Abstract
The objective of this research was to examine the potential for intercalation of trichloroethene (TCE) by clay minerals associated with aquifer sediments. Sediment samples were collected from a field site in Tucson, AZ. Two widely used Montmorillonite specimen clays were employed as controls. X-ray diffraction, conducted with a controlled-environment chamber, was used to characterize smectite interlayer d-spacing for three treatments (bulk air-dry sample, sample mixed with synthetic groundwater, sample mixed with TCE-saturated synthetic groundwater). The results show that the d-spacing measured for the samples treated with TCE-saturated synthetic groundwater are larger (~26%) than those of the untreated samples for all field samples as well as the specimen clays. These results indicate that TCE was intercalated by the clay minerals, which may have contributed to the extensive elution tailing observed in prior miscible-displacement experiments conducted with this sediment.
Keywords: Smectite, montmorillonite, clay, sorption, X-ray diffraction
INTRODUCTION
Pump-and-treat systems are widely used to remediate hazardous waste sites wherein groundwater is contaminated by compounds such as trichloroethene (TCE). It is well known that removal of contaminant mass by pump and treat becomes less effective over time, with a persistent mass discharge causing greatly extended operational periods. One mechanism potentially responsible for this persistent mass discharge is “back diffusion”, wherein dissolved contaminant stored in lower-permeability layers diffuses into the higher-permeability zones that are more readily flushed via pump and treat. Because the lower-permeability layers typically contain high fractions of clay minerals, a question of great interest is whether contaminant-clay interactions may influence the back-diffusion process. For example, intercalation of TCE into the interlayer spaces of clay minerals could potentially exacerbate diffusive mass-transfer limitations.
The potential for TCE intercalation was discussed recently by Aggarwal et al. (2006), who examined the impact of interlayer cation on the relative magnitude of sorption of TCE by homoionic saponite (a smectite clay). Results from their analysis show that the interlayer space is 3, 3, and 6 Å for Cs-saturated, K-saturated, and Ca-saturated saponite, respectively. They noted that TCE, a planar molecule, is approximately 3 to 3.5 Å thick, sufficiently small to fit within the interlayer space. Modeling by Farrell et al. (2002) indicated that TCE sorption was energetically most favorable in pores that are minimally large enough to accommodate a TCE molecule. Spaces of this size range are present in the interlayer spaces of clay minerals. Using molecular dynamics simulations, Teppen et al. (1998) showed that it was possible for TCE molecules to exist in the interlayer spaces of pyrophyllite clay.
Amarasinghe et al. (2009) observed that the d-spacing increased from 9.83 Ǻ to 12.18 Ǻ when liquid TCE was mixed with Na-Montmorillonite (SWy-2). Intercalation into interlayer spaces of smectites has been documented for several other organic compounds. Boyd et al. (2001) reported the intercalation of substituted nitrobenzenes in smectite clays SAz-1 and SWy-2. In a study on the thermodynamics of nitroaromatic compound adsorption from water by homoionic K+ and Ca2+ smectite SWy-2 (Ca-SWy-2 and K-SWy-2), Li et al. (2004) suggested that nitroaromatic compounds are sorbed strongly by K-smectites because they form inner- and/or outer-sphere complexes with K+ cations located in clay interlayer spaces. Sheng et al. (2001, 2002) reported intercalation of several pesticides by a number of smectite clays.
The objective of this research was to examine the potential for intercalation of TCE by clay minerals associated with aquifer sediments. Sediment samples were collected from a field site in Tucson, AZ. Two widely used Montmorillonite specimen clays were used as controls. X-ray diffraction (XRD), conducted with a controlled-environment chamber, was used to characterize smectite interlayer d-spacing for three treatments (bulk air-dry sample, sample mixed with synthetic groundwater, sample mixed with TCE-saturated synthetic groundwater).
MATERIALS AND METHODS
Materials
An electrolyte solution composed of Ca(NO3)2, CaCl2, MgSO4, NaHCO3, and NaCl was used for all experiments. This solution, referred to as the synthetic groundwater solution, was developed to represent the chemical composition of the groundwater at the field site. The concentrations used for the Ca(NO3)2, CaCl2, MgSO4, NaHCO3, and NaCl were 9 mg/L, 85 mg/L, 124 mg/L, 171 mg/L, and 20 mg/L, respectively. Trichloroethene (ACS reagent grade, ≥ 99.5% purity) was obtained from Sigma-Aldrich Chemical Company. The reference clay controls were obtained from the Clay Minerals Society (Chantilly, Virginia), and comprised Na-Montmorillonite (SWy-2) from Wyoming and Ca-Montmorillonite (STx-1b) from Texas.
Sediments used in this study were collected from two sites within the Tucson International Airport Area Superfund complex, located in Tucson, Arizona. Sediment samples were collected from locations at Air Force Pl ant 44 (AFP44) and the Three Hangars Complex at the Tucson International Airport. Sediments were collected from a depth interval of 44 to 46 m BGS (Below Ground Surface), designated the “finer zone”, as well as a depth interval of 46 to 48 m BGS, designated the “coarser zone”, for a core collected from well M-72 borehole at AFP44. A sample was also collected from boring B-119 in the depth interval 41.9 to 43 m BGS at AFP44. Another field sample was collected from the depth interval 28.9 to 30 m BGS from boring CRA-26 at the Three Hangars Complex.
For the 44 to 46 m BGS M-72 sample, the average sand, silt, and clay fractions were 89.5% (±0.6%), 4.1% (±0.6%), and 6.4% (±1.0%), respectively. For the 46 to 48 m BGS M-72 sample, the average sand, silt, and clay fractions were 97.1%, 1.2%, and 1.7%, respectively. The average percent total carbon and organic carbon equaled 0.06% (±0.01%) and 0.03% (±0.009%), respectively, for these samples. For the borehole B-119 sample, the average sand, silt, and clay fractions were 52.5%, 26.5%, and 21.0%, respectively. For the borehole CRA-26 sample, the average sand, silt, and clay fractions were 0.1%, 36.4%, and 63.5%, respectively.
Subsamples of the sediments were analyzed by XRD to qualitatively identify clay mineral composition. Analysis of the material from borehole B-119 shows that the clay minerals present in the clay-sized fraction (<2 μm) are kaolinite, muscovite/illite, smectite, and minor amounts of chlorite, and that the non-clay minerals present in the clay-sized fraction are quartz and feldspar. Analysis of the clay size fraction from CRA-26 shows that the same clay minerals and non-clay minerals are present. Analysis of the sediment collected from borehole M-72 showed large amounts of smectite, with smaller amounts of mica and kaolinite.
Additionally, quantitative analysis of the clay and non-clay materials was performed for samples from boreholes B-119 and CRA-26. The sample from CRA-26 contained 17.6% quartz, 5.4% potassium feldspar (microcline), 3.5% plagioclase (oligoclase), 4.4% calcite, 4.8% kaolinite, 42.6% muscovite/illite, and 21.6% smectite (montmorillonite). The sample from B-119 contained 42.3% quartz, 10.7% potassium feldspar (microcline), 12.8% plagioclase (oligoclase), 3.7% calcite, 1.3% kaolinite, 21.1% muscovite/illite, and 8.0% smectite (montmorillonite).
Methods
For the XRD experiments, sediment and specimen-clay subsamples were subjected to two treatments: synthetic groundwater and TCE-saturated synthetic groundwater (~1100 mg/L TCE). In addition, an untreated (air-dry) subsample was analyzed with XRD to develop a baseline diffractogram for each sediment and specimen clay. Prior to treatment, the sediment samples were air dried and ground to a uniform fine consistency. The sediment samples (~ 1-5 g) were added to 20 mL glass gas-tight vials, after which ~15 mL of solution was added. The samples were incubated for 1 day prior to analysis with XRD. The bottles were shaken periodically to ensure adequate mixing of the solution and sediment.
Sediment samples were subjected to XRD analysis with a PANalytical X’Pert MPD Pro powder diffractometer. Measurements were made with copper Kα radiation, a variable divergence slit, and a graphite post-diffraction monochromator. A gas-tight sample environment around the samples was provided by an Anton Paar TTK 450 sample stage operated at ambient temperature and saturated with TCE vapor during the relevant experiment s. An approximately 150 mg moist sample was placed in the chamber. The samples were still moist, but to a lesser extent, at the end of the analysis.
Samples were scanned from 1 to 22 °2θ using a step size of 0.04° and a count time of 8s. Occasional samples were scanned to a larger final diffraction angle to confirm the identity of minerals, especially non-clays. For all samp les, the diffractograms showed quartz peaks at positions that were close to the expected position (20.88 °2θ), providing an internal check on diffraction angle. Jade© and High-Score© software were used to analyze the XRD data to determine specific locations (°2θ) of peaks. The °2θ peak locations obtained during the XRD measurement were used to calculate the d-spacing using standard methods (e.g., Stanjek et al, 2004).
RESULTS AND DISCUSSION
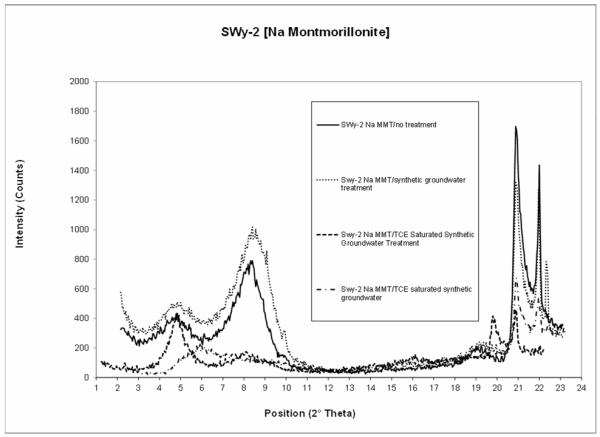
The results of the XRD measurements for Na-Montmorillonite (SWy-2) are presented in Figure 1. The baseline scan for untreated Na-Montmorillonite, shown as the solid line in Figure 1, shows a smectite peak at 8.35 °2θ. This measurement was used to calculate a d-spacing of 10.6 Å. Chipera et al (2001) measured a d-spacing of 11.2 Ǻ for SWy-2. Diffractograms of smectite clays exhibit variability due to relative humidity and other factors (Chipera et al, 2001). Considering this variability, the d-spacing of the smectite clay measured in the current experiment is within the range of literature values quoted by Chipera et al (2001). The XRD measurements for the treated SWy-2 samples are compared to this baseline scan to determine if a change in the d-spacing occurred as a result of treatment.
Figure 1.
Results of the XRD measurements for the SWy-2 (Na montmorillonite) samples.
One of the TCE-saturated synthetic groundwater treatments shows a well-defined smectite peak at 4.85 °2θ with a d-spacing of 18.2 Ǻ. There is no significant smectite peak present at or near the 8.35 °2θ position, which was the peak location observed for the baseline untreated scan. The difference between the d-spacing values for the baseline untreated sample and the sample treated with TCE-satu rated synthetic groundwater is 7.6 Ǻ. Figure 1 shows the results of a second sample that was treated with TCE-saturated synthetic groundwater. A smectite peak with a d-spacing of 15.2 Ǻ is present at 5.83 °2θ. Similar to the prior scan, there was no smectite peak present at 8.35 °2θ. The difference in d-spacing of this sample treated with TCE-saturated synthetic groundwater and the untreated sample is 4.6 Ǻ. The observed increases in the d-spacing indicate that the SWy-2 expanded when it was treated with TCE-saturated synthetic groundwater.
The diffractogram for the treatment where the SWy-2 was exposed to synthetic groundwater with no TCE (Figure 1) is similar to that of the untreated sample (the baseline scan). This indicates that the presence of the synthetic groundwater solution alone caused no measurable expansion for the SWy-2. The similarity of d-spacings for the untreated baseline sample and the sample treated with synthetic groundwater is expected given that both samples have a common origin and therefore are likely to have identical interlayer ion compositions, and that the baseline sample was air-dried rather than oven-dried. These results in conjunction with the increase in d-spacing observed for the samples treated with TCE-saturated groundwater indicate that TCE was intercalated within the interlayer space of the clay mineral.
Similar results were obtained for the Ca-montmorillonite (STx-1b) sample. The STx-1b base scan with no treatment (the blue line Figure S1) shows a smectite peak at 6.90 °2θ with a d-spacing of 12.8 Ǻ. There was no significant change for the sample treated with synthetic groundwater, which had a d-spacing of 12.3 Ǻ. In contrast, when the STx-1b was treated with TCE-saturated synthetic groundwater, the smectite peak appeared at 5.72 °2 θ, and the d-spacing was 15.4 Ǻ.
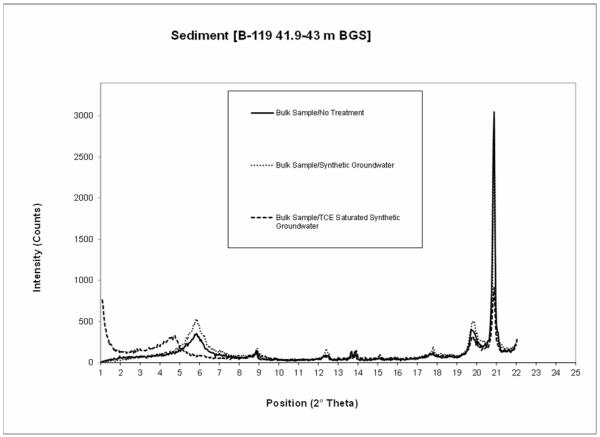
For the B-119 sediment, the smectite peak was at 5.83 °2θ, with a d-spacing of 15.2 Ǻ for the untreated sample (Figure 2). For the TCE-saturated synthetic groundwater treatment, the smectite peak occurred at 4.76 °2θ, and the d-spacing was 18.6 Ǻ. Conversely, there was no change observed for the synthetic groundwater treatment in comparison to the untreated sample.
Figure 2.
Results of the XRD measurements for the B-119 (41.91 to 43 m BGS) samples.
For the CRA-26 sediment, the smectite peak was present at 5.97 °2θ, and the d-spacing was 14.8 Ǻ for the untreated sample (Figure S2). For the TCE-saturated synthetic groundwater treatment, the smectite peak occurred at 4.41 °2θ, and the d spacing was 20.0 Ǻ. Again, there was no change observed for the synthetic groundwater treatment in comparison to the untreated sample.
For the M-72 44 to 46 m BGS sediment, the smectite peak was present at 5.85 °2θ, and the d-spacing was 15.1 Ǻ for the untreated sample (Figure S3). For the TCE-saturated synthetic groundwater treatment, the smectite peak occurred at 4.70 °2θ, and the d-spacing was 18.8 Ǻ. For the synthetic groundwater treatment, the smectite peak occurred at 6.22 °2θ and had a d-spacing of 14.2 Ǻ, which is a slight decrease compared to the control.
For the M-72 46 to 48 m BGS sediment, the smectite peak was present at 5.88 °2θ, and the d-spacing was 15.0 Ǻ for the untreated sample (Figure S4). For the TCE-saturated synthetic groundwater treatment, the smectite peak occurred at 4.62 °2θ, and the d-spacing was 19.1 Ǻ. Conversely, there was no significant change observed for the synthetic groundwater treatment in comparison to the untreated sample.
The results obtained for the four sets of sediments are consistent with those obtained for the two specimen clays. For all cases, the d-spacing was observed to increase for the treatment with TCE-saturated synthetic groundwater, whereas no significant change was observed for the treatment with synthetic groundwater alone. The increase in the d-spacing ranged from approximately 3 to 5 Ǻ for the sediments, similar to that observed for the specimen clays. This is consistent with the thickness of the TCE molecule. These results i ndicate that TCE was intercalated by the clay minerals. The kinetics of the TCE-clay interaction was not investigated in our study. However, the apparent observed intercalation obviously occurred within the treatment period (24 hours), which provides an outer-bound for the reaction time scale. Amarasinghe et al. (2009) reported that the interaction between liquid TCE and clay was almost instantaneous for their system.
The results of prior miscible-displacement experiments conducted with these sediments show that TCE elution exhibits extensive tailing (see Figure S5), wherein more than 100 pore volumes of water flushing were required to reduce TCE concentrations to the 1 ug/L range (Johnson et al., 2009). This is despite the fact that sorption of TCE by these sediments is quite low, with retardation factors less than 1.2. This behavior indicates that some type of recalcitrant interaction is influencing TCE desorption and transport. The results of the present study suggest that the observed behavior may in part be influenced by intercalation of TCE by clay minerals present in the sediment. Such interactions could potentially exacerbate diffusive mass-transfer limitations at the field scale and, for example, impact plume removal via pump and treat.
SUMMARY
The potential intercalation of solutes into interlayers of clay minerals is a question of interest regarding contaminant transport, fate, and remediation. Prior studies have focused primarily on use of specimen clays. In this study, the potential for TCE intercalation by clay mineral components of aquifer sediments was examined. Four sets of sediment samples were collected from a field site in Tucson, AZ. Two widely used Montmorillonite specimen clays were used as controls. X-ray diffraction, conducted with a controlled-environment chamber, was used to characterize smectite interlayer d-spacing for three treatments (bulk air-dry sample, sample mixed with synthetic groundwater, sample mixed with TCE-saturated synthetic groundwater). The results of the XRD analysis show a greater d-spacing for the samples treated with TCE-saturated synthetic groundwater for all field samples as well as the specimen clays. These results indicate that TCE was intercalated by the clay minerals, which may have contributed to the extensive elution tailing observed in prior miscible-displacement experiments conducted with this sediment. A question of interest that merits further investigation is the characteristic time associated with transfer of TCE out of the interlayer domain.
Supplementary Material
Acknowledgements
This research was supported by funding provided by the US DOD Strategic Environmental Research and Development Program (ER-1614) and the NIEHS Superfund Research Program (ES04940). The XRD analysis was performed at the Environmental Molecular Science Laboratory, a national scientific user facility sponsored by the Department of Energy’s Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory in Richland, Washington. The authors would also like to thank Dr. Mercer Meding at the Center for Environmental Physics and Mineralogy (CEPM) at the University of Arizona.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal V, Li H, Boyd SA, Teppen BJ. Enhanced Sorption of Trichloroethene by Smectite Clay Exchanged with Cs+ Environmental Science and Technology. 2006;40:894–899. doi: 10.1021/es0500411. [DOI] [PubMed] [Google Scholar]
- Amarasinghe PM, Katti KS, Katti DR. Nature of Organic Fluid-Montmorillonite Interactions: An FTIR Spectroscopic Study. Journal of Colloid and Interface Science. 2009;337:97–105. doi: 10.1016/j.jcis.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Borden D, Giese RF. Baseline Studies of the Clay Minerals Society Source Clays: Cation Exchange Capacity Measurements by the Ammonia-Electrode Method. Clays and Clay Minerals. 2001;49:444–445. [Google Scholar]
- Boyd SA, Sheng G, Teppen BJ, Johnston CT. Mechanisms for the Adsorption of Substituted Nitrobenzenes by Smectite Clays. Environmental Science and Technology. 2001;35:4227–4234. doi: 10.1021/es010663w. [DOI] [PubMed] [Google Scholar]
- Charles SM, Teppen BJ, Li H, Boyd SA. Fractional Availability of Smectite Surfaces in Soils for Adsorption of Nitroaromatic Compounds in Relation to Soil and Solute Properties. Soil Science Society of America Journal. 2008;72:586–594. [Google Scholar]
- Chipera SJ, Bish DL. Baseline Studies of the Clay Minerals Society Source Clays: Powder X-Ray Diffraction Analysis. Clays and Clay Minerals. 2001;49:398–409. [Google Scholar]
- Evangelou VP. Environmen tal Soil and Water Chemistry. John Wiley and Sons; New York: 1998. [Google Scholar]
- Farrell J, Luo J, Blowers P, Curry J. Experimental and Molecular Mechanics and Ab Initio Investigation of Activated Adsorption and Desorption of Trichloroethylene in Mineral Micropores. Environmental Science and Technology. 2002;36:1524–1531. doi: 10.1021/es011172e. [DOI] [PubMed] [Google Scholar]
- Johnson GR, Norris DK, Brusseau ML. Mass Removal and Low-Concentration Tailing of Trichloroethene in Freshly-Amended, Synthetically Aged, and Field-Contaminated Aquifer Material. Chemosphere. 2009;75:542–548. doi: 10.1016/j.chemosphere.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloprogge JT, Komarneni S, Amonette JE. Synthesis of Smectite Clay Minerals: A Critical Review. Clays and Clay Minerals. 1999;47:529–554. [Google Scholar]
- Li H, Teppen BJ, Johnston CT, Boyd S. Thermodynamics of Nitroaromatic Compound Adsorption from Water by Smectite Clay. Environmental Science and Technology. 2004;38:5433–5442. doi: 10.1021/es035054y. [DOI] [PubMed] [Google Scholar]
- Mercer JW, Cohen RM. A Review of Immiscible Fluids in the Subsurface. Journal of Contaminant Hydrology. 1990;6(2):107–163. [Google Scholar]
- 13.Pignatello JJ, Xing B. Mechanisms of Slow Sorption of Organic Chemicals to Natural Particles. Environmental Science and Technology. 1996;30:1–11. [Google Scholar]
- Sato T, Watanabe T, Otsuk R. Effects of Layer Charge, Charge Location, and Energy Change on Expansion of Propertie s of Dioctahedral Smectites. Clays and Clay Minerals. 1992;40:103–113. [Google Scholar]
- Stanjek H, Hausler W. Basics of X-Ray Diffraction. Hyperfine Interactions. 2004;154:107–119. [Google Scholar]
- Sheng G, Johnston CT, Teppen BJ, Boyd SA. Potential Contributions of Smectite Clays and Organic Matter to Pesticide Retention in Soils. Journal of Agricultural and Food Chemistry. 2001;49:2899–2907. doi: 10.1021/jf001485d. [DOI] [PubMed] [Google Scholar]
- Sheng G, Johnston CT, Teppen BJ, Boyd SA. Adsorption of Dinitrophenol Herbicides from Water by Montmorillonites. Clays and Clay Minerals. 2002;50:25–34. [Google Scholar]
- Teppen BJ, Yu C, Miller DM, Schafer L. Molecular Dynamics Simulations of Sorption of Organic Compounds at the Clay Mineral/Aqueous Solution Interface. Journal of Computational Chemistry. 1998;19:144–153. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.