Abstract
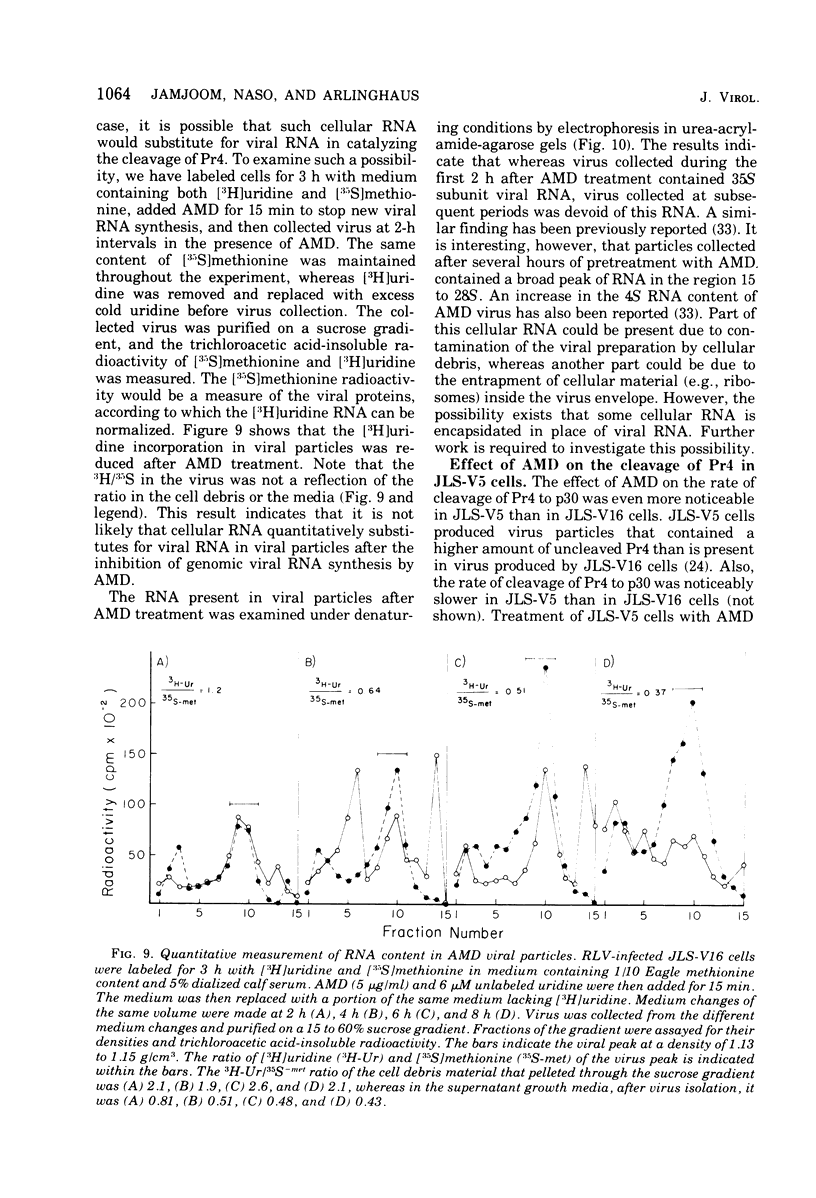
The cleavage of an intracellular 67,000- to 70,000-dalton precursor, termed Pr4 to Rauscher leukemia virus (RLV) p30 protein proceeded at a slower rate when virus-producing cells were treated with actinomycin D (AMD). Treatment with AMD also caused a slight accumulation of Pr4 in purified early virus particles produced by a cell line which usually produces virions that contain little Pr4. The cleavage of other intracellular viral precursor polypeptides was not affected by treatment with AMD. Treatment of infected cells with cycloheximide, on the other hand, allowed the cleavage of Pr4 to proceed at the usual rate for a short period of time before further cleavage was drastically slowed or prevented. The cleavage of several other viral precursor polypeptides was also inhibited by treatment with cycloheximide. Different lines of evidence suggest that the mechanism of action of AMD is not due to a possible indirect effect on protein synthesis. Thus, the rate of cleavage of Pr4 was not affected by the length of pretreatment with AMD between 1 to 8 h. In addition, the combined effect of AMD and cycloheximide, at their maximal inhibitory concentrations, was greater than the effect of either drug alone, indicating the involvement of two at least partially different mechanisms in the action of AMD and cycloheximide. Furthermore, AMD did not affect the pulse labeling of viral precursor polypeptides. These results suggest that the interaction with viral RNA, whose production is inhibited by AMD, accelerates the cleavage of Pr4 to p30 during virus assembly. A hypothetical model is presented to illustrate th possible advantages of having a step in virus assembly in which genomic RNA interacts with a precursor to capsid proteins before the cleavage of that precursor.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Arcement L. J., Karshin W. L., Naso R. B., Jamjoom G., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins: presence of p30 and envelope p15 sequences in precursor polypeptides. Virology. 1976 Feb;69(2):763–774. doi: 10.1016/0042-6822(76)90504-3. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Synthesis of the RNA of RNA-containing tumor viruses. I. The interval between synthesis and envelopment. Virology. 1970 Mar;40(3):494–504. doi: 10.1016/0042-6822(70)90192-3. [DOI] [PubMed] [Google Scholar]
- Bases R. E., King A. S. Inhibition of Rauscher murine leukemia virus growth in vitro by actinomycin D. Virology. 1967 Jun;32(2):175–183. doi: 10.1016/0042-6822(67)90268-1. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Phillips L. A., Kramer M. J., Haapala D. K., Peebles P. T., Nomura S., Fischinger P. J. Transformation of mouse 3T3 cells by murine sarcoma virus: release of virus-like particles in the absence of replicating murine leukemia helper virus. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1520–1524. doi: 10.1073/pnas.68.7.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Davis J., Scherer M., Tsai W. P., Long C. Low-molecular- weight Rauscher leukemia virus protein with preferential binding for single-stranded RNA and DNA. J Virol. 1976 May;18(2):709–718. doi: 10.1128/jvi.18.2.709-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Inhibition of mouse leukemia virus (MLV) replication by actinomycin D. Virology. 1967 Apr;31(4):742–746. doi: 10.1016/0042-6822(67)90211-5. [DOI] [PubMed] [Google Scholar]
- East J. L., Allen P. T., Knesek J. E., Chan J. C., Bowen J. M., Dmochowski L. Structural rearrangement and subunit composition of RNA from released Soehner-Dmochowski murine sarcoma virions. J Virol. 1973 May;11(5):709–720. doi: 10.1128/jvi.11.5.709-720.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert W. A., Franke W. W., Scheer U. Nucleocytoplasmic translocation of RNA in Tetrahymena pyriformis and its inhibition by actinomycin D and cycloheximide. Exp Cell Res. 1975 Aug;94(1):31–46. doi: 10.1016/0014-4827(75)90528-5. [DOI] [PubMed] [Google Scholar]
- Eisenman R., Vogt V. M., Diggelmann H. Synthesis of avian RNA tumor virus structural proteins. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1067–1075. doi: 10.1101/sqb.1974.039.01.122. [DOI] [PubMed] [Google Scholar]
- Fakan S. Inhibition of nucleolar RNP synthesis by cycloheximide as studied by high resolution radioautography. J Ultrastruct Res. 1971 Mar;34(5):586–596. doi: 10.1016/s0022-5320(71)80065-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Tomas C. B., Baltimore D. Morphogenesis of poliovirus. II. Demonstration of a new intermediate, the proviron. J Virol. 1973 Nov;12(5):1122–1130. doi: 10.1128/jvi.12.5.1122-1130.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner E., Tress E. Isolation of a ribonucleoprotein structure from oncornaviruses. J Virol. 1973 Dec;12(6):1612–1615. doi: 10.1128/jvi.12.6.1612-1615.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R. W., Stone M. P., Joklik W. K. Separation of single-stranded ribonucleic acids by acrylamide-agarose-urea gel electrophoresis. Anal Biochem. 1974 Jun;59(2):599–609. doi: 10.1016/0003-2697(74)90313-3. [DOI] [PubMed] [Google Scholar]
- Goldstein E. S., Penman S. Regulation of protein synthesis in mammalian cells. V. Further studies on the effect of actinomycin D on translation control in HeLa cells. J Mol Biol. 1973 Oct 25;80(2):243–254. doi: 10.1016/0022-2836(73)90170-8. [DOI] [PubMed] [Google Scholar]
- Green M., Cartas M. The genome of RNA tumor viruses contains polyadenylic acid sequences. Proc Natl Acad Sci U S A. 1972 Apr;69(4):791–794. doi: 10.1073/pnas.69.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Fry M. Post-translational cleavage of polypeptide chains: role in assembly. Annu Rev Biochem. 1975;44:775–797. doi: 10.1146/annurev.bi.44.070175.004015. [DOI] [PubMed] [Google Scholar]
- Hohn B., Wurtz M., Klein B., Lustig A., Hohn T. Phage lambda DNA packaging, in vitro. J Supramol Struct. 1974;2(2-4):302–317. doi: 10.1002/jss.400020220. [DOI] [PubMed] [Google Scholar]
- Hohn T., Hohn B. Structure and assembly of simple RNA bacteriophages. Adv Virus Res. 1970;16:43–98. doi: 10.1016/s0065-3527(08)60021-4. [DOI] [PubMed] [Google Scholar]
- Jamjoom G., Karshin W. L., Naso R. B., Arcement L. J., Arlinghaus R. B. Proteins of Rauscher murine leukemia virus: resolution of a 70,000-dalton, Nonglycosylated polypeptide containing p30 peptide sequences. Virology. 1975 Nov;68(1):135–145. doi: 10.1016/0042-6822(75)90155-5. [DOI] [PubMed] [Google Scholar]
- Klug A. The polymorphism of tobacco mosaic virus protein and its significance for the assembly of the virus. Ciba Found Symp. 1972;7:207–215. doi: 10.1002/9780470719909.ch12. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Amos L. A., Klug A. Correlation between structural transformation and cleavage of the major head protein of T4 bacteriophage. Cell. 1976 Feb;7(2):191–203. doi: 10.1016/0092-8674(76)90018-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levin J. G., Grimley P. M., Ramseur J. M., Berezesky I. K. Deficiency of 60 to 70S RNA in murine leukemia virus particles assembled in cells treated with actinomycin D. J Virol. 1974 Jul;14(1):152–161. doi: 10.1128/jvi.14.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Rosenak M. J. Synthesis of murine leukemia virus proteins associated with virions assembled in actinomycin D-treated cells: evidence for persistence of viral messenger RNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1154–1158. doi: 10.1073/pnas.73.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger G. G., Klein R. A., Gilden R. V., Hatanaka M. The effect of cordycepin on cell transformation by RNA tumor viruses. Virology. 1973 Oct;55(2):524–526. doi: 10.1016/0042-6822(73)90195-5. [DOI] [PubMed] [Google Scholar]
- Matthews K. S., Cole R. D. Shell formation by capsid protein of f2 bacteriophage. J Mol Biol. 1972 Mar 14;65(1):1–15. doi: 10.1016/0022-2836(72)90487-1. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins. Cell. 1975 Jan;4(1):31–36. doi: 10.1016/0092-8674(75)90130-0. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Karshin W. L., Jamjoom G. A., Arlinghaus R. B. A fucose-deficient glycoprotein precursor to Rauscher leukemia virus gp69/71. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2326–2330. doi: 10.1073/pnas.73.7.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Long C. W., Gilden R. V. Isolation of murine type-C virus p30 precursor protein by DNA-cellulose chromatography. Virology. 1976 Jul 15;72(2):523–526. doi: 10.1016/0042-6822(76)90182-3. [DOI] [PubMed] [Google Scholar]
- Paskind M. P., Weinberg R. A., Baltimore D. Dependence of Moloney murine leukemia virus production on cell growth. Virology. 1975 Sep;67(1):242–248. doi: 10.1016/0042-6822(75)90421-3. [DOI] [PubMed] [Google Scholar]
- Phillips L. A., Hollis V. W., Jr, Bassin R. H., Fischinger P. J. Characterization of RNA from noninfectious virions produced by sarcoma positive-leukemia negative transformed 3T3 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):3002–3006. doi: 10.1073/pnas.70.10.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M., Myerson D., Wallace J. Enucleation of mammalian cells with cytochalasin B. Exp Cell Res. 1972;71(2):480–485. doi: 10.1016/0014-4827(72)90322-9. [DOI] [PubMed] [Google Scholar]
- Ross J., Tronick S. R., Scolnick E. M. Polyadenylate rich RNA in the 70 S RNA of murine leukemia-sarcoma virus. Virology. 1972 Jul;49(1):230–235. doi: 10.1016/s0042-6822(72)80025-4. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., LATHAM H., DARNELL J. E. Demonstration of an unstable RNA and of a precursor to ribosomal RNA in HeLa cells. Proc Natl Acad Sci U S A. 1963 Feb 15;49:240–248. doi: 10.1073/pnas.49.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Specific binding of the type C viral core protein p12 with purified viral RNA. Cell. 1976 Jan;7(1):21–32. doi: 10.1016/0092-8674(76)90251-8. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Syrewicz J. J., Naso R. B., Wang C. S., Arlinghaus R. B. Purification of large amounts of murine ribonucleic acid tumor viruses produced in roller bottle cultures. Appl Microbiol. 1972 Sep;24(3):488–494. doi: 10.1128/am.24.3.488-494.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMIN H. M. THE EFFECTS OF ACTINOMYCIN D ON GROWTH OF ROUS SARCOMA VIRUS IN VITRO. Virology. 1963 Aug;20:577–582. doi: 10.1016/0042-6822(63)90282-4. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Bratt M. A. Effect of cordycepin (3'-deoxyadenosine) on virus-specific RNA species synthesized in Newcastle disease virus-infected cells. J Virol. 1975 Dec;16(6):1575–1583. doi: 10.1128/jvi.16.6.1575-1583.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems M., Penman M., Penman S. The regulation of RNA synthesis and processing in the nucleolus during inhibition of protein synthesis. J Cell Biol. 1969 Apr;41(1):177–187. doi: 10.1083/jcb.41.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Ting R. C., Paran M., Gallo R. C. Cordycepin inhibits induction of murine leukovirus production by 5-iodo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3820–3824. doi: 10.1073/pnas.69.12.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]