Abstract
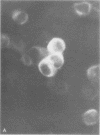
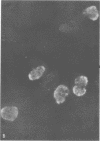
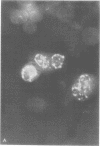
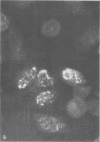
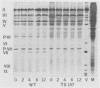
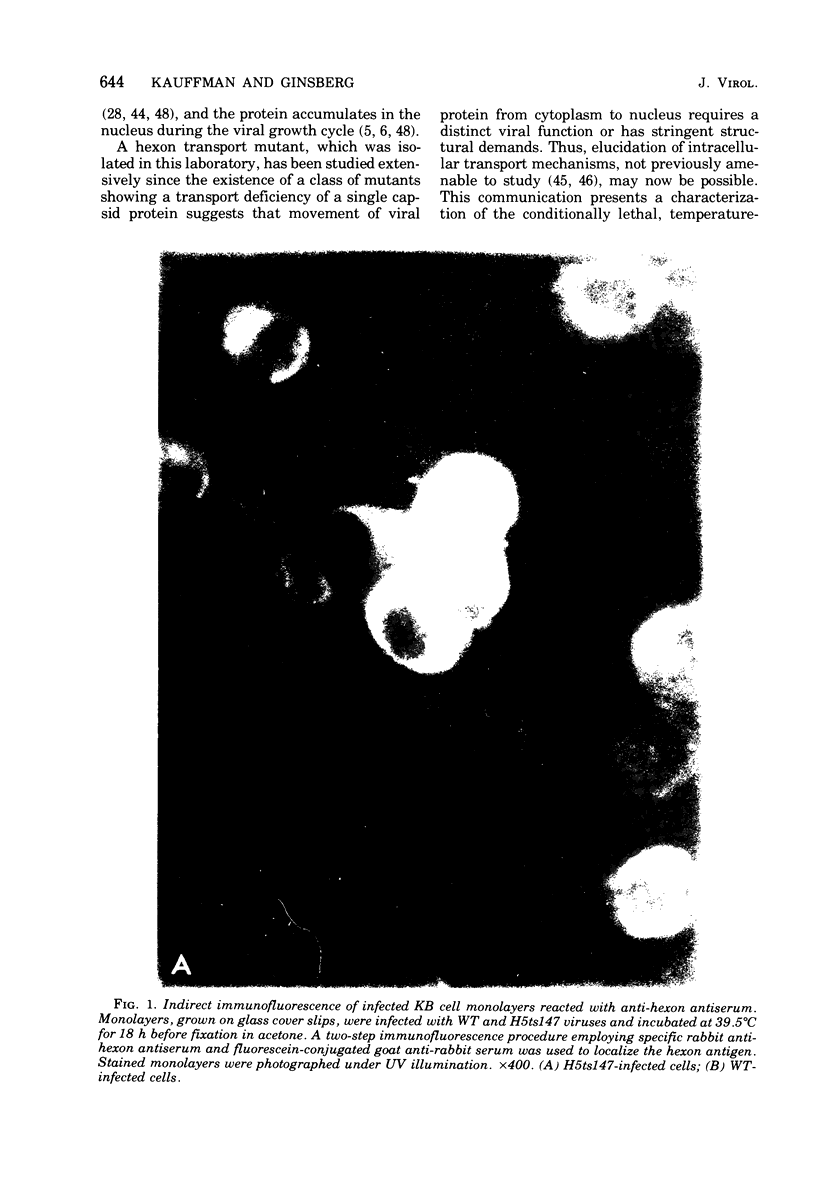
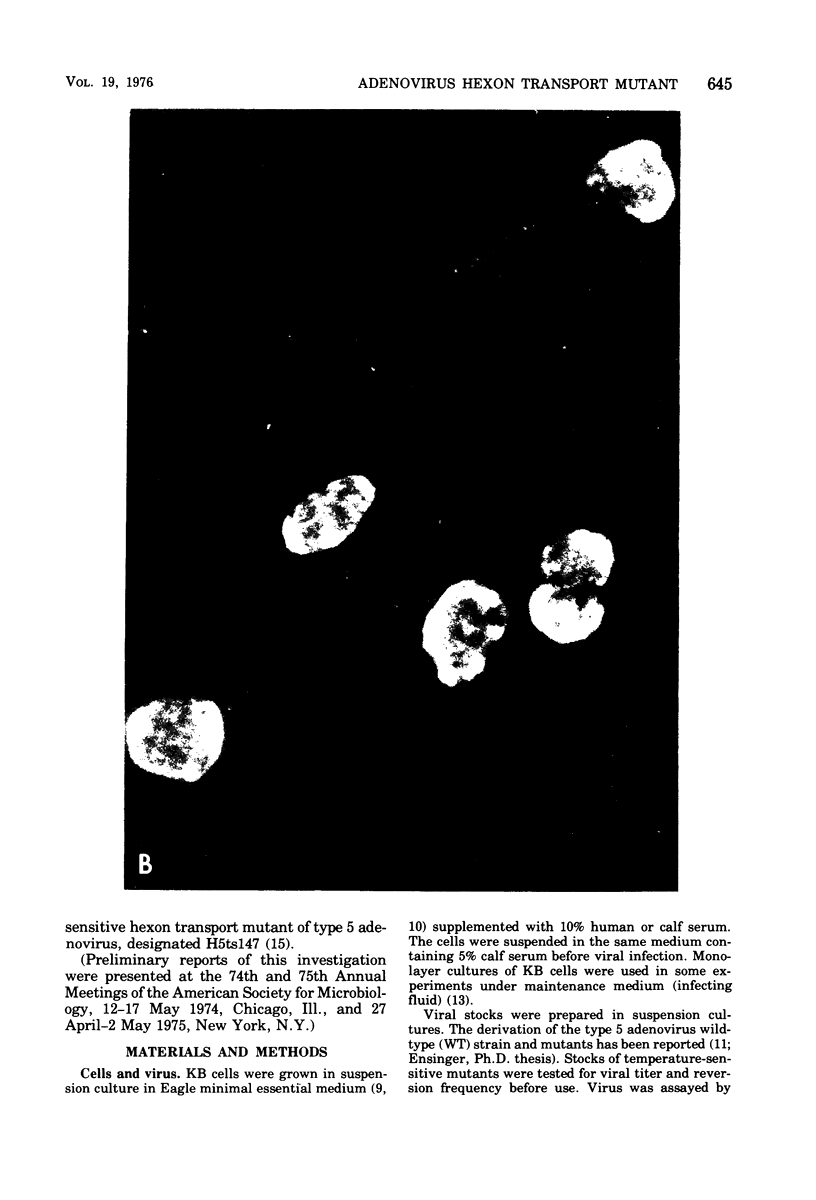
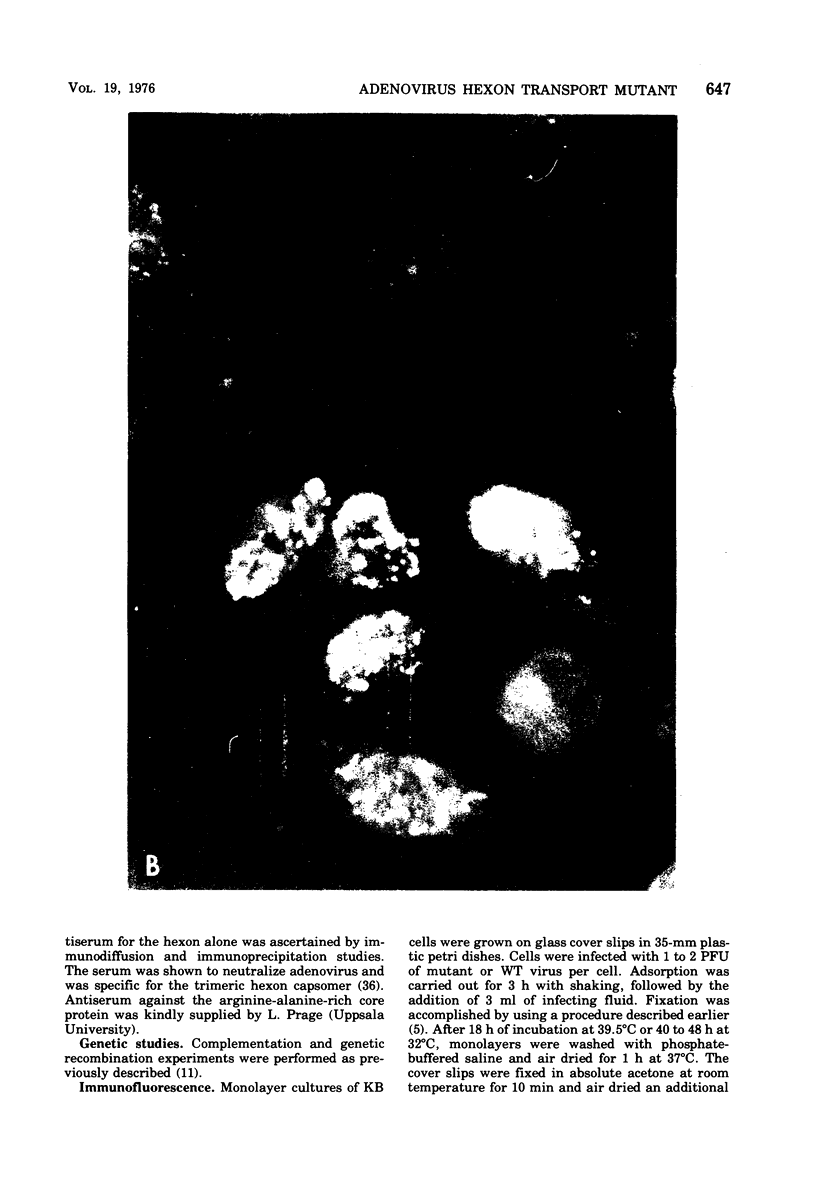
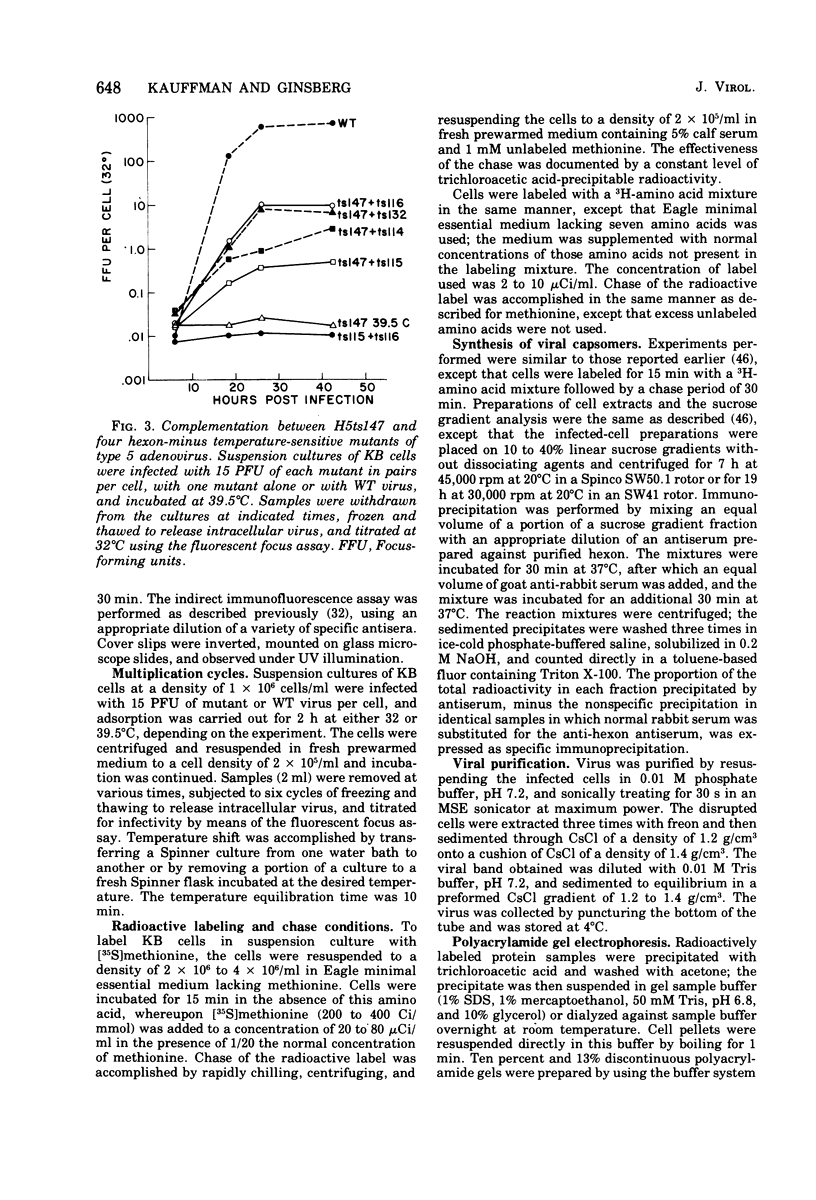
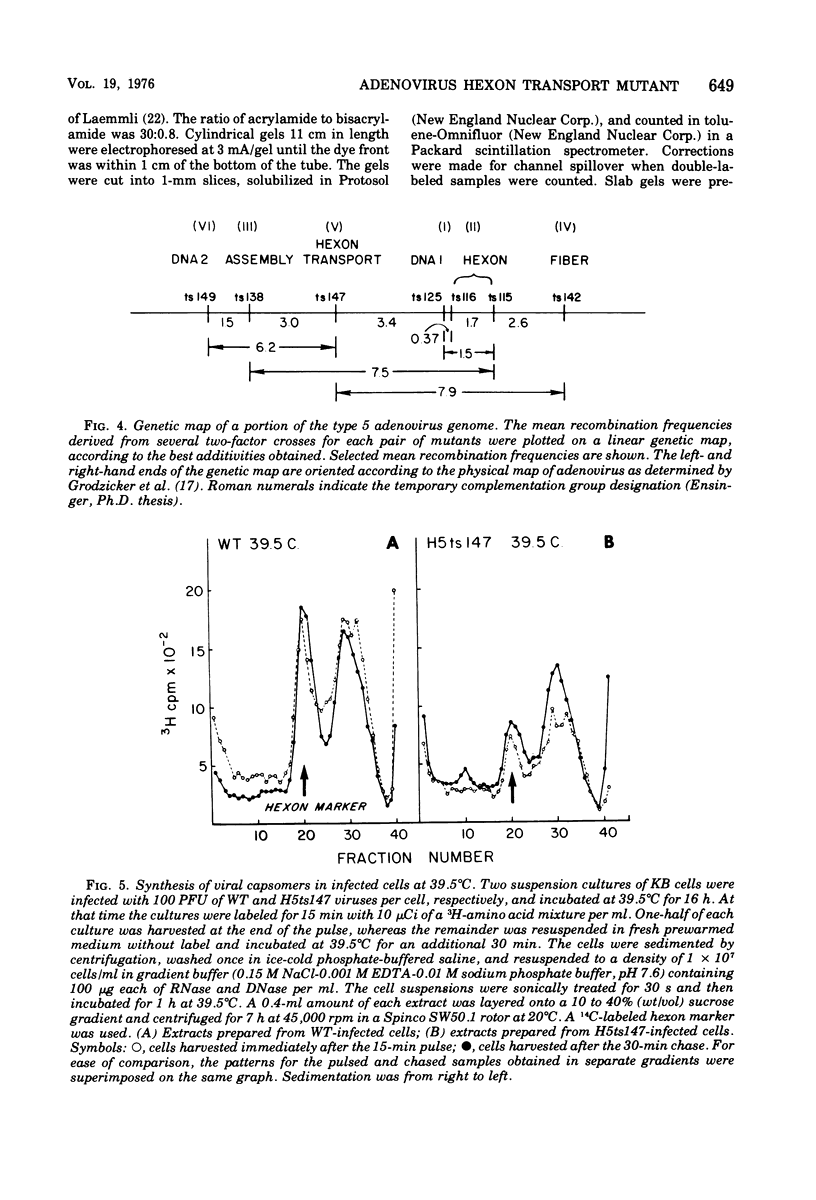
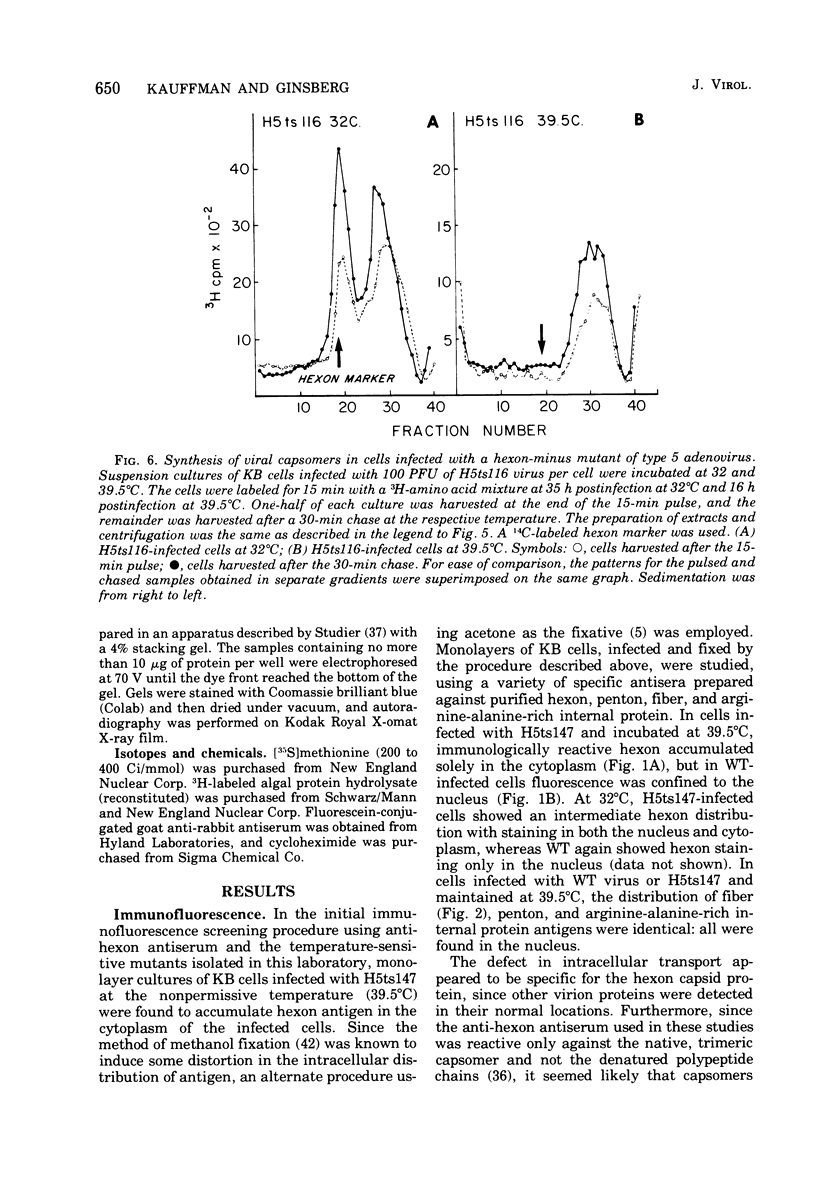
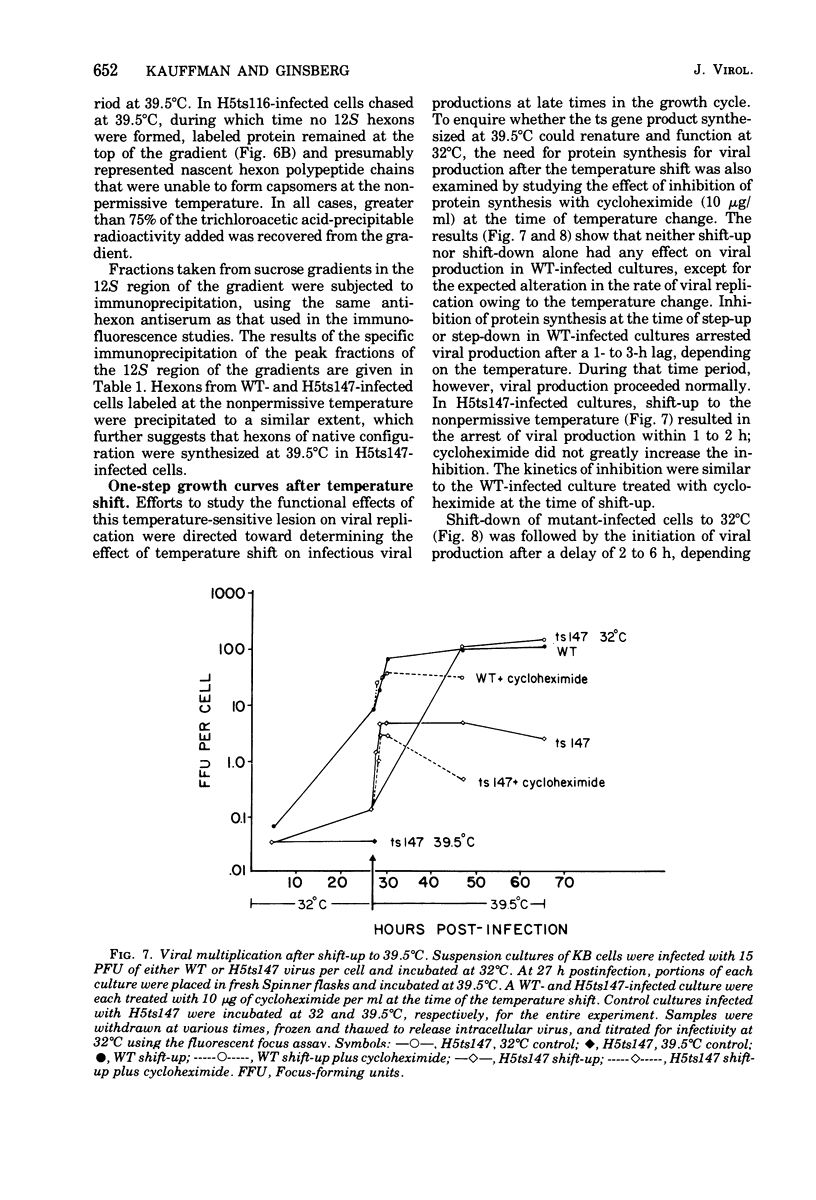
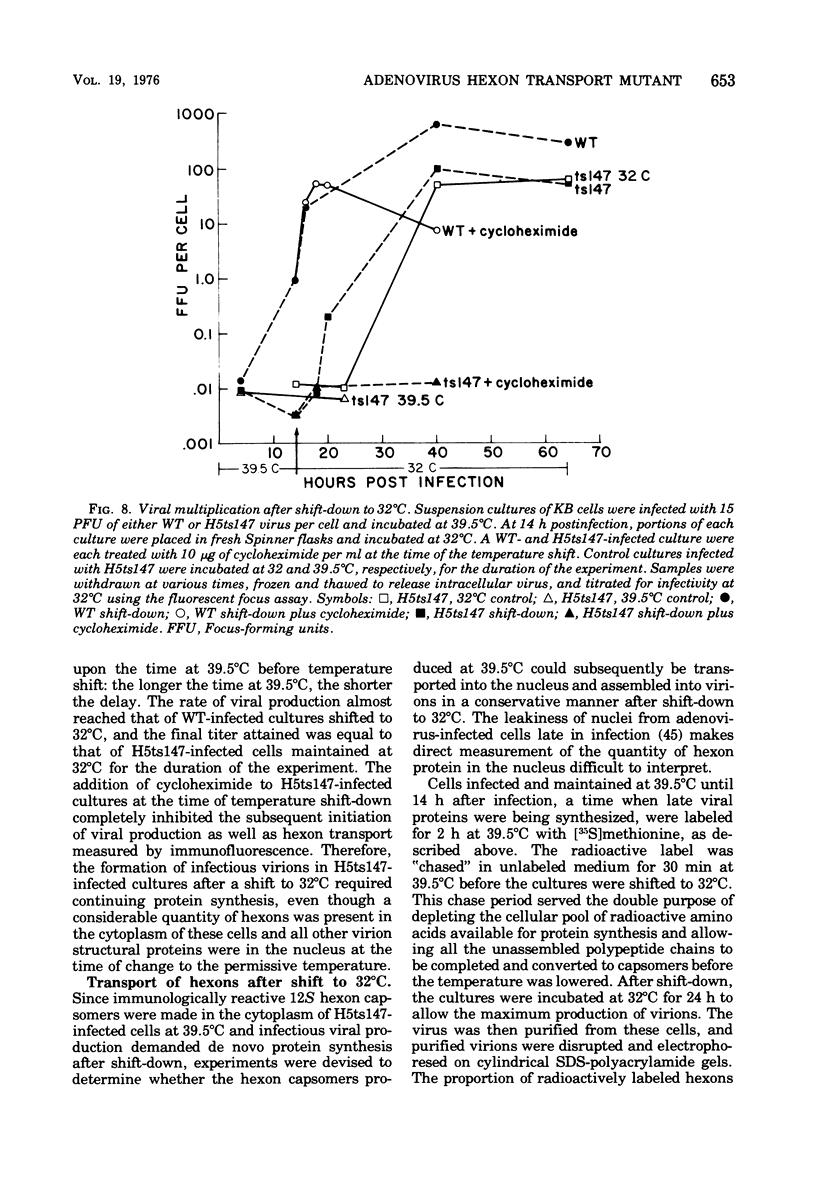
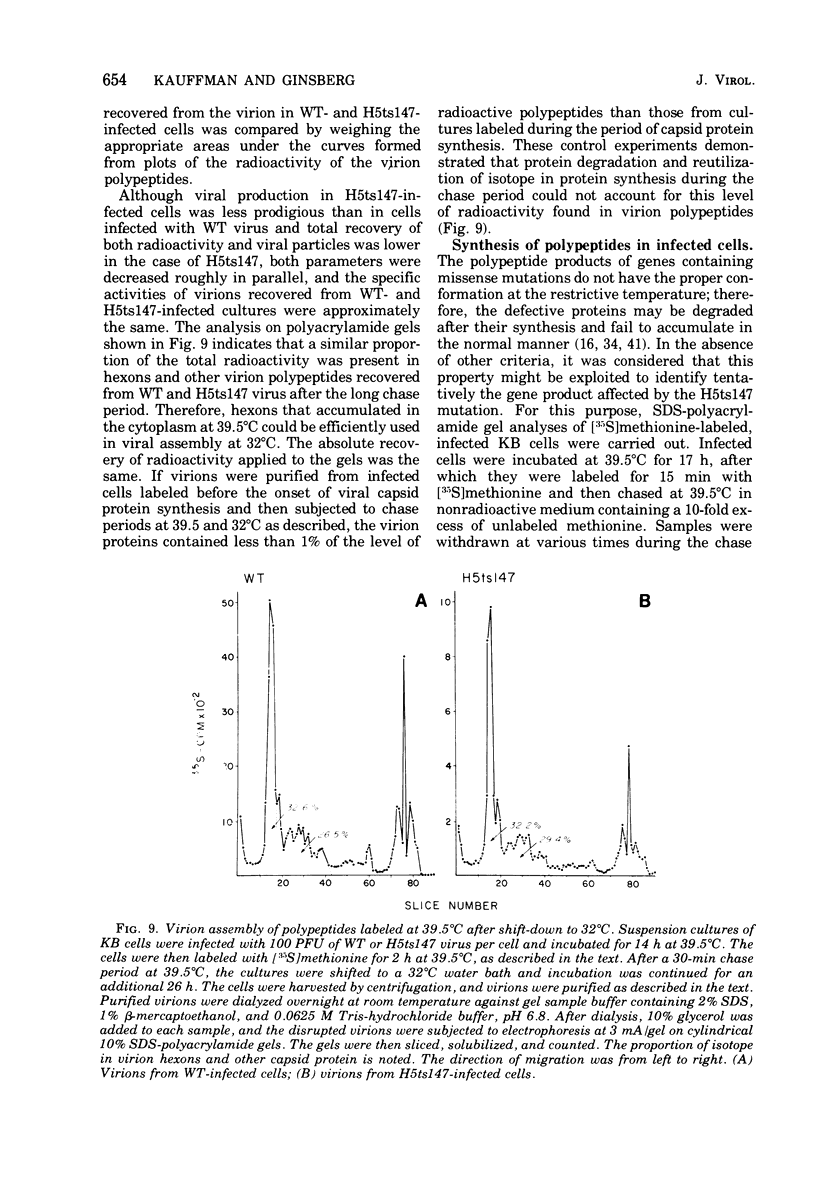
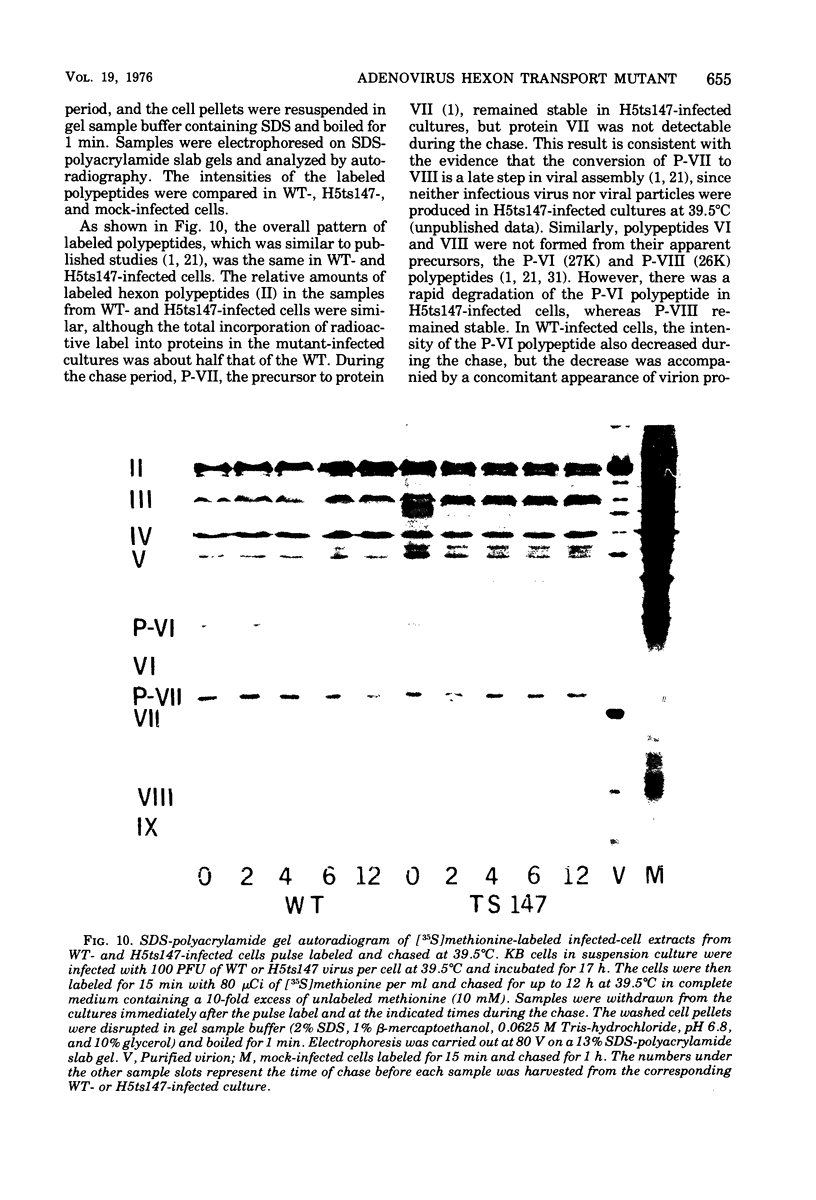
Infection of KB cells at 39.5 degrees C with H5ts147, a temperature-sensitive (ts) mutant of type 5 adenovirus, resulted in the cytoplasmic accumulation of hexon antigen; all other virion proteins measured, however, were normally transported into the nucleus. Immunofluorescence techniques were used to study the intracellular location of viral proteins. Genetic studies revealed that H5ts147 was the single member of a nonoverlapping complementation group and occupied a unique locus on the adenovirus genetic map, distinct from mutants that failed to produce immunologically reactive hexons at 39.5 degrees C ("hexon-minus" mutants). Sedimentation studies of extracts of H5ts147-infected cells cultured and labeled at 39.5 degrees C revealed the production of 12S hexon capsomers (the native, trimeric structures), which were immunoprecipitable to the same extent as hexons synthesized in wild type (WT)-infected cells. In contrast, only 3.4S polypeptide chains were found in extracts of cells infected with the class of mutants unable to produce immunologically reactive hexon protein at 39.5 degrees C. Hexons synthesized in H5ts147-infected cells at 39.5 degrees C were capable of being assembled into virions, to the same extent as hexons synthesized in WT-infected cells, when the temperature was shifted down to the permissive temperature, 32 degrees C. Infectious virus production was initiated within 2 to 6 h after shift-down to 32 degrees C; de novo protein synthesis was required to allow this increase in viral titer. If ts147-infected cells were shifted up to 39.5 degrees C late in the viral multiplication cycle, viral production was arrested within 1 to 2 h. The kinetics of shutoff was similar to that of a WT-infected culture treated with cycloheximide at the time of shift-up. The P-VI nonvirion polypeptide, the precursor to virion protein VI, was unstable at 39.5 degrees C, whereas the hexon polypeptide was not degraded during the chase. It appears that there is a structural requirement for the transport of hexons into the nucleus more stringent than the acquisition of immunological reactivity and folding into the 12S form.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNSTEIN H., EDGAR R. S., DENHARDT G. H. INTRAGENIC COMPLEMENTATION AMONG TEMPERATURE SENSITIVE MUTANTS OF BACTERIOPHAGE T4D. Genetics. 1965 Jun;51:987–1002. doi: 10.1093/genetics/51.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER G. S., DENNY F. W., Jr, GINSBERG H. S. Intracellular localization of type 4 adenovirus. II. Cytological and fluorescein-labelled antibody studies. J Exp Med. 1959 Jan 1;109(1):85–96. doi: 10.1084/jem.109.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER G. S., LEUCHTENBERGER C., GINSBERG H. S. Cytological and cytochemical studies of HeLa cells infected with adeno-viruses. J Exp Med. 1957 Mar 1;105(3):195–216. doi: 10.1084/jem.105.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts R. F., Tachovsky T. G., Hare J. D. Studies on the mechanism of cytoplasmic antigen accumulation following infection with a new variant of polyoma virus. J Gen Virol. 1972 Jul;16(1):29–38. doi: 10.1099/0022-1317-16-1-29. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Guentzel M. J., Rapp F. Variants of defective simian papovavirus 40 (PARA) characterized by cytoplasmic localization of simian papovavirus 40 tumor antigen. J Virol. 1969 Nov;4(5):632–641. doi: 10.1128/jvi.4.5.632-641.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bégin M., Weber J. Genetic analysis of adenovirus type 2. I. Isolation and genetic characterization of temperature-sensitive mutants. J Virol. 1975 Jan;15(1):1–7. doi: 10.1128/jvi.15.1.1-7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornick G., Sigler P. B., Ginsberg H. S. Mass of protein in the asymmetric unit of hexon crystals--a new method. J Mol Biol. 1973 Feb 5;73(4):533–538. doi: 10.1016/0022-2836(73)90099-5. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc Soc Exp Biol Med. 1955 Jul;89(3):362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Pettersson U., Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973 Mar;52(1):130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- GINSBERG H. S., GOLD E., JORDAN W. S., Jr Tryptose phosphate broth as supplementary factor for maintenance of HeLa cell tissue cultures. Proc Soc Exp Biol Med. 1955 May;89(1):66–71. doi: 10.3181/00379727-89-21718. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Pereira H. G., Valentine R. C., Wilcox W. C. A proposed terminology for the adenovirus antigens and virion morphological subunits. Virology. 1966 Apr;28(4):782–783. doi: 10.1016/0042-6822(66)90271-6. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Williams J. F., Doerfler W. H., Shimojo H. Proposed nomenclature for mutants of adenoviruses. J Virol. 1973 Sep;12(3):663–664. doi: 10.1128/jvi.12.3.663-664.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Williams J., Sharp P., Sambrook J. Physical mapping of temperature-sensitive mutations of adenoviruses. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):439–446. doi: 10.1101/sqb.1974.039.01.056. [DOI] [PubMed] [Google Scholar]
- Hare J. D. A new type of variation among the polyoma viruses characterized by cytoplasmic accumulation of capsid antigen. Virology. 1970 Apr;40(4):978–988. doi: 10.1016/0042-6822(70)90144-3. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Maizel J. V., Jr The polypeptides of adenovirus. V. Young virions, structural intermediate between top components and aged virions. Virology. 1974 Feb;57(2):409–424. doi: 10.1016/0042-6822(74)90181-0. [DOI] [PubMed] [Google Scholar]
- Ishibashi M. Retention or viral antigen in the cytoplasm of cells infected with temperature-sensitive mutants of an avian adenovirus. Proc Natl Acad Sci U S A. 1970 Feb;65(2):304–309. doi: 10.1073/pnas.65.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M. Temperature-sensitive conditional-lethal mutants of an avian adenovirus (CELO). I. Isolation and characterization. Virology. 1971 Jul;45(1):42–52. doi: 10.1016/0042-6822(71)90111-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledinko N. Temperature-sensitive mutants of adenovirus type 12 defective in viral DNA synthesis. J Virol. 1974 Sep;14(3):457–468. doi: 10.1128/jvi.14.3.457-468.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. II. Soluble proteins, cores, top components and the structure of the virion. Virology. 1968 Sep;36(1):126–136. doi: 10.1016/0042-6822(68)90122-0. [DOI] [PubMed] [Google Scholar]
- Mautner V., Williams J., Sambrook J., Sharp P. A., Grodzicker T. The location of the genes coding for hexon and fiber proteins in adenovirus DNA. Cell. 1975 May;5(1):93–99. doi: 10.1016/0092-8674(75)90097-5. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. Mitochondrial and nuclear interaction. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):351–352. doi: 10.1083/jcb.2.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg B., Saborio J., Persson T., Everitt E., Philipson L. Identification of the in vitro translation products of adenovirus mRNA by immunoprecipitation. J Virol. 1975 Jan;15(1):199–207. doi: 10.1128/jvi.15.1.199-207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPSON L. Adenovirus assay by the fluorescent cell-counting procedure. Virology. 1961 Nov;15:263–268. doi: 10.1016/0042-6822(61)90357-9. [DOI] [PubMed] [Google Scholar]
- Russel W. C., Newman C., Williams J. F. Characterization of temperature-sensitive mutants of adenovirus type 5--serology. J Gen Virol. 1972 Dec;17(3):265–279. doi: 10.1099/0022-1317-17-3-265. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Skehel J. J., Williams J. F. Characterization of temperature-sensitive mutants of adenovirus type 5: synthesis of polypeptides in infected cells. J Gen Virol. 1974 Aug;24(2):247–259. doi: 10.1099/0022-1317-24-2-247. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Irisawa J., Shimojo H. Isolation and a preliminary characterization of temperature-sensitive mutants of adenovirus 12. Virology. 1972 Jul;49(1):1–11. doi: 10.1016/s0042-6822(72)80002-3. [DOI] [PubMed] [Google Scholar]
- Stinski M. F., Ginsberg H. S. Antibody to the type 5 adenovirus hexon polypeptide: detection of nascent polypeptides in the cytoplasm of infected KB cells. Intervirology. 1974;4(4):226–236. doi: 10.1159/000149967. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Suzuki E., Shimojo H. A temperature-sensitive mutant of adenovirus 31, defective in viral deoxyribonucleic acid replication. Virology. 1971 Feb;43(2):488–494. doi: 10.1016/0042-6822(71)90320-5. [DOI] [PubMed] [Google Scholar]
- Suzuki E., Shimojo H., Moritsugu Y. Isolation and a preliminary characterization of temperature-sensitive mutants of adenovirus 31. Virology. 1972 Aug;49(2):426–438. doi: 10.1016/0042-6822(72)90495-3. [DOI] [PubMed] [Google Scholar]
- Takahashi M. Isolation of conditional lethal mutants (temperature sensitive and host-dependent mutants) of adenovirus type 5. Virology. 1972 Sep;49(3):815–817. doi: 10.1016/0042-6822(72)90540-5. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Robb J. A., Widmer C., Ozer H. L. Altered protein metabolism in infection by the late tsB11 mutant of simian virus 40. J Virol. 1974 Oct;14(4):997–1007. doi: 10.1128/jvi.14.4.997-1007.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel J. F., Smith K. O. Fluorescent focus assay of viruses on cell monolayers in plastic Petri plates. Proc Soc Exp Biol Med. 1967 Jul;125(3):892–895. doi: 10.3181/00379727-125-32232. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Green M. Biochemical studies on adenovirus multiplication, xi. Evidence of a cytoplasmic site for the synthesis of viral-coded proteins. Proc Natl Acad Sci U S A. 1966 Jul;56(1):243–246. doi: 10.1073/pnas.56.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Pereira H. G. Antigens and structure of the adenovirus. J Mol Biol. 1965 Aug;13(1):13–20. doi: 10.1016/s0022-2836(65)80076-6. [DOI] [PubMed] [Google Scholar]
- Velicer L. F., Ginsberg H. S. Cytoplasmic synthesis of type 5 adenovirus capsid proteins. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1264–1271. doi: 10.1073/pnas.61.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer L. F., Ginsberg H. S. Synthesis, transport, and morphogenesis of type adenovirus capsid proteins. J Virol. 1970 Mar;5(3):338–352. doi: 10.1128/jvi.5.3.338-352.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. STRUCTURE OF TYPE 5 ADENOVIRUS. I. ANTIGENIC RELATIONSHIP OF VIRUS-STRUCTURAL PROTEINS TO VIRUS-SPECIFIC SOLUBLE ANTIGENS FROM INFECTED CELLS. J Exp Med. 1963 Aug 1;118:295–306. doi: 10.1084/jem.118.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer C., Robb J. A. Simian virus 40-host cell interactions. II. Cytoplasmic and nucleolar accumulation of simian virus 40 virion protein. J Virol. 1974 Dec;14(6):1530–1546. doi: 10.1128/jvi.14.6.1530-1546.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. F., Gharpure M., Ustacelebi S., McDonald S. Isolation of temperature-sensitive mutants of adenovirus type 5. J Gen Virol. 1971 May;11(2):95–101. doi: 10.1099/0022-1317-11-2-95. [DOI] [PubMed] [Google Scholar]
- Williams J., Grodzicker T., Sharp P., Sambrook J. Adenovirus recombination: physical mapping of crossover events. Cell. 1975 Feb;4(2):113–119. doi: 10.1016/0092-8674(75)90117-8. [DOI] [PubMed] [Google Scholar]
- Willians J. F., Young C. S., Austin P. E. Genetic analysis of human adenovirus type 5 in permissive and nonpermissive cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):427–437. doi: 10.1101/sqb.1974.039.01.055. [DOI] [PubMed] [Google Scholar]