied by pharmaceutical industry | 4 | 0.79 (0.53 to 1.17) | 62 | Random effects |
| Supplied by pharmaceutical industry | 3 | 0.67 (0.42 to 1.07)* | 86 | Random effects |
| Primary prevention | 7 | 0.72 (0.54 to 0.95)* | 76 | Random effects |
| Not supplied by pharmaceutical industry | 3 | 0.79 (0.42 to 1.50) | 55 | Random effects |
| Supplied by pharmaceutical industry | 4 | 0.63 (0.42 to 0.97)* | 82 | Random effects |
| Secondary prevention | 5 | 0.79 (0.61 to 1.02) | 80 | Random effects |
| Not supplied by pharmaceutical industry | 4 | 0.79 (0.53 to 1.17) | 62 | Random effects |
| Supplied by pharmaceutical industry | 8 | 0.73 (0.59 to 0.92)* | 82 | Random effects |
| β carotene | 4 | 0.95 (0.80 to 1.14) | 52 | Random effects |
| Selenium | 3 | 0.87 (0.59 to 1.28) | 0 | Fixed effects |
| Fatal myocardial infarction | 9 | 1.02 (0.92 to 1.12) | 43 | Fixed effects |
| Vitamin B6 | 1 | 1.00 (0.75 to 1.33) | NA | NA |
| Vitamin B12 | 1 | 1.00 (0.75 to 1.33) | NA | NA |
| Folic acid | 1 | 1.00 (0.75 to 1.33) | NA | NA |
| Vitamin E† | 3 | 0.57 (0.32 to 1.03) | 0 | Fixed effects |
| β carotene | 1 | 1.05 (0.95 to 1.17) | NA | NA |
| Selenium | 1 | 1.12 (0.43 to 2.87) | NA | NA |
| Non-fatal myocardial infarction | 13 | 0.83 (0.66 to 1.04) | 89 | Random effects |
| Vitamin B6 | 3 | 1.08 (0.90 to 1.30) | 22 | Fixed effects |
| Vitamin B12 | 4 | 1.03 (0.93 to 1.14) | 1 | Fixed effects |
| Folic acid | 4 | 1.03 (0.93 to 1.14) | 1 | Fixed effects |
| Vitamin C† | 4 | 0.85 (0.70 to 1.04) | 0 | Random effects |
| Vitamin E† | 9 | 0.57 (0.32 to 1.03) | 0 | Fixed effects |
| β carotene | 4 | 0.95 (0.80 to 1.14) | 52 | Random effects |
| Selenium | 3 | 0.82 (0.53 to 1.27) | 0 | Fixed effects |
| Stroke | 32 | 0.97 (0.93 to 1.02) | 0 | Fixed effects |
| Vitamin B6 | 12 | 0.93 (0.85 to 1.01) | 13 | Fixed effects |
| Vitamin B12 | 5 | 0.91 (0.80 to 1.03) | 8 | Fixed effects |
| Folic acid | 7 | 0.90 (0.79 to 1.01) | 19 | Fixed effects |
| Vitamin C† | 4 | 0.98 (0.88 to 1.09) | 0 | Fixed effects |
| Vitamin D | 5 | 1.00 (0.88 to 1.13) | 6 | Fixed effects |
| Vitamin E† | 12 | 1.00 (0.93 to 1.09) | 20 | Fixed effects |
| β carotene | 2 | 0.98 (0.89 to 1.07) | 0 | Fixed effects |
| Selenium | 1 | 1.09 (0.68 to 1.72) | NA | NA |
| Transient ischaemic attack | 5 | 1.12 (0.97 to 1.30) | 0 | Fixed effects |
| Vitamin B6 | 2 | 1.12 (0.88 to 1.42) | 0 | Fixed effects |
| Vitamin B12 | 2 | 1.12 (0.88 to 1.42) | 0 | Fixed effects |
| Folic acid | 2 | 1.12 (0.88 to 1.42) | 0 | Fixed effects |
| Vitamin D | 1 | 1.12 (0.96 to 1.42) | NA | NA |
| Vitamin E† | 2 | 0.93 (0.59 to 1.47) | 0 | Fixed effects |
NA=not applicable.
*P≤0.05.
†For subgroup meta-analysis of vitamin C and vitamin E, we used data from 2008 PHS2 article83 because data were not available in 2012 PHS article.
Table 4.
Efficacy of vitamin and antioxidant supplements in prevention of major cardiovascular events in subgroup meta-analysis by various factors
| Factor | No of trials | Relative risk (95% CI) | Heterogeneity, I2 (%) | Model |
|---|---|---|---|---|
| Study design: | ||||
| RDBPCT | 45 | 1.00 (0.98 to 1.02) | 46 | Fixed effects |
| OLRCT | 5 | 0.98 (0.89 to 1.07) | 0 | Fixed effects |
| Methodological quality: | ||||
| High quality (Jadad score 5) | 24 | 0.99 (0.96 to 1.03) | 45 | Fixed effects |
| Low quality (Jadad score ≤4) | 23 | 1.01 (0.98 to 1.03) | 32 | Fixed effects |
| Duration of treatment (years): | ||||
| <5 | 34 | 0.97 (0.90 to 1.04) | 52 | Random effects |
| ≥5 | 16 | 1.01 (0.98 to 1.03) | 0 | Fixed effects |
| Funding source: | ||||
| Pharmaceutical industry | 5 | 1.01 (0.96 to 1.07) | 0 | Fixed effects |
| Independent organisation | 42 | 1.00 (0.98 to 1.02) | 42 | Fixed effects |
| Supply source for supplements: | ||||
| Pharmaceutical industry | 29 | 0.99 (0.97 to 1.02) | 33 | Fixed effects |
| Not pharmaceutical industry | 18 | 1.01 (0.98 to 1.05) | 47 | Fixed effects |
| Type of control: | ||||
| Placebo | 44 | 1.00 (0.98 to 1.02) | 46 | Fixed effects |
| No placebo | 6 | 0.97 (0.89 to 1.06) | 0 | Fixed effects |
| No of participants in each trial: | ||||
| <10 000 | 40 | 0.97 (0.94 to 1.01) | 40 | Fixed effects |
| ≥10 000 | 10 | 1.02 (0.99 to 1.04) | 39 | Fixed effects |
RDBPCT=randomised, double blind, placebo-controlled trial; OLRCT=open label, randomised controlled trial.
Fig 3 Efficacy of vitamin A supplements in prevention of major cardiovascular events in subgroup meta-analysis of randomised controlled trials
Fig 4 Efficacy of vitamins B6 and B12 and folic acid supplements in prevention of major cardiovascular events in subgroup meta-analysis of randomised controlled trials
Fig 5 Efficacy of vitamins C, D, and E supplements in prevention of major cardiovascular events in subgroup meta-analysis of randomised controlled trials. For subgroup meta-analysis of vitamin C and vitamin E, we used data from 2008 PHS2 article83 because data were not available in 2012 PHS article
Fig 6 Efficacy of β carotene and selenium supplements in prevention of major cardiovascular events in subgroup meta-analysis of randomised controlled trials
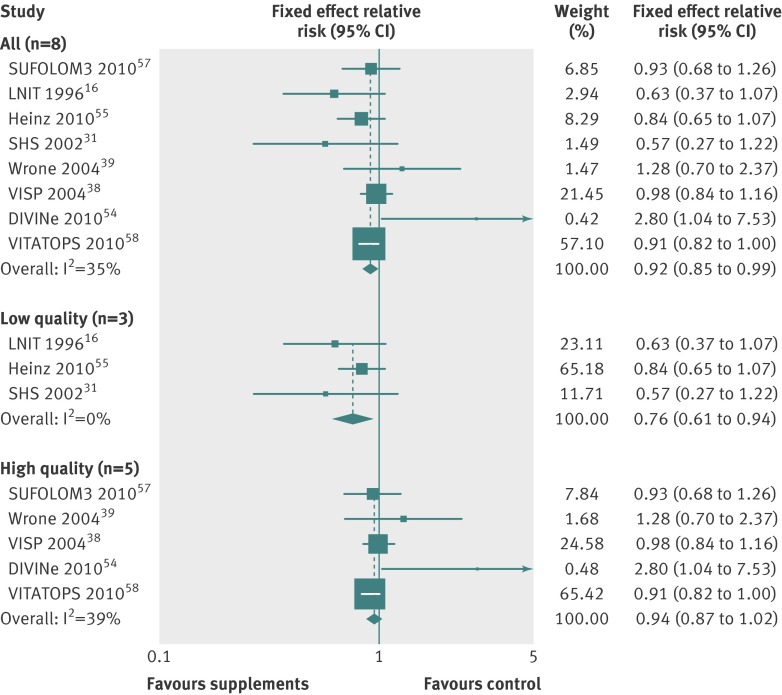
Fig 7 Efficacy of low dose vitamin B6 supplements in prevention of major cardiovascular events in subgroup meta-analysis of randomised controlled trials by study quality according to Jadad scale
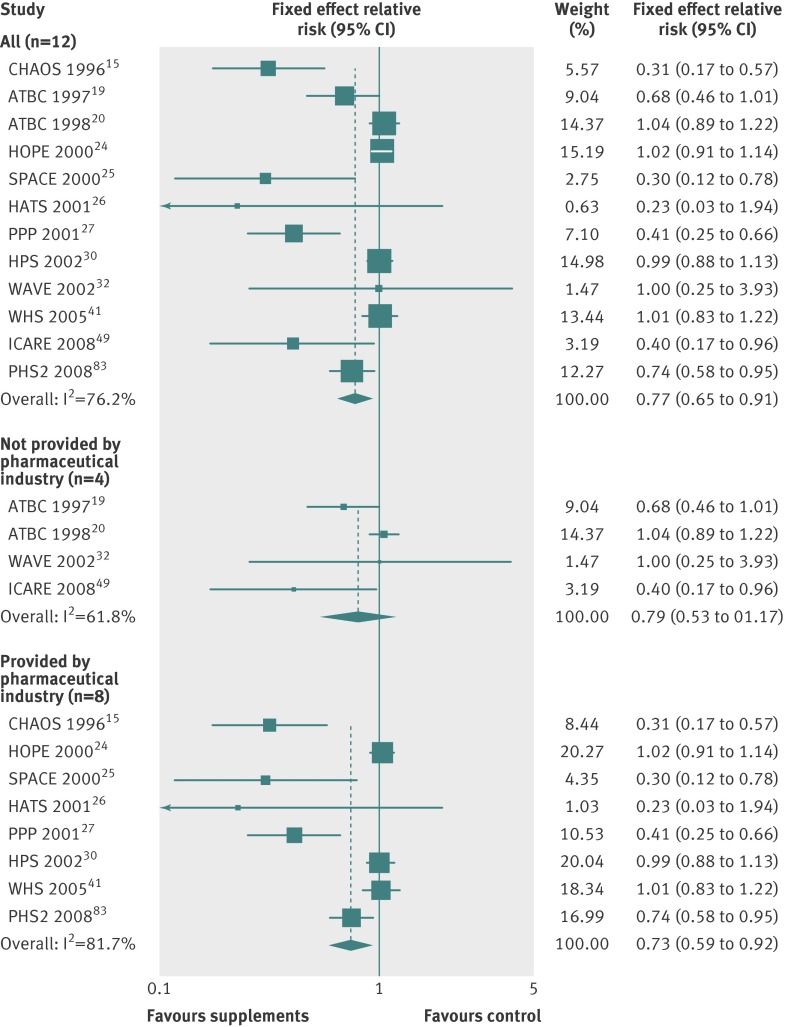
In the subgroup meta-analysis of high quality randomised controlled trials within each category of low dose vitamin B6 (relative risk 0.94, 95% confidence interval 0.87 to 1.02; I2=39%; fixed effects model, table 2 and fig 7) and angina (1.01, 0.86 to 1.18; I2=57%; random effects model, table 3), however, beneficial or harmful effects disappeared. Also, even though vitamin B6 supplementation was associated with decreased risks of cardiovascular death in high quality trials, and vitamin E supplementation with a decreased risk of myocardial infarction, those beneficial effects were seen only in trials supplied with supplements by pharmaceutical industry (fig 8 and table 3).
Fig 8 Efficacy of vitamin E supplements in prevention of myocardial infarction in subgroup meta-analysis of randomised controlled trials according to provider of supplements (pharmaceutical industry or other). For subgroup meta-analysis of vitamin E, we used data from 2008 PHS2 article83 because data were not available in 2012 PHS article
In the subgroup meta-analysis by the number of participants in each trial, vitamin or antioxidant supplements showed a trend toward a decreased (but not significant) risk of major cardiovascular events (relative risk 0.97, 95% confidence interval 0.94 to 1.01; I2=40%) in the subgroup meta-analysis of trials with <10 000 participants, while those supplements showed an increased (but not significant) risk of the major cardiovascular events (1.02, 0.99 to 1.04; I2=39%) in the subgroup meta-analysis of those with ≥10 000 participants (table 4).
Table 5 shows the efficacy of vitamin and antioxidant supplements given singly or in combination with other vitamin or antioxidant supplements on major cardiovascular events in subgroup meta-analyses. We found no significant beneficial effect of vitamin and antioxidant supplements in most of the subgroup meta-analyses, while only vitamin E supplements had a marginally significant decreased efficacy for the major cardiovascular events in high quality trials (relative risk 0.95, 95% confidence interval 0.90 to 1.00; I2=45%).
Table 5.
Efficacy of vitamin and antioxidant supplements given singly or combined with other vitamin or antioxidant supplements in prevention of major cardiovascular events in subgroup meta-analysis
| Factor | No of trials | Relative risk (95% CI) | Heterogeneity, I2 (%) | Model |
|---|---|---|---|---|
| All | 50 | 1.00 (0.98 to 1.02) | 42 | Fixed effects |
| Vitamin A: | ||||
| Given singly | NA | — | — | — |
| Combined with others | 2 | 0.98 (0.45 to 2.16) | 87 | Random effects |
| Vitamin B6: | ||||
| Given singly | NA | — | — | — |
| Combined with others | 16 | 0.96 (0.92 to 1.01) | 33 | Fixed effects |
| Low quality trials | 5 | 0.94 (0.73 to 1.21) | 66 | Random effects |
| High quality trials | 11 | 0.96 (0.91 to 1.01) | 1 | Fixed effects |
| Vitamin B12: | ||||
| Given singly | NA | — | — | — |
| Combined with others | 17 | 0.99 (0.95 to 1.02) | 37 | Fixed effects |
| Low quality trials | 5 | 0.94 (0.73 to 1.21) | 66 | Random effects |
| High quality trials | 12 | 0.98 (0.95 to 1.02) | 18 | Fixed effects |
| Folic acid: | ||||
| Given singly | 4 | 1.02 (0.84 to 1.23) | 47 | Fixed effects |
| Combined with others | 17 | 0.99 (0.95 to 1.02) | 37 | Fixed effects |
| Given singly or combined | 21 | 0.99 (0.95 to 1.02) | 35 | Fixed effects |
| Low quality trials | 8 | 0.99 (0.90 to 1.08) | 49 | Fixed effects |
| High quality trials | 12 | 0.98 (0.95 to 1.02) | 18 | Fixed effects |
| Vitamin C*: | ||||
| Given singly | NA | — | — | — |
| Combined with others | 7 | 0.99 (0.94 to 1.06) | 16 | Fixed effects |
| Low quality trials | 4 | 0.99 (0.94 to 1.04) | 44 | Fixed effects |
| High quality trials | 3 | 0.99 (0.88 to 1.11) | 0 | Fixed effects |
| Vitamin D | ||||
| Given singly | 2 | 0.95 (0.86 to 1.05) | 11 | Fixed effects |
| Combined with others | 5 | 1.04 (0.99 to 1.10) | 0 | Fixed effects |
| Given singly or combined | 7 | 1.02 (0.98 to 1.07) | 23 | Fixed effects |
| Low quality trials | 5 | 1.02 (0.98 to 1.08) | 47 | Fixed effects |
| High quality trials | 2 | 1.01 (0.45 to 2.27) | 0 | Fixed effects |
| Vitamin E*: | ||||
| Given singly | 10 | 0.93 (0.85 to 1.01) | 57 | Random effects |
| Combined with others | 7 | 0.99 (0.94 to 1.04) | 15 | Fixed effects |
| Given singly or combined | 17 | 0.97 (0.94 to 1.01) | 44 | Fixed effects |
| Low quality trials | 9 | 0.99 (0.95 to 1.03) | 43 | Fixed effects |
| High quality trials | 8 | 0.95 (0.90 to 1.00)† | 45 | Fixed effects |
| β carotene: | ||||
| Given singly | 5 | 1.02 (0.96 to 1.08) | 31 | Fixed effects |
| Combined with others | 6 | 1.00 (0.81 to 1.23) | 70 | Random effects |
| Given singly or combined | 11 | 1.04 (0.96 to 1.12) | 55 | Random effects |
| Low quality trials | 6 | 0.99 (0.95 to 1.03) | 30 | Fixed effects |
| High quality trials | 5 | 1.13 (0.98 to 1.29) | 64 | Random effects |
| Selenium: | ||||
| Given singly | 3 | 0.34 (0.06 to 2.05) | 70 | Random effects |
| Combined with others | 4 | 0.88 (0.72 to 1.08) | 26 | Fixed effects |
| Given singly or combined | 7 | 0.91 (0.77 to 1.06) | 47 | Fixed effects |
| Low quality trials | 4 | 0.91 (0.73 to 1.12) | 43 | Fixed effects |
| High quality trials | 1 | 0.98 (0.77 to 1.24) | NA | NA |
NA=not applicable.
*For subgroup meta-analysis of vitamin C and vitamin E, we used data from 2008 PHS2 article83 because data were not available in 2012 PHS article.
†P≤0.05.
Discussion
Summary of main findings
In this large scale meta-analysis of randomised controlled trials, we found no evidence to support the use of vitamin or antioxidant supplements for the primary or secondary prevention of major cardiovascular events. Furthermore, these supplements were not associated with any reduced risk of the such events in the subgroup meta-analyses according to various factors such as type of vitamins and antioxidants, type of cardiovascular outcomes, study design, methodological quality, duration of treatment, funding source, provider of supplements, type of control, number of participants in each trial, and supplements given singly or in combination with other vitamins or antioxidant supplements.
Strengths of the current meta-analysis
Our main findings are consistent with those of previous meta-analyses that investigated the association between the use of vitamin B,7 61 62 vitamin D,63 vitamin E,5 64 65 β carotene,5 folic acid,7 8 61 62 66 or selenium6 and cardiovascular diseases in randomised controlled trials.
Our findings, however, are inconsistent with those of previous in vivo animal studies that suggested vitamins or antioxidants inhibit the development of atherosclerosis67 68 69 70 and in vitro laboratory studies that indicated vitamins and antioxidants reduce lipid peroxidation and free radical damage, and finally inhibit atheroslcerosis.71 72 73 The findings from animal and laboratory studies are associated with the oxidative modification hypothesis of atherosclerosis. This hypothesis, which claims that the oxidation of low density lipoprotein cholesterol initiates atherosclerosis, could explain these associations.70 It suggests that accumulated low density lipoprotein in the subendothelial space of arteries is oxidised to minimally modified low density lipoprotein by vascular cells, which then induces accumulation of monocytes and macrophages, which stimulate further peroxdiation of low density lipoprotein.74 This reaction makes oxidised low density lipoprotein more negatively charged and completely oxidised.75 The uptake of completely oxidised low density lipoprotein leads to massive uptake of cholesterol by the macrophages.70 It also stimulates the binding of monocytes to the endothelium, promotes the release of lipids and lysosomal enzymes, and thus enhances the progression of atherosclerosis.70 76
Our meta-analysis indicates that there is a discrepancy in findings between in vivo animal or in vitro laboratory studies and randomised controlled trials with regard to the association between vitamin or antioxidants (natural forms in fruit and vegetables or synthetic forms) and cardiovascular disease. Several theories could explain this discrepancy. Firstly, preclinical studies such as animal studies and in vitro laboratory studies might not represent the biological processes in the human body.73 Thus, even though vitamins or antioxidant substances show benefits against a certain disease in preclinical studies, they might show no benefit or could be harmful under clinical circumstances. Secondly, the beneficial effects of vitamin or antioxidant supplements might be related to the timing of their administration. For example, the beneficial effects vitamin C occur in the early stages of atherosclerosis,73 and once the atherosclerotic plaque has developed it has no beneficial effect.77 In the trials we included in our analysis, the mean age of participants ranged from 49 to 82, the ages at which atherosclerotic plaques or changes might be already formed.73
We found a similar discrepancy in findings between case-control studies and randomised controlled trials. This could be explained by methodological biases of case-control studies. Case-control studies use retrospective assessment of each participant’s information on fruit and vegetable consumption and are thus susceptible to two potential biases: recall and selection.
Even though cohort studies are less biased than case-control studies, some important methodological issues might explain the differences in findings between cohort studies and randomised controlled trials. The diet assessment tools such as the food frequency questionnaire might not precisely assess an individual’s long term diet or might not provide sufficient information on fruit and vegetable consumption. Also, and more importantly, the use of vitamin or antioxidant supplements in randomised controlled trials should not be seen as equivalent to the intake of fruit and vegetables in cohort studies, which contain other various micronutrients as well as specific nutritional substances. Beneficial effects of vitamin or antioxidant supplements on cardiovascular disease might be obtained from the combination of various nutrients, not from one or several specific nutrients.
Strengths and weaknesses in relation to other meta-analyses
Our findings are similar to those of the previous meta-analysis of randomised controlled trials on the association between vitamin or antioxidant supplementation and other outcomes such as mortality and cancer. In 2007, Bjelakovic et al reported that vitamin A, vitamin E, or β carotene supplements were associated with increased mortality in a meta-analysis of 47 low bias (high quality) trials with 180 938 participants, while vitamin C and selenium were not associated with mortality.78 Regarding the negative effect of antioxidant supplements on mortality, they suggested that the elimination of free radicals in the human body through antioxidant supplementation interferes with essential defensive mechanisms such as apoptosis, phagocytosis, and detoxification and might lead to an increased mortality.78 Their updated meta-analysis including the recently published trials had similar findings on this issue and suggested that antioxidant supplements should be considered as medicinal products and should undergo sufficient evaluation before they are marketed.79 In 2010, Myung et al reported that antioxidant supplements had no primary or secondary preventive effect on cancer and even increased the risk of bladder cancer in a meta-analysis of 22 randomised controlled trials.80
Because of these discrepancies in results between preclinical studies and clinical trials, the findings from preclinical studies on the effects or actions of vitamin or antioxidant substances should not be directly applied to humans.
In the meantime, when we performed subgroup meta-analyses by quality (high v low), dose (low v high), and supplements given singly or in combination with other supplements, we found no overall association between vitamin or antioxidant supplements and the risk of major cardiovascular events, while vitamin and antioxidant supplementation were associated with a marginally increased risk of angina pectoris, and low dose vitamin B6 supplementation with a slightly decreased risk of major cardiovascular events. In the subgroup meta-analysis of high quality randomised controlled trials within each category, however, beneficial or harmful effects disappeared. We cannot therefore conclude that vitamin and antioxidant supplements are harmful for angina pectoris or that vitamin B6 supplements are beneficial for major cardiovascular events. Also, even though vitamin B6 supplementation was associated with a decreased risk of cardiovascular mortality in high quality trials, and vitamin E supplementation was associated with a decreased risk of myocardial infarction, those beneficial effects were shown only in trials with supplements provided by the pharmaceutical industry. So we cannot completely exclude the possibility that this might have influenced the respective trial design, results, or interpretations.
We also found that there was a trend toward an increased (not significant) risk of major cardiovascular events for the supplementation group in subgroup meta-analysis of trials with ≥10 000 participants, while there was a trend toward a decreased (not significant) risk in subgroup meta-analysis of trials with <10 000 participants. Given that a larger sample size is more accurate than a smaller size, we cannot exclude that vitamin or antioxidant supplementation might be associated with an increase in the risk of cardiovascular disease. Further large scale trials are needed to confirm this.
Weaknesses of the current meta-analysis
There are several limitations in the current study. Firstly, we investigated the association only between synthetic vitamin and antioxidant supplements and cardiovascular disease. Thus, our findings could not be directly applied to fruit and vegetables rich in natural vitamins or antioxidants or natural vitamins derived or extracted from plants. Secondly, we were unable to evaluate whether vitamin and antioxidant supplementation would be beneficial against cardiovascular disease for populations who are deficient in vitamins or antioxidants at baseline. Further randomised controlled trials in those populations are needed. Thirdly, we used the Jadad scale to assess the methodological quality of the trials, which has been criticised because it is subject to the generic problems of scales, has a strong emphasis on reporting rather than conduct, and does not cover concealment of allocation, one of the important biases in randomised controlled trials.81 As an alternative, the Cochrane Risk of Bias (RoB) tool has been used to evaluate internal validity of randomised controlled trials since 2008. Hartling et al, however, reported that the inter-rater agreement varied substantially across domains in the Cochrane tool, and it took considerably longer to complete than the Jadad scale.82 Further validated tools for the assessment of quality are needed. Finally, we assessed the methodological quality of the trials based only on the data presented in each article. Thus, we might not have assessed the actual performance or biases of each trial.
Meaning of the findings
In summary, we found no evidence to support the use of vitamin or antioxidant supplements in the prevention of cardiovascular disease. Also, recent meta-analyses have shown that vitamin or antioxidant supplements are associated with increased mortality and have no preventive effect on cancer or were even associated with increases in some types of cancer. Most countries permit the pharmaceutical or food industry to sell these supplements under the name of functional food or medical food, and many people take vitamin or antioxidant supplements in the belief that they improve their health. Based on recent meta-analyses of randomised controlled trials, including the current study, however, governments and regulating agencies for food and drugs should consider vitamin and antioxidant supplements as medicinal products and strictly evaluate their efficacy and safety before marketing.
Unanswered questions and future research
Further randomised controlled trials are required to determine whether vitamin and antioxidant supplementation would be beneficial against cardiovascular disease for people who are deficient in vitamins or antioxidants at baseline. Regarding the assessment of methodological quality of each trial, further validated tools that could assess the actual performance or biases of each trial should be developed.
What is already known on this topic
Over the past few decades, observational epidemiological studies have reported that intake of fruit and vegetables rich in various vitamins and antioxidants is associated with a reduced risk of cardiovascular disease
Unlike the evidence for the benefits of fruit and vegetables, however, randomised controlled trials have had inconsistent results regarding benefits of vitamin or antioxidant supplements
Even though several meta-analyses of randomised controlled trials have been published, those involved individual vitamin or antioxidant supplements, and there has been no published comprehensive meta-analysis that reviewed this topic all at once in one report
What this study adds
This large scale meta-analysis of randomised controlled trials suggests that there is no evidence to support the use of vitamin or antioxidant supplements for the primary or secondary prevention of major cardiovascular events
Governments and regulating agencies for food and drug should consider vitamin and antioxidant supplements as medicinal products and strictly evaluate their efficacy and safety before marketing
Contributors: BC and S-KM were responsible for the initial plan, study design, conducting the study, data interpretation, and manuscript drafting. S-KM was responsible for statistical analysis. S-KM, WJ, and S-WO were responsible for data collection, data extraction, and data interpretation. SMP, B-KK, and B-J P were responsible for data interpretation and manuscript drafting. BC and S-KM are guarantors.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
Cite this as: BMJ 2013;346:f10
Web Extra. Extra material supplied by the author
Appendix 1: Full details of 50 included trials
References
- 1.Mendis S, Lindholm LH, Anderson SG, Alwan A, Koju R, Onwubere BJ, et al. Total cardiovascular risk approach to improve efficiency of cardiovascular prevention in resource constrain settings. J Clin Epidemiol 2011;64:1451-62. [DOI] [PubMed] [Google Scholar]
- 2.Dauchet L, Amouyel P, Dallongeville J. Fruits, vegetables and coronary heart disease. Nat Rev Cardiol 2009;6:599-608. [DOI] [PubMed] [Google Scholar]
- 3.Lock K, Pomerleau J, Causer L, Altmann DR, McKee M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull World Health Organ 2005;83:100-8. [PMC free article] [PubMed] [Google Scholar]
- 4.Bhupathiraju SN, Tucker KL. Coronary heart disease prevention: nutrients, foods, and dietary patterns. Clin Chim Acta 2011;412:1493-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003;361:2017-23. [DOI] [PubMed] [Google Scholar]
- 6.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr 2006;84:762-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine-lowering therapy with folic acid in stroke prevention: a meta-analysis. Stroke 2010;41:1205-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin X, Huo Y, Langman CB, Hou F, Chen Y, Matossian D, et al. Folic acid therapy and cardiovascular disease in ESRD or advanced chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol 2011;6:482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
- 11.Korpela H, Kumpulainen J, Jussila E, Kemilä S, Kääriäinen M, Kääriäinen T, et al. Effect of selenium supplementation after acute myocardial infarction. Res Commun Chem Pathol Pharmacol 1989;65:249-52. [PubMed] [Google Scholar]
- 12.Kuklinski B, Weissenbacher E, Fahnrich A. Coenzyme Q10 and antioxidants in acute myocardial infarction. Mol Aspects Med 1994;15suppl:s143-7. [DOI] [PubMed]
- 13.Steiner M, Glantz M, Lekos A. Vitamin E plus aspirin compared with aspirin alone in patients with transient ischemic attacks. Am J Clin Nutr 1995;62:1381-4S. [DOI] [PubMed] [Google Scholar]
- 14.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334:1150-5. [DOI] [PubMed] [Google Scholar]
- 15.Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 1996;347:781-6. [DOI] [PubMed] [Google Scholar]
- 16.Mark SD, Wang W, Fraumeni JF Jr, Li JY, Taylor PR, Wang GQ, et al. Lowered risks of hypertension and cerebrovascular disease after vitamin/mineral supplementation: the Linxian Nutrition Intervention Trial. Am J Epidemiol 1996;143:658-64. [DOI] [PubMed] [Google Scholar]
- 17.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 1996;334:1145-9. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg ER, Baron JA, Karagas MR, Stukel TA, Nierenberg DW, Stevens MM, et al. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA 1996;275:699-703. [DOI] [PubMed] [Google Scholar]
- 19.Rapola JM, Virtamo J, Ripatti S, Huttenen JK, Albanes D, Taylor PR, et al. Randomised trial of alpha-tocopherol and beta-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet 1997;349:1715-20. [DOI] [PubMed] [Google Scholar]
- 20.Virtamo J, Rapola JM, Ripatti S, Heinonen OP, Taylor PR, Albanes D, et al. Effect of vitamin E and beta carotene on the incidence of primary nonfatal myocardial infarction and fatal coronary heart disease. Arch Intern Med 1998;158:668-75. [DOI] [PubMed] [Google Scholar]
- 21.GISSI-Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999;354:447-55. [PubMed] [Google Scholar]
- 22.Komulainen M, Kröger H, Tuppurainen MT, Heikkinen AM, Alhava E, Honkanen R, et al. Prevention of femoral and lumbar bone loss with hormone replacement therapy and vitamin D3 in early postmenopausal women: a population-based 5-year randomised trial. J Clin Endocrinol Metab 1999;84:546-52. [DOI] [PubMed] [Google Scholar]
- 23.Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999;354:723-9. [DOI] [PubMed] [Google Scholar]
- 24.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000;342:154-60. [DOI] [PubMed] [Google Scholar]
- 25.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet 2000;356:1213-8. [DOI] [PubMed] [Google Scholar]
- 26.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001;345:1583-92. [DOI] [PubMed] [Google Scholar]
- 27.De Gaetano G; Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet 2001;357:89-95. [DOI] [PubMed] [Google Scholar]
- 28.You WC, Chang YS, Heinrich J, Ma JL, Liu WD, Zhang L, et al. An intervention trial to inhibit the progression of precancerous gastric lesions: compliance, serum micronutrients and S-allyl cysteine levels, and toxicity. Eur J Cancer Prev 2001;10:257-63. [DOI] [PubMed] [Google Scholar]
- 29.Rafiee P, Shi Y, Kong X, Pritchard KA Jr, Tweddell JS, Litwin SB, et al. Activation of protein kinases in chronically hypoxic infant human and rabbit hearts: role in cardioprotection. Circulation 2002;106:239-45. [DOI] [PubMed] [Google Scholar]
- 30.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:23-33.12114037 [Google Scholar]
- 31.Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomised controlled trial. JAMA 2002;288:973-9. [DOI] [PubMed] [Google Scholar]
- 32.Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR, Rogers WJ, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomised controlled trial. JAMA 2002;288:2432-40. [DOI] [PubMed] [Google Scholar]
- 33.Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: effects on clinical outcomes. J Am Coll Cardiol 2003;41:2105-13. [DOI] [PubMed] [Google Scholar]
- 34.Righetti M, Ferrario GM, Milani S, Serbelloni P, La Rosa L, Uccellini M, et al. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit 2003;9:PI19-24. [PubMed] [Google Scholar]
- 35.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003;326:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange H, Suryapranata H, De Luca G, Börner C, Dille J, Kallmayer K, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med 2004;350:2673-81. [DOI] [PubMed] [Google Scholar]
- 37.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX Study: a randomised, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med 2004;164:2335-42. [DOI] [PubMed] [Google Scholar]
- 38.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomised controlled trial. JAMA 2004;291:565-75. [DOI] [PubMed] [Google Scholar]
- 39.Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomised trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol 2004;15:420-6. [DOI] [PubMed] [Google Scholar]
- 40.Brazier M, Grados F, Kamel S, Mathieu M, Morel A, Maamer M, et al. Clinical and laboratory safety of one year’s use of a combination calcium + vitamin D tablet in ambulatory elderly women with vitamin D insufficiency: results of a multicenter, randomised, double-blind, placebo-controlled study. Clin Ther 2005;27:1885-93. [DOI] [PubMed] [Google Scholar]
- 41.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomised controlled trial. JAMA 2005;294:56-65. [DOI] [PubMed] [Google Scholar]
- 42.Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, et al. Cardiovascular morbidity and mortality in the atherosclerosis and folic acid supplementation trial (ASFAST) in chronic renal failure: a multicenter, randomised, controlled trial. J Am Coll Cardiol 2006;47:1108-16. [DOI] [PubMed] [Google Scholar]
- 43.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 2006;354:1567-77. [DOI] [PubMed] [Google Scholar]
- 44.Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 2006;354:1578-88. [DOI] [PubMed] [Google Scholar]
- 45.Stranges S, Marshall JR, Trevisan M, Natarajan R, Donahue RP, Combs GF, et al. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomised clinical trial. Am J Epidemiol 2006;163:694-9. [DOI] [PubMed] [Google Scholar]
- 46.Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomised controlled trial. JAMA 2007;298:1163-70. [DOI] [PubMed] [Google Scholar]
- 47.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007;115:846-54. [DOI] [PubMed] [Google Scholar]
- 48.Berggren M, Stenvall M, Olofsson B, Gustafson Y. Evaluation of a fall-prevention program in older people after femoral neck fracture: a one-year follow-up. Osteoporos Int 2008;19:801-9. [DOI] [PubMed] [Google Scholar]
- 49.Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol 2008;28:341-7. [DOI] [PubMed] [Google Scholar]
- 50.Prince RL, Austin N, Devine A, Dick IM, Bruce D, Zhu K. Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Arch Intern Med 2008;168:103-8. [DOI] [PubMed] [Google Scholar]
- 51.Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomised trial. JAMA 2008;299:2027-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebbing M, Bleie Ø, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomised controlled trial. JAMA 2008;300:795-804. [DOI] [PubMed] [Google Scholar]
- 53.Hodis HN, Mack WJ, Dustin L, Mahrer PR, Azen SP, Detrano R, et al. High-dose B vitamin supplementation and progression of subclinical atherosclerosis: a randomised controlled trial. Stroke 2009;40:730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al. Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomised controlled trial. JAMA 2010;303:1603-9. [DOI] [PubMed] [Google Scholar]
- 55.Heinz J, Kropf S, Domröse U, Westphal S, Borucki K, Luley C, et al. B vitamins and the risk of total mortality and cardiovascular disease in end-stage renal disease: results of a randomised controlled trial. Circulation 2010;121:1432-8. [DOI] [PubMed] [Google Scholar]
- 56.Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomised trial. JAMA 2010;303:2486-94. [DOI] [PubMed] [Google Scholar]
- 57.Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol 2010;9:855-65. [DOI] [PubMed] [Google Scholar]
- 59.Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, et al. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the folic acid for vascular outcome reduction in transplantation trial. Circulation 2011;123:1763-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, Schavartz M, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomised controlled trial. JAMA 2012;308:1751-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomised trials involving 37 485 individuals. Arch Intern Med 2010;170:1622-31. [DOI] [PubMed] [Google Scholar]
- 62.Mei W, Rong Y, Jinming L, Yongjun L, Hui Z. Effect of homocysteine interventions on the risk of cardiocerebrovascular events: a meta-analysis of randomised controlled trials. Int J Clin Pract 2010;64:208-15. [DOI] [PubMed] [Google Scholar]
- 63.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011;96:1931-42. [DOI] [PubMed] [Google Scholar]
- 64.Eidelman RS, Hollar D, Hebert PR, Lamas GA, Hennekens CH. Randomised trials of vitamin E in the treatment and prevention of cardiovascular disease. Arch Intern Med 2004;164:1552-6. [DOI] [PubMed] [Google Scholar]
- 65.Shekelle PG, Morton SC, Jungvig LK, Udani J, Spar M, Tu W, et al. Effect of supplemental vitamin E for the prevention and treatment of cardiovascular disease. J Gen Intern Med 2004;19:380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou YH, Tang JY, Wu MJ, Lu J, Wei X, Qin YY, et al. Effect of folic acid supplementation on cardiovascular outcomes: a systematic review and meta-analysis. PLoS One 2011;6:e25142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parker RA, Sabrah T, Cap M, Gill BT. Relation of vascular oxidative stress, alpha-tocopherol, and hypercholesterolemia to early atherosclerosis in hamsters. Arterioscler Thromb Vasc Biol 1995;15:349-58. [DOI] [PubMed] [Google Scholar]
- 68.Pratico D, Tangirala RK, Rader DJ, Rokach J, FitzGerald GA. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat Med 1998;4:1189-92. [DOI] [PubMed] [Google Scholar]
- 69.Crawford RS, Kirk EA, Rosenfeld ME, LeBoeuf RC, Chait A. Dietary antioxidants inhibit development of fatty streak lesions in the LDL receptor-deficient mouse. Arterioscler Thromb Vasc Biol 1998;18:1506-13. [DOI] [PubMed] [Google Scholar]
- 70.Diaz MN, Frei B, Vita JA, Keaney JF Jr. Antioxidants and atherosclerotic heart disease. N Engl J Med 1997;337:408-16. [DOI] [PubMed] [Google Scholar]
- 71.Ricciarelli R, Zingg JM, Azzi A. Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation 2000;102:82-7. [DOI] [PubMed] [Google Scholar]
- 72.Ulrich-Merzenich G, Metzner C, Schiermeyer B, Vetter H. Vitamin C and vitamin E antagonistically modulate human vascular endothelial and smooth muscle cell DNA synthesis and proliferation. Eur J Nutr 2002;41:27-34. [DOI] [PubMed] [Google Scholar]
- 73.Farbstein D, Kozak-Blickstein A, Levy AP. Antioxidant vitamins and their use in preventing cardiovascular disease. Molecules 2010;15:8098-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A 1987;84:2995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henriksen T, Mahoney EM, Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A 1981;78:6499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Nerem RM. The pathogenesis of atherosclerosis: an overview. Clin Cardiol 1991;14:I1-16. [DOI] [PubMed] [Google Scholar]
- 77.Aguirre R, May JM. Inflammation in the vascular bed: importance of vitamin C. Pharmacol Ther 2008;119:96-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomised trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007;297:842-57. [DOI] [PubMed] [Google Scholar]
- 79.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 2012;3:CD007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Myung SK, Kim Y, Ju W, Choi HJ, Bae WK. Effects of antioxidant supplements on cancer prevention: meta-analysis of randomised controlled trials. Ann Oncol 2010;21:166-79. [DOI] [PubMed] [Google Scholar]
- 81.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.0.0, February 2008. www.cochrane-handbook.org.
- 82.Hartling L, Ospina M, Liang Y, Dryden DM, Hooton N, Krebs Seida J, et al. Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ 2009;339:b4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomised controlled trial. JAMA 2008;300:2123-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Full details of 50 included trials