Abstract
Human induced pluripotent stem (iPS) cells may represent the ideal cell source for research and applications in regenerative medicine. However, standard culture conditions that depend on the use of undefined substrates and xenogeneic medium components represent a significant obstacle to clinical translation. Recently, we reported a defined culture system for human embryonic stem (ES) cells using a fully defined synthetic polymer coating, poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide] (PMEDSAH), in conjunction with xeno-free and defined culture medium. Here we tested the hypothesis that iPS cells grown in this defined culture system can be differentiated into mesenchymal stem cells (iPS-MSCs). Human iPS cells were cultured on PMEDSAH and differentiated into functional MSCs, as confirmed by expression of characteristic MSC markers (CD166+, CD105+, CD73+, CD44+, CD34− and CD45−) and their ability to differentiate in vitro into adipogenic, chondrogenic and osteoblastic lineages. To demonstrate the potential of iPS-MSCs to regenerate bone in vivo, the newly derived cells were induced to osteoblast differentiation for 4 days and transplanted into calvaria defects in immoncompromised mice for 8 weeks. MicroCT analysis and histology demonstrated de novo bone formation in the calvaria defects for animals treated with iPS-MSCs, but not for the control group. Moreover, positive staining for human nuclear antigen and human mitochondria monoclonal antibodies unambiguously confirmed the participation of the transplanted human iPS-MSCs in the regenerated bone. These results confirmed that human iPS cells grown in a defined and xeno-free system have the capability to differentiate into functional MSCs with the ability to form bone in vivo.
INTRODUCTION
Induced-pluripotent stem (iPS) cells and embryonic stem (ES) cells have the ability to undergo self-renewal and differentiate into every cell type in the body1–3, and therefore represent a potential renewable cell-source for cell therapies and regenerative medicine. Both pluripotent stem cell sources can further give rise to progenitor cells, such as mesenchymal stem cells (MSCs), that themselves have the capability to differentiate into mesoderm derivatives, such as bone, fat, cartilage, tendon and muscle4–6. In addition, MSCs have important immunomodulatory and engraftment-promoting properties7. While MSCs can be isolated from bone marrow, adipose tissue, umbilical cord blood, umbilical cord stroma (Wharton’s jelly), placenta and other tissues and organs, the harvesting procedures are invasive, expensive and laborious. Direct derivation of MSCs from pluripotent stem cells represents an effective alternative to obtain larger populations of progenitor cells that are needed for cell therapies and/or regenerative medicine.
Like human ES cells, human iPS cells will need to be cultured in clinically compliant conditions, if broader translation into clinical practice is intended. Although, co-culture with human feeder cells represent a xeno-free option for the in vitro expansion of pluripotent stem cells8, such human feeder cell environments are undefined, may contain pathogens and will require expensive and labor-consuming screening. Similarly, extracellular matrix coatings made of undefined animal derived proteins such as matrigel, vitronectin, fibronectin or laminin are also expensive, may be immunologically incompatible with humans, have batch to batch variation, and will require extensive pre-transplant screening.
To overcome some of limitations of human feeder cells or animal-derived extracellular matrices, synthetic cell culture substrates for pluripotent stem cells that are devoid of xenogeneic components have recently been developed9–14. Some of these substrates are based on recombinant proteins and/or peptides and thus are hampered by well-known problems of polypeptide matrices such as difficulties in sterilization, propensity to degrade and the high cost of production.
Alternatively, cell culture coatings based on synthetic polymers can be reproducibly fabricated, are inexpensive and highly manipulable, and thus represent a valuable option to expand pluripotent stem cells. Recently we reported the development of a fully defined synthetic polymer coating made of poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide] (PMEDSAH), that in combination with human-cell conditioned, or chemically defined medium supports the long-term culture and self-renewal of undifferentiated human ES cells14, 15. This pluripotent culture system makes use of a fully synthetic polymer as the structural motifs in cell-substrate interactions (i.e., no peptides, sugars, or proteins) and therefore provides a xenogeneic-free environment.
In this study, we tested the hypothesis that patient specific iPS cells can continuously proliferate (15 passages) on PMEDSAH in an undifferentiated state and yet will be capable of subsequent lineage-specific differentiation as well as regeneration of clinically relevant craniofacial skeletal defects. Importantly, we also demonstrate that human iPS cells cultured in this clinically compliant culture system can be directed toward differentiation into functional MSCs in vitro and bone formation in vivo.
MATERIALS AND METHODS
Generation of induced-pluripotent stem (iPS) cells
Retroviral vectors carrying Klf4, Sox2, Oct3/4 and c-Myc were generated by transient co-transfection (using Addgene plasmids 17217, 17219, 17220, and 17226, and VSV-g envelope plasmid 8454) into Clontech GP2-293 packaging cells. Viral supernatant was harvested after 60 h, filtered and concentrated. Human fibroblasts were cultured in DMEM + 10% FCS with 1× non-essential amino acid supplement (Invitrogen, Carlsbad, CA). To generate iPS cells, two rounds of viral transduction of 30,000 fibroblasts were performed and cells were incubated with virus for another 48 h. After 4 d, cells were passaged on irradiated MEFs in fibroblast medium, and the following day switched to hES cell-medium, which consists of Dulbecco’s modified Eagle Medium (DMEM)/F12 (Invitrogen), 20% knockout serum replacer (Invitrogen), 1 mM L-glutamine (Invitrogen), 1× non-essential amino acid supplement (Invitrogen), 0.1 mM β-mercaptoethanol (Sigma), and 4 ng/ml human recombinant FGF2 (Invitrogen). Cell were cultured in dedicated incubators set at 37°C/5% CO2. The iPS colonies were manually picked and passaged. Immunohistochemistry was used to confirm expression of Nanog, stage-specific embryonic antigen (SSEA)-3/4, Oct3/4, and alkaline phosphatase. Culture of H7-hES cells (WA07, WiCell Research Institute; NIH Registration Number 0061) was performed as described above for human iPS cells.
Illumina Microarray
Total RNA was purified from iPS cells, parental fibroblasts and the H7-hES cells with the RNeasy Mini kit (Qiagen; Valencia, CA) and DNAse-I treatment. A total of 400 ng of RNA was amplified and labeled with the Total Prep RNA amplification kit (Ambion; Austin, TX) and 750 ng of biotin-labeled cRNA was used to hybridize to Illumina HumanHT-12 v4 Expression BeadChip. After washing, chips were coupled with Cy3 and scanned in an Illumina BeadArray Reader (Illumina, Inc., San Diego, CA). Un-normalized summary probe profiles, with associated probe annotation, were output from BeadStudio.
Culture of iPS cells in defined culture conditions
Human iPS cells were cultured on PMEDSAH coated plates with human-cell-conditioned-medium (hCCM, GlobalStem, Inc., Rockville, MD) supplemented with 4 ng/ml of FGF2, as described previously (nat biotech and nat prot). Briefly, PMEDSAH coated plates were pre-incubated with hCCM for at least 48 h at 37°C in 5% CO2 atmosphere before use. Twenty-four h before passaging onto PMEDSAH coated plates hES cell-medium was replaced with hCCM, and passaging was mechanically performed using a sterile pulled-glass pipette or the StemPro EZPassage Disposable Stem Cell Passaging Tool (Invitrogen). Cells were observed every 48 h using a Leica stereomicroscope and differentiated cells were removed mechanically using a sterile pulled-glass pipette, followed by replacement of cell culture medium.
Derivation, culture and characterization of MSCs
To induce differentiation of iPS cells into mesenchymal stem cells (MSCs), embryoid bodies were formed and cultured in suspension for 7 days with hCCM in low-attachment culture dishes. Subsequently, approximately 70 embryoid bodies/well were plated onto 0.1% gelatin-coated 6-well plates in the presence of MSC growth medium (α-MEM, 10% FBS, 200 mM L-glutamine and 10 mM nonessential amino acid supplement and 8 ng/ml FGF2. The outgrowth cells were cultured for up to 2 weeks to reach confluence, then trypsinized and passaged at a ratio of 1: 3. Cells were continually passaged in T-75 flasks until a homogeneous fibroblastic morphology appeared.
Characterization of MSCs involved analysis of cell surface antigens and functional differentiation assays in vitro. Cell surface antigen profiling was performed using fluorescent-activated cell sorting (FACS). MSCs were harvested using trypsin 0.25% EDTA, and after neutralization, single cell suspensions were washed in cold BSA, 0.5% (w/v) (Sigma) in DPBS and incubated at a concentration of 1×106 cells/mL in 1 µg/mL unconjugated goat anti-human IgG (Invitrogen) on ice for 15 min to block nonspecific binding. Samples (2.5×105 cells) were then incubated on ice with the optimal dilution of fluorochrome-conjugated monoclonal antibody (mAb) in 1 µg/mL unconjugated goat anti-human IgG in the dark. All mAbs were of the immunoglobulin G1 (IgG1) isotype. The following conjugated antibodies were used in the analyses: allophycocyanin (APC)-conjugated antibodies against CD44 (fluorescein isothiocyanate (FITC)-conjugated mAbs against CD29, CD90, and CD45, phycoerythrin (PE)-conjugated mAbs against CD49a, CD49e, CD73, CD166 and CD105. All antibodies were from BD Pharmingen (TM, San Jose, CA) except for CD105 which was supplied by eBioscience (San Diego, CA). After 30 min incubation, cells were washed twice with ice cold 0.5% BSA in DPBS. Nonspecific fluorescence was determined by incubating cells with respective fluorochrome conjutages raised against antihuman IgG1. At least 10,000 events were acquired for each sample using a FACSCalibur instrument (Becton Dickinson, San Jose, CA) and cell flow cytometry data were analyzed using CELLQUEST software (Becton Dickinson). The fluorescence histogram for each mAb was displayed alongside the control antibody.
For functional differentiation, human iPS-MSCs at passages 6–7 were induced to differentiate into adipogenic, chondrogenic, and osteogenic lineages in cell-specific culture medium. For osteogenesis, the cultures were incubated in DMEM that was supplemented with 15% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, 12 mM L-glutamine, 10 mM β-glycerophosphate (Sigma, St Louis, MO), 1 nM dexamethasone (Sigma), and 0.5 µM ascorbate-2-phosphate (Sigma). The media was changed 2 times per week for 3 weeks. The cells were fixed with 10% formalin for 20 minutes at RT and stained with Alizarin Red, pH 4.1 (Sigma) for 20 minutes at RT.
For adipogenesis, the cultures were incubated in DMEM that was supplemented with 15% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, 12 mM L-glutamine, 5 µg/ml insulin(Sigma), 50 µM indomethacin (Sigma), 1 ×10−6 M dexamethasone (Sigma), and 0.5 µM 3-isobutyl-1-methylxanthine (IBMX; Sigma). The media was changed 2 times per week for 3 weeks. The cells were fixed with 10% formalin for 20 minutes at RT and stained with Oil Red (Sigma) in ethanol (Sigma) for 20 minutes at RT.
For chondrocyte differentiation, the cultures were incubated in DMEM that was supplemented with 15% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, 12 mM L-glutamine, 5 µg/ml insulin(Sigma), 50 µM indomethacin (Sigma), 1 ×10−6 M dexamethasone (Sigma), and 0.5 µM 3-isobutyl-1-methylxanthine (IBMX; Sigma). The media was changed 2 times per week for 3 times. The cells were fixed with 10% formalin for 20 minutes at RT and stained Safranin O (Sigma) in ethanol (Sigma) for 20 minutes at RT.
Preparation of iPS-MSCs for implantation
The iPS-MSCs were harvested using 0.25% trypsin-0.53 mM EDTA (Gibco), and cell pellets were re-suspended in 5 mg/ml human plasma fibrinogen (Sigma) at a concentration of 2×106 cells per 40 µl. The fibrinogen cell suspension were then pipetted directly into 6 × 3 mm cubic pieces of Gelfoam (Pharmacia & Upjohn Co, NY, NY). Following absorption by the Gelfoam sponges, 5 µl of human thrombin (200 unit/ml; Sigma) was added. Gelation of the fibrin was observed and placed immediately on ice until implantation..
Surgical procedure and cell transplantation in craniofacial defect model
All procedures were approved by the University of Michigan Committee on the Use and Care of Animals. Four 5 week-old female immunocompromised mice (N:NIH-bg-nu-xid; Harlan Sprague Dawley, Inc., NC) were anesthetized with intraperitoneal injections of ketamine, (Ketaset, 75 mg/kg, Fort Dodge Animal Health, IA) and xylazine, (Ansed, 10 mg/kg, Lloyd laboratories, IA). A semilunar scalp incision was made from right to left in the post-auricular area, and a full-thickness flap was elevated. The periosteum overlying the calvarial bone was completely resected. A trephine was used to create a 5-mm craniotomy defect centered on the sagittal sinus and the calvarial disk was removed. One gelfoam sponge with or without human iPS cells was inserted in the calvaria defect per animal. The incisions were closed with 4-0 Chromic Gut suture (Ethicon/Johonson&Jhonson, NJ). All mice were sacrificed 8 weeks after the implantation.
Radiology, histology and micro-CT analyses
Radiographic analysis was performed immediately after the calvaria were harvested with the use of a microradiographic apparatus (Faxitron X-ray Corporation, IL). Then the calvaria were immediately fixed in aqueous buffered zinc formalin, Z fix (Anatech, MI) and then scanned for the micro-CT (µCT) analysis. Calvaria were subsequently decalcified with a 10% EDTA solution for 3 days, dehydrated with gradient alcohols and embedded in paraffin. Coronal sections 5 µm in thickness were cut and stained with hematoxylin and eosin. Micro-CT scanning was performed (will need to get the Scanco scanning energy parameters from the dental school microCT core).
Statistical analyses
All experiments were performed in triplicate and data were expressed as the mean value ± the standard error of the mean (mean ± SEM) and analyzed by one-way analysis of variance. The levels of statistical significance were set at p<0.05.
RESULTS
Derivation of iPS cells and culture in xeno-free conditions
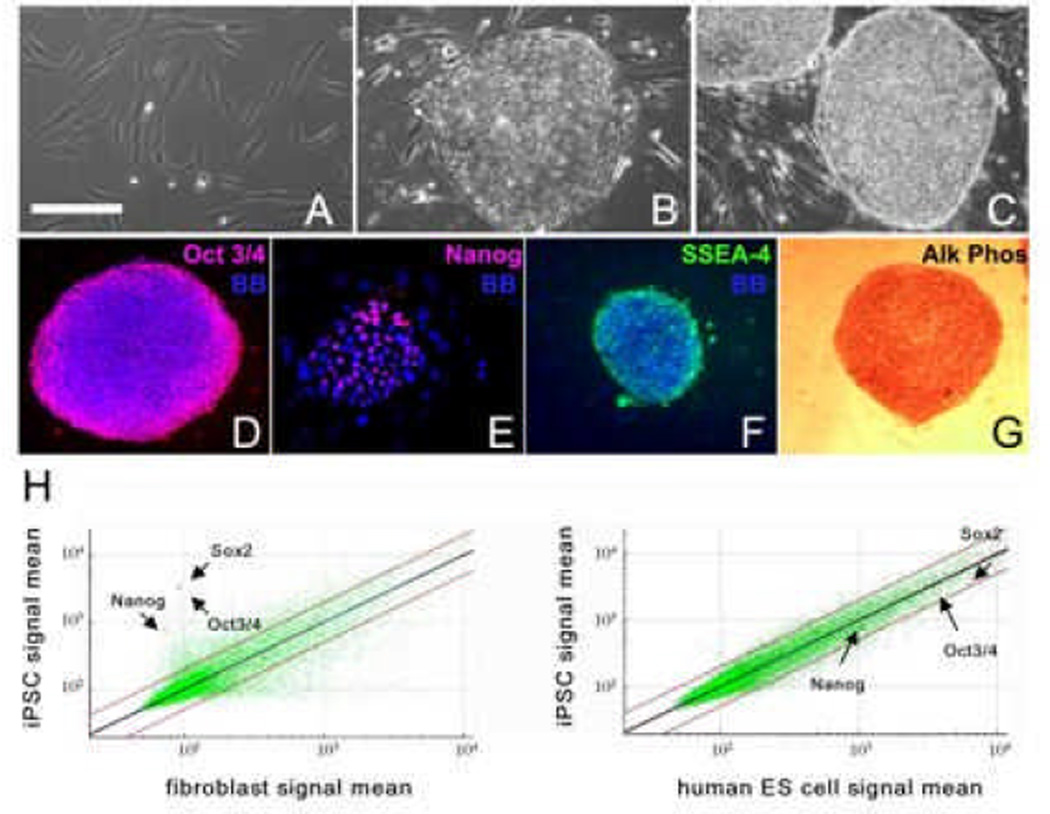
Human fibroblasts were reprogrammed into iPS cells by overexpression of Oct3/4 (also known as PUO5F1), Sox2, Klf4 and c-Myc genes individually packed in retrovirus. After × days in culture on irradiated MEFs, hES cell-like colonies formed and proliferated (Fig 1A–C). The morphology of emerging iPS cell colonies resembled the distinctive characteristic morphology of undifferentiated hES cell colonies with well-defined borders and a high nucleus:cytoplasm ratio3 (Fig. 1B–C). Histochemical analysis revealed continuous expression of Oct3/4, Nanog, SSEA-4 and alkaline phosphatase after 25 passages, showing the proper maintenance of pluripotency of the iPS cell lines on MEFs (Fig. 1D–G). Global gene analysis showed that Nanog, Oct3/4 and Sox2 from iPS cells co-localized in scatter plots with the gene expression patterns of H7-hES cells, while differing from the parental skin fibroblast cell line (Fig. 1H), further confirming successful cellular reprogramming.
Fig 1. Characterization of human IPSC lines.
Phase contrast microscopic images show human fibroblasts (A), an undifferentiated IPS cell colony at passage 30 (B) derived from reprogramming of the fibroblasts in panel A, and an H7 human ES cell colony at passage 45 (C). Immunocytochemistry of IPS cells cultures showing expression of the pluripotency markers Oct4 (D), Nanog (E), and SSEA-4 (F), and histochemical staining for alkaline phosphatase (Alk Phos; G). Bisbenzimide (BB, blue) was used to visualize cell nuclei in D–F. Scale bar: 100 µm in A and E, and 200 µm in B–D, F and G. (H) Scatter plots of microarray data showing global gene expression patterns of human fibroblasts, human IPS cells of the same genetic background, and human ES cells (H7). The position of individual pluripotency genes labeled in the panels is indicated with red dots.
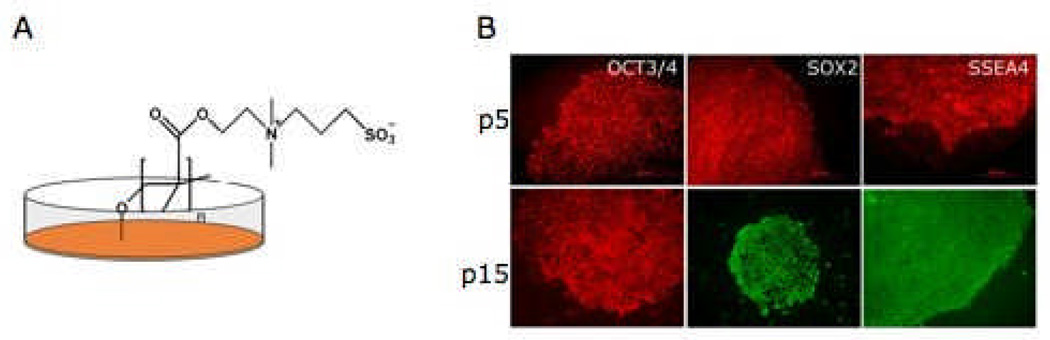
To establish cultures in fully defined and xeno-free conditions, human iPS cells were transitioned to PMEDSAH-coated plates with human cell-conditioned medium (hCCM), as previously described for human ES cells14, 15. The PMEDSAH-coated plates continuously supported attachment, proliferation, self-renewal and maintainance of pluripotency of undifferentiated human iPS cells. These findings were confirmed by rigorous characterization performed every 5 passages, including karyotype analysis (S-Fig 1A), expression of Oct3/4, Sox2 and SSEA-4 (Fig. 2), and formation of embryoid bodies (EB) expressing markers of all three germ layers (data not shown).
Fig 2. Culture of human IPS cells in a clinical compliant culture system.
(A) An Schematic representation of PMEDSAH-coated plates that serve as feeder-free chemically-defined substrate. The chemical structure of PMEDSAH is depicted, showing its carbonyl and sulfonate groups that contribute to the zwitterionic nature of the polymer. Serum-free human cell conditioned medium supplemented with FGF2 provide the soluble components. (B) This clinical compliant culture system supports the long-term growth of human IPS cells, as shown here at passage (p) 5 and 10 colonies expressing undifferentiated markers (OCT3/4, SOX2 and SSEA4) of pluripotent stem cells.
Derivation of mesenchymal stem cells (MSCs) from human iPS cells
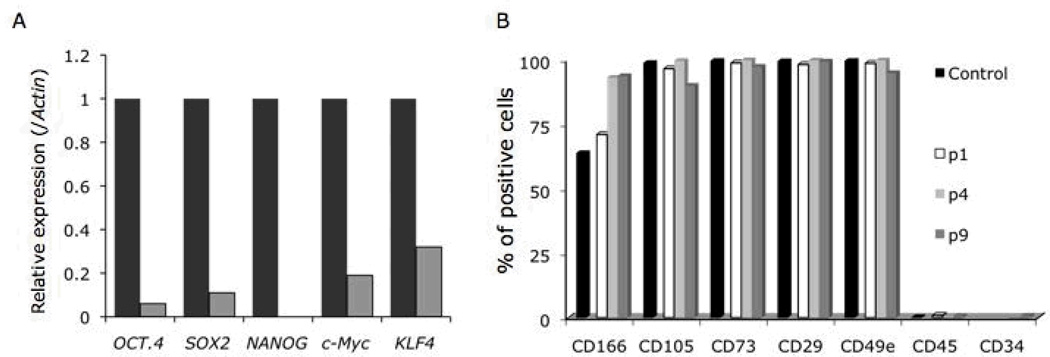
The derivation of human iPS-MSCs was performed following protocols used for the differentiation of human ES cells into MSCs5. Selected colonies were collected to form EBs, while other colonies were maintained on PMEDSAH-coated plates for further propagation as undifferentiated iPS cells. Initially, a heterogeneous cell population developed from EBs and by passage 2, greater than 90% of the total cell population acquired a fibroblast-like morphology that matched the described morphology of derived human ES-MSCs4, 5. Gene analysis by qRT-PCR demonstrated that Oct3/4, Sox2, and Nanog expression was downregulated in the derived iPS-MSCs relative to parental human iPS cells (Fig 3A). Similarly, the expression of c-Myc and Klf4, used in the reprogramming of human fibroblasts into iPS cells was downregulated in the derived iPS-MSCs. As an initial characterization to confirm the derivation MSCs from the iPS cells, fluorescence activated cell sorting (FACS) analysis of cell surface markers present in hMSCs was performed at passages 1, 5, and 9. The immunophenotype of the iPS-MSCs was consistent over all time points studied (CD166+, CD105+, CD73+, CD44+, CD34− and CD45−) (Fig 3B). The derived iPS-MSCs also maintained a normal karyotype over 12 passages (S-Fig 1B).
Fig 3. Characterization of mesenchymal stem cells (MSC) derived from human IPS cells cultured in a clinical compliant system.
After 5 passages of culture in a clinical complain culture system, human IPS cells were differentiated into MSCs. (A) The expression of reprogramming factors (OCT4, SOX2, c-Myc and KLF4) used to generate the lenti-IPS cells was analyzed in the differentiated MSCs (p1; light-grey columns) by qRT-PCR, showing low levels of mRNA compared to human IPS cells (dark-grey columns). Data was normalized to human β-Actin expression. NANOG expression was evaluated as well. (B) The expression of characteristic MSC cell-surface markers was analyzed by flow cytometry at different passages (1, 4 and 9). As control, human bone marrow cells (p1) were used.
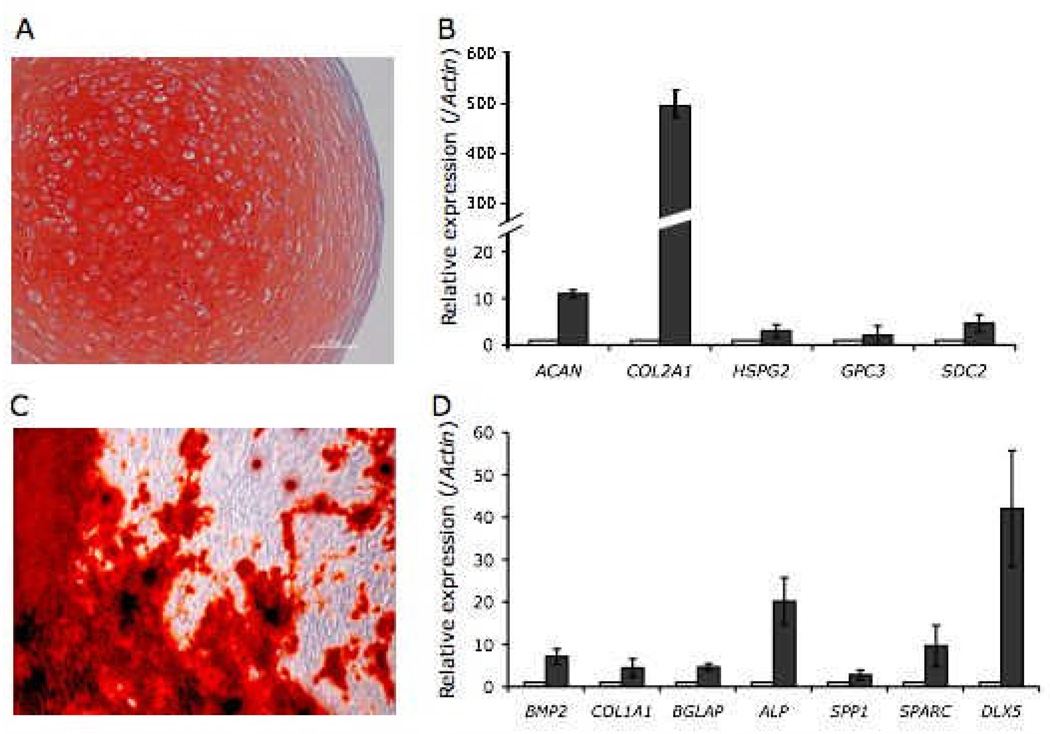
To verify the multilineage differentiation capacity of the iPS-MSCs derived on PMEDSAH, in vitro differentiation directed toward adipogenic, chondrogenic, and osteogenic lineages was performed. Adipogenic differentiation of iPS-MSCs was demonstrated by a 5-fold increase in peroxisome proliferator activated receptor gamma (PPAR-γ) mRNA in iPS-MSCs treated with adipogenic factors as compared controls (S-Fig 2). Chondrogenic differentiation was observed after iPS-MSCs were cultured as micromass pellets in medium containing chondrogenic supplements for four weeks. Safranin O staining of chondrogenic pellets revealed chondrocyte-like cells residing in lacunae surrounded by a well-defined glycosamnioglycan (sGAG) and proteoglycan-rich extracellular matrix (Fig 4A). Gene analysis by qRT-PCR demonstrated increased mRNA levels of genes related to chondrogenesis, such as aggrecan (ACAN), collagen type II alpha 1 (COL2A1), perlecan (HSPG2), glypican 3 (GPC3) and syndecan 2 (SDC2) in chondrogenic pellets compared to iPS-MSCs cultured in 2D and with growth medium (Fig 4B).
Fig 4. In vitro differentiation of human IPS cell–derived mesenchymal stem cells (MSC).
At passage 5, human IPS cell-derived MSCs were induced to differentiated into chondrogenic, osteogenic and adipogenic lineages. (A) Induction into chondrogenic lineage was confirmed by histology and staining of proteoglycans by Safranin ‘O’ staining in pellets, and (B) up-regulation of chondrogenic-related genes: aggrecan (ACAN), collagen type II alpha-1 (COL2A1), periecan (HSPG2), glypican 3 (GPC3) and syndecan 2 (SCD2). (C) Osteogenic differentiation was confirmed by Alizarin Red Staining indicating calcium deposition in mineralized cultures and (D) up-regulation of osteogenic-related genes: bone morphogenetic protein 2 (BMP2), collagen type 1 alpha-1 (COL1A1), osteocalcin (BGLAAP), alkaline phosphatase (ALP), bone sialoprotein 1 (SPP1), osteonectin (SPARC) and distal-less homeobox 5 (DLX5). Adipogenic induction was detected by up-regulation (5.48 ± 0.7 fold-increase; S-Fig 2) of peroxisome proliferator activated receptor gamma (PPAR-γ). Cells were cultured in differentiation medium (dark columns) and compare to control cells cultured in MSC-grow medium (white columns). Samples were analyzed by qRT-PCR, data normalized to human β-Actin expression and results shown are the mean and SEM of three experiments.
Osteogenic differentiation of iPS-MSCs was induced after cells were cultured in medium containing osteogenic supplements for four weeks. By the end of the differentiation assay, calcium deposition was observed in iPS-MSCs cultured with osteogenic medium, but not in cells in growth medium (Fig 4C). In addition, increases in mRNA levels of genes related to osteogenesis such as bone morphogenic protein 2 (BMP2), collagen type I alpha 1 (COL1A1), osteocalcin (BGLAP), alkaline phosphatase (ALP), bone sialoprotein 1 (SPP1), osteonectin (SPARC) and distal-less homeobox 5 (DLX5), was observed in mineralized iPS-MSCs compared to proliferating iPS-MSCs (control group) (Fig 4D). To demonstrate the specificity of iPS-MSC commitment under defined culture conditions, the expression of genes related to chondrogenesis was evaluated in iPS-MSCs directed to osteogenesis, and vice-versa (S-Fig 3). As expected, the expression of chondrogenic-related genes was not changed in osteogenic samples in relation to iPS-MSCs in growth medium. In contrast, a significant increase in expression of DLX5 and SSP1 was observed in chondrogenic samples, while other bone-related genes did not change compared to control iPS-MSCs.
In vivo osteogenic potential of human iPS-MSCs derived on PMEDSAH
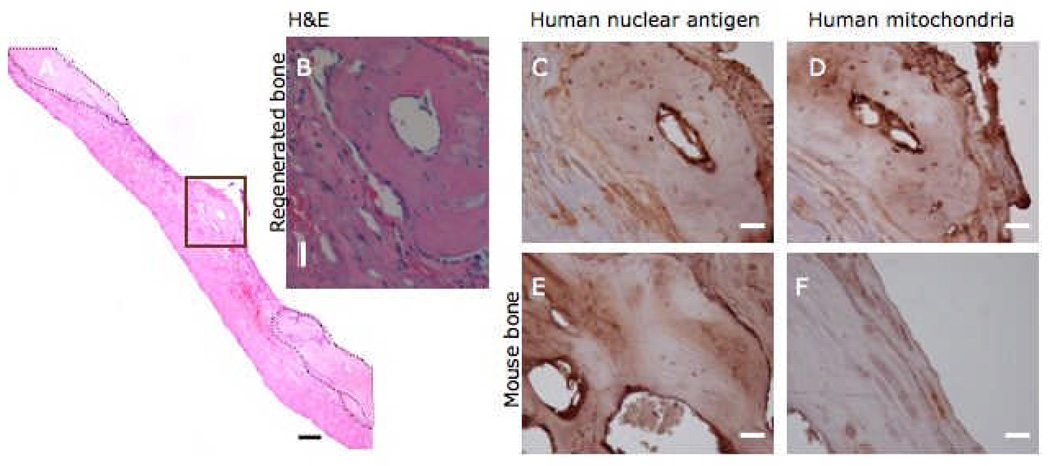
To verify the capability of human iPS-MSCs derived on PMEDSAH to regenerate bone in vivo, cells treated with osteogenic factors for 4 days were transplanted into calvaria defects of immuncompromised mice. After 8 weeks, animals were euthanized and specimens were analyzed by microCT and histology. MicroCT analysis demonstrated a 4.2 fold increase in volume of new bone formed in calvariae transplanted with iPS-MSCs compared to controls. New bone formation was observed within the central region and on the margin of the defect adjacent to the mouse calvaria. Histological evaluation identified osteocytes surrounded by woven bone, proliferating osteoblasts lining the exterior of the newly formed bone, as well as small bone marrow cavities within the newly formed bone (Figure 5A). Fibrous connective tissue filled out the rest of the defect.
Fig 5. In vivo bone formation by human IPS cell–derived mesenchymal stem cells (MSC).
After 4 days of culture in osteogenic medium human IPS cell-MSCs were transplanted into a calvaria defect in SCID mice. Eight weeks later animals were euthanized and skulls processed for microCT analysis, followed by decaicification and preparation for histology. A) Reconstructed image stained with H&E showing an isolated ossicle (highlighted area in brown box and in expanded images B–D) apart from the limits of the calvaria defect (delimited by dashed lines and in expanded images E and F). Positive immunostaining in osteocytes for human nuclear antigen (C) and human mitochondria (D) antibodies confirmed that the origin of the regenerated bone was from transplanted human IPS-MSCs. Notice negative immunostaining for both antibodies in mouse osteocytes (E and F). Scale bar in A = 100 µm, while in B–F = 20 µm.
To determine the contribution of the transplanted human iPS-MSCs in new bone formation, specific anti-human monoclonal antibodies that do not cross-react with murine cells were used. Positive staining for human nuclear antigen and human mitochondria were observed in osteocytes embedded in the newly-formed bone, but not in the surgical margins of the native mouse bone or in the fibrous tissue that filled the defect (Fig 5B–D).
DISCUSSION
The use of MSCs in regenerative medicine has advanced significantly, as demonstrated by numerous stem cell-based clinical trials (www.clinicaltrials.gov) in phase I and phase II currently under way to treat human conditions, such as bone defects, wound repair, myocardial infarction, stroke, diabetes and graft-versus-host-disease for review16. While MSCs can be isolated from several patient tissues, the cell harvesting procedures are invasive, expensive and laborious. Furthermore, the in vitro expansion capacity of isolated MSCs is limited and extensively cultured primary cells may take on phenotypic characteristics that are not consistent with the behavior of natural MSCs in vivo. Thus, iPS-MSCs represent an important alternative source of primary cells. Induced-PS cells have gained attention because they possess pluripotency, self-renewal and differentiation properties, which are similar to ES cells without sharing the ethical concerns associated with hES cells. However, current practices to maintain human iPS cells and human ES cells in an undifferentiated state typically rely on undefined and xenogeneic components that ultimately impede our ability to use these stem cells to treat debilitating human diseases.
Here, we demonstrated that human iPS cells can proliferate in an undifferentiated state on PMEDSAH-coated plates, a synthetic polymer coating devoid of xenogeneic contamination. The cells can be subsequently differentiated into functional MSCs with in vivo bone regeneration capabilities. PMEDSAH-coated plates supported the expansion of undifferentiated human iPS cells. After 15 passages on PMEDSAH-coated plates, iPS cells maintained the expression of transcription factors and cell surface markers associated with pluripotent stem cells, as well as a cell/colony morphology and normal karyotype3. Most importantly, iPS cells cultured on PMEDSAH maintained their pluripotent character. Thus, PMEDSAH-coated culture substrates in combination with human-cell conditioned medium represents a clinical-grade culture system free of xenogeneic contamination for the expansion of human iPS cells.
Going beyond the current state-of-art6, human iPScells cultured on the fully synthetic PMEDSAH substrate under clinically compliant conditions were used to derived MSCs and osteoprogenitors, and transplanted in vivo where they not only survived, but contributed to de novo bone formation. The derived iPS-MSCs expressed similar levels of markers present in hMSCs17, while qRT-PCR revealed that genes associated with pluripotency and reprogramming markers were no longer expressed once the cells were directed to differentiate. This fact, coupled with the critical observation that no teratomas formed in mice treated with transplanted human iPS-MSCs, indicated a reduced tumorigenic risk of this progenitor population compared to non-differentiated iPS cells. The derived human iPS-MSCs were able to differentiate in vitro into adipogenic, chondrogenic and osteogenic lineages. Interestingly, the chondrogenic and osteogenic differentiation of the derived human iPS-MSCs was more pronounced than adipogenic differentiation. Similar observations have been made for different populations of in vivo derived MSCs indicating a variability that depends on the origin of the cell population18, 19. It remains to be determined whether the differences in differentiation levels are inherent to the nature of the derived iPS-MSCs, or influenced by the origin of the parental iPS cells as dermal fibroblasts. In fact, epigenetic memory has been suggested for iPS cells depending on their origin20–22. However, a significant up regulation of PPAR-γ in cultures treated with adipogenic medium does suggest the potential for iPS-MSC differentiation towards adipogenesis. In addition, effective chondrogenic differentiation of human iPS-MSCs was achieved, as confirmed by histological and gene analysis of chondrogenic pellets. Robust deposition of proteoglycans was observed in pellets of iPS-MSCs treated with chondrogenic factors, as indicated by the intensity of the safranin ‘O’ staining. Significant expression of extracellular matrix-related genes present in cartilage suggests the maturity of the chondrogenic pellets obtained from human iPS-MSCs. The in vitro osteogenic differentiation of human iPS-MSCs was also robust, as indicated by calcium deposition and upregulation of genes related to osteogenesis in cultures treated with osteogenic medium. Taken together, the derived human iPS-MSCs described here meet the specifications of a defined MSC population as proposed by the International Society for Cellular Therapy23: (1) adherence to tissue culture plastic under standard culture conditions; (2) an immunophenotype similar to that of human bone marrow stromal cells with low expression of HSC markers; and (3) the ability to undergo in vitro differentiation along the osteogenic, chondrogenic and adipogenic lineages.
To examine the possible use of derived human iPS-MSCs in cell therapies and regenerative medicine, cell-transplantation assays were performed in immunocompromised mice with the goal of developing bone in vivo. Histological and microCT image reconstruction confirmed the formation of new bone within the calvaria defects of mice treated with transplanted human iPS-MSCs after 8 weeks. Randomly distributed and unorganized bone fragments were found within the calvarial defects. Furthermore, positive immunostaining of osteocytes for monoclonal antibodies to human nuclei and human mitochondria confirmed the participation of transplanted human iPS-MSCs in the regenerated bone. Although, the clinical critical size defect was not completely healed, the fact that human iPS-MSCs participated in bone regeneration in vivo in a calvarial defect mouse model suggests these cells became functional MSCs and osteoprogenitors that can be used as a tool for future cell transplantation studies that investigate craniofacial bone development in response to disease and trauma.
In summary, human iPS cells can be cultured on a synthetic polymer coating, PMEDSAH, in a fully defined and clinically-compliant system under xeno-free conditions, and have the capacity to differentiate into functional MSCs both in vitro and in vivo. Future work investigating factors such as the number of transplanted cells, survival and distribution, in vitro osteogenic induction prior to transplantation, and even more importantly, the optimal cell carrier with osteoconductive properties, will need reveal the role that transplanted cells can play in regenerating bone. Additionally, although not a particular goal of this work, it is important to determine whether virus-free and transgene-free iPS cells can be derived on PMEDSAH-coated plates. Taken together, the PMEDSAH culture system and efficient iPS-MSC derivation on this synthetic substrate provides a unique platform upon which one can design future studies on cell-based strategies for bone regeneration.
Supplementary Material
References
- 1.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Lian Q, et al. Derivation of clinically compliant MSCs from CD105+, CD24− differentiated human ESCs. Stem Cells. 2007;25:425–436. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- 5.Brown SE, Tong W, Krebsbach PH. The derivation of mesenchymal stem cells from human embryonic stem cells. Cells Tissues Organs. 2009;189:256–260. doi: 10.1159/000151746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lian Q, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 7.Ozawa K, et al. Cell and gene therapy using mesenchymal stem cells (MSCs) J Autoimmun. 2008;30:121–127. doi: 10.1016/j.jaut.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 9.Nagaoka M, Si-Tayeb K, Akaike T, Duncan SA. Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum. BMC Dev Biol. 2010;10:60. doi: 10.1186/1471-213X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodin S, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28:611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 11.Brafman DA, et al. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials. 2010;31:9135–9144. doi: 10.1016/j.biomaterials.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klim JR, Li L, Wrighton PJ, Piekarczyk MS, Kiessling LL. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Methods. 2010;7:989–994. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melkoumian Z, et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 2010;28:606–610. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 14.Villa-Diaz LG, et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat Biotechnol. 2010;28:581–583. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nandivada H, et al. Fabrication of synthetic polymer coatings and their use in feeder-free culture of human embryonic stem cells. Nat Protoc. 2011;6:1037–1043. doi: 10.1038/nprot.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernardo ME, Pagliara D, Locatelli F. Mesenchymal stromal cell therapy: a revolution in Regenerative Medicine? Bone Marrow Transplant. 2011:1–8. doi: 10.1038/bmt.2011.81. [DOI] [PubMed] [Google Scholar]
- 17.Majumdar MK, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 18.Kaltz N, et al. In vivo osteoprogenitor potency of human stromal cells from different tissues does not correlate with expression of POU5F1 or its pseudogenes. Stem Cells. 2008;26:2419–2424. doi: 10.1634/stemcells.2008-0304. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Li G, Chan KM, Wang Y, Tang PF. Comparison of multipotent differentiation potentials of murine primary bone marrow stromal cells and mesenchymal stem cell line C3H10T1/2. Calcif Tissue Int. 2009;84:56–64. doi: 10.1007/s00223-008-9189-3. [DOI] [PubMed] [Google Scholar]
- 20.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 21.Kim K, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polo JM, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.