Abstract
Purpose
To investigate the feasibility of adaptive dosing and the impact of pharmacogenetic variation on 13-cisRA disposition in high-risk neuroblastoma patients.
Experimental Design
13cisRA (160mg/m2 or 5.33mg/kg/day) was administered to 103 patients ≤21 years and plasma concentrations of 13-cisRA and 4-oxo-13-cisRA quantitated on day 14 of treatment. 71 patients were recruited to a dose adjustment group, targeting a 13-cisRA Cmax of 2μM, with dose increases of 25-50% implemented for patients with Cmax values <2μM. A population pharmacokinetic model was applied and polymorphisms in relevant cytochrome P450 genes analyzed.
Results
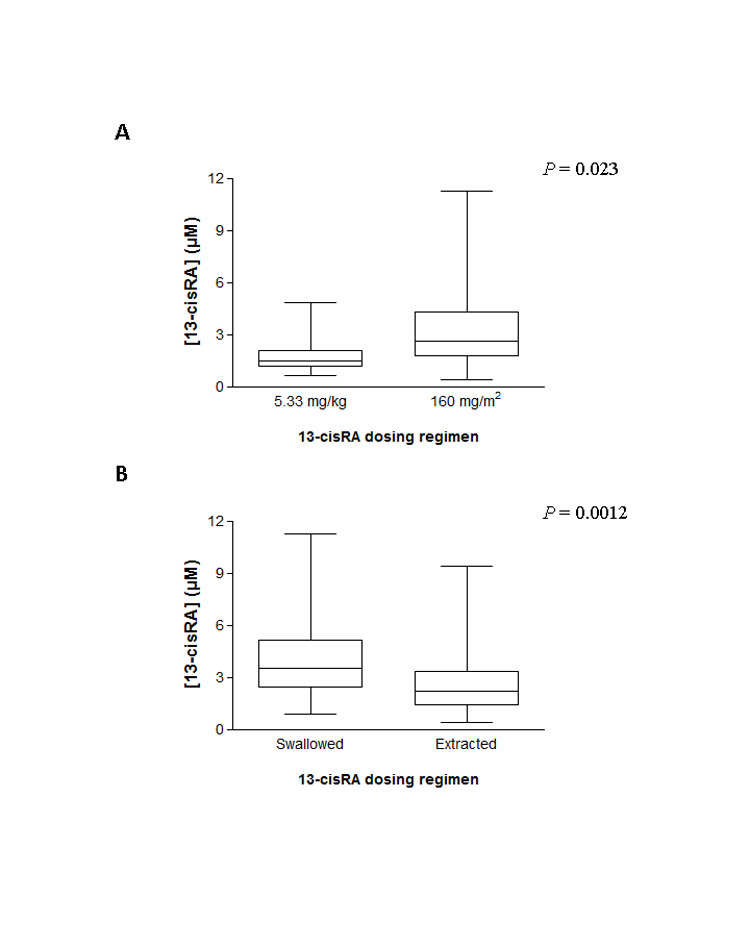
13-cisRA Cmax values ranged from 0.42–11.2μM, with 34/103 (33%) patients failing to achieve a Cmax >2μM. Dose increases carried out in 20 patients in the dose adjustment study group led to concentrations >2μM in 18 patients (90%). 8/11 (73%) patients <12kg, receiving a dose of 5.33mg/kg, failed to achieve a Cmax ≥2μM. Significantly lower Cmax values were observed for patients treated with 5.33mg/kg versus 160mg/m2 (1.9±1.2 versus 3.1±2.0μM; mean±SD; P=0.023). Cmax was higher in patients who swallowed 13-cisRA capsules as compared to receiving the drug extracted from capsules (4.0±2.2 versus 2.6±1.8μM; P=0.0012). The target Cmax was achieved by 93% (25/27) versus 55% (42/76) of patients in these two groups respectively. No clear relationships were found between genetic variants and 13-cisRA pharmacokinetic parameters.
Conclusions
Dosing regimen and method of administration have a marked influence on 13-cisRA plasma concentrations. Body weight-based dosing should not be implemented for children <12kg and pharmacological data support higher doses for children unable to swallow 13-cisRA capsules.
Keywords: 13-cis-retinoic acid, isotretinoin, neuroblastoma, adaptive dosing, pharmacogenetics
INTRODUCTION
Despite remarkable improvements in survival rates for childhood cancer over the past several decades, the treatment of children with high-risk neuroblastoma remains a major challenge. The retinoid drug 13-cis-retinoic acid (13-cisRA; isotretinoin) is now an established component of high-risk neuroblastoma treatment, currently being utilized as maintenance treatment in conjunction with antibody therapy in the US and Europe. The use of 13-cisRA in this setting is supported by the publication of favorable long-term follow up data published from a Children’s Cancer Group study (CCG-3891), demonstrating improved survival rates in patients treated with 13-cisRA following autologous bone marrow transplantation (1, 2). However, despite its widespread use in neuroblastoma for the past decade, there remain a number of drawbacks to its clinical utility.
Previous studies have indicated a significant level of inter-patient variation in 13-cisRA plasma concentrations following standard dosing regimens, with many patients achieving potentially sub-optimal drug exposures (3). In addition, concentrations of the major metabolite 4-oxo-13-cisRA were shown to accumulate to exceed those of the parent compound during the 14 day course of treatment in approximately 70% of patients studied. As 4-oxo-RA metabolites have been shown to be less active than the parent RA in various tumour cell lines, this level of metabolism in vivo could lead to a diminished efficacy of 13-cisRA (4,5). This may be particularly important given that the lowering of retinoid plasma levels due to induced metabolism has been linked with the development of resistance to ATRA in acute promyelocytic leukemia patients (6, 7).
The metabolism of 13-cisRA has previously been characterized in vitro, with cytochrome P450 enzymes (CYPs) including 2C8, 3A7, 4A11, 1B1, 2B6 and 2C9 responsible for the generation of 13-cisRA metabolites including 4-oxo-13-cisRA (8, 9). The expression of many CYPs can vary markedly between individuals, potentially impacting on drug disposition and plasma concentrations of 13-cisRA observed in patients. While in vitro studies have indicated that the presence of CYP2C8.3 or CYP2C8.4 variants are unlikely to explain the high degree of observed interindividual variability in the pharmacokinetics and metabolism of 13cisRA (10), this has not been explored in a clinical setting. In addition it remains possible that other CYP polymorphisms could have a role to play. As well as CYP-mediated phase I metabolism, phase II glucuronidation of 13-cisRA has also recently been characterized, with human UDP-glucuronosyltransferases (UGTs) 1A1, 1A3, 1A7, 1A8 and 1A9 shown to represent the major isoforms responsible for glucuronidation of both 13-cisRA and 4-oxo-13-cisRA in vitro, with a possible additional role for UGT2B7 in glucuronidation of the metabolite (11). It is therefore feasible that common polymorphisms reported in UGT genes could also impact on the pharmacokinetics of 13-cisRA.
While pharmacogenetic variation in key genes responsible for 13-cisRA metabolism may have a role to play in explaining the large inter-patient variability in pharmacokinetics, there are also practical concerns regarding the administration of 13-cisRA to young patients. Due to the large size and number of 13-cisRA capsules required to obtain the specified dose, younger children are physically unable to take the drug unless the capsules are opened and the contents mixed with food prior to administration. This practice raises concerns regarding the actual dose of drug that these patients are receiving. These difficulties were highlighted in a recent case report, indicating that dose modification would be essential to ensure optimal therapy (12).
The current study was designed to investigate the feasibility of carrying out an adaptive dosing approach to 13-cisRA treatment, with dose modifications made following course 1 of treatment for patients achieving Cmax values below a pre-defined minimum cut-off point. While the most appropriate therapeutic window for 13-cisRA exposure has yet to be established, the current approach was aimed to minimize the >10-fold variability in plasma concentrations previously observed with standard dosage regimens. Additional novel data were also generated relating to the pharmacokinetics and pharmacogenetics of 13-cisRA in a high-risk neuroblastoma patient population, providing insight into the potential impact of variation in key genes on 13-cisRA disposition.
MATERIALS AND METHODS
Patient eligibility and details
Study protocols were approved by the UK Trent Multicentre Research Ethics Committee and written informed consent was obtained from patients or parents as appropriate. Patients less than 21 years of age who were receiving 13-cisRA as part of their standard clinical treatment for high-risk neuroblastoma were eligible to participate. The trial was registered through the appropriate clinical trials registries (ISRCTN37126758; ClinicalTrials.gov identifier: NCT00939965) prior to patient recruitment. All patients had a central venous catheter in place to allow for pharmacokinetic sampling. Age and weight together with 13-cisRA administration details were recorded for each patient. The most recent GFR, ALT, bilirubin and creatinine measurements prior to 13-cisRA treatment were obtained from the patients’ notes, in addition to baseline Hb, WBC and platelet counts. Details of concomitant medications being administered prior to and/or in combination with 13-cisRA were recorded.
13-cisRA treatment
Treatment with13-cisRA (Roaccutane brand) was initiated between 80 and 120 days post-myeloablative and radiation therapy as part of a protocol for high-risk neuroblastoma. 13-cisRA was administered orally at a dose of 160mg/m2/day, or 5.33mg/kg for children <12kg, with each course consisting of 14 days of treatment followed by a 14 day break. A total of 6 courses were planned for all patients, during which toxicity was assessed by the National Cancer Institute Common Terminology Criteria of Adverse Events (CTCAE v3). For patients who were unable to swallow 13-cisRA capsules, each capsule was snipped with a pair of scissors and the contents carefully squeezed onto a spoon. Following the opening of all capsules the extracted drug was mixed with food and ingested or mixed with an appropriate diluent and administered via a nasogastric (NG) tube. Patients were not fasted prior to administration. On each study day, administration of the studied dose of 13-cisRA was performed in hospital and was fully documented by a trained research nurse.
For patients studied in the dose adjustment group, the dose of 13-cisRA administered on subsequent study courses was modified based on plasma pharmacokinetics, following analysis of samples obtained on day 14 of the previous course. In the majority of cases this was the first treatment course of 13-cisRA, although some patients were studied on later courses (Table 1). A dose increase of 25% (to 200 mg/m2/day or 6.66 mg/kg/day if child <12 kg) was made for patients attaining 13-cisRA Cmax values of 1.0 - 2.0 μM and who experienced minimal or no toxicity (≤ CTCAE grade 2). A dose increase of 50% (to 240 mg/m2/day or 8.0 mg/kg/day if child <12 kg) was implemented for patients attaining 13-cisRA Cmax values < 1.0 μM and who experienced minimal or no toxicity. The dose was maintained at the standard dose (160 mg/m2/day or 5.33mg/kg/day if child <12 kg) for patients attaining 13-cisRA Cmax values ≥ 2 μM. For those patients where a dose adjustment was implemented, pharmacokinetics and toxicity were again monitored on the following course of treatment. Further dose adjustments were made on subsequent courses as appropriate, depending on 13-cisRA Cmax values achieved at the higher dose, with the aim of achieving concentrations >2μM in all patients. Dose reductions were recommended for patients experiencing specific grade 3 or 4 CTCAE toxicities associated with 13-cisRA use as per standard treatment.
Table 1.
Patient characteristics and 13-cisRA treatment
| Characteristic | No. of patients | % |
|---|---|---|
| Evaluable patients | 103 | |
| Age (years) | ||
| 0-1 | 10 | 10 |
| 2-3 | 37 | 36 |
| 4-5 | 29 | 28 |
| 6-10 | 20 | 19 |
| 11+ | 7 | 7 |
| Sex | ||
| Male | 64 | 62 |
| Female | 39 | 38 |
| Ethnicity | ||
| White British | 83 | 80 |
| White Other | 4 | 4 |
| Pakistani | 3 | 3 |
| Asian Other | 5 | 5 |
| Black Caribbean | 1 | 1 |
| Black African | 2 | 2 |
| Black Other | 2 | 2 |
| Any Mixed Background | 2 | 2 |
| Other | 1 | 1 |
| BW (kg) | ||
| Median | 15.9 | |
| Range | 7.1 – 48.9 | |
| BSA (m2) | ||
| Median | 0.70 | |
| Range | 0.35 – 1.5 | |
| 13-cisRA dose level | ||
| 160 mg/m2 (≥12kg) | 92 | 89 |
| 5.33 mg/kg (<12kg) | 11 | 11 |
| Method of 13-cisRA administration | ||
| Capsules swallowed | 27 | 26 |
| Drug extracted and mixed with food | 53 | 52 |
| Drug extracted and administered via NGT | 23 | 22 |
| Pharmacokinetic data collected | 103 | 100 |
| Course 1 | 60 | 58 |
| Course 2 | 38 | 37 |
| Course 3 | 24 | 23 |
| Course 4 | 13 | 13 |
| Course 5 | 3 | 3 |
| Course 6 | 6 | 6 |
| Pharmacogenetic sample obtained | 73 | 71 |
| Dose adjustment group | ||
| Total number | 71 | |
| Dose increase following 1 course of treatment | 13 | |
| Further dose increases on additional course(s) | 7 | |
| 25% dose increase | 14 | |
| >25% dose increase | 6 |
Abbreviations: BW, body weight; BSA, body surface area; NGT, nasogastric tube.
Blood sampling and analysis
A single 5ml blood sample was taken from each patient prior to the first course of 13-cisRA treatment, transferred to an EDTA tube and stored at –20°C for pharmacogenetic analysis. Blood samples for measurement of concentrations of 13-cisRA and metabolites were obtained from a central line prior to administration and at 1, 2, 4 and 6 hours post-administration. Samples were obtained on day 14 of the study treatment course following administration of the first dose of 13-cisRA on the particular study day. For patients who required a 13-cisRA dose increase, samples for pharmacokinetic analysis were also obtained as detailed above on day 14 of treatment at the higher dose and on one additional course of treatment at the individualized dose. These additional samples were collected to confirm the consistent attainment of Cmax values >2μM on more than one course of treatment. Blood samples (5ml) were collected in heparinized tubes and centrifuged at 1,200g for 10min at 4°C. Plasma was separated and frozen at −20°C, prior to analysis using a high performance liquid chromatography (HPLC) assay, with a limit of quantitation of 0.02μg/ml for all retinoids. This analytical assay allowed for individual quantification of 13-cisRA and the metabolite 4-oxo-13-cisRA as previously described (3). All blood and plasma samples were wrapped in aluminum foil to protect them from light and sample handling was carried out in dim light. The assay was validated for linearity, reproducibility and stability of the analytes according to standard practice (13).
Pharmacogenetics
DNA was extracted from whole blood using a Qiagen QIAamp® DNA Blood Maxi Kit or purified from lymphocytes using a Qiagen QIAamp® DNA Mini Kit. All kits were used according to the manufacturer’s instructions. DNA was quantified using a NanoDrop ND-1000 UV-Vis Spectrophotometer (Wilmington, US) and stored at −20°C prior to pharmacogenetic analysis. Genotyping for CYP2C8*3, CYP2C8*4, CYP3A5*3, CYP3A7*1C, CYP3A7*2 and UGT2B7*2 alleles was performed with the use of TaqMan® probes. For completeness, both SNPs (K139R and R399K) contributing to the CYP2C8*3 genotype were analysed, and as expected were found to be in complete linkage disequilibrium. For the CYP2C8*3 (R139K), CYP2C8*3 (K399R), CYP2C8*4, CYP3A5*3, CYP3A7*2 and UGT2B7*2 alleles, primers and TaqMan® probes were designed by Applied Biosystems (TaqMan® Assays-by-Design, Applied Biosystems). For the CYP3A7*1C allele, primers and TaqMan® probes were custom designed and synthesized by Applied Biosystems. The TA indel variant of UGT1A1 was studied by fragment analysis.
Pharmacokinetics
A population pharmacokinetic model was fitted to all 13-cisRA data obtained from the first available course of treatment, based on a model previously reported (3). As patients in the current report were studied on day 14 of treatment, as opposed to day 1, the model was modified to allow for non-zero concentrations at the time of 13-cisRA administration. In summary, a one-compartment model with modified zero-order absorption and an absorption lag time was used. The model assumes that the appearance of drug in a dose compartment is described by a zero-order process over a fixed duration (D1). Absorption into a central observation compartment was described by a first-order process with rate parameter Ka. Non-zero concentrations at the time of dosing were modeled by a steady state infusion dose into the observation compartment, ending at time 0, and having an unknown rate. The unknown rate, R2, was modeled. All pharmacokinetic parameters were allowed to vary across the population and, in addition, covariance parameters were included for CL and V; and for Ka, ALAG and D1. Non-compartmental, trapezoidal estimates of AUC0-6h were determined using Stata/SE (StataCorp. 2009. Stata Statistical Software: Release 11.2. College Station, TX: StataCorp LP.). Relationships between covariates including gender, age, weight, body surface area, GFR and baseline ALT, bilirubin and creatinine levels and 13-cisRA pharmacokinetics were assessed by visual examination of plots against empirical Bayes estimates of pharmacokinetic parameters.
Statistical analysis
For the analysis of pharmacogenetic data, overall differences between groups were assessed with the Mann-Whitney and Kruskal-Wallis tests using GraphPad Prism version 5.0 software (GraphPad Software, Inc., San Diego, CA). The Mann Whitney test was used to determine differences between 13-cisRA Cmax values in patients receiving different dosing regimens and methods of drug administration. Analysis of linkage disequilibrium was performed using Fisher’s exact test (2-sided) for general contingency tables with SPSS version 15.0 software (SPSS Inc., Chicago, IL). Statistical significance was given for P values < 0.05.
RESULTS
Patient characteristics and treatment
A total of 103 children with high-risk neuroblastoma were recruited over a period of 7.5 years between August, 2004 and January, 2012. Of these 103 patients, 71 were recruited to the 13-cisRA dose adjustment study group. The additional 32 patients were studied on a single cycle of 13-cisRA treatment and contributed to the population pharmacokinetic model and pharmacogenetic data analysis. The overall study population had a median age of 4.3 years (range 0.8–20.5) and included 64 male and 39 female patients. Patient characteristics for the 103 evaluable patients are given in Table 1. 13cisRA was extracted from capsules and administered with food in 53 patients and by NG tube in 23 patients. For those patients extracting 13-cisRA from capsules and administering the drug with food, yoghurt, ice cream or milk was used; drug extracted from capsules and administered by NG tube was mixed with olive oil or milk. The remaining 27 patients were able to swallow the 13-cisRA capsules.
Pharmacokinetics
The population pharmacokinetic model provided an appropriate fit to the data. Supplementary Figure 1 shows observed 13-cisRA plasma concentrations together with individual predictions from four patients chosen to represent the diversity of response. Mean population pharmacokinetic parameters were: apparent clearance 0.24 l/min (14.5 l/h); apparent volume of distribution 63 l; absorption lag time 20 min; zero-order duration 62 min; absorption rate, Ka 0.026 1/min and steady state infusion rate, R2 0.15 1/min. There was large inter-individual variability associated with all of these parameters, in particular the parameters representing the absorption process, as shown in Table 2. Non-compartmental, trapezoidal estimates of AUC0-6h ranged from 1.9-33.9μM.h, with a median value of 9.7μM.h. Covariates including gender, age, weight, body surface area, GFR and baseline ALT, bilirubin and creatinine levels were not observed to have a significant effect on 13-cisRA pharmacokinetics. In addition, concurrent administration of other medications had no impact on variability in 13-cisRA pharmacokinetic parameters. A positive linear relationship was observed between 13-cisRA Cmax and AUC0-6h on study day 14 (r2 = 0.8418), supporting the use of the Cmax value for individualization of 13-cisRA dose (Supplementary Figure 2).
Table 2.
13-cisRA population pharmacokinetic parameters
| Mean | 95% bootstrap confidence interval for mean |
Coefficient of variation (%) |
95% confidence bootstrap interval for CV |
|
|---|---|---|---|---|
| CL/F (l/min) | 0.24 | (0.21, 0.27) | 45 | (34, 56) |
| V/F (l) | 63 | (51, 77) | 64 | (49, 78) |
| KA (1/min) | 0.026 | (0.015, 0.033) | 227 | (183, 263) |
| ALAG (min) | 20 | (13, 29) | 100 | (72, 132) |
| D1 (min) | 62 | (38, 68) | 137 | (123, 176) |
| R2 (1/min) | 0.15 | (0.13, 0.19) | 72 | (55, 84) |
Abbreviations: CL/F, apparent clearance; V/F, apparent volume of distribution; KA, absorption rate; ALAG, absorption lag time; D1, absorption duration; R2, rate of unknown steady-state infusion dose into central compartment, ending at time 0.
Oxidative metabolism
Extensive accumulation of 4-oxo-13-cisRA occurred in all patients, with peak plasma concentrations higher than those of 13-cisRA on day 14 of treatment in 64/96 (67%) patients for whom data were available. Cmax values for the 4-oxo-13-cisRA metabolite ranged from 0.48-14.3μM as compared to a concentration range of 0.40-11.2μM for 13-cisRA. Comparable 4-oxo-13-cisRA levels on day 14 of treatment were observed in subsequent courses where studied. No other retinoic acid metabolites were detected in plasma samples of patients receiving 13-cisRA.
Pharmacogenetics
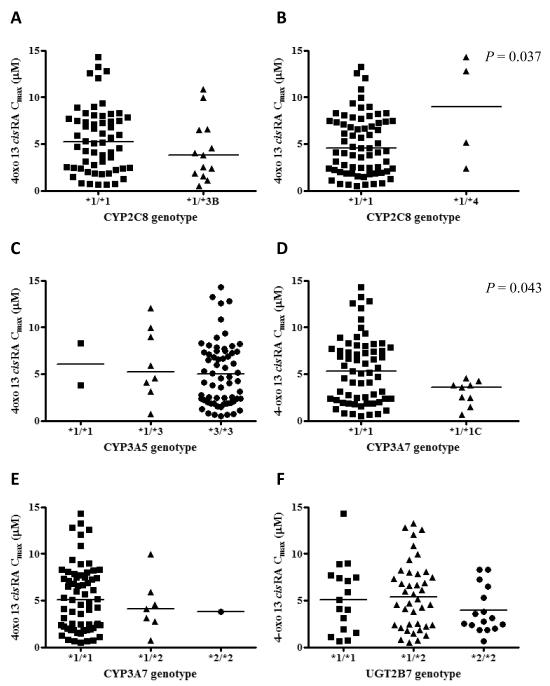
The impact of pharmacogenetic variation on 13-cisRA pharmacokinetics was investigated in a total of 73 patients, for whom both pharmacogenetic and pharmacokinetic data were available. Six SNPs were analyzed in four genes of putative relevance for 13-cisRA disposition. The allele frequencies for CYP2C8*3, CYP2C8*4, CYP3A5*3, CYP3A7*1C, CYP3A7*2 and UGT2B7*2 were 8.9%, 2.8%, 91.8%, 6.2%, 6.2% and 49.3%, respectively. Four different UGT1A1 promoter (TA)n genotypes were identified due to the presence of (TA)5, (TA)6 and (TA)7 repeats. Of the 73 samples evaluated, 25 (34%) were homozygous for the 6/6 genotype (UGT1A1*1), 42 (58%) were heterozygous for the 6/7 genotype (UGT1A1*1/*28) and 4 (5%) were homozygous for the 7/7 genotype (UGT1A1*28). The remaining two patients were homozygous for the rare 5/5 genotype (UGT1A1*36). The frequencies reported for all polymorphisms were in accordance with those observed previously in Caucasian populations (14-17) and were consistent with Hardy-Weinberg equilibrium. Relationships between day 14 13-cisRA AUC0-6h, day 14 4-oxo-13-cisRA Cmax and ratio of 13-cisRA Cmax /4-oxo-13-cisRA Cmax and the studied genetic variants were investigated. No statistically significant relationships were found between any of the genetic variants and 13-cisRA AUC0-6h or ratio of 13-cisRA Cmax /4-oxo-13-cisRA Cmax. Significant differences in day 14 4-oxo-13-cisRA Cmax were observed for the CYP2C8*4 and CYP3A7*1C polymorphisms (P=0.037 and P=0.043 respectively). Relationships between genotype for CYP2C8*3, CYP2C8*4, CYP3A5*3, CYP3A7*1C, CYP3A7*2 and UGT2B7*2 and day 14 4-oxo-13-cisRA Cmax values are shown in Figure 1.
Figure 1.
Effect of CYP2C8*3 (A), CYP2C8*4 (B), CYP3A5*3 (C), CYP3A7*1C (D), CYP3A7*2 (E) and UGT2B7*2 (F) genotypes on peak plasma concentrations of 4-oxo-13-cisRA on day 14 of treatment with 13-cisRA in 73 patients with high-risk neuroblastoma
13-cisRA dose adjustment
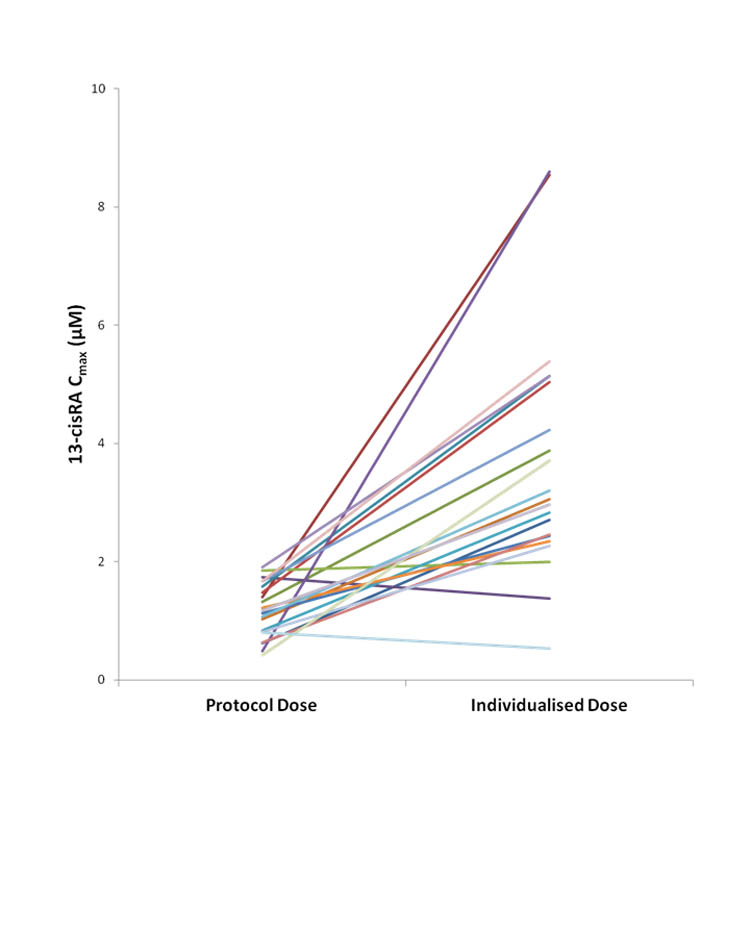
A total of 71 patients were recruited to the 13-cisRA dose adjustment group, with doses of 13-cisRA administered on course 2 of treatment based on plasma pharmacokinetics on course 1. Within this group 13-cisRA Cmax values ranged from 0.42 – 11.2μM, with a total of 24/71 (34%) patients failing to achieve a target Cmax ≥2μM on course 1 of treatment. Dose increases and additional pharmacokinetic studies were carried out in 20 of these 24 patients, with no additional pharmacokinetic data obtained for the additional 4 patients due to loss of central line access or disease relapse. A dose increase of 25% was implemented in the 14 patients attaining 13-cisRA Cmax values of 1.0 - 2.0μM on treatment course 1, with a 50% dose increase implemented in the 6 patients attaining 13-cisRA Cmax values < 1.0μM. On course 2, Cmax values ≥2μM were achieved in 12 (60%) patients. A further 6 patients (30%) achieved the target Cmax following further 25% dose increases on course 3 (4 patients) or course 4 (2 patients). The remaining two patients did not achieve the target Cmax despite several dose increases. Cmax values obtained on course 1 following the protocol-based dose and at the individualized dose are shown in Figure 2 for all patients where dose adjustments were carried out. Pharmacokinetic samples were obtained on an additional course of treatment at the individualized dose in a total of 12 patients, to confirm the plasma concentrations achieved at the increased dose. Eleven of 12 patients maintained the Cmax above 2μM, with values ranging from 1.75 – 5.94 μM.
Figure 2.
Peak plasma concentrations (Cmax) of 13-cisRA observed with protocol-based dosing and following dose increases to identify an individualized dose for all patients with initial Cmax values <2μM (n=20)
Effect of body weight-based 13-cisRA dosing in children <12kg
The overall patient cohort included a total of 11 patients <12kg who received a 13-cisRA dose of 5.33mg/kg, with 8 (73%) of these patients failing to achieve the target Cmax of ≥2μM. Six of these 8 patients were studied as part of the dose adjustment cohort and all attained the target Cmax on course 2 or 3 of treatment at an increased dose. Table 3 shows the initial protocol-based doses and final individualized 13-cisRA doses administered to patients <12kg recruited to the dose adjustment study cohort. A dose level of 5.33mg/kg was equivalent to a daily dose of 100-122mg/m2 in these younger patients, representing 24-38% dose reductions as compared to the standard dose of 160mg/m2. After dose adjustment to the target Cmax, final individualized doses were equivalent to 109-167mg/m2. A significant difference in Cmax values of 3.1 ± 2.0μM versus 1.9 ± 1.2μM (mean ± SD; P = 0.0228) was observed in patients >12kg receiving a dose of 160mg/m2 (n=92) as compared to patients <12kg receiving a dose of 5.33mg/kg (n=11), respectively (Figure 3A).
Table 3.
Initial protocol-based doses (5.33 mg/kg) and final individualized doses required to achieve 13-cisRA Cmax values >2μM observed for patients <12kg treated as part of the dose adjustment patient cohort
| Patient No. |
BW | BSA | Daily dose |
Cmax (μM) |
||||
|---|---|---|---|---|---|---|---|---|
| Initial (mg) |
Initial (mg/m2) |
Individualized (mg) |
Individualized (mg/m2) |
Initial dose |
Individualized dose |
|||
| 1 | 11.8 | 0.56 | 60 | 107 | 100 | 167 | 1.1 | 3.4 |
| 2 | 11.6 | 0.55 | 60 | 109 | n/a | n/a | 3.2 | n/a |
| 8 | 10.9 | 0.53 | 60 | 113 | 80 | 143 | 1.4 | 8.5 |
| 11 | 7.6 | 0.38 | 40 | 105 | 50 | 119 | 1.3 | 3.9 |
| 17 | 8.2 | 0.4 | 40 | 100 | 70 | 159 | 1.1 | 2.4 |
| 23 | 11.9 | 0.53 | 60 | 113 | 80 | 151 | 1.5 | 5.0 |
| 29 | 10.9 | 0.49 | 60 | 122 | 80 | 163 | 1.8 | 2.0 |
| 48 | 10.7 | 0.51 | 60 | 118 | n/a | n/a | 4.8 | n/a |
| 50 | 10.7 | 0.52 | 60 | 115 | n/a | n/a | 2.1 | n/a |
Abbreviations: BW, body weight (kg); BSA, body surface area (m2); n/a, no adjustment. No grade 3/4 toxicity was reported in these patients at either the initial or individualized dose levels.
Figure 3.
Peak plasma concentrations (Cmax) of 13-cisRA achieved in patients >12kg treated on a 160mg/m2 dosing regimen (n=92) as compared to those <12kg treated on a 5.33mg/kg dosing regimen (n=11) (A) and in patients who swallowed 13-cisRA capsules (n=27) as compared to those patients where the drug was extracted from capsules (n=76) (B)
Effect of method of 13-cisRA administration
All 14 patients within the dose adjustment study cohort who swallowed 13cisRA capsules achieved the target Cmax, as compared to 21/39 (54%) when the drug was extracted and mixed with food or 12/18 patients (67%) where the extracted material was administered via NG tube. Considering all patients for whom pharmacokinetic data were obtained, the target Cmax was achieved by 93% (25/27) of patients who swallowed capsules as compared to 55% (42/76) of patients unable to swallow the capsules. A significantly higher Cmax value of 4.0 ± 2.2μM was observed in patients who swallowed capsules as compared to 2.6 ± 1.8μM in patients who required the drug to be extracted prior to administration (mean ± SD; P=0.0012; Figure 3B). Comparable results were seen if the dataset was restricted to include only those patients who received a dose of 160mg/m2 (Cmax values of 4.0 ± 2.8 vs 2.2 ± 1.9μM in patients who swallowed capsules vs drug extraction; P=0.006). These data were supported by mean trough plasma levels, determined immediately prior to the dose administered on day 14 of treatment, which were also higher for patients who swallowed capsules (1.14μM vs 0.71μM). For patients where the drug was extracted prior to administration, Cmax values tended to be higher if the drug was administered via NG tube following extraction, as opposed to being mixed with food (Cmax values of 3.4 ± 2.4μM and 2.3 ± 1.4μM, respectively), although this difference did not reach statistical significance. For patients who had the drug mixed with food, no relationships were observed between the type of food used for administration and 13-cisRA Cmax, although numbers of patients were small in some cases. Of interest, one patient who required the drug to be extracted and mixed with food on course 1 but then swallowed the capsules on course 2, achieved a 3-fold higher Cmax on course 2 of treatment (5.4 vs 1.7μM).
13cisRA levels and toxicity
Treatment was reasonably well tolerated, although 25/103 (24%) patients had persistent grade 3/4 hematological toxicity following previous myeloablative therapy. The most common grade 3/4 toxicities experienced on courses where pharmacokinetic studies were carried out were infection in 11/103 (11%) patients, elevated ALT in 5/103 (5%) patients and nausea and vomiting in 3/103 (3%) patients. Although patients commonly experienced some form of mild skin toxicity, only 5/103 (5%) patients experienced CTC grade 3/4 skin toxicity or cheilitis. Importantly, no patients reported grade 3/4 hypercalcaemia, a dose limiting toxicity previously reported in a Phase I study in high-risk neuroblastoma patients (18). There was no evidence to suggest that any of the toxicities observed were linked to the pharmacokinetics of 13-cisRA or its metabolite. In patients studied on the 13-cisRA dose adjustment cohort, no relationship was observed between 13-cisRA Cmax or 4-oxo-13-cisRA Cmax and incidence of toxicity at higher individualized 13-cisRA doses. However, one patient who received several 13-cisRA dose adjustments, with the dose increasing from 160mg/m2 to 290mg/m2, experienced skin toxicity and behavioral changes which negated further dose increases.
DISCUSSION
Despite the proven clinical benefits of utilizing 13-cisRA following high-dose myeloablative chemotherapy for the treatment of high-risk neuroblastoma, a significant number of patients still suffer relapse within 5 years of retinoid treatment (2). While this may be related to factors such as tumor biology in some cases, the previously reported high degree of variability in the pharmacokinetics and metabolism of 13-cisRA would suggest that further improvements based on individualization of dosing or schedules may be feasible. There are a number of factors relating to the clinical pharmacology of 13-cisRA which, taken together, provide a strong case for the benefit of a therapeutic monitoring approach to ensure that uniform plasma concentrations are achieved in all patients: (i) low dose, continuous use of 13-cisRA has previously been shown to provide limited or no clinical benefit in neuroblastoma patients (19, 20), suggesting that dose intensity and therefore plasma concentrations of drug are important determinants of efficacy; (ii) the current 13-cisRA dosing regimen of 160mg/m2/day results in marked variation in plasma concentrations between patients, but limited intra-patient variability between treatment courses (3); (iii) as 13-cisRA is given as repeated cycles, patients may be exposed to sub-therapeutic concentrations of drug for the entire 6 month treatment period. As a clearly defined therapeutic window for 13-cisRA exposure has yet to be established, the current approach was designed very much as a feasibility study to minimize the marked variability in plasma concentrations previously observed with standard protocol-based dosage regimens. The minimum Cmax value of 2μM being targeted in the current study was supported by published preclinical and clinical data. While it can be difficult to compare between in vitro and in vivo studies, preclinical studies in neuroblastoma cell lines have shown that 13-cisRA concentrations of 2-10μM are required for growth arrest and effects on retinoid biological response markers (21, 22). A Phase I study of 13-cisRA in neuroblastoma patients, which determined the maximum tolerated dose (MTD) of 160mg/m2, reported mean serum levels between 4.1 ± 2.7μM and 7.2 ± 5.3μM, with a marked increase in grade 3/4 clinical toxicity observed at concentrations >10μM (23). Targeting peak plasma concentrations between 2-10μM therefore would seem an appropriate therapeutic window for 13-cisRA in this patient population. However, it should be noted that the current trial represents a feasibility study to reduce the variability in 13-cisRA exposure between patients, as opposed to a study designed to define the most appropriate target therapeutic window.
Pharmacokinetic data generated in the current study were analyzed using a modified one-compartment, zero-order absorption model combined with an absorption lag time, a model previously shown to be the most appropriate approach for 13-cisRA in a comparable clinical setting (3). The model was further developed to allow for non-zero concentrations at the time of 13-cisRA administration on day 14 of treatment, providing a good fit to the data. Population pharmacokinetic model parameters from 103 patients studied were comparable to those generated in the previous study, reporting preliminary results from a limited dataset (3). As reported in this previous study, conventional dosing of 13-cisRA at 160 mg/m2 (5.33 mg/kg in children <12kg) was associated with significant interpatient variation in 13-cisRA pharmacokinetics, with >20-fold variability in 13-cisRA Cmax and AUC. As 13-cisRA treatment approaches are based on body weight or surface area-based dosing, it is clearly a concern that neither of these covariates were observed to have a significant effect on 13-cisRA pharmacokinetics in the current study.
Based on findings from both in vitro and in vivo studies, there is a good rationale for hypothesizing that drug metabolism plays a key role in influencing 13-cisRA pharmacokinetics following drug administration. A number of commonly-expressed CYP enzymes responsible for the metabolism of 13-cisRA have been characterized. The current study investigated the extent of metabolism of 13-cisRA in a relatively large pediatric neuroblastoma patient cohort. The potential influence on the pharmacokinetic profile of 13-cisRA of common SNPs affecting enzymes responsible for 13-cisRA metabolism was explored. Although statistically significant differences in day 14 4-oxo-13-cisRA Cmax values were observed for CYP2C8*4 and CYP3A7*1C polymorphisms, overall these studies failed to show any clear impact of pharmacogenetics in determining peak plasma concentrations of 13-cisRA. Of particular note, functionally relevant SNPs in CYP and UGT enzymes studied did not appear to significantly impact on the ratio of parent drug to metabolite, a parameter which should be unaffected by confounding variables such as 13-cisRA dose level and/or method of administration. However, bearing in mind the overall patient numbers and relatively small numbers of patients in certain genotype groups, these findings should be seen as preliminary data which may help to guide future research in this area.
The results obtained in the current study highlight a number of important factors relating to the administration of 13-cisRA. These include both the appropriateness of dosing based on body weight for smaller patients as well as problems relating to the lack of availability of an appropriate 13-cisRA drug formulation. A 13-cisRA dose level of 5.33mg/kg is currently recommended for children <12kg, which represents a significant number of neuroblastoma patients. As compared to a dose level of 160mg/m2, a dose of 5.33mg/kg administered to children <12kg in the current study equated to 13-cisRA dose reductions of 24-38%. A total of 11/103 (11%) patients received this reduced dose level, with Cmax values below 2μM observed in 73% of these patients and a significantly lower mean 13-cisRA Cmax achieved relative to children receiving 160mg/m2. Dose increases of 25 or 50% implemented in patients receiving an initial dose of 5.33mg/kg resulted in the achievement of plasma concentrations >2 μM in all cases, with the final individualized doses approximately equivalent to the standard surface area-based dose of 160mg/m2. These dose increases were well tolerated in all patients. These data would strongly suggest that a 13-cisRA dosage regimen of 5.33mg/kg should not be implemented for children below 12kg. These findings have implications beyond the dosing of neuroblastoma patients with 13-cisRA, with dosing based on body weight utilized for the vast majority of anticancer drugs used in paediatric oncology in infants and very young children. In addition to the implicit dose reduction that is often seen when shifting from body surface area to body weight-based dosing, additional dose reductions may also be recommended for patients below specified cut-off points, for example an age of 6 months or 1 year, or a body weight of 10 or 12kg (24). Although the implementation of variable cut-off points and dose reductions may be based on sound evidence for certain anticancer drugs, in many cases the scientific rationale behind the dosing regimens used is limited. The current study data would suggest that further studies are warranted to consider whether more rational approaches to dosing in infant patients should be established for other chemotherapeutics.
Again related to the fact that children diagnosed with high-risk neuroblastoma are commonly aged between 1 and 5 years, the administration of 13-cisRA capsules can represent a considerable practical problem. The current study very much highlights this issue, with only 27 out of 103 (26%) patients able to swallow the capsules. This cohort included a small number of patients who chewed and swallowed the capsules as opposed to swallowing capsules whole. For the remainder of patients, 13-cisRA was extracted from the capsules and either mixed with food or administered via NG tube. Target Cmax values were achieved by 93% of patients who swallowed capsules as compared to 55% of patients unable to swallow capsules. Bearing in mind the potential loss of drug during handling, it is unsurprising that mean Cmax values achieved in these patients were lower than in those patients able to swallow the capsules (2.6 ± 1.8 vs 4.0 ± 2.2 μM; P=0.0012). These data were supported by trough levels determined immediately prior to the dose administered on day 14 of treatment, which were also higher for patients who swallowed capsules (1.14μM vs 0.71μM). While these data clearly point to the method of administration as being a major factor influencing 13-cisRA plasma concentrations, it should be noted that these patients were generally the younger patients recruited to the study. As such it can not be excluded that other factors, such as differences in drug absorption, may also have a role to play. It is unclear whether or not this administration problem was an issue for younger children recruited to the Phase I study of 13-cisRA in neuroblastoma patients, which reported mean serum levels between 4.1 ± 2.7μM and 7.2 ± 5.3μM at the MTD of 160mg/m2 (23). While plasma concentrations observed in the current study are generally in agreement with these previously reported levels, the 13-cisRA Cmax range of 0.4-11.2μM includes a relatively large number of patients who achieved plasma concentrations clearly below the minimum concentrations reported in the Phase I trial.
It is also important to consider the potential impact of compliance on the results obtained. When a family is told that their child is not receiving a sufficient dose of drug, it is a natural reaction for the family to make increased efforts to maximize extraction from the capsule. It is therefore almost inevitable that a more thorough and meticulous approach to administering the drug will occur on the following course of treatment at the increased dose level. Indeed, factors relating to drug compliance may go some way to explaining comparatively large increases in 13-cisRA peak plasma concentrations observed in some patients following a 25 or 50% dose increase. If this is the case then it is reassuring that once an individualized dose level has been determined for a particular patient, confirmatory plasma levels obtained on an additional course of treatment show that Cmax values >2μM are being consistently achieved at the increased dose level. On a related note, it is also possible that variability is likely to be higher when 13-cisRA is administered to patients at home, as compared to under the supervision of a trained research nurse on a pharmacokinetic study day.
The current study shows the feasibility of 13-cisRA dose individualization based on Cmax values achieved in individual patients, with marked reduction in inter-patient pharmacokinetic variability and 13-cisRA exposures observed following dose modifications. These data strongly indicate that a standard 13-cisRA dosing regimen of 160mg/m2 is valid for all patients, with no pharmacological rational for implementation of reduced dosing in children <12kg. In addition a 25% dose increase to 200mg/m2 is recommended for children >12kg who are unable to swallow 13-cisRA capsules, when the drug is extracted from the capsules and mixed with food or administered by NG tube. These amended dosing guidelines are likely to provide a more uniform exposure to 13-cisRA across the patient population as a whole, thus allaying concerns of pediatric oncologists that potentially sub-therapeutic plasma concentrations may be achieved in some patients due to formulation and compliance issues. While we anticipate that these approaches may benefit patients receiving 13-cisRA in the short-term, the findings of the current study emphasize a clear need for the availability of an appropriate oral formulation of this drug to facilitate more accurate dosing in children with high-risk neuroblastoma.
Supplementary Material
Translational Relevance.
Following the publication of encouraging data from clinical trials, the use of isotretinoin (13-cisRA) alongside immunotherapy with anti-GD2 is now the established treatment for minimal residual disease in children with high-risk neuroblastoma. However, marked inter-patient variability in 13-cisRA pharmacokinetics may lead to some children receiving sub-therapeutic drug concentrations. In this study we have shown the feasibility of adaptive 13-cisRA dosing, based on individual patient drug exposure, which markedly reduces the variability observed within this patient population. Results strongly indicate that reduced body weight-based dosing should not be implemented for children <12kg and that higher doses may be beneficial for children unable to swallow 13-cisRA capsules, where the drug is extracted prior to administration. These data are significant in that these two patient groups represent a total of 74% of the studied patient population. While we strive to develop innovative therapies for children with poor prognosis tumour types such as high-risk neuroblastoma, it is essential that those treatments available are optimally utilized in all patients. The findings of the current study highlight the challenges faced in treating younger children and the need for appropriate pharmaceutical formulations of medicines for use in all paediatric patient populations.
Acknowledgements
We thank the patients, research nurses and clinicians who participated in the study at the following clinical centres: Royal Victoria Infirmary, Newcastle upon Tyne; Great Ormond Street Hospital, London; Manchester Children’s Hospital; Leeds General Infirmary; Royal Liverpool Children’s Hospital; Royal Marsden Hospital, Surrey; Addenbrooke’s Hospital, Cambridge; Bristol Royal Hospital for Children; John Radcliffe Hospital, Oxford; Royal Hospital for Sick Children, Glasgow; Birmingham Children’s Hospital; Southampton General Hospital; Royal Aberdeen Children’s Hospital; Nottingham Children’s Hospital.
Grant support This research was supported by Cancer Research UK and by the North of England Children’s Cancer Research Fund.
The study was supported by funding from Cancer Research UK.
Footnotes
This work was presented in part at the 102nd Annual AACR meeting in Orlando, April 2011, and the Advances in Neuroblastoma Research (ANR 2012) conference in Toronto, June 2012.
The authors have no potential conflicts of interest to disclose.
References
- 1.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 2.Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a Children’s Oncology Group study. J Clin Oncol. 2009;27:1007–13. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veal GJ, Cole M, Errington J, Pearson ADJ, Foot ABM, Whyman G, Boddy AV. Pharmacokinetics and metabolism of 13-cis-retinoic acid (isotretinoin) in children with high-risk neuroblastoma – a study of the United Kingdom Children’s Cancer Study Group. Br J Cancer. 2007;96:424–31. doi: 10.1038/sj.bjc.6603554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Leede BM, van den Brink CE, Pijnappel WWM, Sonneveld E, van der Saag PT, van der Berg B. Autoinduction of retinoic acid metabolism to polar derivatives with decreased biological activity in retinoic acid-sensitive, but not in retinoic acid resistant human breast cancer cells. J Biol Chem. 1997;272:17921–8. doi: 10.1074/jbc.272.29.17921. [DOI] [PubMed] [Google Scholar]
- 5.Kim SY, Yoo SJ, Kwon HJ, Kim SH, Byun Y, Lee K-S. Retinoic acid 4-hydroxylase-mediated catabolism of all-trans retinoic acid and the cell proliferation in head and neck squamous cell carcinoma. Metabolism. 2002;51:477–81. doi: 10.1053/meta.2002.31335. [DOI] [PubMed] [Google Scholar]
- 6.Muindi J, Frankel SR, Miller WH, Jakubowski A, Scheinberg DA, Young CW, et al. Continuous treatment with all-trans retinoic acid causes a progressive reduction in plasma drug concentrations - implications for relapse and retinoid resistance in patients with acute promyelocytic leukemia. Blood. 1992;79:299–303. [PubMed] [Google Scholar]
- 7.Miller VA, Rigas JR, Muindi JR, Tong WP, Venkatraman E, Kris MG, et al. Modulation of all-trans retinoic acid pharmacokinetics by liarozole. Cancer Chemother Pharmacol. 1994;34:522–6. doi: 10.1007/BF00685665. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Fantel AG, Juchau MR. Catalysis of the 4-hydroxylation of retinoic acids by cyp3a7 in human fetal hepatic tissues. Drug Metab Dispos. 2000;28:1051–7. [PubMed] [Google Scholar]
- 9.Marill J, Capron CC, Idres N, Chabot GG. Human cytochrome P450s involved in the metabolism of 9-cis- and 13-cis-retinoic acids. Biochem Pharmacol. 2002;63:933–43. doi: 10.1016/s0006-2952(01)00925-x. [DOI] [PubMed] [Google Scholar]
- 10.Rowbotham SE, Boddy AV, Redfern CP, Veal GJ, Daly AK. Relevance of non-synonymous CYP2C8 polymorphisms to 13-cis retinoic acid and paclitaxel hydroxylation. Drug Metab Dispos. 2010;38:1261–6. doi: 10.1124/dmd.109.030866. [DOI] [PubMed] [Google Scholar]
- 11.Rowbotham SE, Illingworth NA, Daly AK, Veal GJ, Boddy AV. Role of UDP-glucuronosyltransferase isoforms in 13-cis retinoic acid metabolism in humans. Drug Metab Dispos. 2010;38:1211–7. doi: 10.1124/dmd.109.031625. [DOI] [PubMed] [Google Scholar]
- 12.Bauters TG, Laureys G, Van de Velde V, Benoit Y, Robays H. Practical implications for the administration of 13-cis retinoic acid in pediatric oncology. Int J Clin Pharm. 2011;33:597–8. doi: 10.1007/s11096-011-9519-9. [DOI] [PubMed] [Google Scholar]
- 13.Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, et al. Analytical methods validation - bioavailability, bioequivalence, and pharmacokinetic studies. Journal of Pharmaceutical Sciences. 1992;81:309–12. doi: 10.1007/BF03189968. [DOI] [PubMed] [Google Scholar]
- 14.Bhasker CR, McKinnon W, Stone A, Lo AC, Kubota T, Ishizaki T, Miners JO. Genetic polymorphism of UDP-glucuronosyltransferase 2B7 (UGT2B7) at amino acid 268: ethnic diversity of alleles and potential clinical significance. Pharmacogenetics. 2000;10:679–85. doi: 10.1097/00008571-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 16.Bahadur N, Leathart JB, Mutch E, Steimel-Crespi D, Dunn SA, Gilissen R, et al. CYP2C8 polymorphisms in Caucasians and their relationship with paclitaxel 6alpha-hydroxylase activity in human liver microsomes. Biochem Pharmacol. 2002;64:1579–89. doi: 10.1016/s0006-2952(02)01354-0. [DOI] [PubMed] [Google Scholar]
- 17.Xie HG, Wood AJ, Kim RB, Stein CM, Wilkinson GR. Genetic variability in CYP3A5 and its possible consequences. Pharmacogenomics. 2004;5:243–72. doi: 10.1517/phgs.5.3.243.29833. [DOI] [PubMed] [Google Scholar]
- 18.Villablanca JG, Khan AA, Avramis VI, Seeger RC, Matthay KK, Ramsay NKC, Reynolds CP. Phase I trial of 13-cis-retinoic acid in children with neuroblastoma following bone marrow transplantation. J Clin Oncol. 1995;13:894–901. doi: 10.1200/JCO.1995.13.4.894. [DOI] [PubMed] [Google Scholar]
- 19.Kohler JA, Imeson J, Ellershaw C, Lie SO. A randomized trial of 13-cis retinoic acid in children with advanced neuroblastoma after high dose therapy. Br J Cancer. 2000;83:1124–7. doi: 10.1054/bjoc.2000.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthay KK, Reynolds CP. Is there a role for retinoids to treat minimal residual disease in neuroblastoma? Br J Cancer. 2000;83:1121–3. doi: 10.1054/bjoc.2000.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds CP, Schindler PF, Jones DM, Gentile JL, Proffitt RT, Einhorn PA. Comparison of 13-cis-retinoic acid to trans-retinoic acid using human neuroblastoma cell lines. Prog Clin Biol Res. 1994;385:237–44. [PubMed] [Google Scholar]
- 22.Veal GJ, Errington J, Redfern CPF, Pearson ADJ, Boddy AV. Influence of isomerisation on the growth inhibitory effects and cellular activity of 13-cis and all-trans retinoic acid in neuroblastoma cells. Biochem Pharmacol. 2002;63:207–15. doi: 10.1016/s0006-2952(01)00844-9. [DOI] [PubMed] [Google Scholar]
- 23.Khan AA, Villablanca JG, Reynolds CP, Avramis VI. Pharmacokinetic studies of 13-cis-retinoic acid in pediatric patients with neuroblastoma following bone marrow transplantation. Cancer Chemother Pharmacol. 1996;39:34–41. doi: 10.1007/s002800050535. [DOI] [PubMed] [Google Scholar]
- 24.Veal GJ, Boddy AV. Chemotherapy in newborns and preterm babies. Semin Fetal Neonatal Med. 2012;17:243–8. doi: 10.1016/j.siny.2012.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.